Pruritus Is Associated with an Increased Risk for the Diagnosis of Autoimmune Skin Blistering Diseases: A Propensity-Matched Global Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Database
2.2. Study Population and Definition of Eligible Patients
2.3. Statistical Analysis
3. Results
3.1. Description of Cohorts
3.1.1. Patients with Pruritus
3.1.2. Patients with AIBD
3.2. Pruritus Is Associated with A Higher Risk for Subsequent AIBD Diagnosis
3.2.1. Pruritus Is Associated with a Higher Risk for Subsequent AIBD Diagnosis
3.2.2. The Majority of AIBDs Are Diagnosed within 6 Months after the Initial Pruritus Manifestation
3.3. AIBDs Are Associated with an Increased Risk for the Subsequent Diagnosis of Pruritus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ludwig, R.J.; Vanhoorelbeke, K.; Leypoldt, F.; Kaya, Z.; Bieber, K.M.; McLachlan, S.; Komorowski, L.; Luo, J.; Cabral-Marques, O.; Hammers, C.M.; et al. Mechanisms of autoantibody-induced pathology. Front. Immunol. 2017, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Kasperkiewicz, M.; Joly, J. Pemphigus. Lancet 2019, 394, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Zillikens, D. Pemphigoid diseases. Lancet 2013, 381, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Reunala, T.; Hervonen, K.; Salmi, T. Dermatitis Herpetiformis: An Update on Diagnosis and Management. Am. J. Clin. Dermatol. 2021, 22, 329–338. [Google Scholar] [CrossRef]
- Beek, N.; Weidinger, A.; Schneider, S.; Kleinheinz, A.; Gläser, R.; Holtsche, M.; Georg, A.; Hammers, C.; Hübner, F.; Lima, A.; et al. Incidence of pemphigoid diseases in Northern Germany in 2016—First data from the Schleswig-Holstein Registry of Autoimmune Bullous Diseases. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Smeeth, L.; Hubbard, R.; Fleming, K.M.; Smith, C.J.P.; West, J. Bullous pemphigoid and pemphigus vulgaris—incidence and mortality in the UK: Population based cohort study. BMJ 2008, 337, a180. [Google Scholar] [CrossRef]
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Lamberts, A.; Yale, M.; Grando, S.; Horváth, B.; Zillikens, D.; Jonkman, M. Unmet Needs in Pemphigoid Diseases: An International Survey Amongst Patients, Clinicians and Researchers. Acta Derm. Venereol. 2019, 99, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Zeidler, C.; Augustin, M.; Darsow, U.; Kremer, A.E.; Legat, F.J.; Koschmieder, S.; Kupfer, J.; Mettang, T.; Metz, M.; et al. S2k Leitlinie: Diagnostik und Therapie des chronischen Pruritus. JDDG J. Dtsch. Dermatol. Ges. 2022, 20, 1386–1402. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Berger, T.; Fassett, M. Neuroimmune interactions in chronic itch of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2019, 34, 239–250. [Google Scholar] [CrossRef]
- Schmelz, M. Itch Processing in the Skin. Front. Med. 2019, 6, 167. [Google Scholar] [CrossRef]
- Kim, B.S.; Inan, S.; Ständer, S.; Sciascia, T.; Szepietowski, J.C.; Yosipovitch, G. Role of kappa-opioid and mu-opioid receptors in pruritus: Peripheral and central itch circuits. Exp. Dermatol. 2022, 31, 1900–1907. [Google Scholar] [CrossRef]
- Sheahan, T.D.; Warwick, C.A.; Fanien, L.G.; Ross, S.E. The Neurokinin-1 Receptor is Expressed with Gastrin-Releasing Peptide Receptor in Spinal Interneurons and Modulates Itch. J. Neurosci. 2020, 40, 8816–8830. [Google Scholar] [CrossRef]
- Wilzopolski, J.; Kietzmann, M.; Mishra, S.; Stark, H.; Bäumer, W.; Rossbach, K. TRPV1 and TRPA1 Channels Are Both Involved Downstream of Histamine-Induced Itch. Biomolecules 2021, 11, 1166. [Google Scholar] [CrossRef] [PubMed]
- Trier, A.M.; Kim, B.S. Structural insights into MRGPRX2: A new vision of itch and allergy. J. Allergy Clin. Immunol. 2022, 149, 1221. [Google Scholar] [CrossRef]
- Lebonvallet, N.; Fluhr, J.W.; Le Gall-Ianotto, C.; Leschiera, R.; Talagas, M.; Reux, A.; Bataille, A.; Brun, C.; Oddos, T.; Pennec, J.; et al. A re-innervated in vitro skin model of non-histaminergic itch and skin neurogenic inflammation: PAR2-, TRPV1- and TRPA1-agonist induced functionality. Ski. Health Dis. 2021, 1, e66. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Yokozeki, H.; Karasuyama, H.; Satoh, T. IL-31–generating network in atopic dermatitis comprising macrophages, basophils, thymic stromal lymphopoietin, and periostin. J. Allergy Clin. Immunol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Ruppenstein, A.; Limberg, M.M.; Loser, K.; Kremer, A.E.; Homey, B.; Raap, U. Involvement of Neuro-Immune Interactions in Pruritus With Special Focus on Receptor Expressions. Front. Med. 2021, 8, 627985. [Google Scholar] [CrossRef]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef]
- Rüdrich, U.; Gehring, M.; Papakonstantinou, E.; Illerhaus, A.; Engmann, J.; Kapp, A.; Hartmann, K.; Meyer, N.; Gibbs, B.; Raap, U. Eosinophils are a Major Source of Interleukin-31 in Bullous Pemphigoid. Acta Derm. Venereol. 2018, 98, 766–771. [Google Scholar] [CrossRef]
- Guseva, P.D.; Rüdrich, U.; Kotnik, N.; Gehring, M.; Patsinakidis, N.; Agelopoulos, K.; Ständer, S.; Homey, B.; Kapp, A.; Gibbs, B.F.; et al. Neuronal branching of sensory neurons is associated with BDNF-positive eosinophils in atopic dermatitis. Clin. Exp. Allergy 2020, 50, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Briand, C.; Gourier, G.; Poizeau, F.; Jelti, L.; Bachelerie, M.; Quéreux, G.; Jeudy, G.; Acquitter, M.; Ingen-Housz-Oro, S.; Caux, F.; et al. Characteristics of Pruritus in Bullous Pemphigoid and Impact on Quality of Life: A Prospective Cohort Study. Acta Derm. Venereol. 2020, 100, adv00320. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, A.; Meijer, J.M.; Pas, H.H.; Diercks, G.F.; Horváth, B.; Jonkman, M.F. Nonbullous pemphigoid: Insights in clinical and diagnostic findings, treatment responses, and prognosis. J. Am. Acad. Dermatol. 2019, 81, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.M.; Lamberts, A.; Luijendijk, H.J.; Diercks, G.F.H.; Pas, H.H.; Zuidema, S.U.; Jonkman, M.F. Prevalence of Pemphigoid as a Potentially Unrecognized Cause of Pruritus in Nursing Home Residents. JAMA Dermatol. 2019, 155, 1423–1424. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, S.Z.; Chams-Davatchi, C.; Daneshpazhooh, M.; Valikhani, M.; Esmaili, N. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J. Dermatol. 2011, 39, 141–144. [Google Scholar] [CrossRef]
- Rolader, R.; Daugherty, L.N.; Liu, Y.; Feldman, R.J. Prevalence and predictors of pruritus in pemphigus compared with bullous pemphigoid: A cross-sectional study. J. Am. Acad. Dermatol. 2020, 83, 251–254. [Google Scholar] [CrossRef]
- Kulczycka-Siennicka, L.; Cynkier, A.; Waszczykowska, E.; Woźniacka, A.; Żebrowska, A. The Role of Intereukin-31 in Pathogenesis of Itch and Its Intensity in a Course of Bullous Pemphigoid and Dermatitis Herpetiformis. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.F.M.; DeGrazia, T.; Al Shamekh, S.; Feldman, R.M. Itch-related quality of life impact across 3 autoimmune blistering diseases: A retrospective cohort study. Itch 2020, 5, e39. [Google Scholar] [CrossRef]
- Abdat, R.; Waldman, R.A.; de Bedout, V.; Czernik, A.; Mcleod, M.; King, B.; Gordon, S.; Ahmed, R.; Nichols, A.; Rothe, M.; et al. Dupilumab as a novel therapy for bullous pemphigoid: A multicenter case series. J. Am. Acad. Dermatol. 2020, 83, 46–52. [Google Scholar] [CrossRef]
- Valenti, M.; De Giacomo, P.; Lavecchia, A.; Valenti, G. A severe case of IgA bullous pemphigoid successfully treated with dupi-lumab. Dermatol Ther. 2022, 35, e15890. [Google Scholar] [CrossRef]
- van Beek, N.; Kridin, K.; Bühler, E.; Kochan, A.S.; Ständer, S.; Ludwig, R.J.; Zillikens, D.; Schmidt, E.; Günther, C. Evaluation of Site- and Autoantigen-Specific Characteristics of Mucous Membrane Pemphigoid. JAMA Dermatol. 2022, 158, 84. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Sugita, K.; Horie, T.; Yamamoto, O. Dual role of basophils in the pathogenesis of bullous pemphigoid elucidated by pathological and ultrastructural studies. Eur. J. Dermatol. 2022, 32, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Raap, U.; Gehring, M.; Kleiner, S.; Rüdrich, U.; Eiz-Vesper, B.; Haas, H.; Kapp, A.; Gibbs, B.F. Human basophils are a source of—And are differentially activated by–IL-31. Clin. Exp. Allergy 2017, 47, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.-C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Raap, U.; Wieczorek, D.; Gehring, M.; Pauls, I.; Ständer, S.; Kapp, A.; Wedi, B. Increased levels of serum IL-31 in chronic spontaneous urticaria. Exp. Dermatol. 2010, 19, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Raap, U.; Weißmantel, S.; Gehring, M.; Eisenberg, A.M.; Kapp, A.; Fölster-Holst, R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr. Allergy Immunol. 2012, 23, 285–288. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kursewicz, C.D.; Fayne, R.A.; Nanda, S.; Shah, S.M.; Nattkemper, L.; Yokozeki, H.; Yosipovitch, G. Pathophysiologic mechanisms of itch in bullous pemphigoid. J. Am. Acad. Dermatol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Nemmer, J.M.; Kuchner, M.; Datsi, A.; Oláh, P.; Julia, V.; Raap, U.; Homey, B. Interleukin-31 Signaling Bridges the Gap Between Immune Cells, the Nervous System and Epithelial Tissues. Front. Med. 2021, 8, 639097. [Google Scholar] [CrossRef]
- Touho, H.; Hino, A.; Suzuki, K.; Kubo, S.; Hirakawa, K. Coagulation fibrinolysis Abnormalities in Acute Stage Hypertensive Intracerebral Hemorrhage and Head Injury (Preliminary Report). Neurol. Medico-Chir. 1985, 25, 203–208. [Google Scholar] [CrossRef]
- Li, S.-Z.; Jin, X.-X.; Ge, X.-L.; Zuo, Y.-G.; Jin, H.-Z. Thymic Stromal Lymphopoietin Is Implicated in the Pathogenesis of Bullous Pemphigoid by Dendritic Cells. J. Immunol. Res. 2020, 2020, 4594630. [Google Scholar] [CrossRef]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xian, D.; Yang, L.; Xiong, X.; Lai, R.; Zhong, J. Pruritus: Progress toward Pathogenesis and Treatment. BioMed Res. Int. 2018, 2018, 9625936. [Google Scholar] [CrossRef]
- Mollanazar, N.K.; Smith, P.K.; Yosipovitch, G. Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out? Clin. Rev. Allergy Immunol. 2015, 51, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S. The translational revolution of itch. Neuron 2022, 110, 2209–2214. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, O.; Soares, G.B.; Yosipovitch, G. Transient Receptor Potential Channels and Itch. Int. J. Mol. Sci. 2022, 24, 420. [Google Scholar] [CrossRef] [PubMed]
- Schaper-Gerhardt, K.; Köther, B.; Wolff, L.; Kabatas, A.; Gehring, M.; Nikolouli, E.; Mommert, S.; Werfel, T.; Gutzmer, R. The H4 R is highly expressed on eosinophils from AD patients and IL-4 upregulates expression and function via the JAK/STAT path-way. Allergy 2021, 76, 1261–1264. [Google Scholar] [CrossRef]
- Takamura, S.; Teraki, Y. Treatment of bullous pemphigoid with dupilumab: Dupilumab exerts its effect by primarily suppressing T-helper 2 cytokines. J. Dermatol. 2022, 49, 845–850. [Google Scholar] [CrossRef]
- Chen, S.; Zhan, S.; Hua, C.; Tang, Y.; Cheng, H. A Novel Combined Use of Dupilumab for Treatment of Aggressive Refractory Pemphigus Vulgaris Complicated With Pulmonary Tuberculosis: A Case Report and the RNA-seq Analysis. Front. Immunol. 2022, 13, 234. [Google Scholar] [CrossRef]
- Ji-Xu, A.; Artounian, K.; Fung, M.A.; Le, S.T.; Maverakis, E. Omalizumab as adjuvant therapy for pemphigus vulgaris. Dermatol. Ther. 2022, 35, e15646. [Google Scholar] [CrossRef]
- Vassallo, C.; Somenzi, A.; De Amici, M.; Barruscotti, S.; Brazzelli, V. Omalizumab as a corticosteroid-sparing agent in the treatment of bullous pemphigoid. Dermatol. Ther. 2022, 35, e15946. [Google Scholar] [CrossRef]
- Lamberts, A.; Kotnik, N.; Diercks, G.; Meijer, J.; Di Zenzo, G.; Pas, H.; Jonkman, M.; Gibbs, B.; Raap, U.; Horváth, B. IgE autoantibodies in serum and skin of non-bullous and bullous pemphigoid patients. J. Eur. Acad. Dermatol. Venereol. 2020, 35, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Jang, J.; Lee, A.; Min, S.Y.; Lee, S.G.; Kim, S.-C.; Shin, J.; Kim, J.H. Clinical impact and a prognostic marker of early rituximab treatment after rituximab reimbursement in Korean pemphigus patients. Front. Immunol. 2022, 13, 4007. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, A.; Mimouni, T.; Hodak, E.; Gdalevich, M.; Oren-Shabtai, M.; Levi, A.; Mimouni, D.; Leshem, Y.A. Early rituximab treatment is associated with increased and sustained remission in pemphigus patients: A retrospective cohort of 99 patients. Dermatol. Ther. 2022, 35, e15397. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Pruritus | Controls | Pruritus * | Controls * | Pruritus ** | Controls ** |
|---|---|---|---|---|---|---|
| Number of participants | 1,720,045 | 1,720,045 | 1,657,855 | 1,657,855 | 1,312,591 | 1,312,591 |
| Age in years (SD) | 43.3 ± 23.8 | 43.3 ± 23.8 | 42 ± 22.7 | 42 ± 22.7 | 43.9 ± 22.3 | 43.9 ± 22.3 |
| Female (%) | 65.869 | 65.869 | 67.242 | 67.242 | 65.579 | 65.579 |
| Not Hispanic or Latino (%) | 69.503 | 69.503 | 74.389 | 74.389 | 73.053 | 73.053 |
| White (%) | 64.205 | 64.205 | 65.526 | 65.526 | 64.81 | 64.81 |
| Disease | Cases/Controls | Number of Participants | Age in Years (SD) | Female (%) | Not Hispanic or Latino (%) | White (%) |
|---|---|---|---|---|---|---|
| Bullous pemphigoid | Cases | 16,019 | 72.7 ± 14.9 | 53.543 | 60.690 | 64.068 |
| Controls | 16,019 | 72.7 ± 14.9 | 53.543 | 60.678% | 64.068% | |
| Bullous pemphigoid * | Cases | 13,398 | 71.8 ± 15.7 | 55.486 | 56.046% | 56.523% |
| Controls | 13,398 | 71.8 ± 15.7 | 55.486 | 56.046% | 56.523% | |
| Mucous membrane pemphigoid | Cases | 4165 | 66.1 ± 14.9 | 61.345 | 68.884% | 73.661% |
| Controls | 4165 | 66.1 ± 14.9 | 61.345% | 68.884% | 73.661% | |
| Epidermolysis bullosa acquisita | Cases | 1234 | 56.4 ± 19 | 52.107% | 48.947% | 69.287% |
| Controls | 1234 | 56.3 ± 19.3 | 52.107% | 48.298% | 69.854% | |
| Dermatitis herpetiformis | Cases | 7791 | 49.6 ± 21.3 | 56.219% | 64.934% | 73.084% |
| Controls | 7791 | 49.6 ± 21.3 | 56.219% | 64.934% | 73.084% | |
| Dermatitis herpetiformis * | Cases | 6926 | 47.6 ± 21.4 | 58.88% | 67.196% | 69.448% |
| Controls | 6926 | 47.6 ± 21.4 | 58.88% | 67.196% | 69.448% | |
| Lichen planus pemphigoides | Cases | 158 | 56.6 ± 19.2 | 63.291% | 68.987% | 50% |
| Controls | 158 | 56.6 ± 19.2 | 60.127% | 70.253% | 50.633% | |
| Pemphigus vulgaris | Cases | 6243 | 56.2 ± 18.2 | 57.168% | 60.628% | 56.159% |
| Controls | 6243 | 56.2 ± 18.2 | 57.168% | 60.628% | 56.159% | |
| Pemphigus vulgaris * | Cases | 5685 | 53.8 ± 18.1 | 58.54% | 60.088% | 51.645% |
| Controls | 5685 | 53.8 ± 18.1 | 58.54% | 60.088% | 51.645% | |
| Pemphigus foliaceous | Cases | 4161 | 55.4 ± 19.1 | 55.756% | 62.437% | 56.381% |
| Controls | 4161 | 55.4 ± 19.1 | 55.756% | 62.437% | 56.381% | |
| Paraneoplastic pemphigus | Cases | 176 | 59.1 ± 18.1 | 53.977% | 69.886% | 68.75% |
| Controls | 176 | 58.9 ± 18.4 | 52.273% | 72.159% | 68.75% |
| Disease | ICD10 Code | N of Eligible Participants | N of Outcomes | Risk, % | N of Eligible Participants * | N of Outcomes | Risk, % | Risk Difference (95% Confidence Interval), % | Hazard Ratio (95% Confidence Interval) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
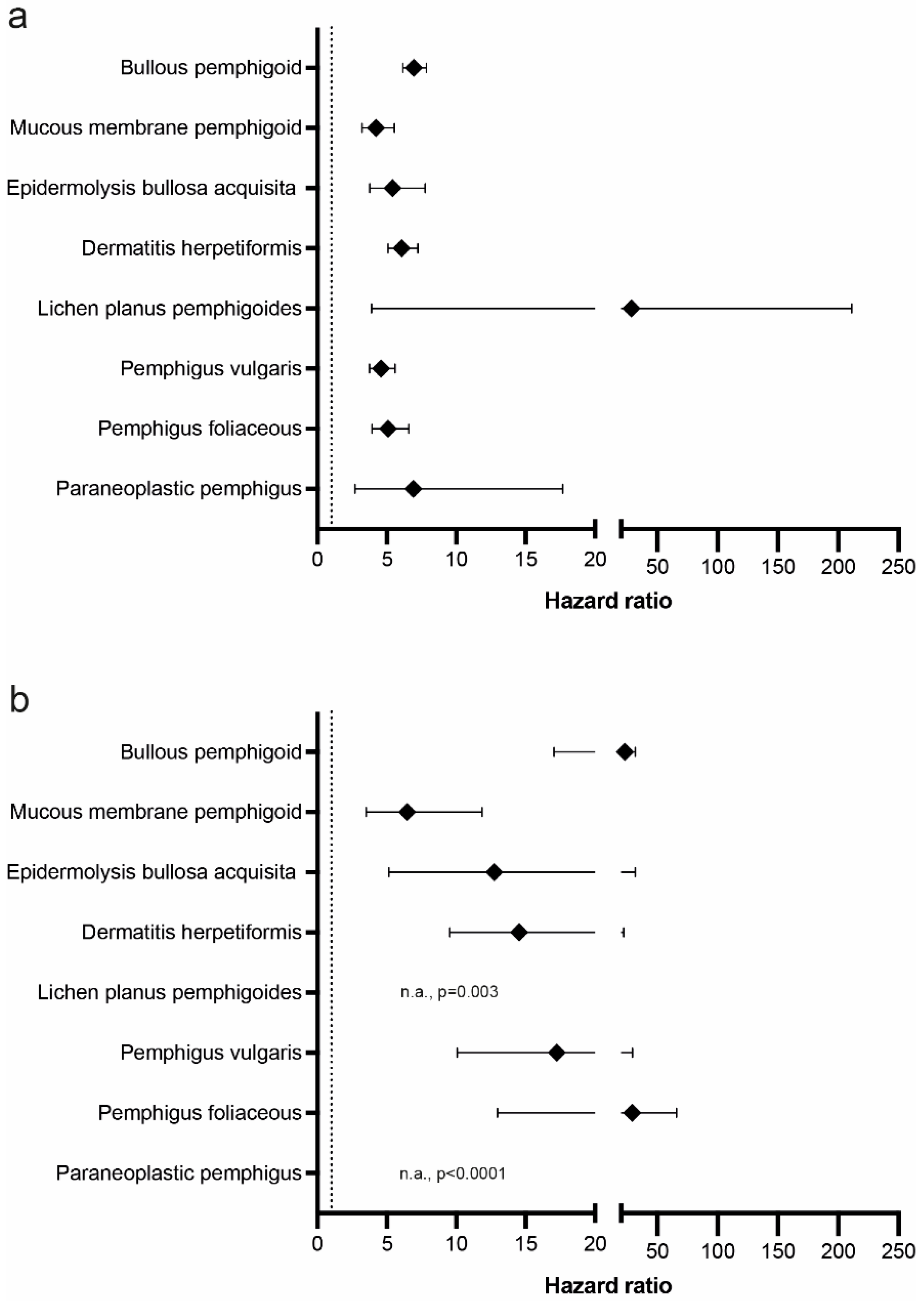
| Bullous pemphigoid | L12.0 | 1,717,744 | 2003 | 0.117 | 1,719,818 | 300 | 0.017 | 0.099% (0.094%, 0.105%) | 6.952 (6.158, 7.85) | <0.0001 |
| Bullous pemphigoid * | L12.0 | 1,656,086 | 1561 | 0.094% | 1,657,705 | 238 | 0.014% | 0.08% (0.075%, 0.085%) | 6.794 (5.927, 7.788) | <0.0001 |
| Bullous pemphigoid ** | L12.0 | 1,311,489 | 747 | 0.057% | 1,312,478 | 159 | 0.012% | 0.045% (0.04%, 0.049%) | 4.878 (4.11, 5.789) | <0.0001 |
| Mucous membrane pemphigoid | L12.1 | 1,719,520 | 256 | 0.015% | 1,719,972 | 65 | 0.004% | 0.011% (0.009%, 0.013%) | 4.219 (3.213, 5.54) | <0.0001 |
| Epidermolysis bullosa acquisita | L12.3 | 1,719,859 | 180 | 0.01% | 1,720,024 | 35 | 0.002% | 0.008% (0.007%, 0.01%) | 5.402 (3.76, 7.76) | <0.0001 |
| Dermatitis herpetiformis | L13.0 | 1,718,888 | 843 | 0.049% | 1,719,881 | 146 | 0.008% | 0.041% (0.037%, 0.044%) | 6.068 (5.09, 7.234) | <0.0001 |
| Dermatitis herpetiformis * | L13.0 | 1,656,838 | 803 | 0.048% | 1,657,717 | 152 | 0.009% | 0.039% (0.036%, 0.043%) | 5.545 (4.662, 6.596) | <0.0001 |
| Dermatitis herpetiformis ** | L13.0 | 1,312,162 | 284 | 0.022% | 1,312,520 | 85 | 0.006% | 0.015% (0.012%, 0.018%) | 3.509 (2.753, 4.472) | <0.0001 |
| Lichen planus pemphigoides | L43.1 | 1,720,023 | 27 | 0.002% | 1,720,045 | 10 | 0.001% | 0.001% (0%, 0.002%) | 28.705 (3.9, 211.299) | <0.0001 |
| Pemphigus vulgaris | L10.0 or L10.1 | 1,719,203 | 522 | 0.03% | 1,719,927 | 119 | 0.007% | 0.023% (0.021%, 0.026%) | 4.582 (3.754, 5.592) | <0.0001 |
| Pemphigus vulgaris * | L10.0 or L10.1 | 1,657,121 | 404 | 0.024% | 1,657,758 | 89 | 0.005% | 0.019% (0.016%, 0.022%) | 4.749 (3.775, 5.976) | <0.0001 |
| Pemphigus vulgaris ** | L10.0 or L10.1 | 1,312,179 | 244 | 0.019% | 1,312,504 | 66 | 0.005% | 0.014% (0.011%, 0.016%) | 3.857 (2.938, 5.063) | <0.0001 |
| Pemphigus foliaceous | L10.2 or L10.4 | 1,719,417 | 343 | 0.02% | 1,719,955 | 70 | 0.004% | 0.016% (0.014%, 0.018%) | 5.093 (3.938, 6.587) | <0.0001 |
| Paraneoplastic pemphigus | L10.81 | 1,720,022 | 34 | 0.002% | 1,720,040 | 10 | 0.001% | 0.001% (0.001%, 0.002%) | 6.913 (2.703, 17.678) | <0.0001 |
| Disease | ICD10 Code | N of Eligible Participants | N of Outcomes | Risk, % | N of Eligible Participants * | N of Outcomes | Risk, % | Risk Difference (95% Confidence Interval), % | Hazard Ratio (95% Confidence Interval) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Bullous pemphigoid | L12.0 | 1,717,744 | 973 | 0.057 | 1,719,818 | 41 | 0.002 | 0.054 (0.051, 0.058) | 23.293 (17.042, 31.838) | <0.0001 |
| Mucous membrane pemphigoid | L12.1 | 1,719,520 | 79 | 0.005% | 1,719,972 | 12 | 0.001% | 0.004% (0.003%, 0.005%) | 6.459 (3.519, 11.855) | <0.0001 |
| Epidermolysis bullosa acquisita | L12.3 | 1,719,859 | 65 | 0.004% | 1,720,024 | 10 | 0.001% | 0.003% (0.002%, 0.004%) | 12.756 (5.137, 31.679) | <0.0001 |
| Dermatitis herpetiformis | L13.0 | 1,718,888 | 341 | 0.02% | 1,719,881 | 23 | 0.001% | 0.019% (0.016%, 0.021%) | 14.535 (9.529, 22.171) | <0.0001 |
| Lichen planus pemphigoides | L43.1 | 1,718,397 | 10 | 0.001% | 1,718,416 | 0 | 0% | 0.001% (0%, 0.001%) | n.a. | 0.0030 |
| Pemphigus vulgaris | L10.0 or L10.1 | 1,717,578 | 246 | 0.014% | 1,718,294 | 14 | 0.001% | 0.014% (0.012%, 0.015%) | 17.247 (10.066, 29.553) | <0.0001 |
| Pemphigus foliaceous | L10.2 or L10.4 | 1,717,792 | 179 | 0.01% | 1,718,326 | 10 | 0.001% | 0.01% (0.008%, 0.011%) | 29.265 (12.974, 66.013) | <0.0001 |
| Paraneoplastic pemphigus | L10.81 | 1,718,397 | 21 | 0.001% | 1,718,419 | 0 | 0% | 0.001% (0.001%, 0.002) | n.a. | <0.0001 |
| Disease | ICD10 Code | N of Eligible Participants | N of Outcomes | Risk, % | N of Eligible Participants * | N of Outcomes | Risk, % | Risk Difference (95% Confidence Interval), % | Hazard Ratio (95% Confidence Interval) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Bullous pemphigoid | L12.0 | 13,740 | 467 | 3.399 | 15,608 | 110 | 0.705 | 2.694 (2.364, 3.02%) | 5.084 (4.13, 6.257) | < 0.0001 |
| Bullous pemphigoid * | L12.0 | 11,643 | 346 | 2.972% | 13,048 | 79 | 0.605% | 2.366% (2.03%, 2.702%) | 5.093 (3.989, 6.504) | < 0.0001 |
| Mucous membrane pemphigoid | L12.1 | 3888 | 79 | 2.032% | 4,053 | 26 | 0.642% | 1.39% (0.883%, 1.897%) | 3.223 (2.069, 5.02) | <0.0001 |
| Epidermolysis bullosa acquisita | L12.3 | 1031 | 32 | 3.104% | 1,199 | 10 | 0.834% | 2.27% (1.093%, 3.447%) | 6.018 (2.516, 14.393) | <0.0001 |
| Dermatitis herpetiformis | L13.0 | 6532 | 197 | 3.016% | 7,638 | 37 | 0.484% | 2.532% (2.088%, 2.975%) | 6.263 (4.409, 8.899) | <0.0001 |
| Dermatitis herpetiformis * | L13.0 | 5731 | 175 | 3.054% | 6,761 | 37 | 0.547% | 2.506% (2.027%, 2.985%) | 5.632 (3.951, 8.03) | <0.0001 |
| Lichen planus pemphigoides | L43.1 | 127 | 10 | 7.874% | 154 | 10 | 6.494% | 1.381% (−4.71%, 7.471%) | 7.042 (0.848, 58.497) | 0.0350 |
| Pemphigus vulgaris | L10.0 or L10.1 | 5803 | 115 | 1.982% | 6,109 | 29 | 0.475% | 1.507% (1.109%, 1.905%) | 4.198 (2.793, 6.308) | <0.0001 |
| Pemphigus vulgaris * | L10.0 or L10.1 | 5334 | 87 | 1.631% | 5,544 | 22 | 0.397% | 1.234% (0.856%, 1.612%) | 4.043 (2.533, 6.454) | <0.0001 |
| Pemphigus foliaceous | L10.2 or L10.4 | 3895 | 84 | 2.157% | 4,068 | 25 | 0.615% | 1.542% (1.027%, 2.058%) | 3.471 (2.221, 5.424) | <0.0001 |
| Paraneoplastic pemphigus | L10.81 | 146 | 10 | 6.849% | 169 | 10 | 5.917% | - | - | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raap, U.; Limberg, M.M.; Kridin, K.; Ludwig, R.J. Pruritus Is Associated with an Increased Risk for the Diagnosis of Autoimmune Skin Blistering Diseases: A Propensity-Matched Global Study. Biomolecules 2023, 13, 485. https://doi.org/10.3390/biom13030485
Raap U, Limberg MM, Kridin K, Ludwig RJ. Pruritus Is Associated with an Increased Risk for the Diagnosis of Autoimmune Skin Blistering Diseases: A Propensity-Matched Global Study. Biomolecules. 2023; 13(3):485. https://doi.org/10.3390/biom13030485
Chicago/Turabian StyleRaap, Ulrike, Maren M. Limberg, Khalaf Kridin, and Ralf J. Ludwig. 2023. "Pruritus Is Associated with an Increased Risk for the Diagnosis of Autoimmune Skin Blistering Diseases: A Propensity-Matched Global Study" Biomolecules 13, no. 3: 485. https://doi.org/10.3390/biom13030485
APA StyleRaap, U., Limberg, M. M., Kridin, K., & Ludwig, R. J. (2023). Pruritus Is Associated with an Increased Risk for the Diagnosis of Autoimmune Skin Blistering Diseases: A Propensity-Matched Global Study. Biomolecules, 13(3), 485. https://doi.org/10.3390/biom13030485







