Abstract
Autophagy is a highly conserved process in eukaryotes that degrades and recycles damaged cells in plants and is involved in plant growth, development, senescence, and resistance to external stress. Top-rot disease (TRD) in Rosa roxburghii fruits caused by Colletotrichum fructicola often leads to huge yield losses. However, little information is available about the autophagy underlying the defense response to TRD. Here, we identified a total of 40 R. roxburghii autophagy-related genes (RrATGs), which were highly homologous to Arabidopsis thaliana ATGs. Transcriptomic data show that RrATGs were involved in the development and ripening processes of R. roxburghii fruits. Gene expression patterns in fruits with different degrees of TRD occurrence suggest that several members of the RrATGs family responded to TRD, of which RrATG18e was significantly up-regulated at the initial infection stage of C. fructicola. Furthermore, exogenous calcium (Ca2+) significantly promoted the mRNA accumulation of RrATG18e and fruit resistance to TRD, suggesting that this gene might be involved in the calcium-mediated TRD defense response. This study provided a better understanding of R. roxburghii autophagy-related genes and their potential roles in disease resistance.
1. Introduction
R. roxburghii is a medicine and food homologous crop, whose fruits are rich in vitro antioxidant substances beneficial to human health, such as total phenols, flavonoids, triterpenes, and L-ascorbic acid [1]. Accordingly, R. roxburghii has been widely cultivated as an economic crop in Southwest China, especially in Guizhou Province, where the cultivated areas have so far exceeded 140,000 hm2 [1]. In recent years, there has been a new fungal disease named TRD in R. roxburghii fruits caused by C. fructicola [2]. At the occurrence beginning of this disease, there were obviously small, dark red diseased spots at the junction of the top fruit pulp and sepals, whereas in the later developmental stage, the pulp became dark brown and rotten, and the fruit was highly prone to drop at pre-harvest. TRD has caused serious yield losses and quality declines in R. roxburghii production in China every year [2].
Plants growing in nature are exposed to many adverse biotic and abiotic stresses such as drought, cold, salt, and pathogens. Unfortunately, they cannot choose their desired survival environments by moving, hence they have evolved a sophisticated immune system to fight against various stresses [3,4]. Based on how the immune response is triggered, the innate immune system of plants is divided into two categories: pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) [5,6]. ETI is a plant-specific defense response that can accelerate and amplify the PTI response and trigger the hypersensitive response (HR) to cell death of the host cell to stop the pathogen from multiplying [3,7]. This cell death linked to genetics might be essential for resistance to plant diseases [8]. ‘Autophagic cell death’ is often defined as a type of cell death by morphological criteria, which would contribute to inhibiting mycelia elongation, especially when the invader is a biotrophic pathogen [7,8].
The main structure of autophagy is the autophagosome, which can form a double-membrane structure to engulf damaged or unwanted macromolecules/organelles to deliver into the vacuole or lysosome for degradation or recycling [9,10]. From yeast to animals and plants, the autophagic process is highly conserved in eukaryotes and controlled by autophagy-related genes (ATGs) [11,12,13]. Based on the roles that core ATG proteins play in the autophagic pathways, ATG proteins are approximately divided into the following functional groups: (i) the ATG1/13 kinase complex; (ii) the PI3K kinase complex; (iii) the ATG9 kinase complex; and (iv) ATG8-PE and ATG12-5 ubiquitination-like conjugation systems [10,14,15]. Up to now, there have been more than 35 ATGs identified in yeast and Arabidopsis, and many homologous ATGs have been identified in other species based on the model plants [10,16,17,18,19,20,21,22,23,24,25,26]. Subsequently, the functions of these ATGs in the plant were gradually demonstrated. For example, OsATG8a/8b improved nitrogen uptake and utilization, contributing to improving the rice grain quality and yield [27,28]. MdATG5a and MdATG10 could enhance the drought/salt stress tolerance of apple plants, and MdATG3b exhibited better growth performance as the nutrient supply was limited [29,30,31]. Besides, ATGs have also been demonstrated to play an important role in the resistance of plants to pathogens. For instance, the silencing of PbrATG8c decreased the resistance to Botryosphaeria dothidea in pear leaves [32]. In Arabidopsis, phosphorylation of AtATG18a compromised the resistance of plants to Botrytis cinerea, whereas overexpression of the AtATG18a dephosphorylation-mimic form promoted the accumulation of autophagosomes and increased plant resistance to B. cinerea, as well as overexpressing AtATG5, AtATG7, and AtATG8a enhanced plant resistance to necrotrophic pathogens [33,34,35]. Moreover, MaATG8s were essential for the resistance of banana plants to Fusarium wilt [22]. In brief, autophagy plays a key role in host–pathogen interactions. However, there is little information available about autophagy-related genes of R. roxburghii in response to TRD.
Ca2+ signaling is one of the important transduction events in plant immunity. Sufficient external Ca2+ is indispensable for transmitting the perception of nonself signals to an intracellular signaling pathway and is also essential for activating antimicrobial responses to inhibit the growth of pathogens [36]. When Arabidopsis plants were grown in a low Ca2+ medium, the reduction of PTI responses was examined [37]. Similarly, the high-vigor maize seeds grew better without being affected by pathogens due to the higher concentration of free Ca2+ in the cytoplasm and nucleus [38]. Recently, the most prevalent approach to reducing the damage caused by various kinds of stresses was to increase intracellular Ca2+ concentration through the application of exogenous calcium salt [39]. Spraying calcium chloride before pathogenic inoculation could enhance the resistance of pear leaves to B. dothidea [40]. The application of calcium chloride was advantageous in controlling Phytophthora pistaciae gummosis in commercial pistachio crops [41]. Foliar spraying of exogenous calcium could reduce ozone damage in rice and had better control effects against apple fruit watercore [42,43].
TRD caused by C. fructicola has been one of the most serious diseases of R. roxburghii [2]. Colletotrichum spp. is one of the top 10 fungal pathogens from the international community and causes enormous yield losses to crops every year [44]. At present, chemical fungicides are an effective way to control TRD. However, the long-term use of chemical fungicides inevitably results in potential adverse health effects on ecological environments, wildlife populations, and humans due to their hazardous nature of toxic residues. As a consequence, an environmentally friendly alternative to chemical fungicides needs to be taken into consideration for safely controlling these diseases and potential issues of concern. In this study, a total of 40 RrATGs were identified and were further verified to take part in the response of R. roxburghii fruits to TRD, of which RrATG18e was significantly up-regulated at the initial infection stage of C. fructicola under the condition of exogenous Ca2+. This study would provide new insight into the potential role of RrATGs in the defensive responses of R. roxburghii fruits to TRD.
2. Materials and Methods
2.1. Genome-Wide Identification of ATG Family Genes in R. roxburghii
The corresponding protein sequence of AtATGs was downloaded from the TAIR database (https://www.arabidopsis.org/index.jsp (accessed on 3 March 2022)) based on the ID number of known AtATGs, and a BLASTP was performed with the existing genome of ‘Guinong 5’ on TBtools (v.1.098769) software, with the E-value set to 1 × 10−20, NumofHits set to 5, and NumofAligns was also set to 5 to filter the results. Subsequently, the conserved domain of the candidate protein sequence was analyzed and identified by Pfam at the website of http://pfam.xfam.org/ (accessed on 4 March 2022). All the identified genes were named RrATGs. The amino acid length, molecular weight, and theoretical isoelectric point of proteins of RrATGs were obtained using BioXM2.6. The subcellular localization was predicted using the online tool WoLF PSORT (https://wolfpsort.hgc.jp/ (accessed on 23 September 2022)).
2.2. Bioinformatics Analysis of RrATGs
Using the ATG protein sequences of Arabidopsis thaliana, Nicotiana tabacum, Oryza sativa, Vitis vinifera, and the putative RrATG proteins, a total of five species sequences were submitted to ClustalW for the multiple sequence alignment. The generated file was used to construct a phylogenetic tree through the neighbor-joining method, and bootstrap analyses were carried out in MEGA 7 software (in 1000 replicates). The bootstrap value below 50% was not displayed in the phylogenetic tree. The chromosome localization and collinear analysis were conducted by TBtools (v.1.098769) software. The exon–intron structure of RrATGs was visualized using the GSDS v2.0 (http://gsds.gao-lab.org/ (accessed on 25 September 2022)) online website and put together based on the different functional groups.
2.3. Plant Materials and Treatments
The fruits with different degrees of TRD occurrence were taken from R. roxburghii plants with tree years of 10 in the orchards of Chaxiang Village, Gujiao Town, Longli County, Guizhou Province, China, in 2022 (26°54′ N, 106°95′ E). The healthy and diseased fruits were ranked according to the proportion of fruit spot size to the total surface area of the fruit as follows [45]: grade 0 is no incidence, grade 1 is 1–10%, grade 2 is 11–25%, grade 3 is 26–50%, and grade 4 is >50%. The sampling site was the junction of 1 cm of diseased spot and healthy flesh fruit tissue, i.e., 0.5 cm of diseased flesh fruit tissue and 0.5 cm of healthy flesh fruit tissue. Samples were frozen at −180 °C in an ultra-low temperature refrigerator for RNA extraction.
The pathogen inoculation trials were conducted in the fruit germplasm repository of Guizhou University, Guizhou, China, in 2022 (26°42.408′ N, 106°67.353′ E). Twelve-year-old plants of ‘Guinong 5’ R. roxburghii were selected as in vivo materials. Considering the TRD occurrence period of R. roxburghii fruits in the field, healthy fruits with uniform size were selected to inoculate C. fructicola on July 13. Firstly, the fruit surface was disinfected with 75% ethanol for 15 min and then washed with sterile water. Subsequently, the fruits were sprayed with 2% calcium acetate (Ca2+). Controls were sprayed with an equal amount of double-distilled water (H2O). After 24 h of spraying, the fruits were in vivo wound-inoculated near the sepal end with strain CXCDF-3 activated on potato dextrose agar (PDA) using a pre-prepared sterile needle. Controls were in vivo wound-inoculated with sterilized PDA. A total of 400 fruits were treated with Ca2+ or H2O (control). Ten fruits were sampled for each plot (in three replicates) at thirteen days after inoculation (DPI). The spot area of the diseased fruit was calculated using the elliptical area formula. The tissue (1 cm) was taken from the edge of the spot in the diseased fruit. The tissue samples were immediately transported back to the laboratory and frozen in liquid nitrogen at −180 °C.
2.4. RNA-Seq Analysis of RrATGs Tissue-Specific Expression
Based on the databases of genomic RNA-seq [46,47], the tissue-specific expression profiles of all the identified RrATGs in four different tissues (stem, leaf, flower, and fruit) and in R. roxburghii fruit at different developmental stages were analyzed. The heat map was also plotted using TBtools (v.1.098769).
2.5. RNA Extraction, cDNA Synthesis, and qRT-PCR Analysis
Total RNA was extracted with the RNAprep Pure Plant Kit (Tiangen Biotech Co., Ltd., Beijing, China). RNA integrity was evaluated using agarose gel electrophoresis and a NanoDrop spectrophotometer (Thermo Scientific, Los Angeles, CA, USA). A total of 1 μg high-quality RNA was used as the input material for cDNA synthesis with the PrimeScrip RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Inc., Dalian, China). Real-time quantitative PCR (qRT-PCR) was implemented on the ABI ViiA 7 DX system (Applied Biosystems) using TB Green Premix Ex Taq II (TaKaRa) with the ubiquitin (UBQ) gene as a reference gene to normalize expression data. The specific primer used for qRT-PCR was designed using Primer Premier 5 software and the sequence is listed in Table S1. Each PCR reaction contained 5.0 µL TB Green mix, 0.8 µL primers, and 1.0 µL diluted cDNA in a final volume of 10 µL. The amplification conditions were as follows: 30 s of denaturation at 95 °C, followed by 40 cycles at 95 °C for 5 s and 60 °C for 30 s, then 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s. Each experiment was repeated at least triplicate and each gene was calculated with the 2−ΔΔCT method for the relative expression.
2.6. Total Calcium Content Detection
Ca2+ content (mmol·L−1) was measured using a calcium colorimetric assay kit (Beyotime Biotechnology Co., Ltd., Shanghai, China) [48].
2.7. Statistical Analysis
Experimental data were expressed as the mean ± standard deviation (SD) of three independent replicates. Data were analyzed by analysis of variance (ANOVA), and means were compared using Tukey’s multipole difference test (p < 0.05). All statistical analyses were implemented with the SPSS 20.0 statistical package (IBM SPSS Statistics). Gene expression heat maps were drawn using TBtools software (v.1.098769).
3. Results
3.1. Identification of 40 ATGs in R. roxburghii
Based on the known AtATG amino acid protein sequences as queries, a total of 40 putative RrATGs were identified from the genome of R. roxburghii. These RrATGs showed 49.16% to 91.45% of their sequence identified with AtATGs and had close phylogenetic relationships with other species having homologous ATGs (Table 1 and Figure 1). The RrATG family identified a total of 19 subfamilies. In the RrATG subfamilies, the RrATG2/3/4/5/6/7/9/10/16/20/101, and the RrVPS15/34 had only one member, whereas other subfamilies contained multiple members: seven members in the RrATG18 subfamily, five members in the RrATG8 subfamily, four members in the RrATG1 subfamily and the RrATG12 subfamily, respectively, three members in the RrATG5 subfamily and RrATG13 subfamily, respectively, and two members in the RrTOR subfamily. In contrast to AtATGs, RrATGs were identified with more genes in RrATG5, RrATG12, RrATG13, and RrTOR, but less in the RrATG4, RrATG8, and RrATG18 subfamilies. In addition, bioinformatics analysis results indicate that the length of amino acids ranged from 77 to 2459 aa and the molecular weights ranged from 8.73 to 276.22 kD. The RrTORa possesses the maximum amino acids and molecular weights of all RrATG proteins. This information suggests the RrATGs identified might exist in significant variations with potential functional differentiation. The prediction of subcellular location results shows that most RrATGs were predicted to localize to the cytoplasm and nucleus, accounting for more than 50%, followed by mitochondria, chloroplasts, Golgi, plasma membrane, endoplasmic reticulum, extracellular, and the cytoskeleton. In addition to the RrATG1 subfamily, there are differences in the subcellular localization of the RrATG subfamilies, which contain several members, especially the RrATG18 subfamily, which has seven genes that are not in the same location. The significantly various subcellular localization could mean that they played various roles in the autophagic process.

Table 1.
Related information of autophagy-related genes (ATGs) in R. roxburghii.
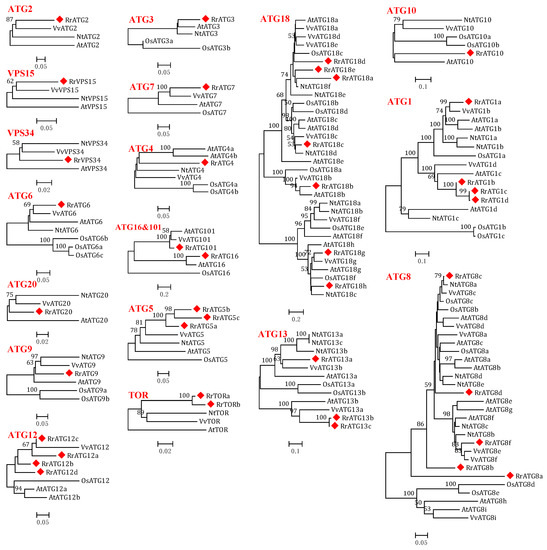
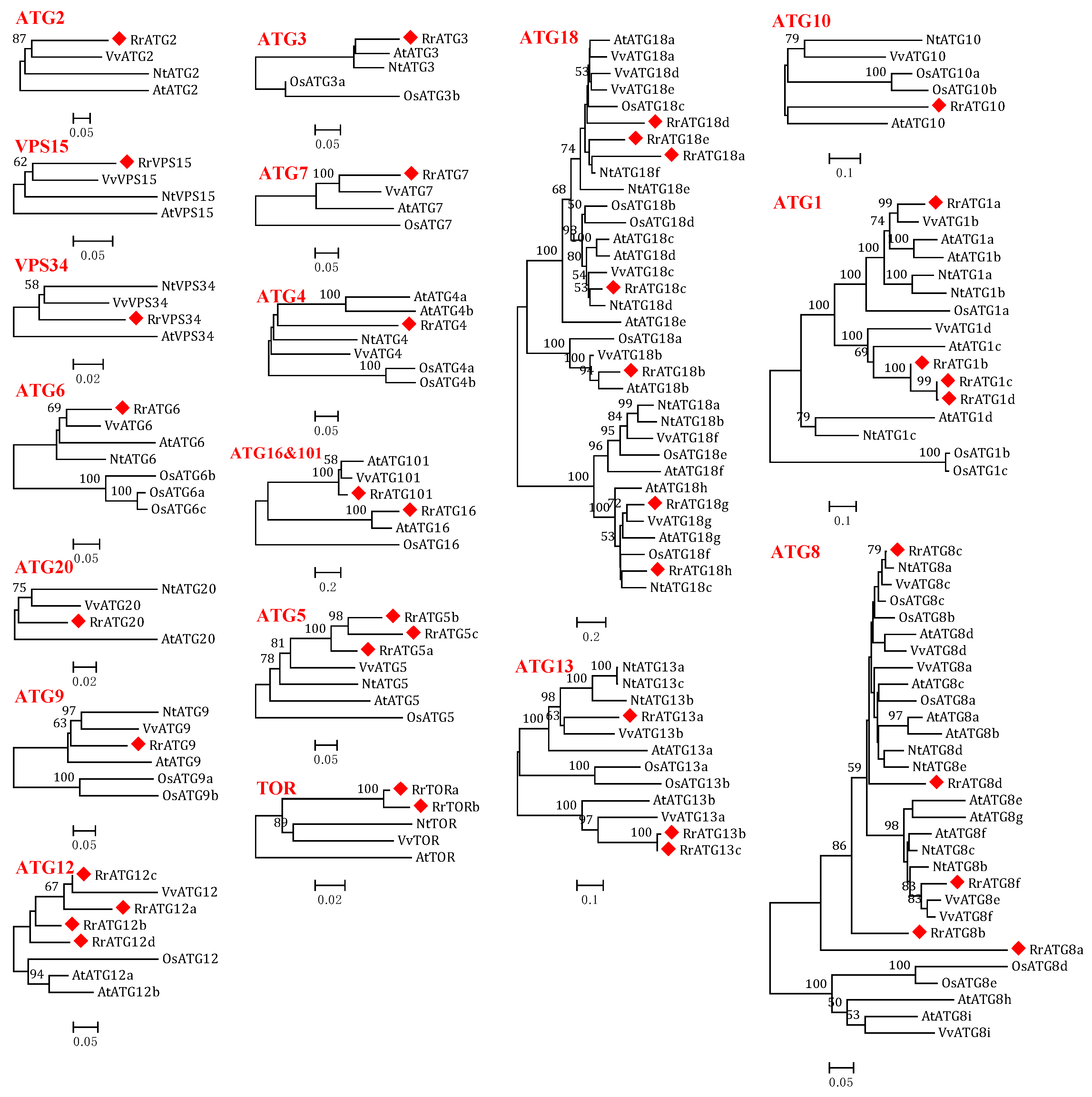
Figure 1.
Phylogenetic trees were constructed using the neighbor-joining method with 1000 bootstrap values for ATG protein sequences of five species: R. roxburghii, Arabidopsis thaliana, Vitis vinifera, Nicotiana tabacum, and Oryza sativa. RrATGs are marked with red squares and bootstrap values below 50% are not shown in the phylogenetic trees.
3.2. Bioinformatics Analysis of RrATGs
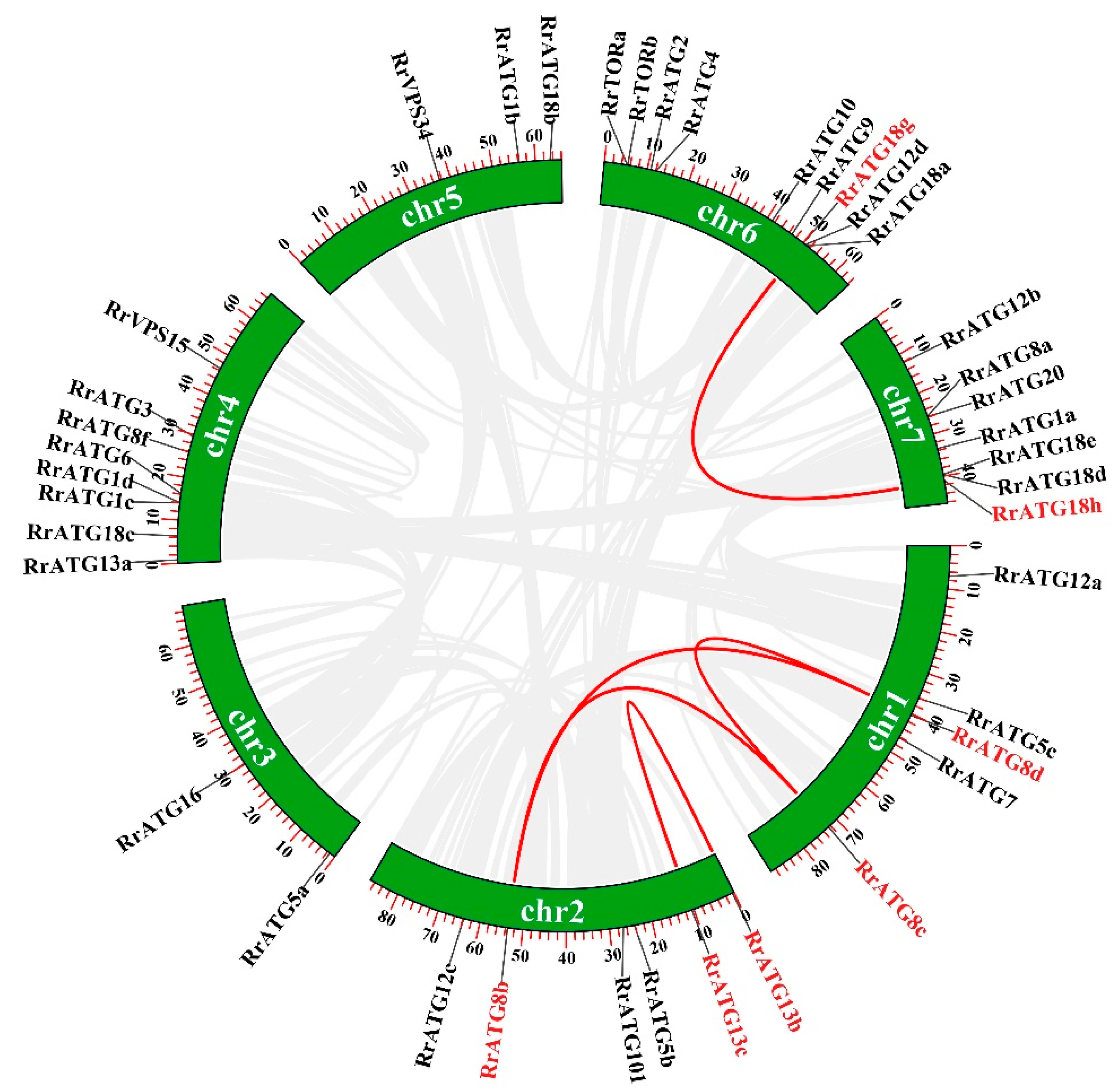
To assess the evolutionary relationships of RrATGs we used R. roxburghii, Arabidopsis thaliana, Nicotiana tabacum, Oryza sativa, and Vitis vinifera autophagy-proteins to construct the neighbor-joining phylogenetic trees. As shown in Figure 1, most RrATGs were clustered in one branch and showed close homology with Vitis vinifera. Some multiple members of the RrATG subfamily were clustered in one branch (RrATG1, RrATG5, and RrATG12 superfamily), containing the least identity value RrATG1d. Whereas other multiple members were clustered in two or three branches. For instance, the RrATG13 subfamily with five members was clustered in two branches, and the RrATG18 subfamily with seven members was clustered in three branches. In total, RrATGs had similar evolutionary relationships but were not consistent with function. Furthermore, chromosome location showed that seven chromosomes distributed all RrATGs, whose size was indicated by their relative length (Figure 2). Chromosome 6 (chr6) contained the greatest number of RrATGs (9); the minimum was chromosome 3 (chr3), which only contained two genes. In addition, the multiple members of the RrATG subfamilies were not localized on the same chromosome except for the RrTOR subfamily. The RrATG12 subfamily has four members spread over four chromosomes. Segmental duplication events play an important role in the evolution of the family. As shown in Figure 2, five pairs of genes were predicted to be segmental duplications, accounting for about 25% of all RrATGs. The RrATG8b and RrATG8c, RrATG8b and RrATG8d, RrATG8c and RrATG8d, RrATG13b and RrATG13c, and RrATG18g and RrATG18h had collinear correlations, which were linked with red color, respectively. The chromosomal distribution and segmental duplication provided further evidence for the wide functional divergence.
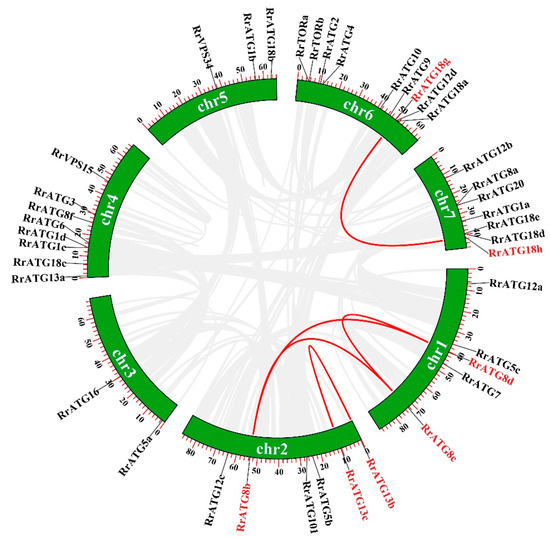
Figure 2.
Chromosome distribution and gene replication of RrATG genes. Genes that had segmental duplications were highlighted in red and pairwise collinearity was linked by red lines.
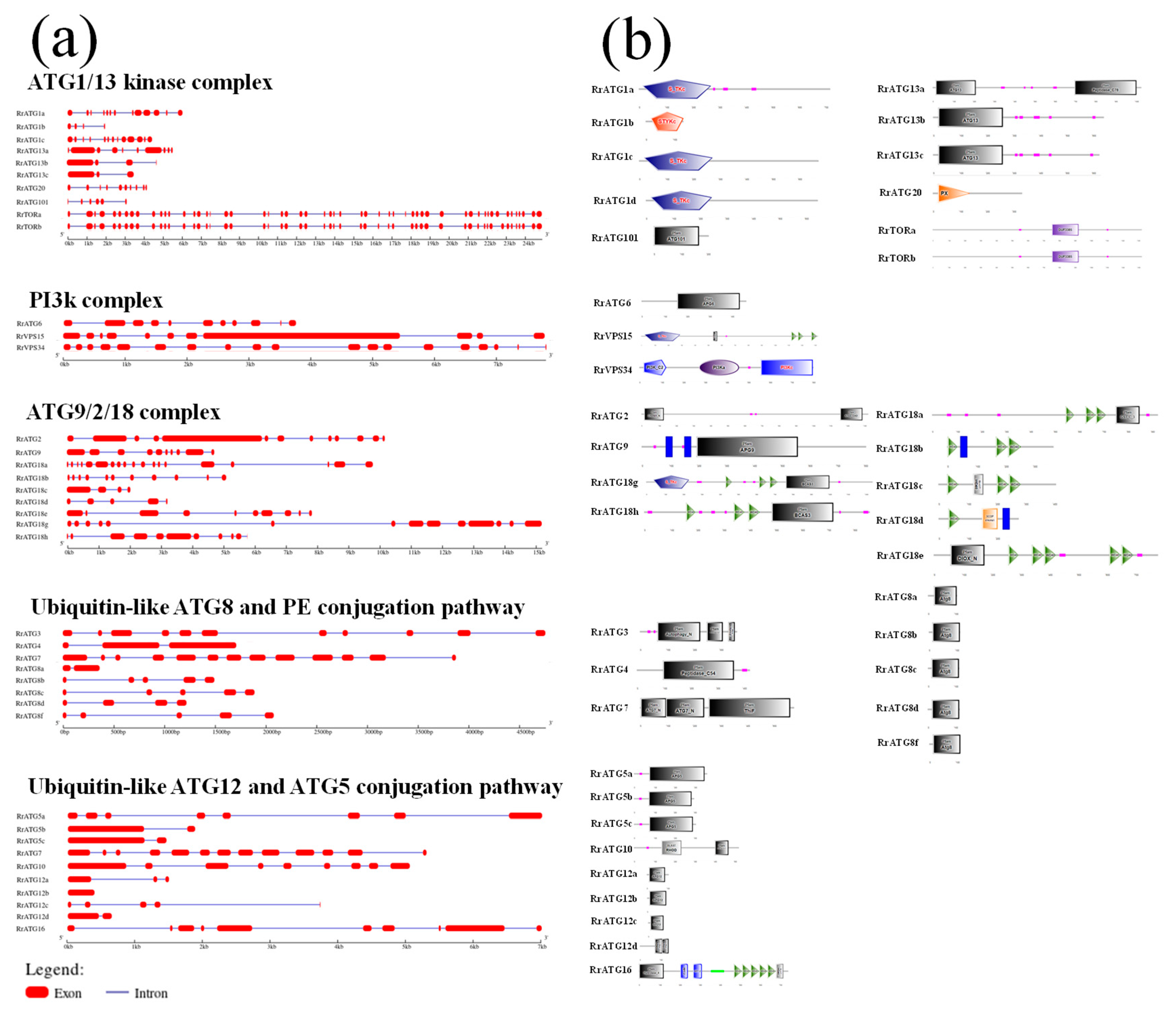
The exon–intron structure of RrATGs was predicted by the GSDS v2.0 online website, as shown in Figure 3a. Fifty-five exons were found in RrTORa, fifty-four in RrTORb, and other genes ranged from one to nineteen. Moreover, all RrATGs were predicted to be introns except for RrATG12b. Given the importance of conserved domains for assessing protein function, the SMART program was used to visualize the conserved domains of RrATG proteins (Figure 3b). From the predicted visualization, we discovered that a lot of RrATGs had their own ATG domains, for example, RrATG5, RrATG6, RrATG7, RrATG8, RrATG9, RrATG12, RrATG13, and RrATG101. Besides, the same functional group in the RrATGs subfamily usually contains similar conserved domains. Serine/threonine protein kinases emerged from the RrATG1 subfamily, and WD40 domains existed in all members of the RrATG18 subfamily. However, the members of the RrATG18 subfamily were still divided into two groups because the special breast carcinoma amplified sequence 3 (BCAS3) was only encoded by RrATG18g and RrATG18h. Notably, RrATG18e has a specific DIOX_N conserved domain, which means it may have a particular protein function. Additionally, the phox homology (PX) domain, PI3K, chorein N, peptidase C54, peptidase C78, ThiF, RHOD, Hydrolase 4, and DUF3385 were depicted in RrATGs. The number of conserved domains indicates that each RrATG protein may play various roles in regulating autophagy processes.

Figure 3.
Gene structures and conserved domains of RrATGs. (a) Exon–intron of RrATGs, exons indicated by red squares and introns indicated by blue lines. (b) The predicted proteins conserved domain of RrATGs.
3.3. RNA-Seq Analyses of RrATGs in Different Tissues and Developmental Stage-Specific Expressions
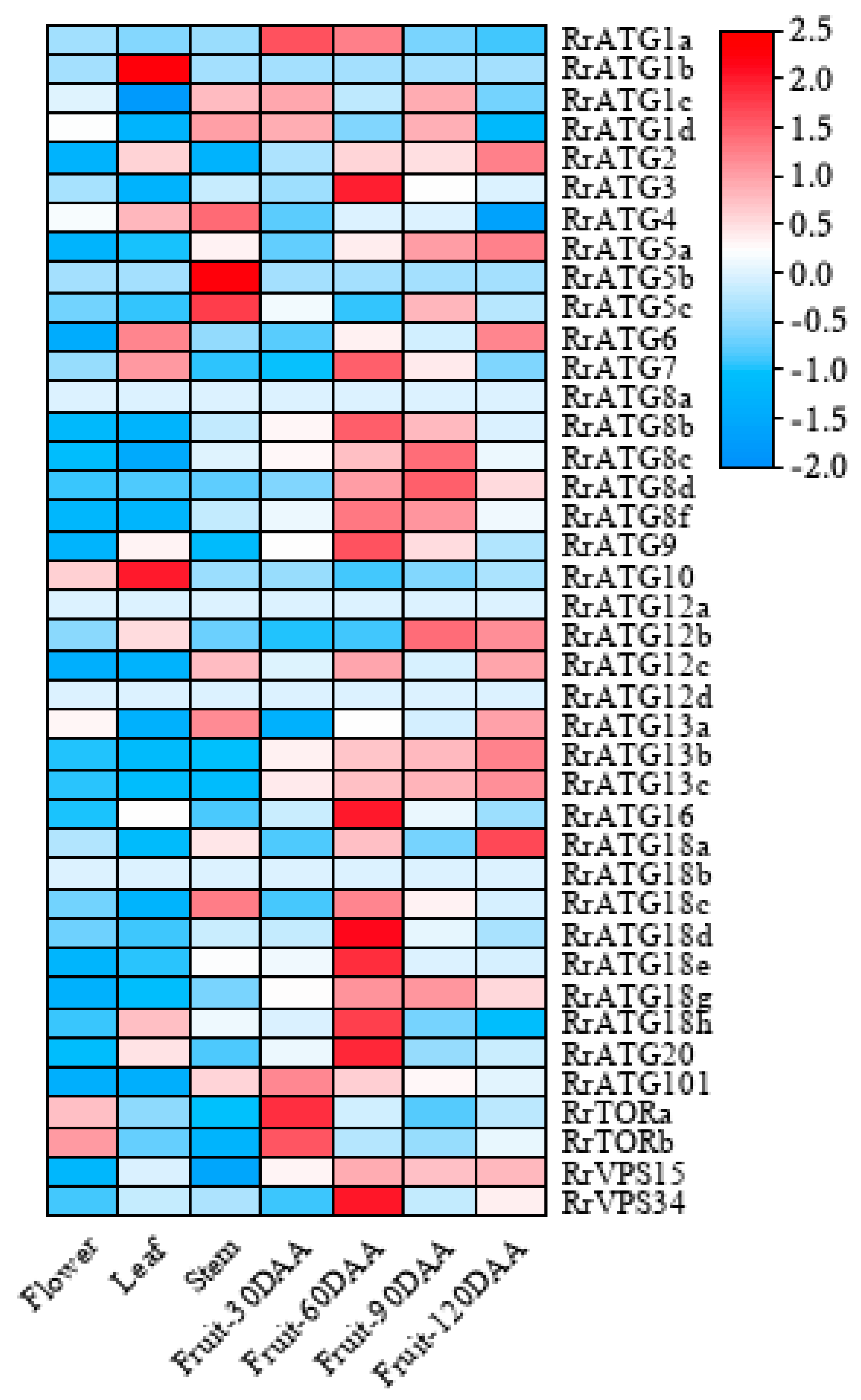
To understand the importance of RrATGs in a plant’s growth and development, the expression levels of 40 RrATGs in various tissues (flower, leaf, stem, fruit), and at different fruit developmental stages (30, 60, 90, and 120 days after anthesis) were retrieved from the genomic RNA-seq databases. As exhibited in Figure 4, in which RrATG1b, RrATG5b, RrATG8a, RrATG12a, RrATG12d, and RrATG18b displayed extremely low relative expression levels in every tissue mentioned, while other genes in the same RrATG subfamily showed higher expression levels. This suggests that the members of the same subfamily had significant tissue specificity, implying that they had functional differences. Among the four different tissues, no RrATGs had the highest expression in flowers, suggesting that RrATGs were less involved in the developmental process of flowers. RrATG1b and RrATG10 had higher expression in leaves than in other tissues; probably they played more roles in leaf development than other tissues. RrATG4, RrATG5b, and RrATG5c had higher expression in the stem, while the remaining genes were expressed higher in fruits, indicating that most RrATGs were more involved in the ripening process of fruits. Different RrATGs were differentially expressed at different developmental stages of fruits. RrATG1a, RrATG101, and RrTOR were more highly expressed in fruits 30 days after anthesis, indicating that they were mainly involved in the development of young fruits. As well as the RrATG3, RrATG7, RrATG16, RrATG20, and RrVPS34, most members of the RrATG8/18 subfamily were expressed centrally during mid-fruit development. The RrATGs mainly involved in fruit ripening were members of the RrATG12 and RrATG13 subfamilies. In conclusion, these data suggest that RrATGs had tissue-specific and spatiotemporal expression properties, which were involved in the growth and developmental processes of R. roxburghii, mediating the ripening process of fruits.
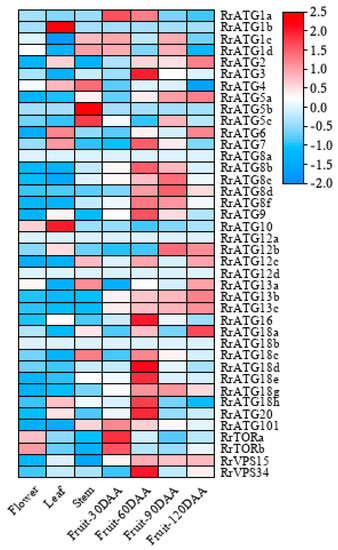
Figure 4.
Expression patterns of RrATGs in different tissues and different fruit developmental stages. Raw data from genomic RNA-seq databases in R. roxburghii. DAA was represented days after anthesis. Red and blue represent the higher and lower expression levels in each row, respectively.
3.4. Expression Profiles of RrATGs with Different TRD Grades of R. roxburghii Fruits
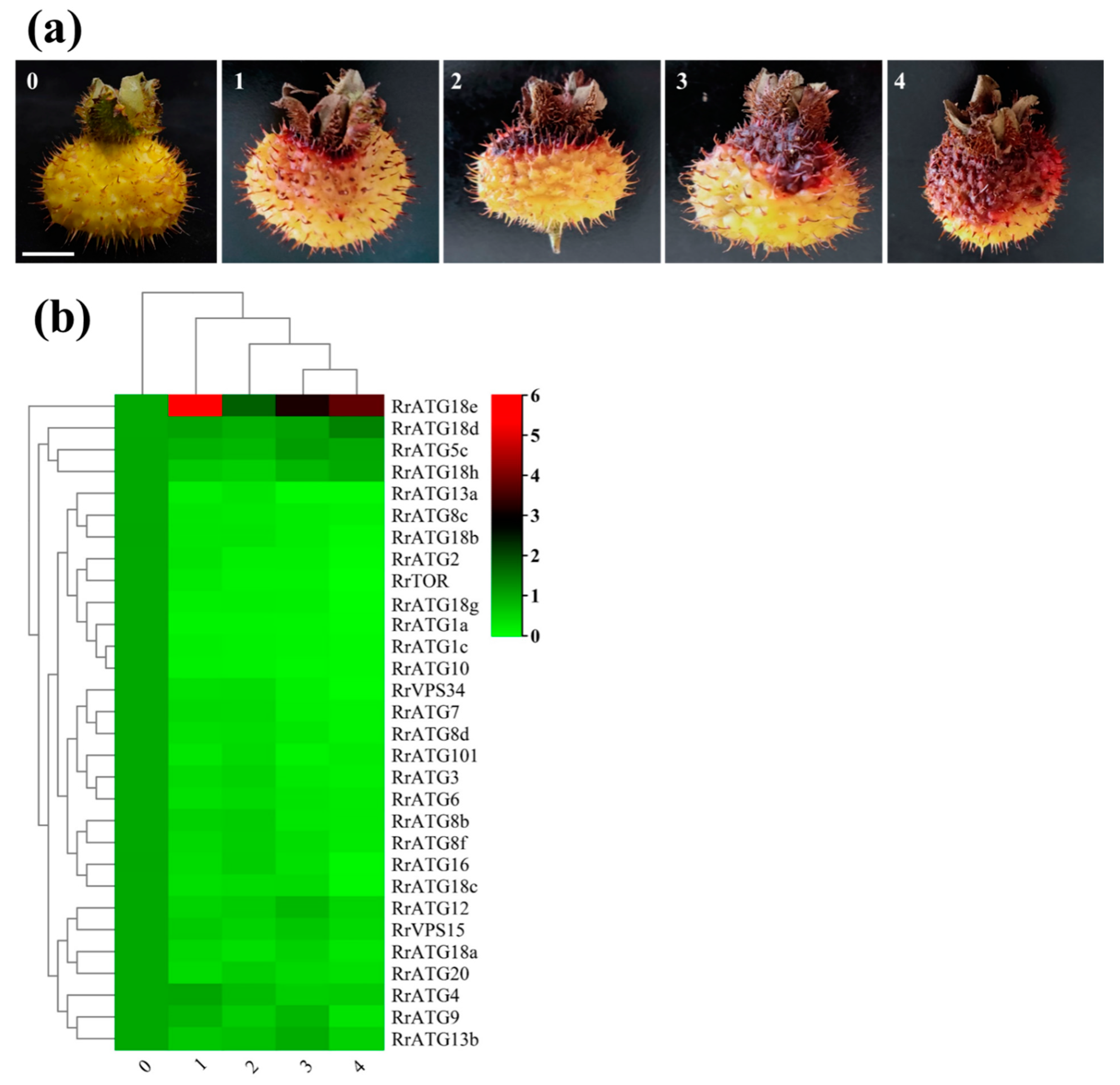
To explore the mechanism of RrATGs in response to the pathogenesis of R. roxburghii TRD, the expression levels of RrATGs in fruits with different grades of TRD were evaluated. The q-PCR results are presented in Figure 5b. Using the expression in healthy fruits (0 grade) as a template, the expression of most RrATGs was significantly decreased after infection by C. fructicola, except for RrATG18e, which was significantly up-regulated at the early stage of fruit susceptibility. This indicates that RrATG18e might play a key role in the early resistance to TRD. The expression of RrATG18e decreased slowly but was still significantly higher than other genes as the fruit disease progressed, suggesting that RrATG18e plays a central role in response to C. fructicola infection in R. roxburghii fruits. In addition, the relative expression of some RrATGs also deserves our attention. The expression of RrATG5c, RrATG18d, and RrATG18h in different grades of TRD fruits showed similar trends, with a slight decrease followed by an increase, of which RrATG18d showed a greater increase in expression in grade 4 TRD fruits. The expression of the RrATG4 gene did not decrease significantly in grade 1 TRD fruits, and its expression only decreased slowly with the increase in disease index. The expression of RrATG12b, RrATG12c, RrATG9, RrATG13b, and RrATG13c rose in grade 3 TRD fruits compared to grade 2 TRD fruits. While RrATG1 subfamily members and RrATG10 genes were particularly low in expression in grades 1–4 TRD fruits, other genes with floating decreasing expression would be minimally expressed in grade 4 TRD fruits. The above trends in gene expression indicate that different RrATG genes responded differently in different grades of TRD fruits.
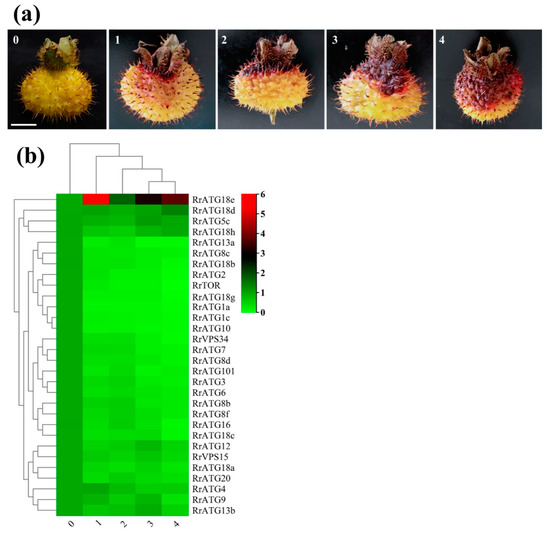
Figure 5.
Schematic diagram and expression of RrATGs in fruits with different grades of TRD occurrence. (a) Grade 0 is no incidence, grade 1 is 1–10%, grade 2 is 11–25%, grade 3 is 26–50%, and grade 4 is >50% the proportion of fruit spot size to the total surface area of the fruit, respectively. Bar = 1 cm. (b) Heat map showed the corresponding expression levels of RrATGs in diseased fruits of different grades, and the expression levels of healthy fruits were considered as ‘1’, red and green represent the higher and lower expression levels, respectively.
3.5. Field Control Effect of 2% Calcium Acetate (Ca2+) against TRD in R. roxburghii Fruits
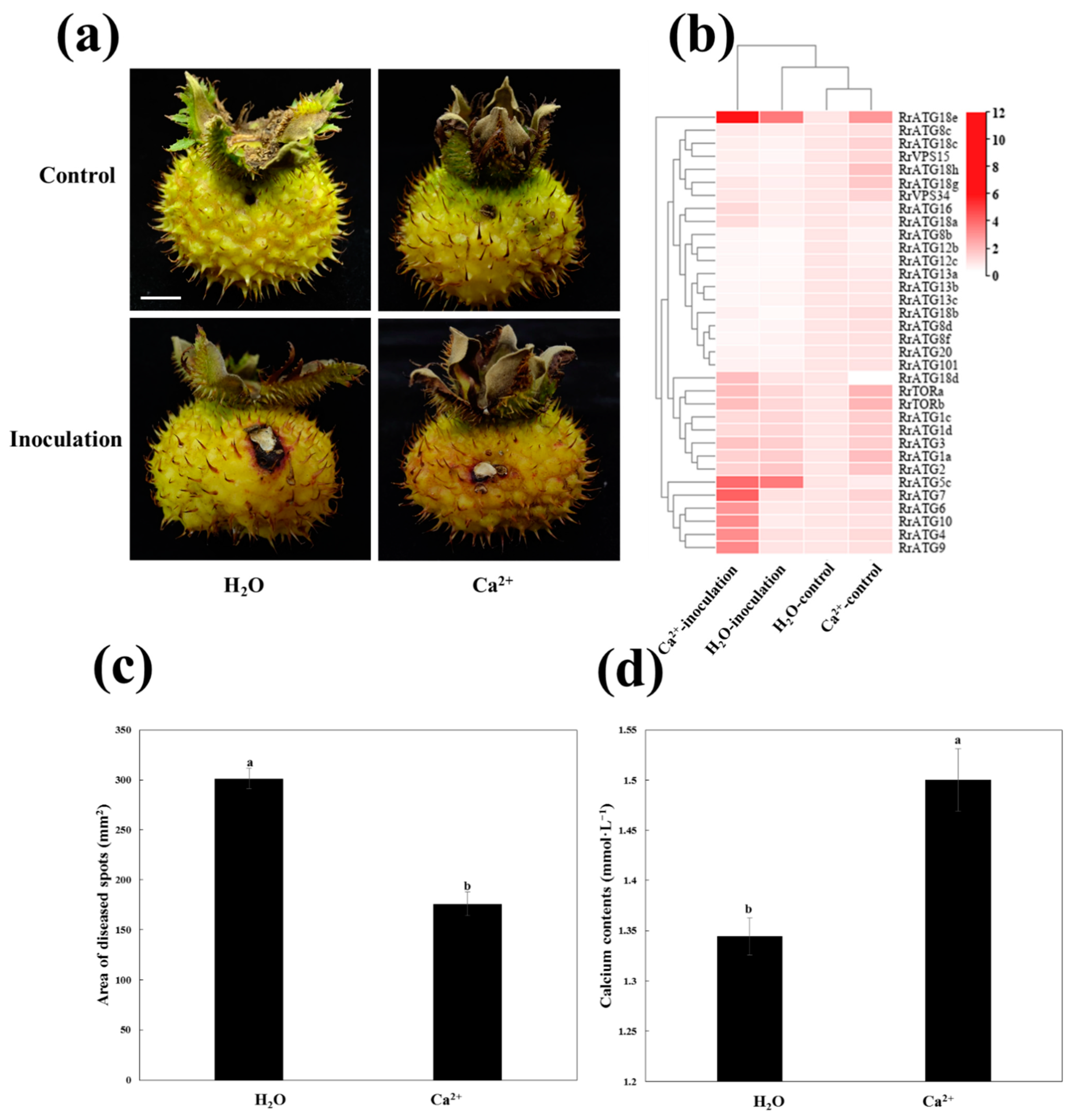
The previous study had shown that the application of exogenous Ca2+ could inhibit the infection of B. dothidea in pear leaves [40]. The same results were obtained when exogenous Ca2+ was applied to enhance the resistance of R. roxburghii fruits to C. fructicola infection. As shown in Figure 6, the area of disease spots of Ca2+-treated fruits was significantly lower than that of H2O-treated fruits after the fruits were infected with C. fructicola at 13 DPI (Figure 6c). At the same time, the total calcium content levels showed that the fruits with 2% Ca2+ treatment were significantly higher than those of the H2O-treated fruits (Figure 6d).
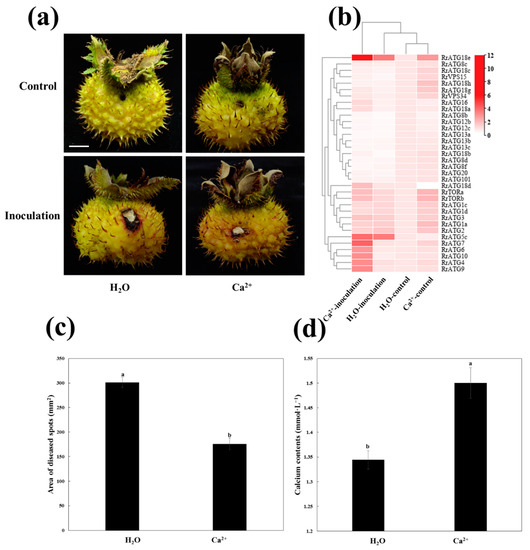
Figure 6.
Schematic diagram of Ca2+ enhanced the resistance of R. roxburghii fruits to TRD. (a) Phenotypes of fruits inoculated with C. fructicola and control PDA after H2O and Ca2+ treatments at 13 DPI. Bar = 1 cm. (b) The heat map showed the expression of RrATGs under different treatments at 13 DPI. The H2O-control represented the expression of inoculated control PDA after H2O-treated, the H2O-inoculation represented the expression of inoculated C. fructicola PDA after H2O-treated, the Ca2+-control represented the expression of inoculated control PDA after Ca2+-treated, the Ca2+-inoculation represented the expression of inoculated C. fructicola PDA after Ca2+-treated. The expression of H2O-control was considered “1”, red and white represented the higher and lower expression levels, respectively. (c) The area of diseased spots of fruits inoculated with C. fructicola PDA after H2O and Ca2+ treatments at 13 DPI. (d) Total calcium content of fruits after H2O and Ca2+ treatments at 13 DPI. Data are means of standard errors of three replicates. The letters on the column denote significant differences (p < 0.05, ANOVA) between H2O and Ca2+ treatments.
3.6. Expression Profiles of RrATGs under C. fructicola Infection after Ca2+ Treatment
To further explore the potential relationship between Ca2+, autophagy, and C. fructicola infection in R. roxburghii fruits, the changes in the expression patterns of RrATGs after Ca2+ and H2O treatment at 13 DPI were investigated (Figure 6b). The fruits inoculated with control PDA after treatment with H2O were used as a template. The expression levels of several RrATGs were highly induced when Ca2+-treated fruits were inoculated with control PDA, such as RrATG1/2/3/7/18/TOR/VPS34, suggesting that exogenous Ca2+ could stimulate the expression of some RrATGs even though fruits were not infected by C. fructicola. When the fruits were inoculated with C. fructicola, RrATG5c and RrATG18e gene expression was significantly up-regulated, and RrATG8b/12b/12c/13a/13b/13c/18b/8d/8f/20 were significantly down-regulated under H2O treatment. And Ca2+-treatment promoted not only a significant increase in the expression of RrATG5c and RrATG18e but also promoted the expression of RrATG4/6/7/9/10 genes. However, there was no significant effect on the down-regulated expression of genes when fruits were inoculated with C. fructicola under both Ca2+- and H2O-treated conditions. Notably, RrATG18e expression was sharply up-regulated by 11-fold in fruits inoculated with C. fructicola under Ca2+ treatment. Therefore, RrATG18e might play a comparatively important role in calcium-mediated enhancement of the resistance of R. roxburghii fruits to TRD.
4. Discussion
ETI is the innate immune system of plants, which can enhance plant resistance by effector recognition of pathogenic motifs attached to the cell surface, and leads to the hypersensitive response (HR) to control mycelia growth [49]. Autophagic cell death may be the result of an overactive defense response of HR during the development of resistance, meaning that cell death is essential for resistance, and it makes better sense especially when the invader is C. fructicola (a biotrophic pathogen that prefers a living host) [8]. Accordingly, there was an attempt to understand how autophagy responds when R. roxburghii fruits are infected by C. fructicola. First of all, there were 41 AtATGs used as queries to identify 40 RrATGs, except that RrATG11 was not identified. Among many different species, the ATG1, ATG8, and ATG18 subfamilies were identified as having multiple members: AtATG1/8/18 had 4/9/8 members; CsARG1/8/18 had 2/5/6 members; MtATG1/8/18 had 3/8/8 members; CsATG1/8/18 had 4/8/8 members; VvATG1/8/18 had 2/6/7 members; NtATG1/8/18 had 3/5/6 members; OsATG1/8/18 had 3/7/6 members; and ZmATG1/8/18 had 4/5/9 members [14,17,18,19,23,24,25,26]. Similarly, 4, 5, and 7 members were respectively identified in RrATG1, 8, and 18 subfamilies. Besides, the conserved domains of members of the same subfamily are also extremely similar in these different species. ATG1s encode serine/threonine protein kinases family. ATG8 domain is ubiquitin homologs (UBQ) in the ATG8 subfamily. ATG16 consists of multiple WD40 domains. ATG20 contains a phox homology (PX) domain. VPS15 possesses both the S_TKc domain and the WD40 domain, the former being the structural domain of the protein kinase family that catalyzes protein phosphorylation. VPS34 is the phosphatidylinositol 3 kinase (PI3K) family. TOR, as a conserved phosphatidylinositol kinase-associated protein kinase, always contains a specific rapamycin-binding domain (DUF3385). The important RrATG18 subfamily contains the WD40 structural domain in all members. Like other species, it can be divided into two categories according to the presence or absence of the BCAS3 domain at the C-terminal. These similarities obviously indicate the ATG family remains highly conserved over a long evolutionary period. In the collinearity analysis, RrATGs were found to have a total of 5 collinearity gene pairs. The members of the RrATG8 and RrATG18 subfamilies had a large number of segmental duplication events, indicating those members belonging to their subfamily were mostly derived from gene duplication during evolution [24,26,50].
The importance of autophagy has been widely reported in nutrient cycling. Leaf-senescence-induced autophagy occurs to fully recirculate 75% of the nitrogen stored in the chloroplast [15]. OsATG8a/8b improved rice grain yield and quality by enhancing nitrogen uptake and utilization; AtATG18a was induced and expressed under sucrose and nitrogen starvation during the senescence of Arabidopsis thaliana leaves [27,28,51]. Our transcriptome data analysis shows that the most RrATGs were highly expressed in the middle and late stages of fruit development, which might be the involvement of RrATGs in the development and ripening processes of R. roxburghii fruits through nutrient allocation or material recirculation. Normally, when Colletotrichum appressoria penetrate fruits, their mycelia first attach to the cuticle and uppermost epidermal cell layers of immature fruits to develop and fully erupt when the fruits ripen [52]. The differential expression of the RrATGs with the increased TRD occurrence of fruits under the conditions of nature suggests that different response mechanisms may occur in RrATG genes faced with stress. Previous studies had reported that the overexpression of MdATG5a and MdATG10 enhanced the drought/salt stress tolerance of apple plants, and over-expressed MdATG3b displayed better growth performance when nutrient supplies were limited [29,30,31]. The silencing of PbrATG8c decreased the resistance to B. dothidea in the pear [32]. Likewise, RrATG18e responded positively to C. fructicola infection in different degrees of TRD occurrence, especially at the early infection stage, suggesting that RrATG18e might be a potential key gene for improving the resistance of R. roxburghii fruits to TRD.
Ca2+ signaling events are important transduction events in plant immunity [36]. When a plant initially receives the signal of pathogen infection, the higher intracellular Ca2+ concentration can induce an immediate and strong defense response to enhance the plant’s resistance. The most prevalent approach is to apply exogenous calcium salt to plant trees [39]. Since TRD of R. roxburghii caused by C. fructicola usually occurs sporadically in early July and in vitro culture tests of C. fructicola have shown that it is suitable for the growth of TRD at 25 °C [2], the field trials were thus conducted in July. Higher ambient temperatures during the trial may be more favorable for pathogen infection. In this study, the area of disease spots was smaller and the total calcium contents were higher in Ca2+-treated fruits compared with H2O-treated fruits, which might be due to the spraying of exogenous calcium increasing the intracellular Ca2+ concentration to enhance the innate immune response and inhibit mycelia growth. In addition, the expression of RrATG4/5c/6/7/9/10/18e was significantly higher after Ca2+ treatment than H2O treatment under C. fructicola infection, indicating these genes might be the core RrATGs in response to the Ca2+-mediated TRD defense mechanism. Finally, the high expression of RrATG18e in both naturally diseased and C. fructicola-inoculated fruits raised our concern. A positive response of RrATG18e to early C. fructicola infection was clearly observed in natural fruits of TRD, and the mRNA of RrATG18e was highly accumulated in fruits inoculated with C. fructicola at 13 DPI after Ca2+ and H2O treatments, suggesting that RrATG18e may be a core autophagy gene in the autophagic pathway to enhance R. roxburghii resistance to TRD. Moreover, the expression of RrATG18e was also induced higher after Ca2+ treatment under inoculating the control PDA, indicating that RrATG18e could be stimulated by exogenous Ca2+. In conclusion, RrATG18e would be the core RrATGs involved in the calcium-mediated TRD defense response.
AtATG18a protein is critical for autophagosome formation, and its phosphorylation and overexpression have also been shown to play a key role in plant resistance to pathogenic infection [34,51,53]. From the phylogenetic tree, we can see RrATG18e and AtATG18a were constructed on a branch, which suggests they have a close evolutionary relationship and maybe have a similar function. Besides, unlike other members of the RrATG18 subfamily, RrATG18e was predicted in the cytoplasm and had a specific DIOX_N conserved structural domain. Under normal conditions, the free Ca2+ stored in the extracellular space and certain intracellular stores is 10,000-fold higher than the resting cytoplasmic free Ca2+ level. Such a Ca2+ concentration gradient allows pathogens to infect plants by triggering intracytoplasmic Ca2+ spikes. Next, it will transmit immune signals to downstream cellular responses through a decoding mechanism formed by Ca2+ sensors [36]. In addition, the germination of highly viable maize seeds was not affected by F. graminearum infection because it probably had a higher concentration of free Ca2+ in the cytoplasm of the embryonic cells [38]. Moreover, the dependent function of AtATG18a in the cytoplasm was sufficient to induce autophagy and enhance resistance against B. cinerea [34]. Therefore, the above observations suggest that the excellent performance of RrATG18e against C. fructicola under Ca2+ treatment may be related to its being localized in the cytoplasm. However, relevant validation remains to be done in subsequent experiments. Further, the special DIOX_N unique to the RrATG18e may be the key function domain for the defense TRD. This is a highly conserved N-terminal region of proteins with 2-oxoglutarate/Fe(II)-dependent dioxygenase activity and is widely distributed in nature [54]. It can promote the accumulation of flavonoids and positively regulate plant abiotic stress tolerance [55]. Perhaps the response of RrATG18e to C. fructicola infection in R. roxburghii fruits is closely related to this special conserved domain.
5. Conclusions
A total of 40 RrATGs were identified in R. roxburghii, and bioinformatic analysis shows that they were highly homologous to Arabidopsis thaliana autophagy-related genes (AtATGs). Most RrATGs were expressed up-regulated in R. roxburghii fruits at the medium to late stages of fruit development and down-regulated in fruits with TRD. Exogenous Ca2+ treatment enhanced the R. roxburghii fruit resistance to TRD and promoted the mRNA accumulation of RrATGs, of which the highest expression levels of RrATG18e suggest that it might be the core RrATGs involved in the calcium-mediated TRD defense response. In this study, RrATGs were analyzed and initially revealed their involvement in response to C. fructicola infection, which laid the foundation for further studies on the molecular mechanism of R. roxburghii resistance to TRD. Further studies are needed to understand the physiological functions of RrATG18e in R. roxburghii’s resistance to TRD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom13030556/s1, Table S1: Sequences of primers used in qPCR; Table S2: The coding sequences (CDS) of RrATG genes; Table S3: The protein sequences of RrATG proteins.
Author Contributions
H.A. and X.W. constructed the project; H.A., X.W. and K.L. designed the experiments; K.L. and J.L. performed the experiments; K.L. and M.L. analyzed the data; K.L., H.A. and X.W. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Joint Fund of the National Natural Science Foundation of China and the Karst Science Research Center of Guizhou Province (No. U1812401), the National Natural Science Foundation of China (grant no. 32160656), the Science-Technology Support Program of Guizhou Province [grant no. (2020)1Y134, (2021) YB243], the “Hundred” Level Talent Foundation of Guizhou Province (grant no. GCC [2022]023-1), and the Cultivation Program of Guizhou University (grant no. [2019]09].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used or analyzed during the current study available from the corresponding author upon reasonable request.
Conflicts of Interest
We declare that we do not have any commercial or associative interest that represent a conflict of interest in connection with the work submitted.
References
- Wang, L.; Wei, T.; Zheng, L.; Jiang, F.; Ma, W.; Lu, M.; Wu, X.; An, H. Recent advances on main active ingredients, pharmacological activities of Rosa roxbughii and its development and utilization. Foods 2023, 12, 1051. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, Y.; Lu, M.; Wu, X.; An, H. The pathogen of top rot disease in Rosa roxburghii and its effective control fungicides. Horticulturae 2022, 8, 1036. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J. The plant immune system. Nature 2006, 444, 323. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C. The intelligent behavior of plants. Trends Plant Sci. 2016, 21, 286–294. [Google Scholar] [CrossRef]
- Hacquard, S.; Spaepen, S.; Garrido-Oter, R.; Schulze-Lefert, P. Interplay between innate immunity and the plant microbiota. Annu. Rev. Phytopathol. 2017, 55, 565–589. [Google Scholar] [CrossRef]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Eldeiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A. Classification of cell death: Recommendations of the nomenclature committee on cell death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef]
- Künstler, A.; Bacsó, R.; Gullner, G.; Hafez, Y.M.; Király, L. Staying alive-is cell death dispensable for plant disease resistance during the hypersensitive response? Physiol. Mol. Plant Pathol. 2016, 93, 75–84. [Google Scholar] [CrossRef]
- Liu, Y.; Bassham, D.C. Autophagy: Pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 2012, 63, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.T.; Yoshimori, T. A current perspective of autophagosome biogenesis. Cell Res. 2014, 24, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Mehrpour, M.; Esclatine, A.; Beau, I.; Codogno, P. Overview of macroautophagy regulation in mammalian cells. Cell Res. 2010, 20, 748–762. [Google Scholar] [CrossRef]
- Han, S.; Yu, B.; Wang, Y.; Liu, Y. Role of plant autophagy in stress response. Protein Cell. 2011, 2, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, B.; Chen, W. Research progress of ATGs involved in plant immunity and NPR1 metabolism. Int. J. Mol. Sci. 2021, 22, 12093. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, Y.; Niu, Y. An overview of the molecular mechanisms and functions of autophagic pathways in plants. Plant Signal Behav. 2021, 16, 1977527. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ospina, L.; Moison, M.; Yoshimoto, K.; Masclaux-Daubresse, C. Autophagy, plant senescence, and nutrient recycling. J. Exp. Bot. 2014, 65, 3799–3811. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.D.; Haller, E.; Melzer, E.; Kober, K.; Wurster, K.; Stahl, M.; Bassham, D.C.; Vierstra, R.D.; Parker, J.E.; Bautor, J.; et al. Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J. 2011, 66, 818–830. [Google Scholar] [CrossRef]
- Xia, K.; Liu, T.; Ouyang, J.; Wang, R.; Fan, T.; Zhang, M. Genome-wide identification, classification, and expression analysis of autophagy-associated gene homologues in rice (Oryza sativa L.). DNA Res. 2011, 18, 363–377. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, P.; Wang, W.; Zou, J.; Cheng, T.; Peng, X.; Sun, M. A comprehensive, genome-wide analysis of autophagy-related genes identified in tobacco suggests a central role of autophagy in plant response to various environmental cues. DNA Res. 2015, 22, 245–257. [Google Scholar] [CrossRef]
- Li, F.; Chung, T.; Pennington, J.G.; Federico, M.L.; Kaeppler, H.F.; Kaeppler, S.M.; Otegui, M.S.; Vierstra, R.D. Autophagic recycling plays a central role in maize nitrogen remobilization. Plant Cell. 2015, 27, 1389–1408. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Guo, M.; Wang, H.; Lu, J.; Liu, J.; Zhang, C.; Gong, Z.; Lu, M. Autophagy, a conserved mechanism for protein degradation, responds to heat, and other abiotic stresses in Capsicum annuum L. Front. Plant Sci. 2016, 7, 131. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Wang, E.; Hu, L.; Hawkesford, M.J.; Zhong, L.; Chen, Z.; Xu, Z.; Li, L.; Zhou, Y.; et al. Genome-wide analysis of autophagy-associated genes in foxtail millet (Setaria italica L.) and characterization of the function of SiATG8a in conferring tolerance to nitrogen starvation in rice. BMC Genomics 2016, 17, 797. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, W.; Hu, W.; Liu, G.; Wu, C.; Liu, W.; Zeng, H.; He, C.; Shi, H. Genome-wide analysis of autophagy-related genes in banana highlights MaATG8s in cell death and autophagy in immune response to Fusarium wilt. Plant Cell Rep. 2017, 36, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, L.; Fang, X.; Chen, L.; Cui, L.; Fang, J. Genome-wide analysis of autophagy-related genes (ARGs) in grapevine and plant tolerance to copper stress. Planta 2018, 247, 1449–1463. [Google Scholar] [CrossRef]
- Fu, X.; Zhou, X.; Xu, Y.; Hui, Q.; Chun, C.; Ling, L.; Peng, L. Comprehensive analysis of autophagy-related genes in sweet orange (Citrus sinensis) highlights their roles in response to abiotic stresses. Int. J. Mol. Sci. 2020, 21, 2699. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, Z.; Gou, M.; Hu, J.; Wang, Y.; Wang, L.; Wang, Y.; Di, T.; Zhang, X.; Hao, X.; et al. Genome-wide identification, characterization, and expression analysis of tea plant autophagy-related genes (CsARGs) demonstrates that they play diverse roles during development and under abiotic stress. BMC Genomics 2021, 22, 121. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Chen, C.; Guo, X.; Lin, C.; Huang, W.; Chen, L. Genome-wide analysis of autophagy-related genes in Medicago truncatula highlights their roles in seed development and response to drought stress. Sci. Rep. 2021, 11, 22933. [Google Scholar] [CrossRef]
- Fan, T.; Yang, W.; Zeng, X.; Xu, X.; Xu, Y.; Fan, X.; Luo, M.; Tian, C.; Xia, K.; Zhang, M. A rice autophagy gene OsATG8b is involved in nitrogen remobilization and control of grain quality. Front. Plant Sci. 2020, 11, 588. [Google Scholar] [CrossRef]
- Yu, J.; Zhen, X.; Li, X.; Li, N.; Xu, F. Increased autophagy of rice can increase yield and nitrogen use efficiency (NUE). Front. Plant Sci. 2019, 10, 584. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Jia, X.; Ma, F. Apple autophagy-related protein MdATG3s afford tolerance to multiple abiotic stresses. Plant Sci. 2017, 256, 53–64. [Google Scholar] [CrossRef]
- Huo, L.; Guo, Z.; Jia, X.; Sun, X.; Wang, P.; Gong, X.; Ma, F. Increased autophagic activity in roots caused by overexpression of the autophagy-related gene MdATG10 in apple enhances salt tolerance. Plant Sci. 2020, 294, 110444. [Google Scholar] [CrossRef]
- Jia, X.; Jia, X.; Li, T.; Wang, Y.; Sun, X.; Huo, L.; Wang, P.; Che, R.; Gong, X.; Ma, F. MdATG5a induces drought tolerance by improving the antioxidant defenses and promoting starch degradation in apple. Plant Sci. 2021, 312, 111052. [Google Scholar] [CrossRef]
- Sun, X.; Pan, B.; Xu, W.; Chen, Q.; Wang, Y.; Ban, Q.; Xing, C.; Zhang, S. Genome-wide identification and expression analysis of the pear autophagy-related gene PbrATG8 and functional verification of PbrATG8c in Pyrus bretschneideri Rehd. Planta 2021, 253, 14. [Google Scholar] [CrossRef]
- Minina, E.A.; Moschou, P.N.; Vetukuri, R.R.; Sanchez-Vera, V.; Cardoso, C.; Liu, Q.S.; Elander, P.H.; Dalman, K.; Beganovic, M.; Yilmaz, J.L.; et al. Transcriptional stimulation of rate-limiting components of the autophagic pathway improves plant fitness. J. Exp. Bot. 2018, 69, 1415–1432. [Google Scholar] [CrossRef]
- Zhang, B.; Shao, L.; Wang, J.; Zhang, Y.; Guo, X.; Peng, Y.; Cao, Y.; Lai, Z. Phosphorylation of ATG18a by BAK1 suppresses autophagy and attenuates plant resistance against necrotrophic pathogens. Autophagy 2020, 17, 2093–2110. [Google Scholar] [CrossRef] [PubMed]
- Rigault, M.; Citerne, S.; Masclaux-Daubresse, C.; Dellagi, A. Salicylic acid is a key player of Arabidopsis autophagy mutant susceptibility to the necrotrophic bacterium Dickeya dadantii. Sci. Rep 2021, 11, 3624. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef]
- Wang, T.; Hou, C.; Ren, Z.; Wang, C.; Zhao, F.; Dahlbeck, D.; Hu, S.; Zhang, L.; Niu, Q.; Li, L.; et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, X.; Song, X.; Guo, Q.; Yin, Y.; Zhang, C.; Li, Y. High-vigor maize seeds resist Fusarium graminearum infection through stronger Ca2+ signaling. Agriculture 2022, 12, 992. [Google Scholar] [CrossRef]
- Winkler, A.; Knoche, M. Calcium uptake through skins of sweet cherry fruit: Effects of different calcium salts and surfactants. Sci. Hortic. 2021, 276, 109761. [Google Scholar] [CrossRef]
- Sun, X.; Pan, B.; Wang, Y.; Xu, W.; Zhang, S. Exogenous calcium improved resistance to Botryosphaeria dothidea by increasing autophagy activity and salicylic acid level in pear. Mol. Plant Microbe Interact. 2020, 33, 1150–1160. [Google Scholar] [CrossRef]
- Mostowfizadeh-Ghalamfarsa, R.; Hussaini, K.; Ghasemi-Fasaei, R. Effects of calcium salts in controlling Phytophthora pistaciae, the causal agent of pistachio gummosis. Eur. J. Plant Pathol. 2018, 151, 475–485. [Google Scholar] [CrossRef]
- Kittisak, L.; Saengchai, A.; Paitip, T. Effect of calcium acetate and calcium chloride on grain morphology and antioxidant regulation in rice under ozone stress. J. Plant Growth Regul. 2021, 41, 3138–3152. [Google Scholar] [CrossRef]
- Wang, Y. Studies on the Effects of Calcium on Watercore and Sorbitol Content in ‘Yueguan’ Apple Fruit; Shenyang Agricultural University: Shenyang, China, 2018. [Google Scholar]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Kou, X.; Wu, M.; Li, L.; Wang, S.; Xue, Z.; Liu, B. Effects of CaCl2 dipping and pullulan coating on the development of brown spot on ‘Huangguan’ pears during cold storage. Postharvest Biol. Technol. 2015, 99, 63–72. [Google Scholar] [CrossRef]
- Huang, X.; Yan, H.; Zhai, L.; Yang, Z.; Yi, Y. Characterization of the Rosa roxburghii Tratt transcriptome and analysis of MYB genes. PLoS ONE 2019, 14, e0203014. [Google Scholar] [CrossRef]
- Lu, M.; Ma, W.; Liu, Y.; An, H.; Ludlow, R.A. Transcriptome analysis reveals candidate lignin-related genes and transcription factors in Rosa roxburghii during fruit ripening. Plant Mol. Biol. Report. 2020, 38, 331–342. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Zhou, X.; Wei, B.; Zhao, Y.; Ji, S. Calcium treatment alleviates pericarp browning of ‘Nanguo’ pears by regulating the GABA shunt after cold storage. Front. Plant Sci. 2020, 11, 580986. [Google Scholar] [CrossRef] [PubMed]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.P. Calcium signalling in plant biotic interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, L.; Ruan, X.; Chen, D.; Zhu, A.; Chen, C.; Bertrand, D.; Jiao, W.; Hao, B.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 2013, 45, 59–66. [Google Scholar] [CrossRef]
- Xiong, Y.; Contento, A.L.; Bassham, D.C. AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J. 2005, 42, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Alkan, N.; Friedlander, G.; Ment, D.; Prusky, D.; Fluhr, R. Simultaneous transcriptome analysis of Colletotrichum gloeosporioides and tomato fruit pathosystem reveals novel fungal pathogenicity and fruit defense strategies. New Phytol. 2015, 205, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huo, L.; Jia, X.; Che, R.; Gong, X.; Wang, P.; Ma, F. Overexpression of MdATG18a in apple improves resistance to Diplocarpon mali infection by enhancing antioxidant activity and salicylic acid levels. Hortic. Res. 2018, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Kluza, A.; Niedzialkowska, E.; Kurpiewska, K.; Wojdyla, Z.; Quesne, M.; Kot, E.; Porebski, P.J.; Borowski, T. Crystal structure of thebaine 6-O-demethylase from the morphine biosynthesis pathway. J. Struct. Biol. 2018, 202, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Fan, F.; Yu, Q.; Zhang, P. A moss 2-Oxoglutarate/Fe(II)-dependent dioxygenases (2-ODD) gene of flavonoids biosynthesis positively regulates plants abiotic stress tolerance. Front. Plant Sci. 2022, 13, 850062. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).