Exosomal Non-Coding RNAs: Novel Regulators of Macrophage-Linked Intercellular Communication in Lung Cancer and Inflammatory Lung Diseases
Abstract
1. Introduction
2. Overview of Macrophage Polarization Phenotypes
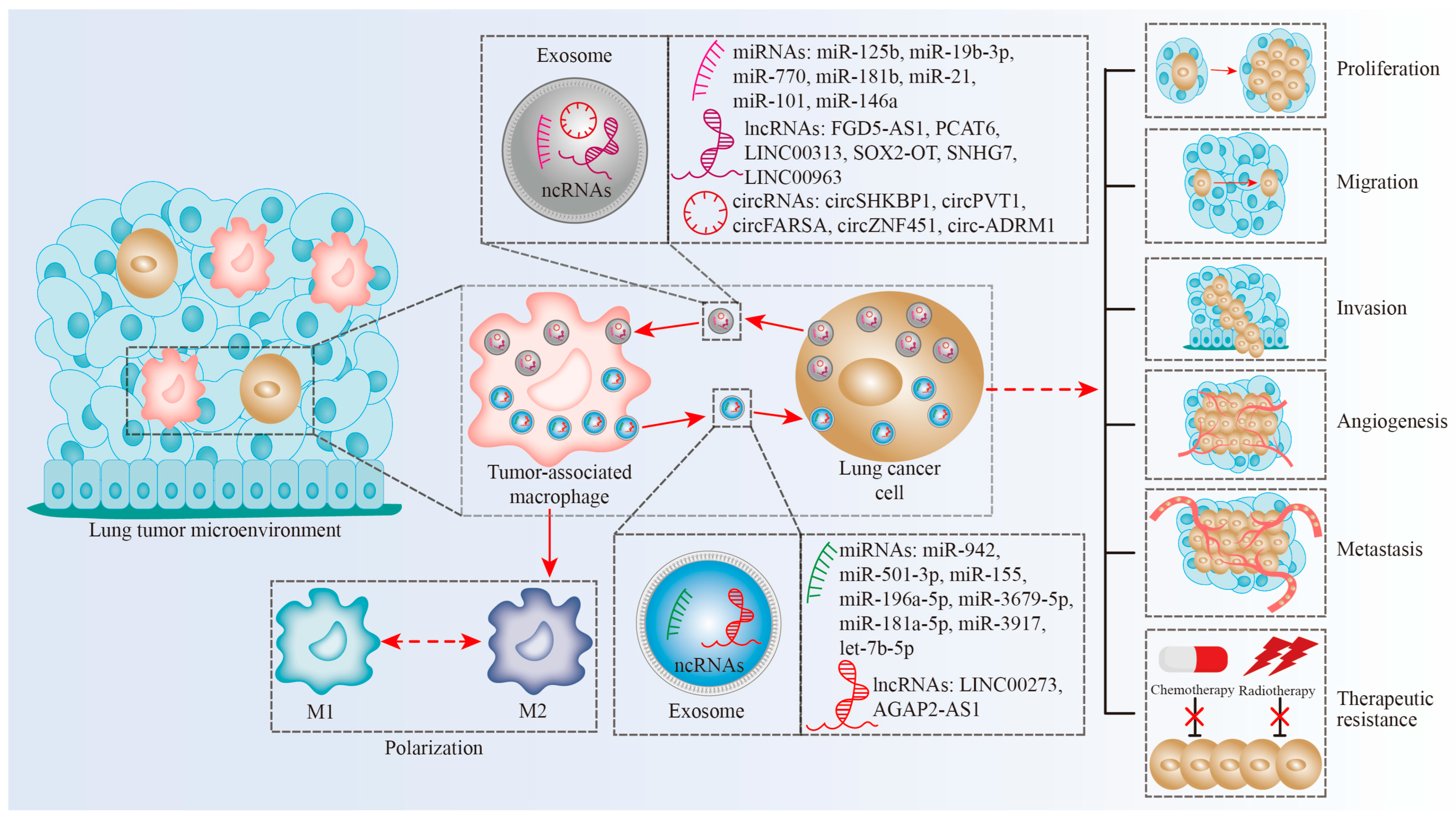
3. Exosomal ncRNAs Regulate Macrophage-Linked Intercellular Communication in Lung Cancer
3.1. The Impacts of Exosomal miRNAs on Macrophage-Linked Intercellular Communication
3.1.1. Exosomal miRNAs from Macrophages Can Be Transferred into Lung Tumor Cells
3.1.2. Exosomal miRNAs from Lung Tumor Cells Can Be Transferred into Macrophages
3.2. The Impacts of Exosomal lncRNAs on Macrophage-Linked Intercellular Communication
3.2.1. Exosomal lncRNAs from Macrophages Can Be Transferred into Lung Tumor Cells
3.2.2. Exosomal lncRNAs from Lung Tumor Cells Can Be Transferred into Macrophages
3.3. The Impacts of Exosomal circRNAs on Macrophage-Linked Intercellular Communication
4. Exosomal ncRNAs Regulate Macrophage-Linked Intercellular Communication in Acute Lung Injury
4.1. Exosomal ncRNAs from Immune Cells Can Be Transferred into Macrophages
4.2. Exosomal ncRNAs from Stem Cells Can Be Transferred into Macrophages
4.3. Exosomal ncRNAs from Epithelial Cells Can Be Transferred into Macrophages
4.4. Exosomal ncRNAs from other Sources Can Be Transferred into Macrophages
5. Exosomal ncRNAs Regulate Macrophage-Linked Intercellular Communication in Pulmonary Fibrosis
5.1. Exosomal ncRNAs from Macrophages Can Be Transferred into Fibroblasts
5.2. Exosomal ncRNAs from Other Sources Can Be Transferred into Macrophages
6. Exosomal ncRNAs Regulate Macrophage-Linked Intercellular Communication in Asthma
7. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; MacDonald, A.S. The impact of the lung environment on macrophage development, activation and function: Diversity in the face of adversity. Mucosal Immunol. 2022, 15, 223–234. [Google Scholar] [CrossRef]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, J.; Westphalen, K. Macrophage-epithelial interactions in pulmonary alveoli. Semin. Immunopathol. 2016, 38, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Matthay, M.A. Extracellular Vesicle Transfer from Mesenchymal Stromal Cells Modulates Macrophage Function in Acute Lung Injury. Basic Science and Clinical Implications. Am. J. Respir. Crit. Care Med. 2017, 196, 1234–1236. [Google Scholar] [CrossRef]
- Fricker, M.; Gibson, P.G. Macrophage dysfunction in the pathogenesis and treatment of asthma. Eur. Respir. J. 2017, 50, 1700196. [Google Scholar] [CrossRef]
- Byrne, A.J.; Maher, T.M.; Lloyd, C.M. Pulmonary Macrophages: A New Therapeutic Pathway in Fibrosing Lung Disease? Trends Mol. Med. 2016, 22, 303–316. [Google Scholar] [CrossRef]
- Uthamacumaran, A. Cancer: A turbulence problem. Neoplasia 2020, 22, 759–769. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Zhang, T.; Gao, Y.; Shi, H.; Song, K.; Chen, W.; Yin, W. Applications of Single-Cell Omics in Tumor Immunology. Front. Immunol. 2021, 12, 697412. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, J.; Liang, M.; Song, M. Development of functional nanomedicines for tumor associated macrophages-focused cancer immunotherapy. Theranostics 2022, 12, 7821–7852. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Brennan, G.P.; Henshall, D.C. MicroRNAs as regulators of brain function and targets for treatment of epilepsy. Nat. Rev. Neurol. 2020, 16, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Karreth, F.A.; Pandolfi, P.P. ceRNA cross-talk in cancer: When ce-bling rivalries go awry. Cancer Discov. 2013, 3, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Li, P.; Wu, M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol. Cancer 2017, 16, 94. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Sun, H.; Wang, J.; Liang, Y.; Wang, Y.; Wong, G. The bioinformatics toolbox for circRNA discovery and analysis. Brief. Bioinform. 2021, 22, 1706–1728. [Google Scholar] [CrossRef]
- Li, C.; Ni, Y.Q.; Xu, H.; Xiang, Q.Y.; Zhao, Y.; Zhan, J.K.; He, J.Y.; Li, S.; Liu, Y.S. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct. Target. Ther. 2021, 6, 383. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Ma, L.; Chen, Y.; Liu, J.; Guo, Y.; Yu, T.; Zhang, L.; Zhu, L.; Shu, Y. Role of exosomal non-coding RNAs from tumor cells and tumor-associated macrophages in the tumor microenvironment. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 3133–3154. [Google Scholar] [CrossRef]
- Fan, Q.; Yang, L.; Zhang, X.; Peng, X.; Wei, S.; Su, D.; Zhai, Z.; Hua, X.; Li, H. The emerging role of exosome-derived non-coding RNAs in cancer biology. Cancer Lett. 2018, 414, 107–115. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Li, C.; Chen, T.; Yang, Q. Exosomal non-coding RNAs: Emerging roles in bilateral communication between cancer cells and macrophages. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 1036–1053. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, X.; Li, H.; Gelinsky, M.; Gu, Z. Tailoring Materials for Modulation of Macrophage Fate. Adv. Mater. 2021, 33, e2004172. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lyon, C.J.; Fletcher, J.K.; Tang, W.; Wan, M.; Hu, T.Y. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm. Sin. B 2021, 11, 1493–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M.; Ericsson, A.C. Function of Macrophages in Disease: Current Understanding on Molecular Mechanisms. Front. Immunol. 2021, 12, 620510. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Chávez-Galán, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages. Front. Immunol. 2015, 6, 263. [Google Scholar] [CrossRef]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Bender, E. Epidemiology: The dominant malignancy. Nature 2014, 513, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Tsai, Y.M.; Lien, C.T.; Kuo, P.L.; Hung, A.J. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1611. [Google Scholar] [CrossRef] [PubMed]
- Morgensztern, D.; Ng, S.H.; Gao, F.; Govindan, R. Trends in stage distribution for patients with non-small cell lung cancer: A National Cancer Database survey. J. Thorac. Oncol. 2010, 5, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.; Chirieac, L.R.; D’Amico, T.A.; DeCamp, M.M.; Dilling, T.J.; Dobelbower, M.; et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2017, 15, 504–535. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Akerley, W.; Bazhenova, L.A.; Borghaei, H.; Camidge, D.R.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Dilling, T.J.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J. Natl. Compr. Cancer Netw. JNCCN 2016, 14, 255–264. [Google Scholar] [CrossRef]
- Wang, N.; Wang, S.; Wang, X.; Zheng, Y.; Yang, B.; Zhang, J.; Pan, B.; Gao, J.; Wang, Z. Research trends in pharmacological modulation of tumor-associated macrophages. Clin. Transl. Med. 2021, 11, e288. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Karger, A.; Nandigama, R.; Stenzinger, A.; Grimminger, F.; Pullamsetti, S.S.; Seeger, W.; Savai, R. Hidden Treasures: Macrophage Long Non-Coding RNAs in Lung Cancer Progression. Cancers 2021, 13, 4127. [Google Scholar] [CrossRef]
- Pritchard, A.; Tousif, S.; Wang, Y.; Hough, K.; Khan, S.; Strenkowski, J.; Chacko, B.K.; Darley-Usmar, V.M.; Deshane, J.S. Lung Tumor Cell-Derived Exosomes Promote M2 Macrophage Polarization. Cells 2020, 9, 1303. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, D.; Lee, H.; Menon, A.A.; Wu, J.; Hu, K.; Jin, Y. Macrophage-derived apoptotic bodies promote the proliferation of the recipient cells via shuttling microRNA-221/222. J. Leukoc. Biol. 2017, 101, 1349–1359. [Google Scholar] [CrossRef]
- Wan, X.; Xie, B.; Sun, H.; Gu, W.; Wang, C.; Deng, Q.; Zhou, S. Exosomes derived from M2 type tumor-associated macrophages promote osimertinib resistance in non-small cell lung cancer through MSTRG.292666.16-miR-6836-5p-MAPK8IP3 axis. Cancer Cell Int. 2022, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Ma, Z.; Yang, F.; Zhao, X.; Jiang, W.; Pan, C.; Li, Z.; Pan, X.; He, Z.; Xu, J.; et al. M2 macrophage-derived exosomes promote lung adenocarcinoma progression by delivering miR-942. Cancer Lett. 2022, 526, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Chen, P.; Zhang, F.; Zhang, N.; Zhu, J.; Wang, X.; Jiang, T. M2 macrophages-derived exosomal microRNA-501-3p promotes the progression of lung cancer via targeting WD repeat domain 82. Cancer Cell Int. 2021, 21, 91. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Ni, Y.; Bian, C.; Huang, J.; Chen, L.; Xie, X.; Wang, J. Tumor-associated macrophages secret exosomal miR-155 and miR-196a-5p to promote metastasis of non-small-cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 1338–1354. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Pan, H.; Wang, Y.; Shi, M.; Yu, H.; Wang, C.; Pan, X.; Chen, Z. Exosomes Derived From Macrophages Enhance Aerobic Glycolysis and Chemoresistance in Lung Cancer by Stabilizing c-Myc via the Inhibition of NEDD4L. Front. Cell Dev. Biol. 2020, 8, 620603. [Google Scholar] [CrossRef]
- Palma, M.; Leroy, C.; Salomé-Desnoulez, S.; Werkmeister, E.; Kong, R.; Mongy, M.; Le Hir, H.; Lejeune, F. A role for AKT1 in nonsense-mediated mRNA decay. Nucleic Acids Res. 2021, 49, 11022–11037. [Google Scholar] [CrossRef]
- Song, S.; Zhao, Y.; Wang, X.; Tong, X.; Chen, X.; Xiong, Q. M2 macrophages-derived exosomal miR-3917 promotes the progression of lung cancer via targeting GRK6. Biol. Chem. 2022, 404, 41–57. [Google Scholar] [CrossRef]
- Wang, X.; Huang, R.; Lu, Z.; Wang, Z.; Chen, X.; Huang, D. Exosomes from M1-polarized macrophages promote apoptosis in lung adenocarcinoma via the miR-181a-5p/ETS1/STK16 axis. Cancer Sci. 2022, 113, 986–1001. [Google Scholar] [CrossRef]
- Peng, J.; Li, S.; Li, B.; Hu, W.; Ding, C. Exosomes derived from M1 macrophages inhibit the proliferation of the A549 and H1299 lung cancer cell lines via the miRNA-let-7b-5p-GNG5 axis. PeerJ 2023, 11, e14608. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, K.; Zhi, Y.; Wu, Y.; Chen, B.; Bai, J.; Wang, X. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin. Transl. Med. 2021, 11, e478. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, S.; Liu, Y.; Han, H.; Gong, M.; Song, Y. The role of exosomal miR-181b in the crosstalk between NSCLC cells and tumor-associated macrophages. Genes Genom. 2022, 44, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wen, J.X.; Cao, X.S.; Zhao, W.; Han, Y.L.; Wen, X.H.; Yan, L.; Zhang, M.; Wang, Y.F.; Hai, L.; et al. Tumor cell-derived exosomal microRNA-146a promotes non-small cell lung cancer cell invasion and proliferation by inhibiting M1 macrophage polarization. Ann. Transl. Med. 2022, 10, 1307. [Google Scholar] [CrossRef]
- Salem, A.; Asselin, M.C.; Reymen, B.; Jackson, A.; Lambin, P.; West, C.M.L.; O’Connor, J.P.B.; Faivre-Finn, C. Targeting Hypoxia to Improve Non-Small Cell Lung Cancer Outcome. J. Natl. Cancer Inst. 2018, 110, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yu, G. Hypoxic lung cancer cell-derived exosomal miR-21 mediates macrophage M2 polarization and promotes cancer cell proliferation through targeting IRF1. World J. Surg. Oncol. 2022, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, P.; Wu, D.; Guan, M.; Weng, X.; Lu, Y.; Zeng, Y.; Chen, R. Hypoxic stress suppresses lung tumor-secreted exosomal miR101 to activate macrophages and induce inflammation. Cell Death Dis. 2021, 12, 776. [Google Scholar] [CrossRef]
- Liu, J.; Luo, R.; Wang, J.; Luan, X.; Wu, D.; Chen, H.; Hou, Q.; Mao, G.; Li, X. Tumor Cell-Derived Exosomal miR-770 Inhibits M2 Macrophage Polarization via Targeting MAP3K1 to Inhibit the Invasion of Non-small Cell Lung Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 679658. [Google Scholar] [CrossRef]
- Trivedi, M.; Talekar, M.; Shah, P.; Ouyang, Q.; Amiji, M. Modification of tumor cell exosome content by transfection with wt-p53 and microRNA-125b expressing plasmid DNA and its effect on macrophage polarization. Oncogenesis 2016, 5, e250. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Adamson, B.; Smogorzewska, A.; Sigoillot, F.D.; King, R.W.; Elledge, S.J. A genome-wide homologous recombination screen identifies the RNA-binding protein RBMX as a component of the DNA-damage response. Nat. Cell Biol. 2012, 14, 318–328. [Google Scholar] [CrossRef]
- Sarkar, A.; Rahaman, A.; Biswas, I.; Mukherjee, G.; Chatterjee, S.; Bhattacharjee, S.; Mandal, D.P. TGFβ mediated LINC00273 upregulation sponges mir200a-3p and promotes invasion and metastasis by activating ZEB1. J. Cell. Physiol. 2020, 235, 7159–7172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sang, Y.; Chen, D.; Wu, X.; Wang, X.; Yang, W.; Chen, Y. M2 macrophage-derived exosomal long non-coding RNA AGAP2-AS1 enhances radiotherapy immunity in lung cancer by reducing microRNA-296 and elevating NOTCH2. Cell Death Dis. 2021, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Li, Q.; Ma, R.; Wang, Z.; Yu, Y.; Liu, H.; Miao, Y.; Jiang, S. Long Noncoding RNA FGD5-AS1 Knockdown Decrease Viability, Migration, and Invasion of Non-Small Cell Lung Cancer (NSCLC) Cells by Regulating the MicroRNA-944/MACC1 Axis. Technol. Cancer Res. Treat. 2021, 20, 1533033821990090. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hong, C.; Qu, J.; Chen, J.; Qin, Z. Knockdown of lncRNA PCAT6 suppresses the growth of non-small cell lung cancer cells by inhibiting macrophages M2 polarization via miR-326/KLF1 axis. Bioengineered 2022, 13, 12834–12846. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, L.; Chen, Y.; Yu, Z.; Zhao, Z. Cancer cell-derived exosomal LINC00313 induces M2 macrophage differentiation in non-small cell lung cancer. Clin. Transl. Oncol. 2022, 24, 2395–2408. [Google Scholar] [CrossRef]
- Yan, D.; Huelse, J.M.; Kireev, D.; Tan, Z.; Chen, L.; Goyal, S.; Wang, X.; Frye, S.V.; Behera, M.; Schneider, F.; et al. MERTK activation drives osimertinib resistance in EGFR-mutant non-small cell lung cancer. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Zhou, D.; Xia, Z.; Xie, M.; Gao, Y.; Yu, Q.; He, B. Exosomal long non-coding RNA SOX2 overlapping transcript enhances the resistance to EGFR-TKIs in non-small cell lung cancer cell line H1975. Hum. Cell 2021, 34, 1478–1489. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, X.; Li, J.; Zheng, Z.; Zhang, J.; Zhao, W.; Liu, L. Tumour-derived exosomal lncRNA-SOX2OT promotes bone metastasis of non-small cell lung cancer by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts. Cell Death Dis. 2021, 12, 662. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Du, L.; Jiang, X.; Yan, S.; Duan, W.; Li, J.; Zhan, Y.; Wang, L.; Zhang, S.; et al. Circulating long noncoding RNA act as potential novel biomarkers for diagnosis and prognosis of non-small cell lung cancer. Mol. Oncol. 2018, 12, 648–658. [Google Scholar] [CrossRef]
- Zheng, C.; Tang, F.; Min, L.; Hornicek, F.; Duan, Z.; Tu, C. PTEN in osteosarcoma: Recent advances and the therapeutic potential. Biochim. Biophys. Acta. Rev. Cancer 2020, 1874, 188405. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, J.; Li, C.; Yuan, Y.; Fang, S.; Liu, W.; Qian, Y.; Ma, J.; Chang, L.; Chen, F.; et al. Exosome-mediated transfer of SNHG7 enhances docetaxel resistance in lung adenocarcinoma. Cancer Lett. 2022, 526, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Xu, B.; Ma, J.; Li, L.; Zhang, L.; Wang, L.; Zhu, J.; Guo, T.; Zhang, H.; Wang, S. LINC00963 promotes the malignancy and metastasis of lung adenocarcinoma by stabilizing Zeb1 and exosomes-induced M2 macrophage polarization. Mol. Med. 2023, 29, 1. [Google Scholar] [CrossRef]
- Chen, W.; Tang, D.; Lin, J.; Huang, X.; Lin, S.; Shen, G.; Dai, Y. Exosomal circSHKBP1 participates in non-small cell lung cancer progression through PKM2-mediated glycolysis. Mol. Ther. Oncolytics 2022, 24, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Song, X. Exosomal circPVT1 derived from lung cancer promotes the progression of lung cancer by targeting miR-124-3p/EZH2 axis and regulating macrophage polarization. Cell Cycle (Georget. Tex.) 2022, 21, 514–530. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.; Li, C.; Xu, C.; Ding, C.; Chen, J.; Zhao, J. Tumor-derived exosomal circFARSA mediates M2 macrophage polarization via the PTEN/PI3K/AKT pathway to promote non-small cell lung cancer metastasis. Cancer Treat. Res. Commun. 2021, 28, 100412. [Google Scholar] [CrossRef] [PubMed]
- Hang, D.; Zhou, J.; Qin, N.; Zhou, W.; Ma, H.; Jin, G.; Hu, Z.; Dai, J.; Shen, H. A novel plasma circular RNA circFARSA is a potential biomarker for non-small cell lung cancer. Cancer Med. 2018, 7, 2783–2791. [Google Scholar] [CrossRef]
- Gao, J.; Ao, Y.Q.; Zhang, L.X.; Deng, J.; Wang, S.; Wang, H.K.; Jiang, J.H.; Ding, J.Y. Exosomal circZNF451 restrains anti-PD1 treatment in lung adenocarcinoma via polarizing macrophages by complexing with TRIM56 and FXR1. J. Exp. Clin. Cancer Res. CR 2022, 41, 295. [Google Scholar] [CrossRef]
- Qian, J.; Li, J.; Ma, H.; Ji, W. Exosomal circ-ADRM1 promotes lung adenocarcinoma progression and induces macrophage M2 polarization through regulating MMP14 mRNA and protein. Anti-Cancer Drugs 2022, 34, 333–343. [Google Scholar] [CrossRef]
- Mokra, D.; Mikolka, P.; Kosutova, P.; Mokry, J. Corticosteroids in Acute Lung Injury: The Dilemma Continues. Int. J. Mol. Sci. 2019, 20, 4765. [Google Scholar] [CrossRef]
- Bhatia, M.; Zemans, R.L.; Jeyaseelan, S. Role of chemokines in the pathogenesis of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2012, 46, 566–572. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Li, Z.G.; Scott, M.J.; Brzóska, T.; Sundd, P.; Li, Y.H.; Billiar, T.R.; Wilson, M.A.; Wang, P.; Fan, J. Lung epithelial cell-derived IL-25 negatively regulates LPS-induced exosome release from macrophages. Mil. Med. Res. 2018, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiao, K.; Xie, L. Advances in the Regulation of Macrophage Polarization by Mesenchymal Stem Cells and Implications for ALI/ARDS Treatment. Front. Immunol. 2022, 13, 928134. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, L.; Li, H.; Ren, W.; Zhuo, R.; Feng, C.; He, Y.; Hu, Y.; Ye, C. Alveolar macrophage-derived exosomal tRF-22-8BWS7K092 activates Hippo signaling pathway to induce ferroptosis in acute lung injury. Int. Immunopharmacol. 2022, 107, 108690. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Zhang, X.; Peng, Z.; Ye, Y.; Liu, R.; Yang, Y.; Chen, Z.; Zhang, Z.; Hu, H.; Yin, S.; et al. Macrophage-derived exosomal aminopeptidase N aggravates sepsis-induced acute lung injury by regulating necroptosis of lung epithelial cell. Commun. Biol. 2022, 5, 543. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of pyroptosis in inflammation and cancer. Cell. Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef]
- Burdette, B.E.; Esparza, A.N.; Zhu, H.; Wang, S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B 2021, 11, 2768–2782. [Google Scholar] [CrossRef]
- Strebovsky, J.; Walker, P.; Lang, R.; Dalpke, A.H. Suppressor of cytokine signaling 1 (SOCS1) limits NFkappaB signaling by decreasing p65 stability within the cell nucleus. FASEB J. 2011, 25, 863–874. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, T.; Zhang, C.; Ji, H.; Tong, X.; Xia, R.; Wang, W.; Ma, Z.; Shi, X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit. Care 2021, 25, 356. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, W.H.; Qiu, Z.M.; Li, Q.G. The Monocyte-Derived Exosomal CLMAT3 Activates the CtBP2-p300-NF-κB Transcriptional Complex to Induce Proinflammatory Cytokines in ALI. Mol. Ther. Nucleic Acids 2020, 21, 1100–1110. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Janockova, J.; Slovinska, L.; Harvanova, D.; Spakova, T.; Rosocha, J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J. Biomed. Sci. 2021, 28, 39. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhang, C.; Lv, N.; Liang, Z.; Ma, T.; Cheng, H.; Xia, Y.; Shi, L. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics 2022, 12, 2928–2947. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, K.; Xie, L. Progress in preclinical studies of macrophage autophagy in the regulation of ALI/ARDS. Front. Immunol. 2022, 13, 922702. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, C.; Wang, Y.; Niu, L.; Jiang, S.; Pan, S. BMSC-Derived Exosomes Ameliorate LPS-Induced Acute Lung Injury by miR-384-5p-Controlled Alveolar Macrophage Autophagy. Oxidative Med. Cell. Longev. 2021, 2021, 9973457. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xing, F.; Fu, Q.; He, B.; Jia, Z.; Du, J.; Li, Y.; Zhang, X.; Chen, X. hUC-MSCs exosomal miR-451 alleviated acute lung injury by modulating macrophage M2 polarization via regulating MIF-PI3K-AKT signaling pathway. Environ. Toxicol. 2022, 37, 2819–2831. [Google Scholar] [CrossRef]
- Liu, F.; Peng, W.; Chen, J.; Xu, Z.; Jiang, R.; Shao, Q.; Zhao, N.; Qian, K. Exosomes Derived From Alveolar Epithelial Cells Promote Alveolar Macrophage Activation Mediated by miR-92a-3p in Sepsis-Induced Acute Lung Injury. Front. Cell. Infect. Microbiol. 2021, 11, 646546. [Google Scholar] [CrossRef]
- Chiang, C.H.; Chuang, C.H.; Liu, S.L.; Lee, T.S.; Kou, Y.R.; Zhang, H. Apocynin attenuates ventilator-induced lung injury in an isolated and perfused rat lung model. Intensive Care Med. 2011, 37, 1360–1367. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W.; Feng, Y.; Xu, Z.; He, Y.; Xiong, Y.; Chen, L.; Li, X.; Liu, J.; Liu, G.; et al. Epithelial-derived exosomes promote M2 macrophage polarization via Notch2/SOCS1 during mechanical ventilation. Int. J. Mol. Med. 2022, 50, 1–16. [Google Scholar] [CrossRef]
- Jiang, K.; Yang, J.; Guo, S.; Zhao, G.; Wu, H.; Deng, G. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 1758–1771. [Google Scholar] [CrossRef]
- He, N.; Tan, H.; Deng, X.; Shu, L.; Qing, B.; Liang, H. MiR-223-3p-loaded exosomes from bronchoalveolar lavage fluid promote alveolar macrophage autophagy and reduce acute lung injury by inhibiting the expression of STK39. Hum. Cell 2022, 35, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhou, J.; Liu, Y.; Xia, R.; Li, Q.; Yan, L.; Chen, Q.; Chen, X.; Jiang, Y.; Chao, G.; et al. Epithelium- and endothelium-derived exosomes regulate the alveolar macrophages by targeting RGS1 mediated calcium signaling-dependent immune response. Cell Death Differ. 2021, 28, 2238–2256. [Google Scholar] [CrossRef]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.; Marchal-Sommé, J.; Marchand-Adam, S.; Quesnel, C.; Borie, R.; Dehoux, M.; Ruffié, C.; Callebert, J.; Launay, J.M.; Hénin, D.; et al. Modulation of bleomycin-induced lung fibrosis by serotonin receptor antagonists in mice. Eur. Respir. J. 2008, 32, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, Y.; Du, S.; Li, S.; Zhang, Y.; Liu, F.; Chen, Y.; Weng, D.; Chen, J. Dioscin Exerts Protective Effects Against Crystalline Silica-induced Pulmonary Fibrosis in Mice. Theranostics 2017, 7, 4255–4275. [Google Scholar] [CrossRef]
- Hettiarachchi, S.U.; Li, Y.H.; Roy, J.; Zhang, F.; Puchulu-Campanella, E.; Lindeman, S.D.; Srinivasarao, M.; Tsoyi, K.; Liang, X.; Ayaub, E.A.; et al. Targeted inhibition of PI3 kinase/mTOR specifically in fibrotic lung fibroblasts suppresses pulmonary fibrosis in experimental models. Sci. Transl. Med. 2020, 12, eaay3724. [Google Scholar] [CrossRef]
- Liu, P.; Miao, K.; Zhang, L.; Mou, Y.; Xu, Y.; Xiong, W.; Yu, J.; Wang, Y. Curdione ameliorates bleomycin-induced pulmonary fibrosis by repressing TGF-β-induced fibroblast to myofibroblast differentiation. Respir. Res. 2020, 21, 58. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lin, X.; Liu, L.; Li, Y.; Li, X.; Deng, Z.; Chen, H.; Chen, H.; Niu, Z.; Li, Z.; et al. Macrophage-derived exosomes mediate silica-induced pulmonary fibrosis by activating fibroblast in an endoplasmic reticulum stress-dependent manner. J. Cell. Mol. Med. 2021, 25, 4466–4477. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, L.; Qin, X.; Ye, Z.; Xie, B.; Hu, Y. Macrophage derived miR-7219-3p-containing exosomes mediate fibroblast trans-differentiation by targeting SPRY1 in silicosis. Toxicology 2022, 479, 153310. [Google Scholar] [CrossRef]
- Wang, D.; Hao, C.; Zhang, L.; Zhang, J.; Liu, S.; Li, Y.; Qu, Y.; Zhao, Y.; Huang, R.; Wei, J.; et al. Exosomal miR-125a-5p derived from silica-exposed macrophages induces fibroblast transdifferentiation. Ecotoxicol. Environ. Saf. 2020, 192, 110253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lao, T.; Qiu, W.; Polverino, F.; Gupta, K.; Guo, F.; Mancini, J.D.; Naing, Z.Z.; Cho, M.H.; Castaldi, P.J.; et al. A Chronic Obstructive Pulmonary Disease Susceptibility Gene, FAM13A, Regulates Protein Stability of β-Catenin. Am. J. Respir. Crit. Care Med. 2016, 194, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.Y.; Zhang, W.H.; Ma, W.T.; Liu, Q.H.; Xing, L.H.; Zhao, G.F. microRNA-328 in exosomes derived from M2 macrophages exerts a promotive effect on the progression of pulmonary fibrosis via FAM13A in a rat model. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Qian, Q.; Ma, Q.; Wang, B.; Qian, Q.; Zhao, C.; Feng, F.; Dong, X. Downregulated miR-129-5p expression inhibits rat pulmonary fibrosis by upregulating STAT1 gene expression in macrophages. Int. Immunopharmacol. 2022, 109, 108880. [Google Scholar] [CrossRef]
- Guiot, J.; Cambier, M.; Boeckx, A.; Henket, M.; Nivelles, O.; Gester, F.; Louis, E.; Malaise, M.; Dequiedt, F.; Louis, R.; et al. Macrophage-derived exosomes attenuate fibrosis in airway epithelial cells through delivery of antifibrotic miR-142-3p. Thorax 2020, 75, 870–881. [Google Scholar] [CrossRef]
- Ban, J.; Liu, F.; Zhang, Q.; Chang, S.; Zeng, X.; Chen, J. Macrophage-derived exosomal lncRNA MSTRG.91634.7 inhibits fibroblasts activation by targeting PINK1 in silica-induced lung fibrosis. Toxicol. Lett. 2023, 372, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ban, J.; Chang, S.; Qu, H.; Chen, J.; Liu, F. The aggravate role of exosomal circRNA11:120406118|12040782 on macrophage pyroptosis through miR-30b-5p/NLRP3 axis in silica-induced lung fibrosis. Int. Immunopharmacol. 2023, 114, 109476. [Google Scholar] [CrossRef]
- Tavallaee, G.; Lively, S.; Rockel, J.S.; Ali, S.A.; Im, M.; Sarda, C.; Mitchell, G.M.; Rossomacha, E.; Nakamura, S.; Potla, P.; et al. Contribution of microRNA-27b-3p to synovial fibrotic responses in knee osteoarthritis. Arthritis Rheumatol. 2022, 74, 1928–1942. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Soman, S.S.; Vijayavenkataraman, S. Applications of 3D Bioprinted-Induced Pluripotent Stem Cells in Healthcare. Int. J. Bioprinting 2020, 6, 280. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Zhang, W.; Chen, Y.; Jin, M.; Yang, Z. Exosomes derived from induced pluripotent stem cells suppresses M2-type macrophages during pulmonary fibrosis via miR-302a-3p/TET1 axis. Int. Immunopharmacol. 2021, 99, 108075. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.J.; Brightling, C. Pathogenesis of asthma: Implications for precision medicine. Clin. Sci. 2017, 131, 1723–1735. [Google Scholar] [CrossRef]
- Zuyderduyn, S.; Sukkar, M.B.; Fust, A.; Dhaliwal, S.; Burgess, J.K. Treating asthma means treating airway smooth muscle cells. Eur. Respir. J. 2008, 32, 265–274. [Google Scholar] [CrossRef]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.L.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. J. Lab. Clin. Med. 2018, 191, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wang, C.; Zhang, J.; Zhang, J.; Gu, Y.; Guo, X.; Zuo, X.; Pan, H.; Hsu, A.C.; Wang, G.; et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res. Ther. 2021, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Deng, C.; Zhou, T.; Hu, J.; Dai, B.; Yi, F.; Tian, N.; Jiang, L.; Dong, X.; Zhu, Q.; et al. MicroRNA-370 carried by M2 macrophage-derived exosomes alleviates asthma progression through inhibiting the FGF1/MAPK/STAT1 axis. Int. J. Biol. Sci. 2021, 17, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wu, Y.; Zhang, Y.; Cheng, Y.; Wu, Y.; Fang, H. Scorpion and centipede alleviates severe asthma through M2 macrophage-derived exosomal miR-30b-5p. Aging 2022, 14, 3921–3940. [Google Scholar] [CrossRef]
- He, H.; Cao, L.; Wang, Z.; Wang, Z.; Miao, J.; Li, X.M.; Miao, M. Sinomenine Relieves Airway Remodeling By Inhibiting Epithelial-Mesenchymal Transition Through Downregulating TGF-β1 and Smad3 Expression In Vitro and In Vivo. Front. Immunol. 2021, 12, 736479. [Google Scholar] [CrossRef]
- Li, X.; Yang, N.; Cheng, Q.; Zhang, H.; Liu, F.; Shang, Y. MiR-21-5p in Macrophage-Derived Exosomes Targets Smad7 to Promote Epithelial Mesenchymal Transition of Airway Epithelial Cells. J. Asthma Allergy 2021, 14, 513–524. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, Y.; Xu, J.; Ge, X.; Hu, Z.; Xiao, J.; Ning, Y.; Dong, Y.; Bai, C. Exosomes from mmu_circ_0001359-Modified ADSCs Attenuate Airway Remodeling by Enhancing FoxO1 Signaling-Mediated M2-like Macrophage Activation. Mol. Ther. Nucleic Acids 2020, 19, 951–960. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: A first-in-man, phase 1, open-label, dose-escalation study. Lancet. Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Yang, P.; Liu, J.; Dai, F.; Xiao, Y.; Zhao, A.; Huang, N. Exosome-Loaded Pro-efferocytic Vascular Stent with Lp-PLA(2)-Triggered Release for Preventing In-Stent Restenosis. ACS Nano 2022, 16, 14925–14941. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Ku, J.; Flojo, R.; Olson, C.; Bengford, D.; Marriott, G. Exosome- and extracellular vesicle-based approaches for the treatment of lysosomal storage disorders. Adv. Drug Deliv. Rev. 2022, 188, 114465. [Google Scholar] [CrossRef]
- Anderson, N.R.; Minutolo, N.G.; Gill, S.; Klichinsky, M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021, 81, 1201–1208. [Google Scholar] [CrossRef]
- Zoulikha, M.; Xiao, Q.; Boafo, G.F.; Sallam, M.A.; Chen, Z.; He, W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm. Sin. B 2022, 12, 600–620. [Google Scholar] [CrossRef]
- Hoy, S.M. Patisiran: First Global Approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, G.; Tian, C.; Jiang, W.; Jin, L.; Zhang, C.; Chen, X. Exosome-mediated small RNA delivery for gene therapy. Wiley Interdiscip. Rev. RNA 2016, 7, 758–771. [Google Scholar] [CrossRef]
- Ren, Y.Z.; Ding, S.S.; Jiang, Y.P.; Wen, H.; Li, T. Application of exosome-derived noncoding RNAs in bone regeneration: Opportunities and challenges. World J. Stem Cells 2022, 14, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long non-coding RNAs: From disease code to drug role. Acta Pharm. Sin. B 2021, 11, 340–354. [Google Scholar] [CrossRef] [PubMed]

| ncRNAs | Donors | Recipients | Targets/Pathways | Effects | Role | References |
|---|---|---|---|---|---|---|
| miR-942 | Macrophages | SPC-A1, H1299 | FOXO1-β-catenin axis | Increased migration, invasion, angiogenesis, and metastasis | Promoter | [52] |
| miR-501-3p | Macrophages | A549, SPC-A1 | WDR82 | Reduced apoptosis and elevated proliferation, migration, and invasion | Promoter | [53] |
| miR-155, miR-196a-5p | Macrophages | A549 | RASSF4 | Enhanced migration, invasion, EMT, and metastasis | Promoter | [55] |
| miR-3679-5p | Macrophages | A549 | NEDD4L | Enhanced aerobic glycolysis and chemoresistance | Promoter | [56] |
| miR-3917 | Macrophages | H1299, A549 | GRK6 | Enhanced proliferation, migration and invasion | Promoter | [58] |
| miR-181a-5p | Macrophages | H1975 | ETS1-STK16-AKT1 axis | Reduced cell viability and enhanced apoptosis | Suppressor | [59] |
| let-7b-5p | Macrophage | A549, H1299 | GNG5 | Suppressed proliferation and enhanced apoptosis | Suppressor | [60] |
| miR-19b-3p | A549, H1975 | Macrophages | PTPRD, STAT3 | Enhanced metastasis | Promoter | [61] |
| miR-146a | H1299 | Macrophages | TRAF-6, IRAK-1 | Promoted invasion and proliferation | Promoter | [63] |
| miR-181b | A549 | Macrophages | JAK2/STAT3 pathway | Enhanced proliferation, migration, and invasion | Promoter | [62] |
| miR-21 | H1299 | Macrophages | IRF1 | Strengthened proliferation | Promoter | [65] |
| miR-101 | A549 | Macrophages | CDK8 | Reduced macrophage inflammation and tumor growth | Suppressor | [66] |
| miR-770 | A549 | Macrophages | MAP3K1 | Repressed invasion and migration | Suppressor | [67] |
| LINC00273 | Macrophages | A549, H1975 | NEDD4-LATS2-YAP axis | Increased metastasis | Promoter | [61] |
| AGAP2-AS1 | Macrophages | A549, H157 | miR-296-Notch2 axis | Induced radiotherapy immunity | Promoter | [72] |
| PCAT6 | A549 | Macrophages | miR-326-KLF1 axis | Enhanced EMT, invasion, and migration | Promoter | [74] |
| LINC00313 | H1299 | Macrophages | miR-135a-3p-STAT6 axis | Enhanced tumor growth | Promoter | [75] |
| SOX2-OT | H1975 | Macrophages | miR-627-3p-Smads axis | Enhanced osimertinib resistance | Promoter | [77] |
| SNHG7 | H1299, SPC-A1 | Macrophages | PTEN/PI3K/AKT pathway | Increased DTX resistance | Promoter | [81] |
| circSHKBP1 | A549 | Macrophages | miR-1294-PKM2 axis | Strengthened tumor growth and metastasis | Promoter | [83] |
| circPVT1 | A549 | Macrophages | miR-124-3p-EZH2 axis | Enhanced proliferation, migration, and invasion | Promoter | [84] |
| circFARSA | A549 | Macrophages | PTEN/PI3K/AKT pathway | Increased invasion and migration | Promoter | [85] |
| circZNF451 | A549, H1299, H1395, H1975 | Macrophages | TRIM56-FXR1-ELF4-IRF4 axis | Suppressed immunotherapy efficacy | Promoter | [87] |
| Disease | ncRNA | Donors | Recipients | Targets/Pathways | Effects | Role | References |
|---|---|---|---|---|---|---|---|
| ALI | miR-30d-5p | PMNs | Raw264.7 macrophages | SOCS1, SIRT1, and NF-κB pathway | Enhanced lung inflammation | Promoter | [99] |
| miR-384-5p | BMSCs | NR8383 macrophages | Beclin-1 | Repressed lung injury | Suppressor | [105] | |
| miR-451 | hUC-MSCs | NR8383 macrophages | MIF-PI3K-AKT axis, | Restrained lung injury | Suppressor | [106] | |
| miR-92a-3p | RLE-6TN cells | NR8383 macrophages | PTEN, NF-κB signal | Strengthened lung inflammation | Promoter | [107] | |
| miR-21a-5p | MLE-12 cells | Raw264.7 macrophages | Notch2, SOCS1 | Suppressed lung injury | Suppressor | [109] | |
| miR-155 | Serum | Raw264.7 macrophages | SHIP1, SOCS1, and NF-κB pathway | Increased lung inflammation | Promoter | [110] | |
| miR-223-3p | BALF | NR8383 macrophages | STK39 | Attenuated lung injury | Suppressor | [111] | |
| miR-223 | Endothelial cells | Tie2+ AMs | RGS1 | Repressed lung injury | Suppressor | [112] | |
| CLMAT3 | Monocytes | U937 macrophages | CtBP2-p300-NF-κB complex | Enhanced lung inflammation | Promoter | [100] | |
| Pulmonary fibrosis | miR-7219-3p | RAW264.7 macrophages | NIH-3T3 cells | Spry1, ERK/MAPK pathway | Enhanced FMT | Promoter | [120] |
| miR-125a-5p | RAW264.7 macrophages | NIH-3T3 and MRC-5 cells | Smurf1, TGF-β signaling pathway | Enhanced FMT | Promoter | [121] | |
| miR-328 | Macrophages | Interstitial fibroblasts | FAM13A | Promoted lung fibrosis | Promoter | [123] | |
| miR-129-5p | Macrophages | Fibroblasts | STAT1 | Enhanced lung fibrosis | Promoter | [124] | |
| miR-142-3p | Macrophages | A549 and MRC5 cells | TGFβ-R1 | Attenuated lung fibrosis | Suppressor | [125] | |
| miR-27b-3p | Type II AECs | Flt3+ AMs | RGS1 | Reduced lung fibrosis | Suppressor | [112] | |
| miR-302a-3p | iPSCs | RAW264.7 macrophages | TET1 | Repressed lung fibrosis | Suppressor | [131] | |
| MSTRG.91634.7 | Macrophages | MRC5 cells | PINK1 | Reduced fibroblast activation | Suppressor | [126] | |
| Asthma | miR-370 | Macrophages | AMSCs | FGF1, MAPK/STAT1 signal pathway | Reduced asthma | Suppressor | [137] |
| miR-30b-5p | RAW264.7 macrophages | Airway epithelial cells | IRF7 | Restrained asthma | Suppressor | [138] | |
| miR-21-5p | NR8383 macrophages | Tracheal epithelial cells | Smad7, TGFβ1/Smad pathway | Enhanced EMT | Promoter | [140] | |
| mmu_circ_0001359 | ADSCs | RAW264.7 macrophages | miR-183-5p-FOXO1 axis | Repressed airway remodeling | Suppressor | [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, X.; Zhong, J.; Zhang, B.; Zhu, T.; Liao, R. Exosomal Non-Coding RNAs: Novel Regulators of Macrophage-Linked Intercellular Communication in Lung Cancer and Inflammatory Lung Diseases. Biomolecules 2023, 13, 536. https://doi.org/10.3390/biom13030536
Lai X, Zhong J, Zhang B, Zhu T, Liao R. Exosomal Non-Coding RNAs: Novel Regulators of Macrophage-Linked Intercellular Communication in Lung Cancer and Inflammatory Lung Diseases. Biomolecules. 2023; 13(3):536. https://doi.org/10.3390/biom13030536
Chicago/Turabian StyleLai, Xingning, Jie Zhong, Boyi Zhang, Tao Zhu, and Ren Liao. 2023. "Exosomal Non-Coding RNAs: Novel Regulators of Macrophage-Linked Intercellular Communication in Lung Cancer and Inflammatory Lung Diseases" Biomolecules 13, no. 3: 536. https://doi.org/10.3390/biom13030536
APA StyleLai, X., Zhong, J., Zhang, B., Zhu, T., & Liao, R. (2023). Exosomal Non-Coding RNAs: Novel Regulators of Macrophage-Linked Intercellular Communication in Lung Cancer and Inflammatory Lung Diseases. Biomolecules, 13(3), 536. https://doi.org/10.3390/biom13030536






