Host Feeding Patterns of Mansonia (Diptera, Culicidae) in Rural Settlements near Porto Velho, State of Rondonia, Brazil
Abstract
1. Introduction
2. Materials and Methods
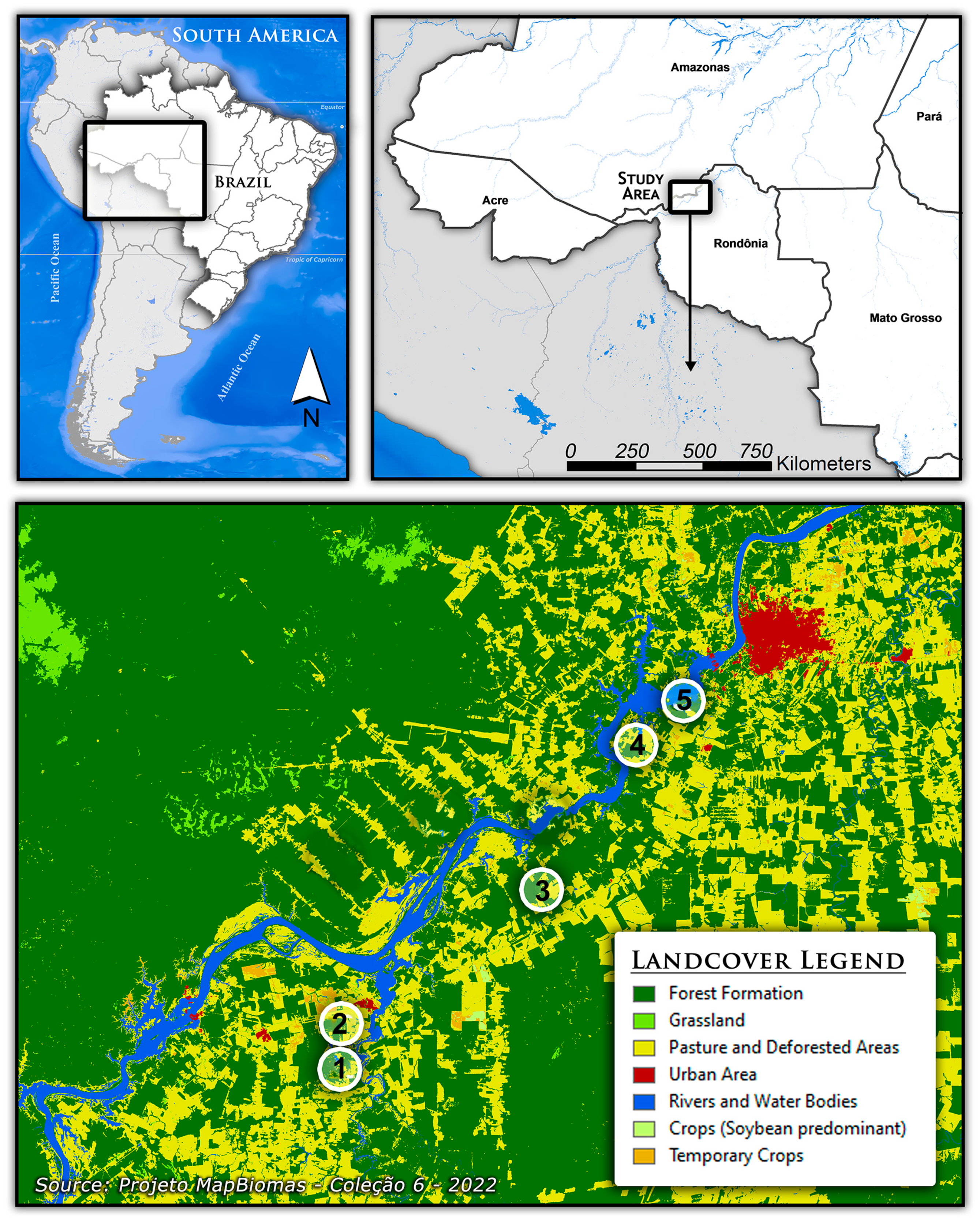
2.1. Study Areas and Field Collections
2.2. Sample Preparation, PCR Conditions for Ingested Blood Meal Identification, and Sequencing
2.3. Ingested Blood Analysis
3. Results
3.1. Sequencing
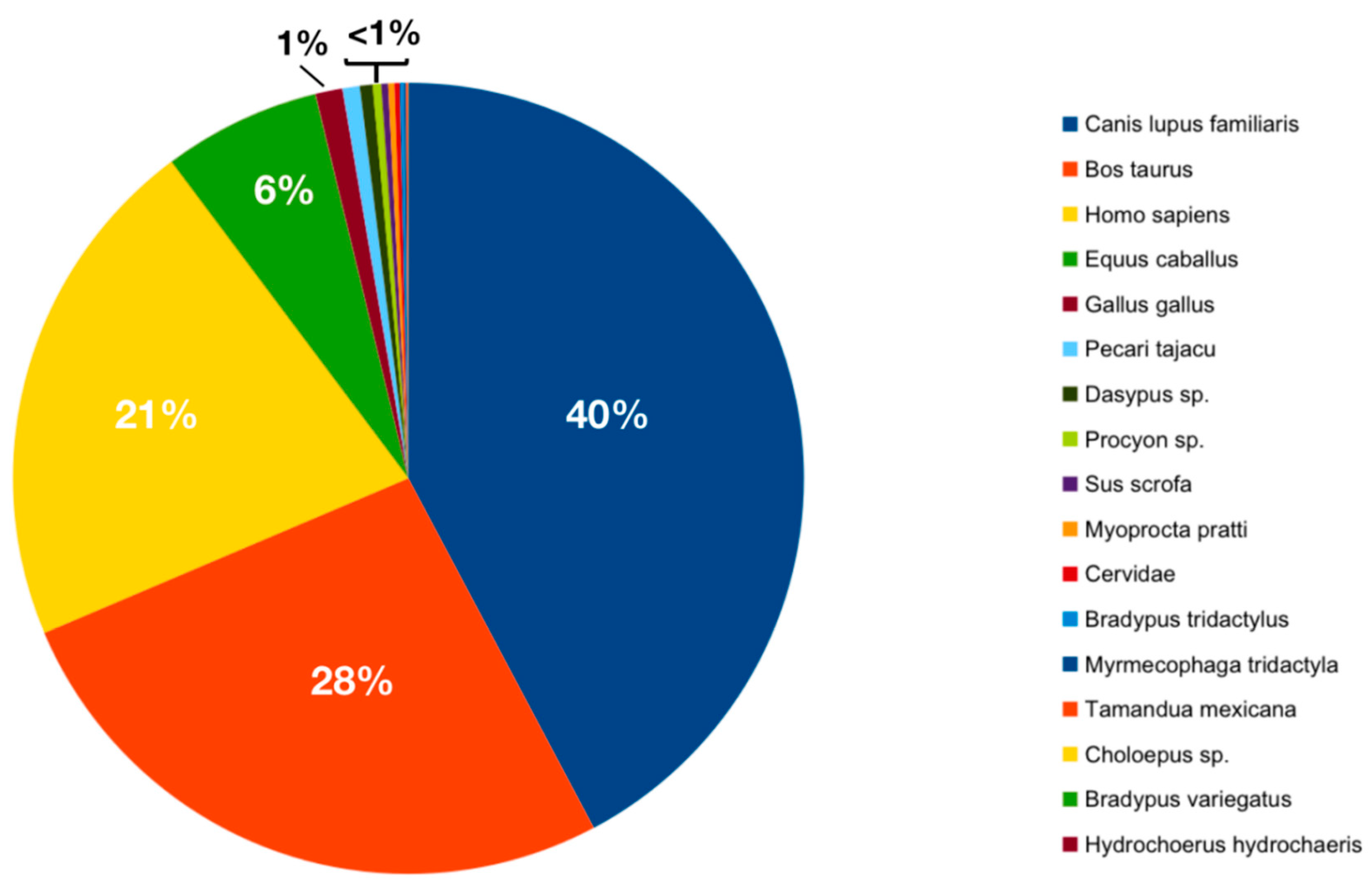
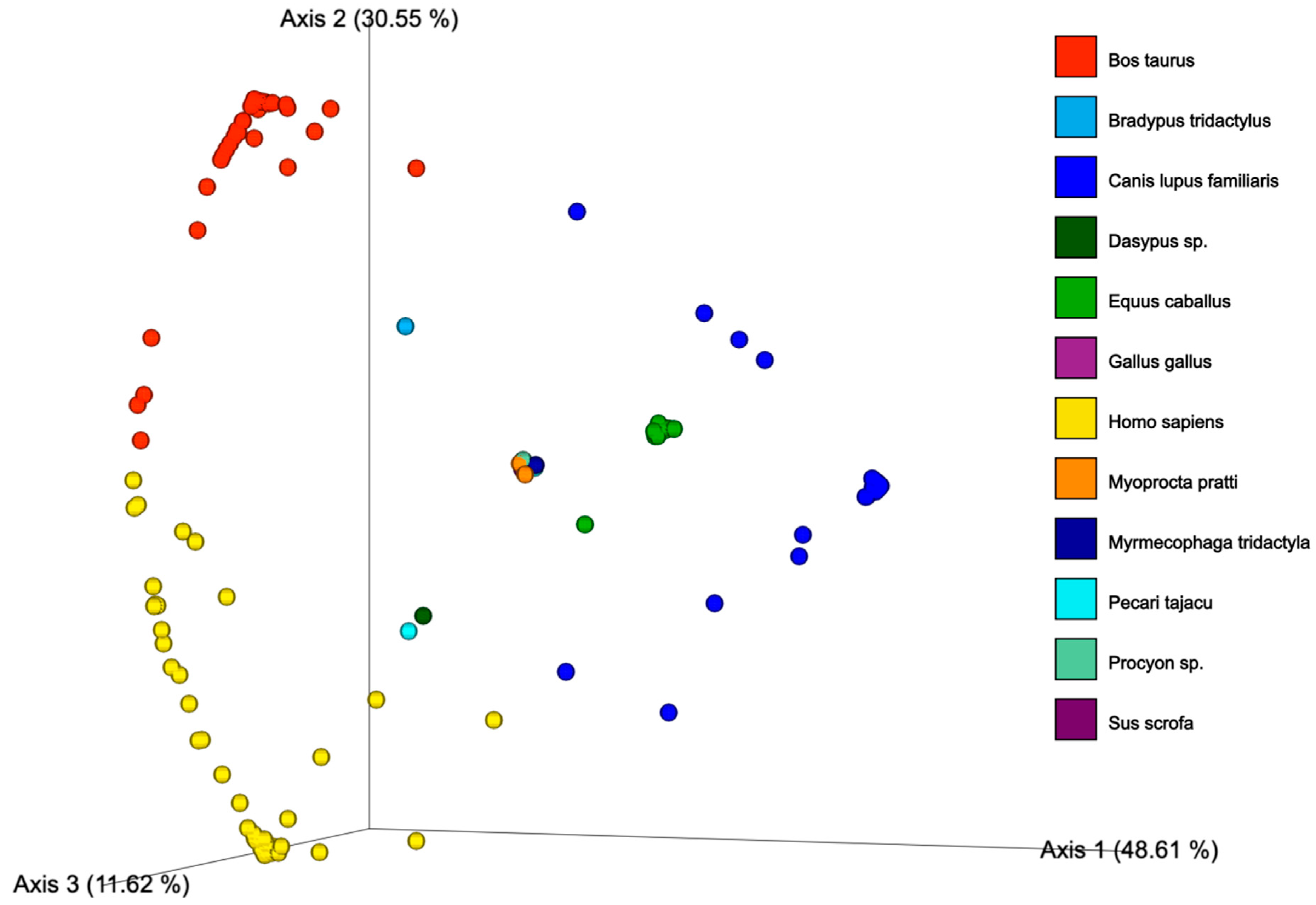
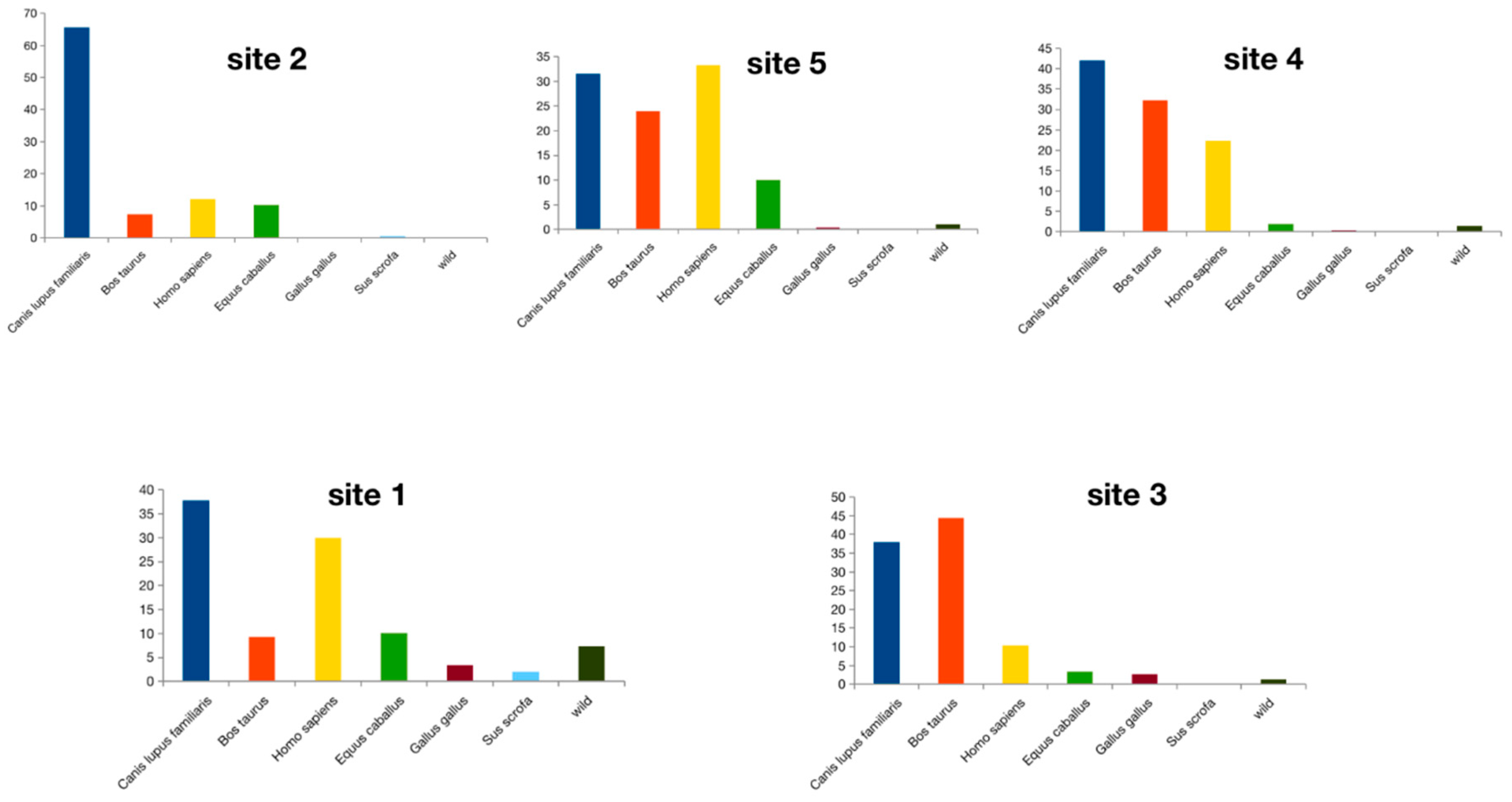
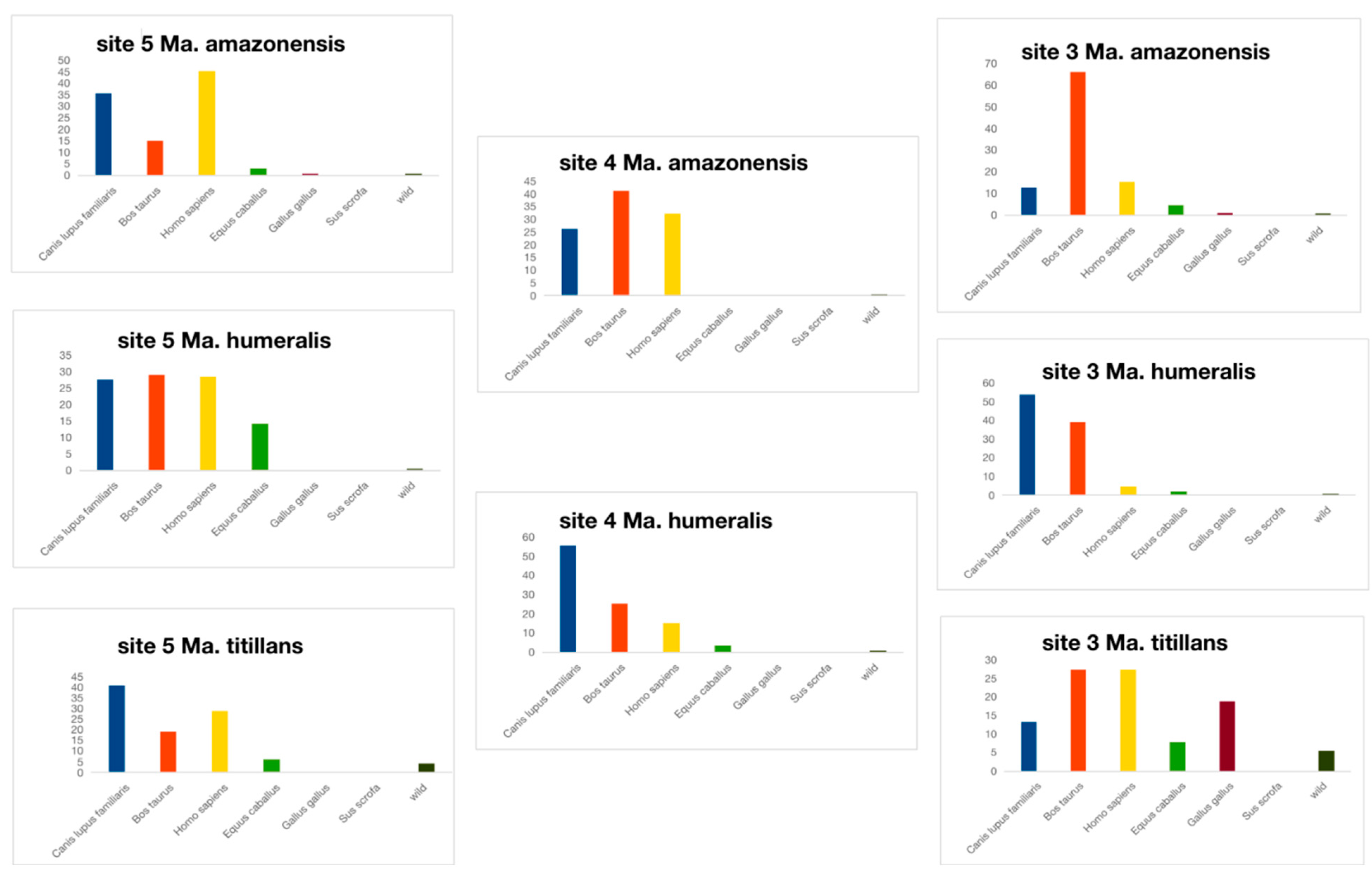
3.2. Ingested Blood Meal Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ronderos, R.A.; Bachmann, A.O. Neotropioal Mansoniini. I. (Diptera-Culicidae). Rev. Soc. Entomol. Argent. 1963, 26, 1–4. [Google Scholar]
- Forattini, O.P. Culicidologia médica: Identificaçäo, biologia e epidemiologia. In Culicidologia Médica: Identificação, Biologia e Epidemiologia; EDUSP: São Paulo, Brazil, 2002; Volume 2. [Google Scholar]
- Harbach, R.E. Mosquito Taxonomic Inventory. 2019. Available online: http://mosquito-taxonomic-inventory.info/ (accessed on 25 September 2022).
- Fikrig, K.; Harrington, L.C. Understanding and interpreting mosquito blood feeding studies: The case of Aedes albopictus. Trends Parasitol. 2021, 37, 959–975. [Google Scholar] [CrossRef]
- Melgarejo-Colmenares, K.; Cardo, M.V.; Vezzani, D. Blood feeding habits of mosquitoes: Hardly a bite in South America. Parasitol. Res. 2022, 121, 1829–1852. [Google Scholar] [CrossRef]
- Turell, M.J.; Jones, J.W.; Sardelis, M.R.; Dohm, D.J.; Coleman, R.E.; Watts, D.M.; Fernandez, R.; Calampa, C.; Klein, T.A. Vector Competence of Peruvian Mosquitoes (Diptera: Culicidae) for Epizootic and Enzootic Strains of Venezuelan Equine Encephalomyelitis Virus. J. Med. Èntomol. 2000, 37, 835–839. [Google Scholar] [CrossRef]
- Mendez, W.; Liria, J.; Navarro, J.C.; Garcia, C.Z.; Freier, J.E.; Salas, R.; Weaver, S.C.; Barrera, R. Spatial dispersion of adult mosquitoes (Diptera: Culicidae) in a sylvatic focus of Venezuelan equine encephalitis virus. J. Med. Èntomol. 2001, 38, 813–821. [Google Scholar] [CrossRef][Green Version]
- Ganjian, N.; Riviere-Cinnamond, A. Mayaro virus in Latin America and the Caribbean. Rev. Panam. Salud Publica 2020, 44, e14. [Google Scholar] [CrossRef]
- Amorim, J.A.; Sa, I.L.R.; Rojas, M.V.R.; Santos Neto, N.F.; Galardo, A.K.R.; Carvalho, D.P.; Ribeiro, K.A.N.; Sallum, M.A.M. Aquatic Macrophytes Hosting Immature Mansonia (Mansonia) Blanchard, 1901 (Diptera, Culicidae) in Porto Velho, Rondonia State, Brazil. J. Med. Entomol. 2022, 59, 631–637. [Google Scholar] [CrossRef]
- Galardo, A.K.R.; Hijjar, A.V.; Falcão, L.L.O.; Carvalho, D.P.; Ribeiro, K.A.N.; Silveira, G.A.; Neto, N.F.S.; Saraiva, J.F. Seasonality and Biting Behavior of Mansonia (Diptera, Culicidae) in Rural Settlements Near Porto Velho, State of Rondônia, Brazil. J. Med. Entomol. 2022, 59, 883–890. [Google Scholar] [CrossRef]
- Luz, S.L.; Lourenço-de-Oliveira, R. Forest Culicinae mosquitoes in the environs of Samuel hydroeletric plant, State of Rondônia, Brazil. Mem. Inst. Oswaldo Cruz. 1996, 91, 427–432. [Google Scholar] [CrossRef]
- Kengne, I.M.; Brissaud, F.; Akoa, A.; Eteme, R.A.; Nya, J.; Ndikefor, A.; Fonkou, T. Mosquito development in a macrophyte-based wastewater treatment plant in Cameroon (Central Africa). Ecol. Eng. 2003, 21, 53–61. [Google Scholar] [CrossRef]
- Allan, S.A.; Day, J.F.; Edman, J.D. Visual ecology of biting flies. Annu. Rev. Entomol. 1987, 32, 297–316. [Google Scholar] [CrossRef]
- Navarro-Silva, M.A.; Barbosa, A.A.; Calado, D. Atividade de Mansonia spp. (Mansoniini, Culicidae) em fragmento florestal na área urbana de Curitiba, Paraná, Brasil. Rev. Bras. Zool. 2004, 21, 243–247. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de La Puente, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef]
- Schiesari, L.; Ilha, P.R.; Negri, D.D.B.; Prado, P.I.; Grillitsch, B. Ponds, puddles, floodplains and dams in the upper Xingu Basin: Could we be witnessing the ‘lentification’ of deforested Amazonia Perspect. Ecol. Conserv. 2020, 18, 61–72. [Google Scholar] [CrossRef]
- Clements, A.N. The Biology of Mosquitoes, Volume 3: Transmission of Viruses and Interactions with Bacteria; CABI Publishing: Wallingford, UK, 2011. [Google Scholar]
- Lyimo, I.N.; Ferguson, H.M. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol. 2009, 25, 189–196. [Google Scholar] [CrossRef]
- Ayala, F.J.; Coluzzi, M. Chromosome speciation: Humans, Drosophila, and mosquitoes. Proc. Natl. Acad. Sci. USA 2005, 102 (Suppl. 1), 6535–6542. [Google Scholar] [CrossRef]
- Takken, W.; Verhulst, N.O. Host Preferences of Blood-Feeding Mosquitoes. Annu. Rev. Èntomol. 2013, 58, 433–453. [Google Scholar] [CrossRef]
- Tempelis, C.H. Host-feeding patterns of mosquitoes, with a review of advances in analysis of blood meals by serology. J. Med. Entomol. 1975, 11, 635–653. [Google Scholar] [CrossRef]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef]
- Zwiebel, L.J.; Takken, W. Olfactory regulation of mosquito–host interactions. Insect Biochem. Mol. Biol. 2004, 34, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A. Why Aedes aegypti is such a good vector. In Dengue and Dengue Hemorrhagic Fever, 2nd ed.; Gubler, D.J., Ooi, E.E., Vasudevan, S., Farrar, J., Eds.; CABI: Wallingford, UK, 2014. [Google Scholar]
- Costantini, C.; Sagnon, N.F.; Della Torre, A.; Coluzzi, M. Mosquito behavioural aspects of vector-human interactions in the Anopheles gambiae complex. Parassitologia 1999, 41, 209–217. [Google Scholar]
- McBride, C.S. Genes and odors underlying the recent evolution of mosquito preference for humans. Curr. Biol. 2016, 26, R41–R46. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Lee, Y.; Ferguson, H.M.; Kreppel, K.S.; Kihonda, A.; Govella, N.J.; Collier, T.C.; Cornel, A.J.; Eskin, E.; Kang, E.Y.; et al. The Genetic Basis of Host Preference and Resting Behavior in the Major African Malaria Vector, Anopheles arabiensis. PLoS Genet. 2016, 12, e1006303. [Google Scholar] [CrossRef]
- Chilaka, N.; Perkins, E.; Tripet, F. Visual and olfactory associative learning in the malaria vector Anopheles gambiae sensu stricto. Malar. J. 2012, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Logue, K.; Keven, J.B.; Cannon, M.V.; Reimer, L.; Siba, P.; Walker, E.D.; Zimmerman, P.A.; Serre, D. Unbiased Characterization of Anopheles Mosquito Blood Meals by Targeted High-Throughput Sequencing. PLoS Negl. Trop. Dis. 2016, 10, e0004512. [Google Scholar] [CrossRef]
- Kieran, T.J.; Gottdenker, N.L.; Varian, C.P.; Saldana, A.; Means, N.; Owens, D.; Calzada, J.E.; Glenn, T.C. Blood meal source characterization using Illumina sequencing in the Chagas disease vector Rhodnius pallescens (Hemiptera: Reduviidae) in Panama. J. Med. Entomol. 2017, 54, 1786–1789. [Google Scholar] [CrossRef]
- Li, D.; Lu, D.; Moran, E.; da Silva, R.F.B. Examining Water Area Changes Accompanying Dam Construction in the Madeira River in the Brazilian Amazon. Water 2020, 12, 1921. [Google Scholar] [CrossRef]
- Cochrane, S.M.V.; Matricardi, E.A.T.; Numata, I.; Lefebvre, P.A. Landsat-based analysis of mega dam flooding impacts in the Amazon compared to associated environmental impact assessments: Upper Madeira river example 2006–2015. Remote Sens. Appl. Soc. Environ. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Ab’Sáber, A.N. Os Domínios de Natureza no Brasil: Potencialidades Paisagísticas; Ateliê Editorial: Cotia, Brasil, 2003; Volume 1. [Google Scholar]
- Nagaki, S.S.; Chaves, L.S.M.; López, R.V.M.; Bergo, E.S.; Laporta, G.Z.; Conn, J.E.; Sallum, M.A.M. Host feeding patterns of Nyssorhynchus darlingi (Diptera: Culicidae) in the Brazilian Amazon. Acta Trop. 2021, 213, 105751. [Google Scholar] [CrossRef]
- Boessenkool, S.; Epp, L.S.; Haile, J.; Bellemain, E.; Edwards, M.; Coissac, E.; Willerslev, E.; Brochmann, C. Blocking human contaminant DNA during PCR allows amplification of rare mammal species from sedimentary ancient DNA. Mol. Ecol. 2011, 21, 1806–1815. [Google Scholar] [CrossRef]
- Beier, J.C.; Perkins, P.V.; Wirtz, R.A.; Koros, J.; Diggs, D.; Gargan, T.P.; Koech, D.K. Blood meal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. J. Med. Entomol. 1988, 25, 9–16. [Google Scholar] [CrossRef]
- Boorman, J.; Mellor, P.S. A latex agglutination test for identification of blood-meals of Culicoides (Diptera: Ceratopogonidae). Bull. Entomol. Res. 1977, 67, 305–311. [Google Scholar] [CrossRef]
- Kent, R.J.; Norris, D.E. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome b. Am. J. Trop. Med. Hyg. 2005, 73, 336–342. [Google Scholar] [CrossRef]
- Gunathilaka, N.; Denipitiya, T.; Hapugoda, M.; Abeyewickreme, W.; Wickremasinghe, R. Determination of the foraging behaviour and blood meal source of malaria vector mosquitoes in Trincomalee District of Sri Lanka using a multiplex real time polymerase chain reaction assay. Malar. J. 2016, 15, 242. [Google Scholar] [CrossRef] [PubMed]
- Hurk, A.F.V.D.; Smith, I.L.; Smith, G.A. Development and evaluation of real-time polymerase chain reaction assays to identify mosquito (Diptera: Culicidae) bloodmeals originating from native Australian mammals. J. Med. Entomol. 2007, 44, 85–92. [Google Scholar] [CrossRef]
- Borland, E.M.; Kading, R.C. Modernizing the Toolkit for Arthropod Bloodmeal Identification. Insects 2021, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Batovska, J.; Lynch, S.E.; Cogan, N.O.I.; Brown, K.; Darbro, J.M.; Kho, E.A.; Blacket, M.J. Effective mosquito and arbovirus surveillance using metabarcoding. Mol. Ecol. Res. 2018, 18, 32–40. [Google Scholar] [CrossRef]
- Verdonschot, P.F.M.; Besse-Lototskaya, A.A. Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica 2014, 45, 69–79. [Google Scholar] [CrossRef]
- De Mello, C.F.; Alencar, J. Dispersion pattern of Mansonia in the surroundings of the Amazon Jirau Hydroelectric Power Plant. Sci. Rep. 2021, 11, 24273. [Google Scholar] [CrossRef]
- Alvarez, M.V.N.; Alonso, D.P.; Kadri, S.M.; Rufalco-Moutinho, P.; Bernardes, I.A.F.; De Mello, A.C.F.; Souto, A.C.; Carrasco-Escobar, G.; Moreno, M.; Gamboa, D.; et al. Nyssorhynchus darlingi genome-wide studies related to microgeographic dispersion and blood-seeking behavior. Parasit. Vectors 2022, 15, 106. [Google Scholar] [CrossRef]
- Campos, M.; Alonso, D.P.; Conn, J.E.; Vinetz, J.M.; Emerson, K.J.; Ribolla, P.E.M. Genetic diversity of Nyssorhynchus (Anopheles) darlingi related to biting behavior in western Amazon. Parasit. Vectors 2019, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Mayagaya, V.; Nkwengulila, G.; Lyimo, I.; Kihonda, J.; Mtambala, H.; Ngonyani, H.; Russell, T.L.; Fetrguson, H.M. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern Tanzania. Malar. J. 2015, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.C.; Monte-Mor, R.L.; Sawyer, D.O.; Singer, B.H. Malaria risk on the Amazon frontier. Proc. Natl. Acad. Sci. USA 2006, 103, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.; Sylla, M.; Badolo, A.; Lutomiah, J.; Ayala, D.; Aribodor, O.; Ibe, N.; Akorli, J.; Otoo, S.; Mutebi, J.-P.; et al. Climate and urbanization drive mosquito preference for humans. Curr. Biol. 2020, 30, 3570–3579. [Google Scholar] [CrossRef] [PubMed]
- Resende AF de Schöngart, J.; Streher, A.S.; Ferreira-Ferreira, J.; Piedade, M.T.F.; Silva, T.S.F. Massive tree mortality from flood pulse disturbances in Amazonian floodplain forests: The collateral effects of hydropower production. Sci. Total Environ. 2019, 659, 587–598. [Google Scholar] [CrossRef]
- Apiwathnasorn, C.; Asavanich, A.; Komalamisra, N.; Samung, Y.; Prummongkol, S.; Kanjanopas, K. The relationship between the abundance of Mansonia mosquitoes inhabiting a peat swamp forest and remotely sensed data. Southeast Asian J. Trop. Med. Public. Health 2006, 37, 463–467. [Google Scholar] [PubMed]
- De Araújo, W.S.; Vieira, T.M.; de Souza, G.A.; Bezerra, I.C.; Corgosinho, P.H.C.; Borges, M.A.Z. Nocturnal Mosquitoes of Pará State in the Brazilian Amazon: Species Composition, Habitat Segregation, and Seasonal Variation. J. Med. Entomol. 2020, 57, 1913–1919. [Google Scholar] [CrossRef]
| Comparisons | Sample Size | Permutations | Pseudo-F | p-Value | q-Value |
|---|---|---|---|---|---|
| site 1 × site 2 | 126 | 999 | 6.978800169 | 0.001 | 0.00250 |
| site 1 × site 5 | 162 | 999 | 0.90541316 | 0.468 | 0.46800 |
| site 2 × site 5 | 196 | 999 | 13.14182445 | 0.001 | 0.00250 |
| site 4 × site 1 | 134 | 999 | 3.841688365 | 0.013 | 0.01625 |
| site 4 × site 2 | 168 | 999 | 9.909787075 | 0.001 | 0.00250 |
| site 4 × site 5 | 204 | 999 | 4.209660355 | 0.012 | 0.01625 |
| site 4 × site 3 | 195 | 999 | 1.078676499 | 0.308 | 0.34222 |
| site 3 × site 1 | 153 | 999 | 7.138065671 | 0.002 | 0.00333 |
| site 3 × site 2 | 187 | 999 | 15.4967486 | 0.001 | 0.00250 |
| site 3 × site 5 | 223 | 999 | 9.598897081 | 0.002 | 0.00333 |
| Comparisons per Group | Sample Size | Permutations | Pseudo-F | p-Value | q-Value |
|---|---|---|---|---|---|
| site 2 | |||||
| Ma. amazonensis × Ma. humeralis | 71 | 999 | 1.003637873 | 0.373 | 0.4303 |
| Ma. humeralis × Ma. titillans | 69 | 999 | 0.5366033 | 0.599 | 0.6305 |
| Ma. amazonensis × Ma. titillans | 12 | 999 | 1.070379585 | 0.32 | 0.3798 |
| site 5 | |||||
| Ma. amazonensis × Ma. humeralis | 98 | 999 | 9.008857428 | 0.001 | 0.0060 |
| Ma. amazonensis × Ma. titillans | 48 | 999 | 4.454286977 | 0.01 | 0.0352 |
| Ma. humeralis × Ma. titillans | 84 | 999 | 10.78372428 | 0.001 | 0.0060 |
| site 4 | |||||
| Ma. amazonensis × Ma. humeralis | 78 | 999 | 4.128920366 | 0.02 | 0.0489 |
| Ma. amazonensis × Ma. titillans | 39 | 999 | 0.395281343 | 0.693 | 0.7107 |
| Ma. humeralis × Ma. titillans | 59 | 999 | 1.185069354 | 0.324 | 0.3798 |
| site 3 | |||||
| Ma. humeralis × Ma. titillans | 75 | 999 | 6.113456897 | 0.003 | 0.0150 |
| Ma. amazonensis × Ma. humeralis | 91 | 999 | 13.35434213 | 0.001 | 0.0060 |
| Ma. amazonensis × Ma. titillans | 48 | 999 | 2.109241498 | 0.109 | 0.1791 |
| site 1 | |||||
| Ma. humeralis × Ma. titillans | 44 | 999 | 1.125252545 | 0.265 | 0.33120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, D.P.; Amorim, J.A.; de Oliveira, T.M.P.; de Sá, I.L.R.; Possebon, F.S.; de Carvalho, D.P.; Ribeiro, K.A.N.; Ribolla, P.E.M.; Sallum, M.A.M. Host Feeding Patterns of Mansonia (Diptera, Culicidae) in Rural Settlements near Porto Velho, State of Rondonia, Brazil. Biomolecules 2023, 13, 553. https://doi.org/10.3390/biom13030553
Alonso DP, Amorim JA, de Oliveira TMP, de Sá ILR, Possebon FS, de Carvalho DP, Ribeiro KAN, Ribolla PEM, Sallum MAM. Host Feeding Patterns of Mansonia (Diptera, Culicidae) in Rural Settlements near Porto Velho, State of Rondonia, Brazil. Biomolecules. 2023; 13(3):553. https://doi.org/10.3390/biom13030553
Chicago/Turabian StyleAlonso, Diego Peres, Jandui Almeida Amorim, Tatiane Marques Porangaba de Oliveira, Ivy Luizi Rodrigues de Sá, Fábio Sossai Possebon, Dario Pires de Carvalho, Kaio Augusto Nabas Ribeiro, Paulo Eduardo Martins Ribolla, and Maria Anice Mureb Sallum. 2023. "Host Feeding Patterns of Mansonia (Diptera, Culicidae) in Rural Settlements near Porto Velho, State of Rondonia, Brazil" Biomolecules 13, no. 3: 553. https://doi.org/10.3390/biom13030553
APA StyleAlonso, D. P., Amorim, J. A., de Oliveira, T. M. P., de Sá, I. L. R., Possebon, F. S., de Carvalho, D. P., Ribeiro, K. A. N., Ribolla, P. E. M., & Sallum, M. A. M. (2023). Host Feeding Patterns of Mansonia (Diptera, Culicidae) in Rural Settlements near Porto Velho, State of Rondonia, Brazil. Biomolecules, 13(3), 553. https://doi.org/10.3390/biom13030553










