Prevalence of Spotted Fever Group Rickettsia and Candidatus Lariskella in Multiple Tick Species from Guizhou Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. Molecular Detection of Rickettsiales Bacteria
2.3. PCR Amplification, Sequencing, Genetic and Phylogenetic Analysis of Key Genes
3. Results
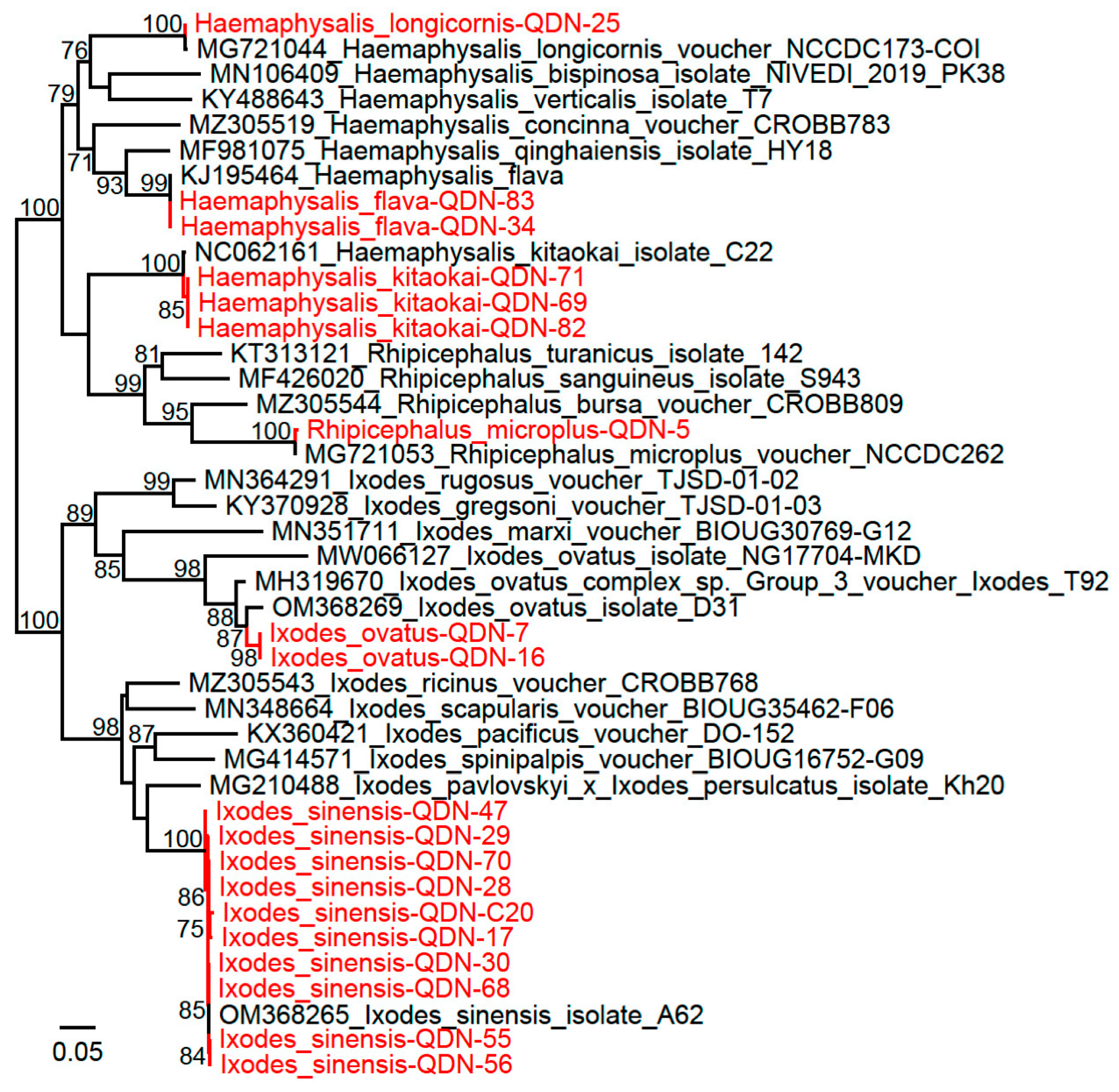
3.1. Sample Collection and Tick Identification
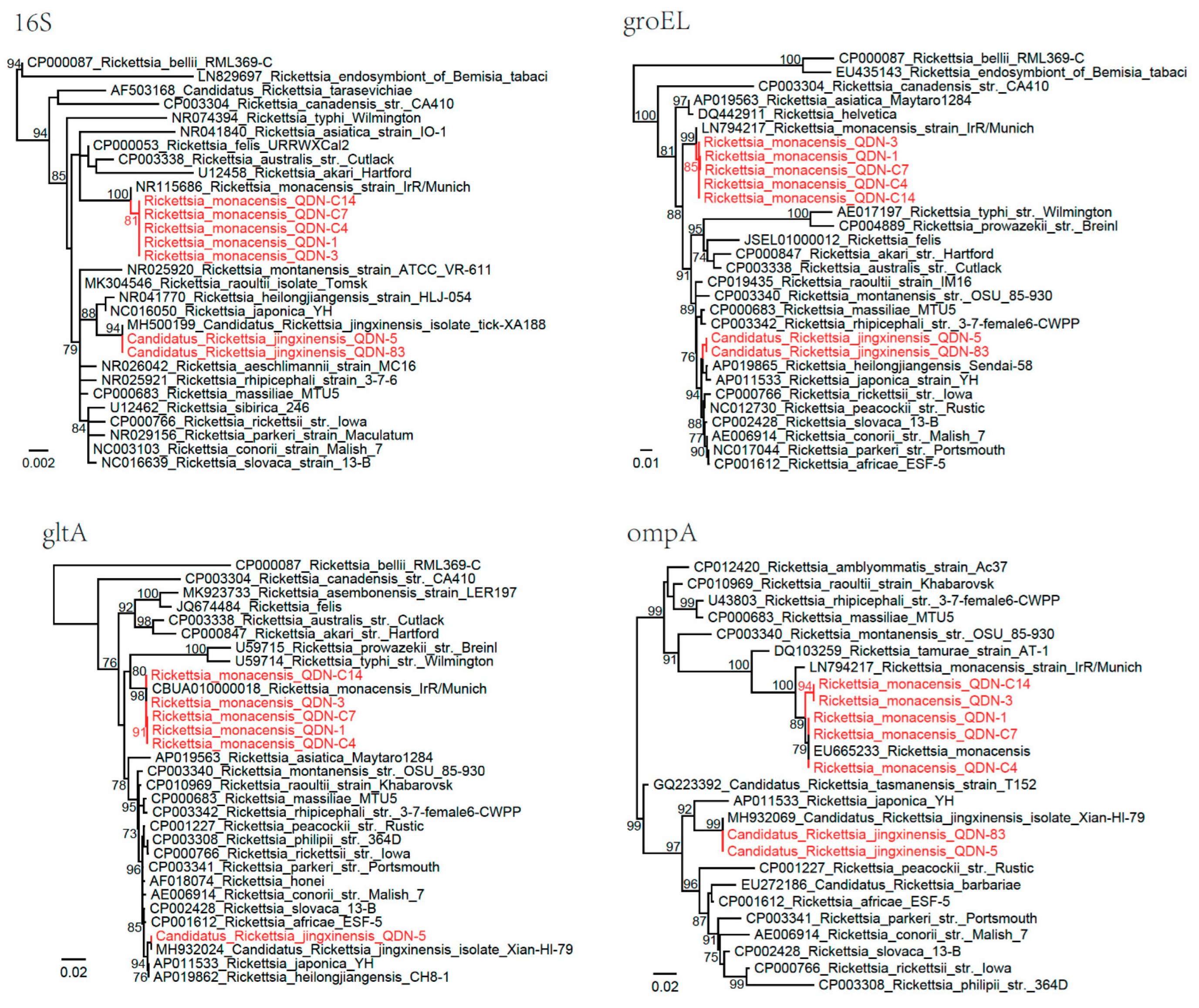
3.2. Detection and Characterization of Rickettsia Isolates
3.3. Detection and Characterization of Candidatus Lariskella Isolates
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mansfield, K.L.; Jizhou, L.; Phipps, L.P.; Johnson, N. Emerging tick-borne viruses in the twenty-first century. Front. Cell Infect. Microbiol. 2017, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, B.; Huang, S.Y.; Wei, F.; Zhu, X.Q. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014, 14, 763–772. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Holmes, E.C.; Hudson, B.J.; Schloeffel, R.; Guillemin, G.J. Human tick-borne diseases in Australia. Front. Cell Infect. Microbiol. 2019, 9, 3. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Ma, J.; Lv, X.L.; Zhang, X.; Han, S.Z.; Wang, Z.D.; Li, L.; Sun, H.T.; Ma, L.X.; Cheng, Z.L.; Shao, J.W.; et al. Identification of a new orthonairovirus associated with human febrile illness in China. Nat. Med. 2021, 27, 434–439. [Google Scholar] [CrossRef]
- Kodama, F.; Yamaguchi, H.; Park, E.; Tatemoto, K.; Sashika, M.; Nakao, R.; Terauchi, Y.; Mizuma, K.; Orba, Y.; Kariwa, H.; et al. A novel nairovirus associated with acute febrile illness in Hokkaido, Japan. Nat. Commun. 2021, 12, 5539. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.B.; Shimoda, H.; Ishijima, K.; Yonemitsu, K.; Minami, S.; Kuroda, Y.; Tatemoto, K.; Mendoza, M.V.; Kuwata, R.; Takano, A.; et al. Zoonotic infection with Oz virus, a novel Thogotovirus. Emerg. Infect. Dis. 2022, 28, 436–439. [Google Scholar] [CrossRef]
- Zhao, G.P.; Wang, Y.X.; Fan, Z.W.; Ji, Y.; Liu, M.J.; Zhang, W.H.; Li, X.L.; Zhou, S.X.; Li, H.; Liang, S.; et al. Mapping ticks and tick-borne pathogens in China. Nat. Commun. 2021, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.L.; Curto, P.; Simões, I. Moonlighting in Rickettsiales: Expanding virulence landscape. Trop. Med. Infect. Dis. 2022, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Low, V.L.; Tan, T.K.; Khoo, J.J.; Lim, F.S.; AbuBakar, S. An overview of rickettsiae in Southeast Asia: Vector-animal-human interface. Acta. Trop. 2020, 202, 105282. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.; Beck, R.; Oteo, J.A.; Pfeffer, M.; Sprong, H. Neoehrlichiosis: An emerging tick-borne zoonosis caused by Candidatus Neoehrlichia mikurensis. Exp. Appl. Acarol. 2016, 68, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Sassera, D.; Beninati, T.; Bandi, C.; Bouman, E.A.P.; Sacchi, L.; Fabbi, M.; Lo, N. ‘Candidatus Midichloria mitochondrii’, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int. J. Syst. Evol. Microbiol. 2006, 56, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M.; Sassera, D.; Epis, S.; Bazzocchi, C.; Vannini, C.; Lo, N.; Sacchi, L.; Fukatsu, T.; Petroni, G.; Bandi, C. “Candidatus Midichloriaceae” fam. nov. (Rickettsiales), an ecologically widespread clade of intracellular alphaproteobacteria. Appl. Environ. Microbiol. 2013, 79, 3241–3248. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.I.; Ivanov, L.I.; Nishikawa, M.; Saito, R.; Sidel’nikov, I.N.; Zdanovskaia, N.I.; Mokretsova, E.V.; Tarasevich, I.V.; Suzuki, H. Microorganism “Montezuma” of the order Rickettsiales: The potential causative agent of tick-borne disease in the Far East of Russia. Z. Mikrobiol. Epidemiol. Immunobiol. 2004, 1, 7–13. [Google Scholar]
- Bazzocchi, C.; Mariconti, M.; Sassera, D.; Rinaldi, L.; Martin, E.; Cringoli, G.; Urbanelli, S.; Genchi, C.; Bandi, C.; Epis, S. Molecular and serological evidence for the circulation of the tick symbiont Midichloria (Rickettsiales: Midichloriaceae) in different mammalian species. Parasit. Vectors. 2013, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Tian, J.; Pan, X.; Qin, X.; Wang, W.; Chen, J.; Guo, W.; Li, K. Identification of Rickettsia spp., Anaplasma spp., and an Ehrlichia canis-like agent in Rhipicephalus microplus from Southwest and South-Central China. Ticks Tick Borne Dis. 2022, 13, 101884. [Google Scholar] [CrossRef]
- Lu, M.; Tian, J.; Zhao, H.; Jiang, H.; Qin, X.; Wang, W.; Li, K. Molecular survey of vector-borne pathogens in ticks, sheep keds, and domestic animals from Ngawa, Southwest China. Pathogens 2022, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Delsuc, F.; Dufayard, J.F.; Gascuel, O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol. Biol. 2009, 537, 113–137. [Google Scholar] [CrossRef]
- Li, L.H.; Zhang, Y.; Wang, J.Z.; Li, X.S.; Yin, S.Q.; Zhu, D.; Xue, J.B.; Li, S.G. High genetic diversity in hard ticks from a China-Myanmar border county. Parasit. Vectors. 2018, 11, 469. [Google Scholar] [CrossRef]
- Bang, M.S.; Kim, C.M.; Pyun, S.H.; Kim, D.M.; Yun, N.R. Molecular investigation of tick-borne pathogens in ticks removed from tick-bitten humans in the southwestern region of the Republic of Korea. PLoS ONE 2021, 16, e0252992. [Google Scholar] [CrossRef]
- Liu, H.; Liang, X.; Wang, H.; Sun, X.; Bai, X.; Hu, B.; Shi, N.; Wang, N.; Zhang, X.; Huang, L.; et al. Molecular evidence of the spotted fever group Rickettsiae in ticks from Yunnan Province, Southwest China. Exp. Appl. Acarol. 2020, 80, 339–348. [Google Scholar] [CrossRef]
- Guo, W.P.; Wang, Y.H.; Lu, Q.; Xu, G.; Luo, Y.; Ni, X.; Zhou, E.M. Molecular detection of spotted fever group rickettsiae in hard ticks, northern China. Transbound. Emerg. Dis. 2019, 66, 1587–1596. [Google Scholar] [CrossRef]
- Simser, J.A.; Palmer, A.T.; Fingerle, V.; Wilske, B.; Kurtti, T.J.; Munderloh, U.G. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl. Environ. Microbiol. 2002, 68, 4559–4566. [Google Scholar] [CrossRef]
- Springer, A.; Montenegro, V.M.; Schicht, S.; Wölfel, S.; Schaper, S.R.; Chitimia-Dobler, L.; Siebert, S.; Strube, C. Detection of Rickettsia monacensis and Rickettsia amblyommatis in ticks collected from dogs in Costa Rica and Nicaragua. Ticks Tick Borne Dis. 2018, 9, 1565–1572. [Google Scholar] [CrossRef]
- Ye, X.; Sun, Y.; Ju, W.; Wang, X.; Cao, W.; Wu, M. Vector competence of the tick Ixodes sinensis (Acari: Ixodidae) for Rickettsia monacensis. Parasit. Vectors. 2014, 7, 512. [Google Scholar] [CrossRef]
- Selmi, R.; Ben Said, M.; Ben Yahia, H.; Abdelaali, H.; Messadi, L. Molecular epidemiology and phylogeny of spotted fever group Rickettsia in camels (Camelus dromedarius) and their infesting ticks from Tunisia. Transbound Emerg. Dis. 2020, 67, 733–744. [Google Scholar] [CrossRef]
- Matei, I.A.; Corduneanu, A.; Sándor, A.D.; Ionică, A.M.; Panait, L.; Kalmár, Z.; Ivan, T.; Papuc, I.; Bouari, C.; Fit, N.; et al. Rickettsia spp. in bats of Romania: High prevalence of Rickettsia monacensis in two insectivorous bat species. Parasit. Vectors. 2021, 14, 107. [Google Scholar] [CrossRef]
- Lu, M.; Li, F.; Liao, Y.; Shen, J.J.; Xu, J.M.; Chen, Y.Z.; Li, J.H.; Holmes, E.C.; Zhang, Y.Z. Epidemiology and diversity of Rickettsiales bacteria in humans and animals in Jiangsu and Jiangxi provinces, China. Sci. Rep. 2019, 9, 13176. [Google Scholar] [CrossRef]
- Guo, W.P.; Tian, J.H.; Lin, X.D.; Ni, X.B.; Chen, X.P.; Liao, Y.; Yang, S.Y.; Dumler, J.S.; Holmes, E.C.; Zhang, Y.Z. Extensive genetic diversity of Rickettsiales bacteria in multiple mosquito species. Sci. Rep. 2016, 6, 38770. [Google Scholar] [CrossRef]
- Matsuura, Y.; Kikuchi, Y.; Meng, X.Y.; Koga, R.; Fukatsu, T. Novel clade of alphaproteobacterial endosymbionts associated with stinkbugs and other arthropods. Appl. Environ. Microbiol. 2012, 78, 4149–4156. [Google Scholar] [CrossRef]

| “Ca. Rickettsia Jingxinensis” | Rickettsia monacensis | “Ca. Lariskella Guizhouensis” | |
|---|---|---|---|
| R. microplus | 1/1 (100%) a | 0/1 (0.00%) | 0/1 (0.00%) |
| H. longicornis | 0/1 (0.00%) | 0/1 (0.00%) | 0/1 (0.00%) |
| H. flava | 1/3 (33.33%) | 1/3 (33.33%) | 0/1 (0.00%) |
| H. kitaokai | 1/3 (33.33%) | 2/3 (66.67%) | 0/3 (0.00%) |
| I. ovatus | 0/4 (0.00%) | 0/4 (0.00%) | 1/4 (25.00%) |
| I. sinensis | 4/101 (3.96%) | 74/101 (73.27%) | 10/101 (9.90%) |
| Total | 7/113 (6.19%) | 77/113 (68.14%) | 11/113 (9.73%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Meng, C.; Zhang, B.; Wang, X.; Tian, J.; Tang, G.; Wang, W.; Li, N.; Li, M.; Xu, X.; et al. Prevalence of Spotted Fever Group Rickettsia and Candidatus Lariskella in Multiple Tick Species from Guizhou Province, China. Biomolecules 2022, 12, 1701. https://doi.org/10.3390/biom12111701
Lu M, Meng C, Zhang B, Wang X, Tian J, Tang G, Wang W, Li N, Li M, Xu X, et al. Prevalence of Spotted Fever Group Rickettsia and Candidatus Lariskella in Multiple Tick Species from Guizhou Province, China. Biomolecules. 2022; 12(11):1701. https://doi.org/10.3390/biom12111701
Chicago/Turabian StyleLu, Miao, Chao Meng, Bing Zhang, Xiao Wang, Junhua Tian, Guangpeng Tang, Wen Wang, Na Li, Mengyao Li, Xiaoyu Xu, and et al. 2022. "Prevalence of Spotted Fever Group Rickettsia and Candidatus Lariskella in Multiple Tick Species from Guizhou Province, China" Biomolecules 12, no. 11: 1701. https://doi.org/10.3390/biom12111701
APA StyleLu, M., Meng, C., Zhang, B., Wang, X., Tian, J., Tang, G., Wang, W., Li, N., Li, M., Xu, X., Sun, Y., Duan, C., Qin, X., & Li, K. (2022). Prevalence of Spotted Fever Group Rickettsia and Candidatus Lariskella in Multiple Tick Species from Guizhou Province, China. Biomolecules, 12(11), 1701. https://doi.org/10.3390/biom12111701







