Therapeutic Oral Application of Carvacrol Alleviates Acute Campylobacteriosis in Mice Harboring a Human Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
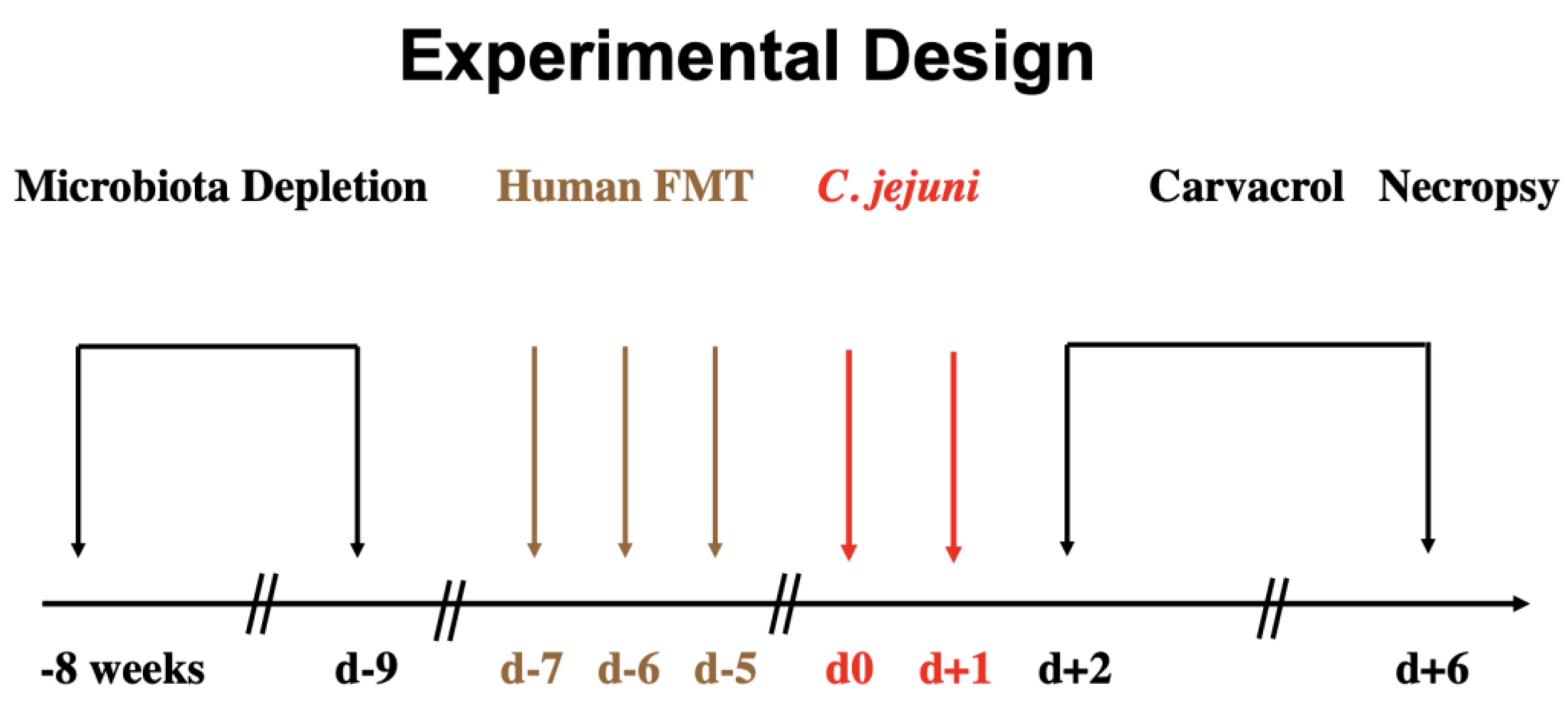
2.1. Secondary Abiotic IL-10−/− Mice
2.2. Human Fecal Microbiota Transplantation
2.3. Campylobacter Jejuni Infection
2.4. Treatment Regimen
2.5. Gastrointestinal C. jejuni Loads
2.6. Gut Microbiota Composition
2.7. Clinical Conditions of Mice
2.8. Sampling Procedures
2.9. Histopathology
2.10. In Situ Immunohistochemistry
2.11. Pro-Inflammatory Mediators
2.12. Statistical Analyses
3. Results
3.1. Gastrointestinal C. jejuni Colonization upon Carvacrol Treatment of Infected IL-10−/− Mice with a Human Gut Microbiota
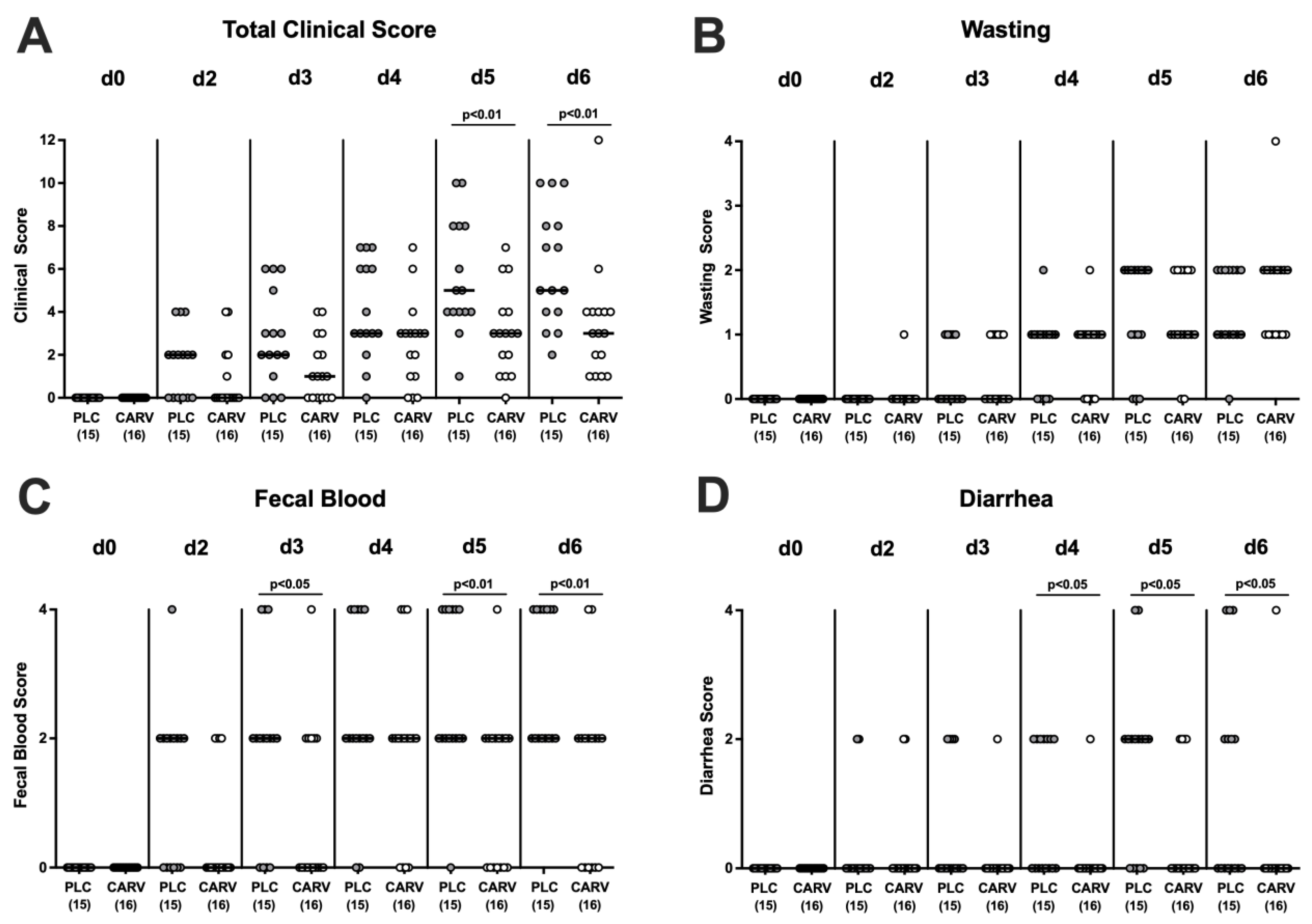
3.2. Clinical Conditions over Time upon Carvacrol Treatment of Infected IL-10−/− Mice with a Human Gut Microbiota
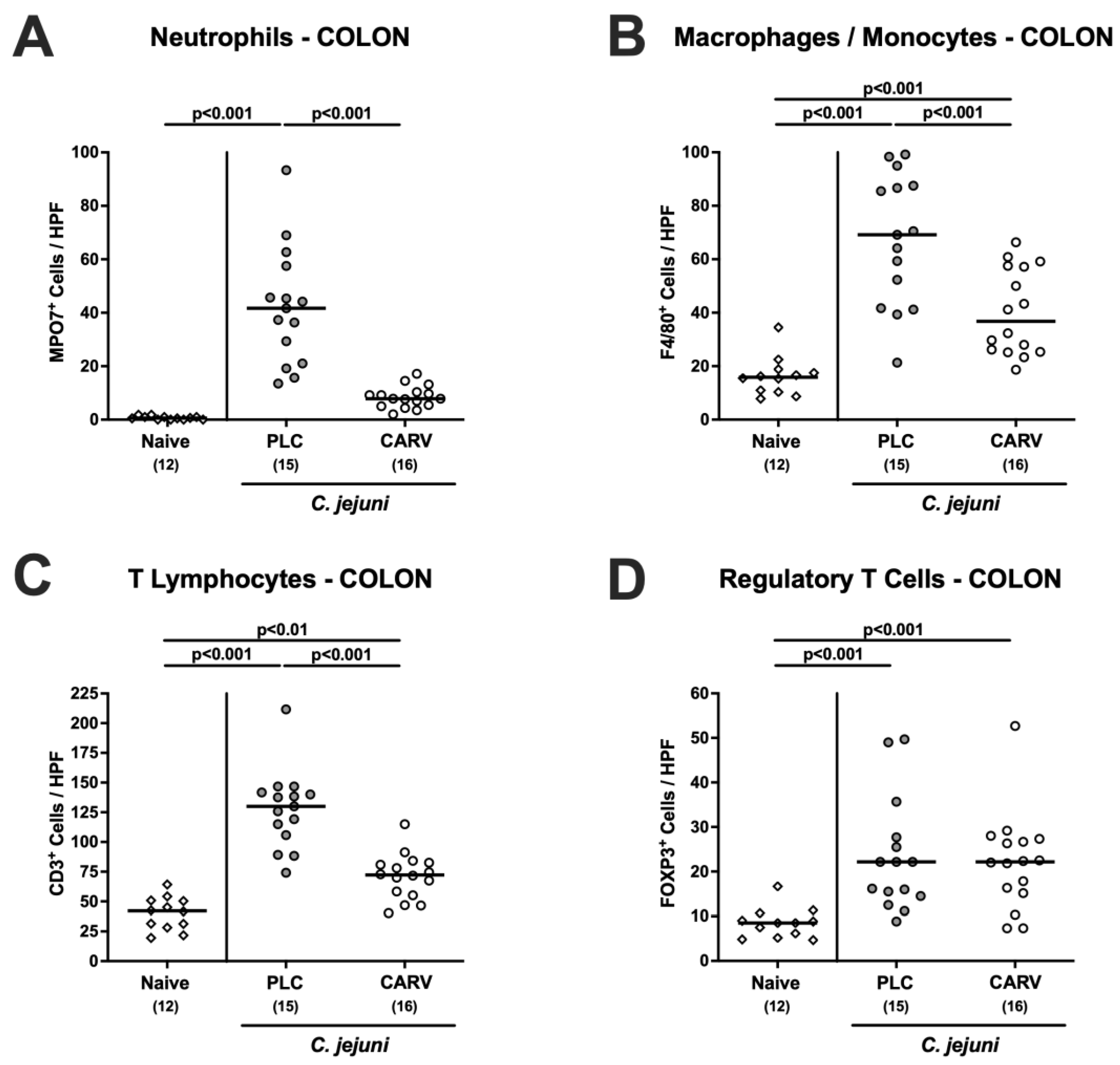
3.3. Microscopic Inflammatory Changes in the Colon upon Carvacrol Treatment of Infected IL-10−/− Mice with a Human Gut Microbiota
3.4. Immune Cell Responses in the Colon upon Carvacrol Treatment of Infected IL-10−/− Mice Harboring a Human Gut Microbiota
3.5. Intestinal Pro-Inflammatory Mediator Secretion upon Carvacrol Treatment of Infected IL-10−/− Mice Harboring a Human Gut Microbiota
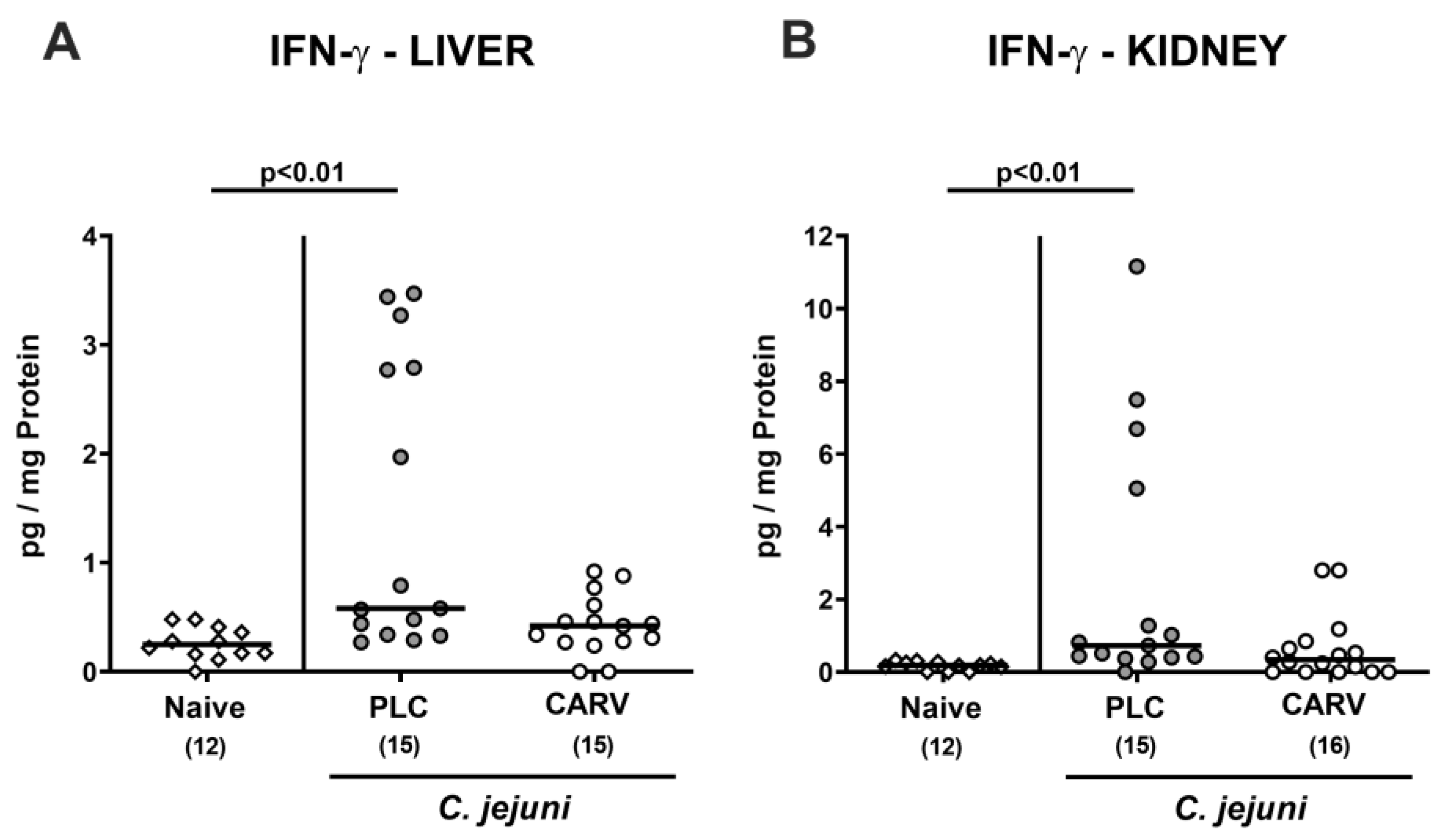
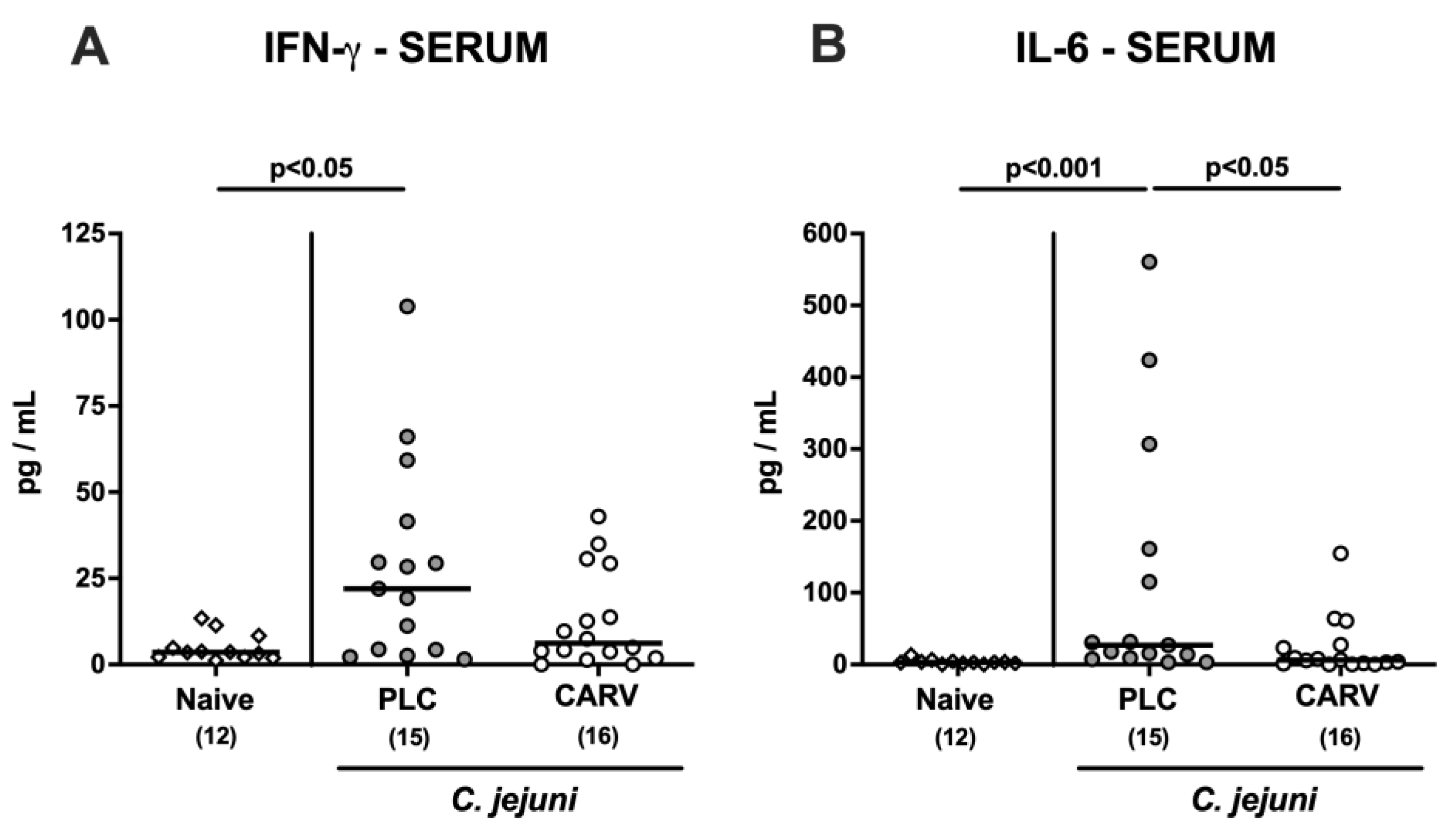
3.6. Extra-Intestinal and Systemic Pro-Inflammatory Cytokine Secretion upon Carvacrol Treatment of Infected IL-10−/− Mice Harboring a Human Gut Microbiota
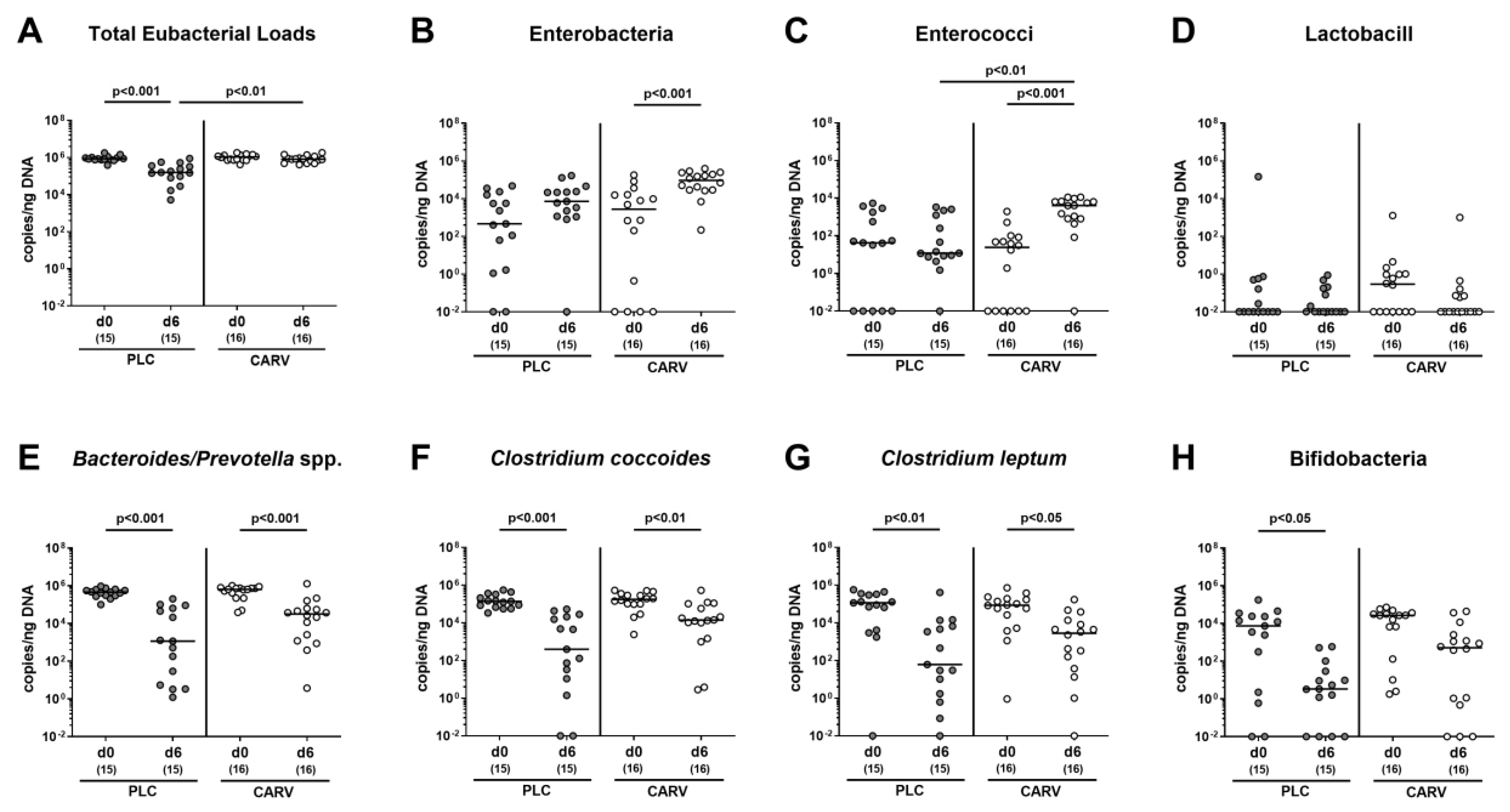
3.7. Impact of Carvacrol Treatment on Fecal Microbiota Composition of Infected IL-10−/− Mice Harboring a Human Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Bronowski, C.; James, C.E.; Winstanley, C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol. Lett. 2014, 356, 8–19. [Google Scholar] [CrossRef]
- Hermans, D.; Pasmans, F.; Messens, W.; Martel, A.; Van Immerseel, F.; Rasschaert, G.; Heyndrickx, M.; Van Deun, K.; Haesebrouck, F. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 2012, 12, 89–98. [Google Scholar] [CrossRef]
- Young, K.T.; Davis, L.M.; Dirita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, N.P.; Kuijf, M.L.; Ang, C.W.; Schiellerup, P.; Krogfelt, K.A.; Jacobs, B.C.; van Belkum, A.; Endtz, H.P.; Bergman, M.P. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 2009, 11, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Klove, S.; Genger, C.; Weschka, D.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Toll-Like Receptor-4 Is Involved in Mediating Intestinal and Extra-Intestinal Inflammation in Campylobacter coli-Infected Secondary Abiotic IL-10−/− Mice. Microorganisms 2020, 8, 1882. [Google Scholar] [CrossRef]
- Cróinín, T.O.; Backert, S. Host epithelial cell invasion by Campylobacter jejuni: Trigger or zipper mechanism? Front. Cell. Infect. Microbiol. 2012, 2, 25. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Novel Clinical Campylobacter jejuni Infection Models Based on Sensitization of Mice to Lipooligosaccharide, a Major Bacterial Factor Triggering Innate Immune Responses in Human Campylobacteriosis. Microorganisms 2020, 8, 482. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Murine Models for the Investigation of Colonization Resistance and Innate Immune Responses in Campylobacter Jejuni Infections. Curr. Top Microbiol. Immunol. 2021, 431, 233–263. [Google Scholar] [CrossRef]
- Bücker, R.; Krug, S.M.; Moos, V.; Bojarski, C.; Schweiger, M.R.; Kerick, M.; Fromm, A.; Janßen, S.; Fromm, M.; Hering, N.A.; et al. Campylobacter jejuni impairs sodium transport and epithelial barrier function via cytokine release in human colon. Mucosal Immunol. 2018, 11, 474–485. [Google Scholar] [CrossRef]
- Mortensen, N.; Jonasson, S.A.; Lavesson, I.V.; Emberland, K.E.; Litleskare, S.; Wensaas, K.A.; Rortveit, G.; Langeland, N.; Hanevik, K. Correction: Characteristics of hospitalized patients during a large waterborne outbreak of Campylobacter jejuni in Norway. PLoS ONE 2021, 16, e0259407. [Google Scholar] [CrossRef]
- Backert, S.; Tegtmeyer, N.; Cróinín, T.Ó.; Boehm, M.; Heimesaat, M.M. Human campylobacteriosis. In Campylobacter; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–25. [Google Scholar]
- Heimesaat, M.M.; Backert, S.; Alter, T.; Bereswill, S. Human Campylobacteriosis-A Serious Infectious Threat in a One Health Perspective. Curr. Top Microbiol. Immunol. 2021, 431, 1–23. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, e07209. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobials in agriculture and the environment: Reducing unnecessary use and waste. Rev. Antimicrob. Resist. 2015, 1, 1–41. [Google Scholar]
- Robinson, T.P.; Bu, D.P.; Carrique-Mas, J.; Fevre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef]
- Anholt, M.; Barkema, H. What is One Health? Can. Vet. J. 2021, 62, 641–644. [Google Scholar]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L.; Leach, D.N. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragr. J. 1999, 14, 322–332. [Google Scholar]
- Friedman, M. Chemistry and multibeneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, T.; Mohammadi, M.; Abdollahpour, F.; Kamarehie, B.; Jafari, A.; Ghaderpoori, A.; Karami, M.A. A comparative study on antibacterial activity of carvacrol and glutaraldehyde on Pseudomonas aeruginosa and Staphylococcus aureus isolates: An in vitro study. J. Environ. Health Sci. Eng. 2021, 19, 475–482. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- Wagle, B.R.; Upadhyay, A.; Upadhyaya, I.; Shrestha, S.; Arsi, K.; Liyanage, R.; Venkitanarayanan, K.; Donoghue, D.J.; Donoghue, A.M. Trans-Cinnamaldehyde, Eugenol and Carvacrol Reduce Campylobacter jejuni Biofilms and Modulate Expression of Select Genes and Proteins. Front. Microbiol. 2019, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Lee, J. Carvacrol-rich oregano oil and thymol-rich thyme red oil inhibit biofilm formation and the virulence of uropathogenic Escherichia coli. J. Appl. Microbiol. 2017, 123, 1420–1428. [Google Scholar] [PubMed]
- Knowles, J.; Roller, S.; Murray, D.B.; Naidu, A. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2005, 71, 797–803. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.; von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef]
- Arsi, K.; Donoghue, A.; Venkitanarayanan, K.; Kollanoor-Johny, A.; Fanatico, A.; Blore, P.; Donoghue, D. The efficacy of the natural plant extracts, thymol and carvacrol against Campylobacter colonization in broiler chickens. J. Food Saf. 2014, 34, 321–325. [Google Scholar]
- Johny, A.K.; Darre, M.; Donoghue, A.; Donoghue, D.; Venkitanarayanan, K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 2010, 19, 237–244. [Google Scholar] [CrossRef]
- Kelly, C.; Gundogdu, O.; Pircalabioru, G.; Cean, A.; Scates, P.; Linton, M.; Pinkerton, L.; Magowan, E.; Stef, L.; Simiz, E.; et al. The In Vitro and In Vivo Effect of Carvacrol in Preventing Campylobacter Infection, Colonization and in Improving Productivity of Chicken Broilers. Foodborne Pathog. Dis. 2017, 14, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Wagle, B.R.; Donoghue, A.M.; Shrestha, S.; Upadhyaya, I.; Arsi, K.; Gupta, A.; Liyanage, R.; Rath, N.C.; Donoghue, D.J.; Upadhyay, A. Carvacrol attenuates Campylobacter jejuni colonization factors and proteome critical for persistence in the chicken gut. Poult. Sci. 2020, 99, 4566–4577. [Google Scholar] [CrossRef]
- Masanta, W.O.; Heimesaat, M.M.; Bereswill, S.; Tareen, A.M.; Lugert, R.; Groß, U.; Zautner, A.E. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin. Dev. Immunol. 2013, 2013, 526860. [Google Scholar] [CrossRef] [PubMed]
- Fiebiger, U.; Bereswill, S.; Heimesaat, M.M. Dissecting the interplay between intestinal microbiota and host immunity in health and disease: Lessons learned from germfree and gnotobiotic animal models. Eur. J. Microbiol. Immunol. 2016, 6, 253–271. [Google Scholar] [CrossRef]
- Bereswill, S.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kuhl, A.A.; Dasti, J.I.; Zautner, A.E.; Munoz, M.; Loddenkemper, C.; et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS ONE 2011, 6, e20953. [Google Scholar] [CrossRef]
- Ellström, P.; Hansson, I.; Nilsson, A.; Rautelin, H.; Olsson Engvall, E. Lipooligosaccharide locus classes and putative virulence genes among chicken and human Campylobacter jejuni isolates. BMC Microbiol. 2016, 16, 116. [Google Scholar] [CrossRef]
- Robertson, S.A.; Care, A.S.; Skinner, R.J. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol. Reprod. 2007, 76, 738–748. [Google Scholar] [CrossRef]
- Haag, L.M.; Fischer, A.; Otto, B.; Plickert, R.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S.; Heimesaat, M.M. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10−/− mice via Toll-like-receptor-2 and -4 signaling. PLoS ONE 2012, 7, e40761. [Google Scholar] [CrossRef]
- Sun, X.; Threadgill, D.; Jobin, C. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterology 2012, 142, 86–95.e85. [Google Scholar] [CrossRef]
- Bereswill, S.; Mousavi, S.; Weschka, D.; Heimesaat, M.M. Disease-Alleviating Effects of Peroral Activated Charcoal Treatment in Acute Murine Campylobacteriosis. Microorganisms 2021, 9, 1424. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Mousavi, S.; Weschka, D.; Bereswill, S. Garlic Essential Oil as Promising Option for the Treatment of Acute Campylobacteriosis-Results from a Preclinical Placebo-Controlled Intervention Study. Microorganisms 2021, 9, 1140. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Weschka, D.; Bereswill, S.; Heimesaat, M.M. Immune-Modulatory Effects upon Oral Application of Cumin-Essential-Oil to Mice Suffering from Acute Campylobacteriosis. Pathogens 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Bereswill, S.; Mousavi, S.; Weschka, D.; Buczkowski, A.; Schmidt, S.; Heimesaat, M.M. Peroral Clove Essential Oil Treatment Ameliorates Acute Campylobacteriosis-Results from a Preclinical Murine Intervention Study. Microorganisms 2021, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Weschka, D.; Bereswill, S.; Heimesaat, M.M. Preclinical Evaluation of Oral Urolithin-A for the Treatment of Acute Campylobacteriosis in Campylobacter jejuni Infected Microbiota-Depleted IL-10−/− Mice. Pathogens 2020, 10, 7. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef]
- Lobo de Sá, F.D.; Backert, S.; Nattramilarasu, P.K.; Mousavi, S.; Sandle, G.I.; Bereswill, S.; Heimesaat, M.M.; Schulzke, J.D.; Bücker, R. Vitamin D Reverses Disruption of Gut Epithelial Barrier Function Caused by Campylobacter jejuni. Int. J. Mol. Sci. 2021, 22, 8872. [Google Scholar] [CrossRef]
- Bereswill, S.; Plickert, R.; Fischer, A.; Kühl, A.A.; Loddenkemper, C.; Batra, A.; Siegmund, B.; Göbel, U.B.; Heimesaat, M.M. What you eat is what you get: Novel Campylobacter models in the quadrangle relationship between nutrition, obesity, microbiota and susceptibility to infection. Eur. J. Microbiol. Immunol. 2011, 1, 237–248. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Genger, C.; Klove, S.; Weschka, D.; Mousavi, S.; Bereswill, S. The Host-Specific Intestinal Microbiota Composition Impacts Campylobacter coli Infection in a Clinical Mouse Model of Campylobacteriosis. Pathogens 2020, 9, 804. [Google Scholar] [CrossRef]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B.; Reading, N.C.; Villablanca, E.J.; Wang, S.; Mora, J.R.; et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef]
- Shayya, N.W.; Foote, M.S.; Langfeld, L.Q.; Du, K.; Bandick, R.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Human microbiota associated IL-10−/− mice: A valuable enterocolitis model to dissect the interactions of Campylobacter jejuni with host immunity and gut microbiota. Eur. J. Microbiol. Immunol. 2023, 12, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Schmidt, A.M.; Escher, U.; Kittler, S.; Kehrenberg, C.; Thunhorst, E.; Bereswill, S.; Heimesaat, M.M. Carvacrol ameliorates acute campylobacteriosis in a clinical murine infection model. Gut Pathog. 2020, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Mousavi, S.; Bandick, R.; Bereswill, S. Campylobacter jejuni infection induces acute enterocolitis in IL-10−/− mice pretreated with ampicillin plus sulbactam. Eur. J. Microbiol. Immunol. 2022, 12, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Fuchs, D.; Struck, D.; Niebergall, J.; Jahn, H.-K.; Dunay, I.R.; Moter, A.; Gescher, D.M. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006, 177, 8785–8795. [Google Scholar]
- Von Klitzing, E.; Öz, F.; Ekmekciu, I.; Escher, U.; Bereswill, S.; Heimesaat, M.M. Comprehensive survey of intestinal microbiota changes in offspring of human microbiota associated mice. Eur. J. Microbiol. Immunol. 2017, 7, 65–75. [Google Scholar]
- Du, K.; Foote, M.S.; Mousavi, S.; Buczkowski, A.; Schmidt, S.; Bereswill, S.; Heimesaat, M.M. Less Pronounced Immunopathological Responses following Oral Butyrate Treatment of Campylobacter jejuni-Infected Mice. Microorganisms 2022, 10, 1953. [Google Scholar] [CrossRef]
- Rausch, S.; Held, J.; Fischer, A.; Heimesaat, M.M.; Kühl, A.A.; Bereswill, S.; Hartmann, S. Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PLoS ONE 2013, 8, e74026. [Google Scholar] [CrossRef]
- Weschka, D.; Mousavi, S.; Biesemeier, N.; Bereswill, S.; Heimesaat, M.M. Survey of Pathogen-Lowering and Immuno-Modulatory Effects Upon Treatment of Campylobacter coli-Infected Secondary Abiotic IL-10−/− Mice with the Probiotic Formulation Aviguard(®). Microorganisms 2021, 9, 1127. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Alutis, M.; Grundmann, U.; Fischer, A.; Tegtmeyer, N.; Böhm, M.; Kühl, A.A.; Göbel, U.B.; Backert, S.; Bereswill, S. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front. Cell. Infect. Microbiol. 2014, 4, 77. [Google Scholar]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557. [Google Scholar]
- Heimesaat, M.M.; Giladi, E.; Kühl, A.A.; Bereswill, S.; Gozes, I. The octapetide NAP alleviates intestinal and extra-intestinal anti-inflammatory sequelae of acute experimental colitis. Peptides 2018, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free. Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Arsi, K.; Wagle, B.R.; Upadhyaya, I.; Shrestha, S.; Donoghue, A.M.; Donoghue, D.J. Trans-cinnamaldehyde, carvacrol, and eugenol reduce Campylobacter jejuni colonization factors and expression of virulence genes in vitro. Front. Microbiol. 2017, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Van Alphen, L.B.; Burt, S.A.; Veenendaal, A.K.; Bleumink-Pluym, N.M.; van Putten, J.P. The natural antimicrobial carvacrol inhibits Campylobacter jejuni motility and infection of epithelial cells. PLoS ONE 2012, 7, e45343. [Google Scholar] [CrossRef]
- Banik, S.; Akter, M.; Corpus Bondad, S.E.; Saito, T.; Hosokawa, T.; Kurasaki, M. Carvacrol inhibits cadmium toxicity through combating against caspase dependent/independent apoptosis in PC12 cells. Food Chem. Toxicol. 2019, 134, 110835. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Oliveira, G.F.; Melo, M.S.; Cavalcanti, S.C.; Antoniolli, A.R.; Bonjardim, L.R.; Silva, F.A.; Santos, J.P.A.; Rocha, R.F.; Moreira, J.C.F. Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin. Pharmacol. Toxicol. 2010, 107, 949–957. [Google Scholar]
- Somensi, N.; Rabelo, T.K.; Guimarães, A.G.; Quintans-Junior, L.J.; de Souza Araújo, A.A.; Moreira, J.C.F.; Gelain, D.P. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef]
- Amirghofran, Z.; Ahmadi, H.; Karimi, M.H.; Kalantar, F.; Gholijani, N.; Malek-Hosseini, Z. In vitro inhibitory effects of thymol and carvacrol on dendritic cell activation and function. Pharm. Biol. 2016, 54, 1125–1132. [Google Scholar] [PubMed]
- Liu, S.; Song, M.; Yun, W.; Lee, J.; Kim, H.; Cho, J. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019, 98, 2026–2033. [Google Scholar]
- Marconi, G.D.; Della Rocca, Y.; Fonticoli, L.; Guarnieri, S.; Carradori, S.; Rajan, T.S.; Pizzicannella, J.; Diomede, F. The Beneficial Effect of Carvacrol in HL-1 Cardiomyocytes Treated with LPS-G: Anti-Inflammatory Pathway Investigations. Antioxidants 2022, 11, 386. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.z.; Gu, C.x.; Liu, G.l.; Tian, K. Carvacrol suppresses inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes. J. Cell. Biochem. 2019, 120, 8169–8176. [Google Scholar] [PubMed]
- Zhao, W.; Deng, C.; Han, Q.; Xu, H.; Chen, Y. Carvacrol may alleviate vascular inflammation in diabetic db/db mice. Int. J. Mol. Med. 2020, 46, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.-h. Inhibitory effect of carvacrol on lipopolysaccharide-induced memory impairment in rats. Korean J. Physiol. Pharmacol. 2020, 24, 27–37. [Google Scholar] [PubMed]
- Kandemir, F.M.; Caglayan, C.; Darendelioğlu, E.; Küçükler, S.; İzol, E.; Kandemir, Ö. Modulatory effects of carvacrol against cadmium-induced hepatotoxicity and nephrotoxicity by molecular targeting regulation. Life Sci. 2021, 277, 119610. [Google Scholar] [PubMed]
- Feng, X.; Jia, A. Protective effect of carvacrol on acute lung injury induced by lipopolysaccharide in mice. Inflammation 2014, 37, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Rangan, P.; Choi, I.; Wei, M.; Navarrete, G.; Guen, E.; Brandhorst, S.; Enyati, N.; Pasia, G.; Maesincee, D.; Ocon, V.; et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep. 2019, 26, 2704–2719.e2706. [Google Scholar] [CrossRef]
- Tojo, R.; Suárez, A.; Clemente, M.G.; de los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Reifenberger, G.; Vicena, V.; Illes, A.; Horvath, G.; Tamas, A.; Fulop, B.D.; Bereswill, S.; Reglodi, D. Intestinal microbiota changes in mice lacking pituitary adenylate cyclase activating polypeptide (PACAP)—bifidobacteria make the difference. Eur. J. Microbiol. Immunol. 2017, 7, 187–199. [Google Scholar] [CrossRef]
- Shang, J.; Yang, S.; Tang, Z.; Chen, Y.; Duan, B.; Meng, X. Bifidobacterium bifidum H3-R2 and its molecular communication within the context of ulcerative colitis. J. Agric. Food Chem. 2022, 70, 11678–11688. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (EFSA FEEDAP Panel); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of an essential oil of Origanum vulgare ssp. hirtum (Link) leetsw. for all poultry species. EFSA J. 2019, 17, e05653. [Google Scholar]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foote, M.S.; Du, K.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Therapeutic Oral Application of Carvacrol Alleviates Acute Campylobacteriosis in Mice Harboring a Human Gut Microbiota. Biomolecules 2023, 13, 320. https://doi.org/10.3390/biom13020320
Foote MS, Du K, Mousavi S, Bereswill S, Heimesaat MM. Therapeutic Oral Application of Carvacrol Alleviates Acute Campylobacteriosis in Mice Harboring a Human Gut Microbiota. Biomolecules. 2023; 13(2):320. https://doi.org/10.3390/biom13020320
Chicago/Turabian StyleFoote, Minnja S., Ke Du, Soraya Mousavi, Stefan Bereswill, and Markus M. Heimesaat. 2023. "Therapeutic Oral Application of Carvacrol Alleviates Acute Campylobacteriosis in Mice Harboring a Human Gut Microbiota" Biomolecules 13, no. 2: 320. https://doi.org/10.3390/biom13020320
APA StyleFoote, M. S., Du, K., Mousavi, S., Bereswill, S., & Heimesaat, M. M. (2023). Therapeutic Oral Application of Carvacrol Alleviates Acute Campylobacteriosis in Mice Harboring a Human Gut Microbiota. Biomolecules, 13(2), 320. https://doi.org/10.3390/biom13020320





