Photobiomodulation for Chemotherapy-Induced Oral Mucositis in Pediatric Patients
Abstract
1. Introduction
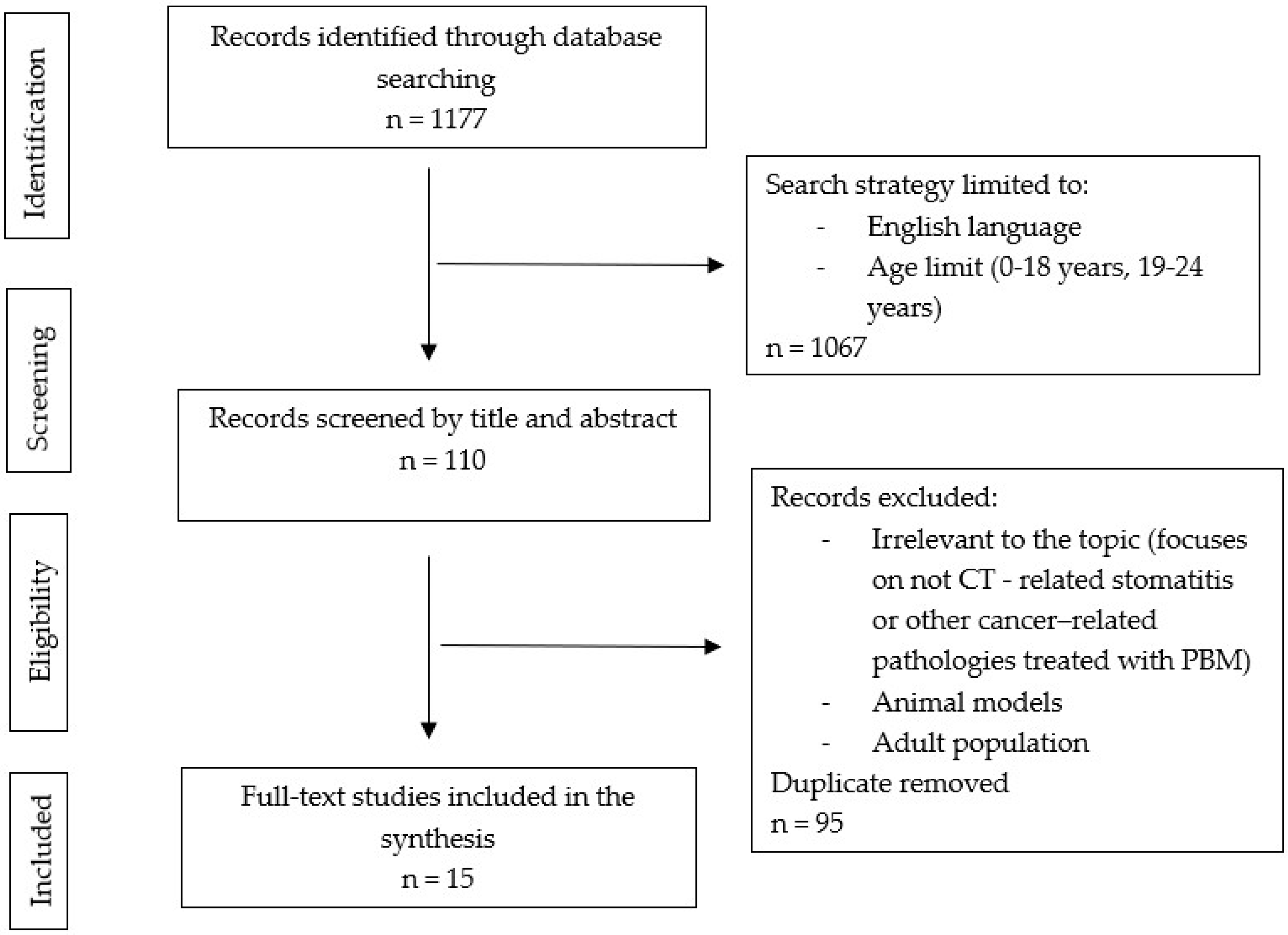
2. Methodology
2.1. Search Strategy
2.2. Study Selection
3. Results
3.1. The Role of Photobiomodulation in the Treatment of Chemotherapy-Induced Oral Mucositis
3.2. The Efficacy of Photobiomodulation Compared to Other Treatment Modalities for Treating Chemotherapy-Induced Oral Mucositis
3.3. The Role of Photobiomodulation in the Prevention of Chemotherapy-induced Oral Mucositis
3.4. In the Search for Optimal Photobiomodulation Protocol
| First Author, Year, (Reference) | Usage | Light Source | Wavelength (nm) | Power (W) | Power Density (W/cm2) | Energy Density (J/cm2) | Energy Per Point (J) | Spot Size (cm2) | Time (s) |
|---|---|---|---|---|---|---|---|---|---|
| Gobbo et al. (2017) [17] | Treatment | GaAlAs (diode) | 660 and 970 combined | 3.2 | 320 | 26.8 | 8 | 1 | 25 per spot |
| Vitale et al. (2017) [18] | Treatment | GaAlAs (diode) | 970 | 3.2 | / | / | / | 1 | 320 per session |
| Karaman et al. (2022) [19] | Treatment | GaAlAs (diode) | 830 | 0.15 | / | 4.5 | / | 1 | 30 per spot |
| Reyad et al. (2022) [8] | Treatment | Diode | 980 | 1.5 | / | 4.5 | / | 30 per spot | |
| Madeiros Filho et al. (2017) [20] | Treatment | AsGaAl and InGaP (diode) | 660 and 808 | 0.1 | / | 4 | / | / | 90 or 10 per area |
| Ribeiro da Silva et al. (2018) [21] | Treatment | InGaAlP (diode) | 660 | 0.1 | 35 or 105 | 0.028 | 30 or 10 per spot | ||
| Noirrit-Esclassan et al. (2019) [26] | Extraoral Treatment | Diode | 635 and 815 combined | 0.15 and 3.85 | 4 | / | / | / | from 30 per 30 cm2 area to 50 for 50 cm2 |
| Intraoral Treatment | Diode | 635 and 815 combined | 0.15 | 6 | / | / | / | 30 per 2 cm2 | |
| Tomaževič et al. (2019) [27] | Treatment | Diode | 810 | 0.25 | / | 8.8 or 15.5 (depending on OM 1 grade) | / | / | 4.4 or 7.8 per spot (depending on OM grade) |
| Treatment | Diode | 810 | 0.5 | / | 8.8 or 15.5 (depending on OM grade) | / | / | 2.2 or 3.9 per spot (depending on OM grade) | |
| Treatment | Diode | 810 | 0.25 | / | 4.4 or 7.75 (depending on OM grade) | / | / | 2.2 or 3.9 per spot (depending on OM grade) | |
| Curra et al. [13] | Treatment | InGaAlP Diode | 660 | 0.1 or 0.04 | / | 6 | / | / | / |
| Fiwek et al. [25] | Treatment | Diode | 635 and 980 combined | 1 | / | 30 | / | / | 30 per spot |
| Treatment | Diode | 635 and 980 combined | 0.1 | / | 2, 4, 8, or 16 (depending on OM grade) | / | / | 20, 40, 80, or 160 per spot (depending on OM grade) | |
| Cavalcanti et al. (2022) [4] | Treatment | GaAlAs and InGaAlP | 660 or 808 (depending on OM grade) | 0.1 | / | 3.3 | / | 0.03 | 10 s per spot |
| Avila-Sanchez et al. (2017) [7] | Prevention and Treatment | Diode | 980 | 0.3 | / | 18 | / | / | / |
| Nunes et al. (2020) [23] | Prevention | GaAIAs InGaAIP (diode) | 660 | 1 | 3.33 | 66.6 | 2 | 0.03 | 20 per spot |
| Miranda-Silva et al. (2021) [24] | Prevention | Diode | 660 | 0.1 | / | 35.7 | 1 | 0.028 | 10 per spot |
| Treatment | Diode | 660 | 0.1 | / | 35.7 | 2 | 0.028 | 20 per spot | |
| Guimaraes et al. (2021) [22] | Treatment and Prevention | InGaAlP (diode) | 660 | 0.1 | / | 2 | 0.6 | 0.03 | 36 per area |
| Treatment and Prevention | LED | 660 | 0.005 | / | 2 | 0.6 | 0.785 | 120 per area |
4. Discussion
4.1. Oral Mucositis—Pathobiology and Clinical Assessment
4.2. Photobiomodulation—Mechanism of Action and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Photobiomodulation and Oral Mucositis: A Systematic Review. Dent. J. 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Oral Mucositis. Anticancer Drugs 2011, 22, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Sonis, S.; Diz, P.D. Oral Mucositis. Oral Dis. 2006, 12, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Leite Cavalcanti, A.; José de Macêdo, D.; Suely Barros Dantas, F.; Dos Santos Menezes, K.; Filipe Bezerra Silva, D.; Alves de Melo Junior, W.; Fabia Cabral Cavalcanti, A. Evaluation of Oral Mucositis Occurrence in Oncologic Patients under Antineoplastic Therapy Submitted to the Low-Level Laser Coadjuvant Therapy. J. Clin. Med. 2018, 7, 90. [Google Scholar] [CrossRef]
- Mazhari, F.; Shirazi, A.S.; Shabzendehdar, M. Management of Oral Mucositis in Pediatric Patients Receiving Cancer Therapy: A Systematic Review and Meta-Analysis. Pediatr. Blood Cancer 2019, 66, e27403. [Google Scholar] [CrossRef]
- He, M.; Zhang, B.; Shen, N.; Wu, N.; Sun, J. A Systematic Review and Meta-Analysis of the Effect of Low-Level Laser Therapy (LLLT) on Chemotherapy-Induced Oral Mucositis in Pediatric and Young Patients. Eur. J. Pediatr. 2018, 177, 7–17. [Google Scholar] [CrossRef]
- Ávila-Sánchez, C.; Purizaca-Bazán, J.P.; Félix-Bermúdez, G.; Ellis-Irigoyen, M.A.; de Lourdes Vega-Vega, M.; Escamilla-Asiaín, G. Impact of a protocol for the prevention and care of oral mucositis in pediatric patients diagnosed with cancer. Gac. Mex. Oncol. 2017, 16, 96–102. [Google Scholar] [CrossRef]
- Reyad, F.A.; Elsayed, N.M.; El Chazli, Y. Photobiomodulation for Chemotherapy-Induced Oral Mucositis in Leukemic Children: A Randomized Controlled Clinical Trial. Oral Dis. 2022. [Google Scholar] [CrossRef]
- Redman, M.G.; Harris, K.; Phillips, B.S. Low-Level Laser Therapy for Oral Mucositis in Children with Cancer. Arch. Dis. Child. 2022, 107, 128–133. [Google Scholar] [CrossRef]
- Alqahtani, S.S.; Khan, S.D. Management of Oral Mucositis in Children. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1648–1657. [Google Scholar] [CrossRef]
- Bowen, J.M.; Wardill, H.R. Advances in the Understanding and Management of Mucositis during Stem Cell Transplantation. Curr. Opin. Support. Palliat. Care 2017, 11, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Anschau, F.; Webster, J.; Capra, M.E.Z.; de Azeredo da Silva, A.L.F.; Stein, A.T. Efficacy of Low-Level Laser for Treatment of Cancer Oral Mucositis: A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2019, 34, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Curra, M.; Gabriel, A.F.; Ferreira, M.B.C.; Martins, M.A.T.; Brunetto, A.T.; Gregianin, L.J.; Martins, M.D. Incidence and Risk Factors for Oral Mucositis in Pediatric Patients Receiving Chemotherapy. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 6243–6251. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, V.A.; Curi, D.S.C.; dos Santos, T.D.D.; de Rezende, R.P.; Lins-Kusterer, L.; Leite-Ribeiro, P. Oral Mucositis in Patients with Hematologic Malignancies Undergoing Chemotherapy. SN Compr. Clin. Med. 2021, 3, 2149–2153. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Miranda-Silva, W.; Gomes-Silva, W.; Zadik, Y.; Yarom, N.; Al-Azri, A.R.; Hong, C.H.L.; Ariyawardana, A.; Saunders, D.P.; Correa, M.E.; Arany, P.R.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis: Sub-Analysis of Current Interventions for the Management of Oral Mucositis in Pediatric Cancer Patients. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 3539–3562. [Google Scholar] [CrossRef]
- Gobbo, M.; Verzegnassi, F.; Ronfani, L.; Zanon, D.; Melchionda, F.; Bagattoni, S.; Majorana, A.; Bardellini, E.; Mura, R.; Piras, A.; et al. Multicenter Randomized, Double-Blind Controlled Trial to Evaluate the Efficacy of Laser Therapy for the Treatment of Severe Oral Mucositis Induced by Chemotherapy in Children: LaMPO RCT. Pediatr. Blood Cancer 2018, 65, e27098. [Google Scholar] [CrossRef]
- Vitale, M.C.; Modaffari, C.; Decembrino, N.; Zhou, F.X.; Zecca, M.; Defabianis, P. Preliminary Study in a New Protocol for the Treatment of Oral Mucositis in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplantation (HSCT) and Chemotherapy (CT). Lasers Med. Sci. 2017, 32, 1423–1428. [Google Scholar] [CrossRef]
- Karaman, K.; Sarica, A.; Tunc, S.K.; Karaman, S. Is Low-Level Laser Therapy a Candidate to Be a Good Alternative in the Treatment of Mucositis in Childhood Leukemia? J. Pediatr. Hematol. Oncol. 2022, 44, e199–e203. [Google Scholar] [CrossRef]
- Medeiros-Filho, J.B.; Maia Filho, E.M.; Ferreira, M.C. Laser and Photochemotherapy for the Treatment of Oral Mucositis in Young Patients: Randomized Clinical Trial. Photodiagn. Photodyn. Ther. 2017, 18, 39–45. [Google Scholar] [CrossRef]
- Ribeiro da Silva, V.C.; da Motta Silveira, F.M.; Barbosa Monteiro, M.G.; da Cruz, M.M.D.; de França Caldas Júnior, A.; Pina Godoy, G. Photodynamic Therapy for Treatment of Oral Mucositis: Pilot Study with Pediatric Patients Undergoing Chemotherapy. Photodiagn. Photodyn. Ther. 2018, 21, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, D.M.; Ota, T.M.N.; Da Silva, D.A.C.; Almeida, F.D.L.D.S.; Schalch, T.D.; Deana, A.M.; Junior, J.M.A.; Fernandes, K.P.S. Low-Level Laser or LED Photobiomodulation on Oral Mucositis in Pediatric Patients under High Doses of Methotrexate: Prospective, Randomized, Controlled Trial. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 6441–6447. [Google Scholar] [CrossRef]
- Nunes, L.F.M.; de Arruda, J.A.A.; Souza, A.F.; Silva, R.C.C.; Lanza, C.R.M.; Kakehasi, F.M.; Mesquita, R.A.; Abreu, L.G.; Travassos, D.V.; Silva, T.A. Prophylactic Photobiomodulation Therapy Using 660 Nm Diode Laser for Oral Mucositis in Paediatric Patients under Chemotherapy: 5-Year Experience from a Brazilian Referral Service. Lasers Med. Sci. 2020, 35, 1857–1866. [Google Scholar] [CrossRef]
- Miranda-Silva, W.; da Fonseca, F.P.; Gomes, A.A.; Mafra, A.B.B.; Rocha, V.; Fregnani, E.R. Oral Mucositis in Paediatric Cancer Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation Preventively Treated with Professional Dental Care and Photobiomodulation: Incidence and Risk Factors. Int. J. Paediatr. Dent. 2022, 32, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Fiwek, P.; Emerich, K.; Irga-Jaworska, N.; Pomiecko, D. Photobiomodulation Treatment in Chemotherapy-Induced Oral Mucositis in Young Haematological Patients-A Pilot Study. Med. Kaunas Lith. 2022, 58, 1023. [Google Scholar] [CrossRef] [PubMed]
- Noirrit-Esclassan, E.; Valera, M.C.; Vignes, E.; Munzer, C.; Bonal, S.; Daries, M.; Vaysse, F.; Puiseux, C.; Castex, M.P.; Boulanger, C.; et al. Photobiomodulation with a Combination of Two Wavelengths in the Treatment of Oral Mucositis in Children: The PEDIALASE Feasibility Study. Arch. Pediatr. Organe Off. Soc. Francaise Pediatr. 2019, 26, 268–274. [Google Scholar] [CrossRef]
- Tomaževič, T.; Potočnik, U.; Cizerl, D.; Jazbec, J. Optimization of Photobiomodulation Protocol for Chemotherapy-Induced Mucositis in Pediatric Patients. Photobiomodul. Photomed. Laser Surg. 2020, 38, 466–471. [Google Scholar] [CrossRef]
- Al-Ansari, S.; Zecha, J.A.E.M.; Barasch, A.; de Lange, J.; Rozema, F.R.; Raber-Durlacher, J.E. Oral Mucositis Induced By Anticancer Therapies. Curr. Oral Health Rep. 2015, 2, 202–211. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B.; et al. Perspectives on Cancer Therapy-Induced Mucosal Injury: Pathogenesis, Measurement, Epidemiology, and Consequences for Patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Sung, L.; Robinson, P.; Treister, N.; Baggott, T.; Gibson, P.; Tissing, W.; Wiernikowski, J.; Brinklow, J.; Dupuis, L.L. Guideline for the Prevention of Oral and Oropharyngeal Mucositis in Children Receiving Treatment for Cancer or Undergoing Haematopoietic Stem Cell Transplantation. BMJ Support. Palliat. Care 2017, 7, 7–16. [Google Scholar] [CrossRef]
- Gibson, F.; Auld, E.M.; Bryan, G.; Coulson, S.; Craig, J.V.; Glenny, A.-M. A Systematic Review of Oral Assessment Instruments: What Can We Recommend to Practitioners in Children’s and Young People’s Cancer Care? Cancer Nurs. 2010, 33, E1–E19. [Google Scholar] [CrossRef] [PubMed]
- De Castro, J.F.L.; Abreu, E.G.F.; Correia, A.V.L.; da Mota Vasconcelos Brasil, C.; da Cruz Perez, D.E.; de Paula Ramos Pedrosa, F. Low-Level Laser in Prevention and Treatment of Oral Mucositis in Pediatric Patients with Acute Lymphoblastic Leukemia. Photomed. Laser Surg. 2013, 31, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-Level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Pyatibrat, L.V.; Kalendo, G.S. Photobiological Modulation of Cell Attachment via Cytochrome c Oxidase. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2004, 3, 211–216. [Google Scholar] [CrossRef]
- Zanotta, N.; Ottaviani, G.; Campisciano, G.; Poropat, A.; Bovenzi, M.; Rupel, K.; Gobbo, M.; Comar, M.; Di Lenarda, R.; Biasotto, M.; et al. Photobiomodulation Modulates Inflammation and Oral Microbiome: A Pilot Study. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2020, 25, 677–684. [Google Scholar] [CrossRef]
- Basso, F.G.; Pansani, T.N.; Soares, D.G.; Scheffel, D.L.; Bagnato, V.S.; de Souza Costa, C.A.; Hebling, J. Biomodulation of Inflammatory Cytokines Related to Oral Mucositis by Low-Level Laser Therapy. Photochem. Photobiol. 2015, 91, 952–956. [Google Scholar] [CrossRef]
- Basso, F.G.; Soares, D.G.; Pansani, T.N.; Cardoso, L.M.; Scheffel, D.L.; de Souza Costa, C.A.; Hebling, J. Proliferation, Migration, and Expression of Oral-Mucosal-Healing-Related Genes by Oral Fibroblasts Receiving Low-Level Laser Therapy after Inflammatory Cytokines Challenge. Lasers Surg. Med. 2016, 48, 1006–1014. [Google Scholar] [CrossRef]
- Chow, R.T.; Armati, P.J. Photobiomodulation: Implications for Anesthesia and Pain Relief. Photomed. Laser Surg. 2016, 34, 599–609. [Google Scholar] [CrossRef]
- Chow, R.T.; David, M.A.; Armati, P.J. 830 Nm Laser Irradiation Induces Varicosity Formation, Reduces Mitochondrial Membrane Potential and Blocks Fast Axonal Flow in Small and Medium Diameter Rat Dorsal Root Ganglion Neurons: Implications for the Analgesic Effects of 830 Nm Laser. J. Peripher. Nerv. Syst. JPNS 2007, 12, 28–39. [Google Scholar] [CrossRef]
- Adnan, A.; Yaroslavsky, A.N.; Carroll, J.D.; Selting, W.; Juliano, A.F.; London, W.B.; Sonis, S.T.; Duncan, C.N.; Treister, N.S. The Path to an Evidence-Based Treatment Protocol for Extraoral Photobiomodulation Therapy for the Prevention of Oral Mucositis. Front. Oral Health 2021, 2, 689386. [Google Scholar] [CrossRef]
- Bezinelli, L.M.; Corrêa, L.; Vogel, C.; Kutner, J.M.; Ribeiro, A.F.; Hamerschlak, N.; de Paula Eduardo, C.; Migliorati, C.A.; de Paula Eduardo, F. Long-Term Safety of Photobiomodulation Therapy for Oral Mucositis in Hematopoietic Cell Transplantation Patients: A 15-Year Retrospective Study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 6891–6902. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Pinto, M.B.; de Lima Gusmão, T.P.; Schmidt-Filho, J.; Jaguar, G.C.; Martins, M.D.; Alves, F.A. Intraoral versus Extraoral Photobiomodulation Therapy in the Prevention of Oral Mucositis in HSCT Patients: A Randomized, Single-Blind, Controlled Clinical Trial. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 6495–6503. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.B.; Ribeiro, A.S.; Rech, A.; Rosa, L.G.N.; Castro, C.G.; Brunetto, A.L. Influence of Low-Energy Laser in the Prevention of Oral Mucositis in Children with Cancer Receiving Chemotherapy. Pediatr. Blood Cancer 2007, 48, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Whelan, H.T.; Connelly, J.F.; Hodgson, B.D.; Barbeau, L.; Post, A.C.; Bullard, G.; Buchmann, E.V.; Kane, M.; Whelan, N.T.; Warwick, A.; et al. NASA Light-Emitting Diodes for the Prevention of Oral Mucositis in Pediatric Bone Marrow Transplant Patients. J. Clin. Laser Med. Surg. 2002, 20, 319–324. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic Dose Response in Low Level Light Therapy-an Update. Dose-Response Publ. Int. Hormesis Soc. 2011, 9, 602–618. [Google Scholar] [CrossRef]
- Abramoff, M.M.F.; Lopes, N.N.F.; Lopes, L.A.; Dib, L.L.; Guilherme, A.; Caran, E.M.; Barreto, A.D.; Lee, M.L.M.; Petrilli, A.S. Low-Level Laser Therapy in the Prevention and Treatment of Chemotherapy-Induced Oral Mucositis in Young Patients. Photomed. Laser Surg. 2008, 26, 393–400. [Google Scholar] [CrossRef]
- Cauwels, R.G.E.C.; Martens, L.C. Low Level Laser Therapy in Oral Mucositis: A Pilot Study. Eur. Arch. Paediatr. Dent. Off. J. Eur. Acad. Paediatr. Dent. 2011, 12, 118–123. [Google Scholar] [CrossRef]
- Amadori, F.; Bardellini, E.; Conti, G.; Pedrini, N.; Schumacher, R.F.; Majorana, A. Low-Level Laser Therapy for Treatment of Chemotherapy-Induced Oral Mucositis in Childhood: A Randomized Double-Blind Controlled Study. Lasers Med. Sci. 2016, 31, 1231–1236. [Google Scholar] [CrossRef]
- Kuhn, A.; Porto, F.A.; Miraglia, P.; Brunetto, A.L. Low-Level Infrared Laser Therapy in Chemotherapy-Induced Oral Mucositis: A Randomized Placebo-Controlled Trial in Children. J. Pediatr. Hematol. Oncol. 2009, 31, 33–37. [Google Scholar] [CrossRef]
- Patel, P.; Robinson, P.D.; Baggott, C.; Gibson, P.; Ljungman, G.; Massey, N.; Ottaviani, G.; Phillips, R.; Revon-Rivière, G.; Treister, N.; et al. Clinical Practice Guideline for the Prevention of Oral and Oropharyngeal Mucositis in Pediatric Cancer and Hematopoietic Stem Cell Transplant Patients: 2021 Update. Eur. J. Cancer Oxf. Engl. 1990 2021, 154, 92–101. [Google Scholar] [CrossRef]

| First Author, Year, (Reference) | Participants (Number) | Underlying Diagnosis | PBM 4 Protocol | Light Application Technique | Treatment Frequency | Outcomes for OM 5 Severity | Outcomes for Oral Pain Reduction |
|---|---|---|---|---|---|---|---|
| Gobbo et al. (2017) [17] | 101 51 laser group, 50 placebo control group | ALL 1, HSCT 2, Lymphoma, AML 3, solid tumors | Diode Laser, 660 and 970 nm, 320 mW/cm2, 26.8 J/cm2, spot size 1 cm2, 25 s per spot, 9 spots total | Irradiation of the entire oral cavity, defocused, non-contact modality | Daily, 4 consecutive days | Significantly lower OM grade on day 7 in the laser group | Significant reduction in pain on day 7 in the laser group |
| Vitale et al. (2017) [18] | 16 8 laser group, 8 placebo control group | Not specified | GaAlAs Laser, 970 nm, 3.2 W, spot size 1 cm2, 320 s per session | Irradiation of the entire oral cavity, defocused modality | Daily, 4 consecutive days | Significantly lower OM grade on day 7 in the laser group | Significant reduction in pain on day 3 in the laser group |
| Karaman et al. (2022) [19] | 40 20 laser group, 20 control group | Leukemia | GaAlAs Laser, 830 nm, 150 mW, 4.5 J/cm2, spot size 1 cm2, 30 s per spot | Irradiation of affected areas only | Every other day, 3 times in total | Significantly lower OM grade on days 3, 5, 6, and 7 in the laser group | Significant reduction in pain in the laser group |
| Reyad et al. (2022) [8] | 44 22 laser group, 22 control group | ALL | Diode Laser, 980 nm, 1.5 W, 4.5 J/cm2, 30 s per spot | Irradiation of affected areas only, non-contact modality | Daily, 4 consecutive days | Significantly lower OM grade on day 14 in the laser group | Significant reduction in pain on day 10 in the laser group |
| First Author, Year, (Reference) | Participants (Number) | Underlying Diagnosis | PBM Protocol | Light Application Technique | Treatment Frequency | Outcomes for OM 4 Severity | Outcomes for Oral Pain Reduction |
|---|---|---|---|---|---|---|---|
| Madeiros Filho et al. (2017) [20] | 15 PDT 1 on one side of the oral cavity, PBM 2 on the other | Leukemia, osteosarcoma, lymphoma, sarcoma, medulloblastoma | InGaP Laser, 660 nm PDT and AsGaAl Laser, 808 nm for PBM, 100 mW, 90 s per site for PDT and 10 s per site for PBM | Irradiation of affected areas only, punctual, contact modality | Daily, 8 consecutive days | Significantly smaller lesions on days 6 to 8 on the side treated with PDT | Not evaluated |
| Ribeiro da Silva et al. (2018) [21] | 29 14 PDT group, 15 PBM group | ALL 3, Non-Hodgkin lymphoma, osteosarcoma | InGaAlP Laser, 660 nm, 100 mW, 107 J/cm2 PDT group and 35 J/cm2 PBM group, spot size 0.028 cm2, 30 s per spot PDT group and 10 s per spot PBM group | Irradiation of affected areas only, punctual, non–contact modality | Daily, until OM healing | No significant difference in the number of sessions required to heal OM between the two groups | No significant difference in the reduction in pain between the two groups |
| Guimaraes et al. (2021) [22] | 80 40 LED group, 40 PBM group | ALL | InGaAlP Laser, 660 nm, 100 mW, 2 J/cm2, spot size 0.03 cm2, 36 s per area LED, 660 nm, 5 mW, 2 J/cm2, spot size 0.785 cm2, 120 s per area | Irradiation of the entire oral cavity, contact modality | Daily, until OM healing or hospital discharge | No significant difference in OM incidence and grade between the two groups | No significant difference in the reduction in pain between the two groups |
| First Author, Year, (Reference) | Participants (Number) | Underlying Diagnosis | PBM 5 Protocol | Light Application Technique | Findings |
|---|---|---|---|---|---|
| Avila-Sanchez et al. (2017) [7] | 157 | Hematological malignancies, solid tumors, CNS 1 tumors | Diode Laser, 980 nm, 300 mW, 18 J/cm2 | Punctual modality | OM occurrence 21.6%, 94% of OM 6 episodes were grade 1 or 2, 4% were grade 3, and 2% were grade 4 on the WHO scale. A higher OM occurrence was associated with ALL and osteosarcoma. |
| Nunes et al. (2020) [23] | 148 | ALL 2, Osteosarcoma, AML 3, HSCT 4, Burkitt lymphoma | InGaAIP, 660 nm, 100 mW, 3.33 W/cm2, 66.6 J/cm2, 20 s per point | Punctual, contact modality | In patients who underwent prophylactic PBM, a lower grade of OM was observed. Higher OM occurrence was observed in HSCT and osteosarcoma patients and those receiving MTX 7. |
| Miranda-Silva et al. (2021) [24] | 49 | HSCT | Diode Laser, 660 nm, 100 mW, 3.57 W/cm2, 35.7 J/cm2, spot size 0.028 cm2, 10 s per point for prevention and 20 s per point for treatment | Irradiation of the entire oral cavity, punctual, non–contact modality | OM occurrence 73.5%; of that, 36.1% were grades 3 or 4 on the WHO 8 scale. The diagnosis of ALL and the use of a myeloablative regiment were associated with OM. |
| He et al. (2017) [6] | 373 | Childhood hematological malignancies and solid tumors | LED or diode lasers, 660–970 nm, 3 mW–3.2 W, 4–72 J/cm2 | Different variety of light application | The odds ratio for developing OM and severe OM (grade 3 or 4 on the WHO scale) after prophylactic PBM is significantly lower compared to placebo (p = 0.01 and p = 0.03). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafner, D.; Hrast, P.; Tomaževič, T.; Jazbec, J.; Kavčič, M. Photobiomodulation for Chemotherapy-Induced Oral Mucositis in Pediatric Patients. Biomolecules 2023, 13, 418. https://doi.org/10.3390/biom13030418
Hafner D, Hrast P, Tomaževič T, Jazbec J, Kavčič M. Photobiomodulation for Chemotherapy-Induced Oral Mucositis in Pediatric Patients. Biomolecules. 2023; 13(3):418. https://doi.org/10.3390/biom13030418
Chicago/Turabian StyleHafner, Daša, Petra Hrast, Tanja Tomaževič, Janez Jazbec, and Marko Kavčič. 2023. "Photobiomodulation for Chemotherapy-Induced Oral Mucositis in Pediatric Patients" Biomolecules 13, no. 3: 418. https://doi.org/10.3390/biom13030418
APA StyleHafner, D., Hrast, P., Tomaževič, T., Jazbec, J., & Kavčič, M. (2023). Photobiomodulation for Chemotherapy-Induced Oral Mucositis in Pediatric Patients. Biomolecules, 13(3), 418. https://doi.org/10.3390/biom13030418





