The Role of a Cytokinin Antagonist in the Progression of Clubroot Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material and Growth Conditions
2.2. Preparation of the Cytokinin Antagonist PI-55
2.3. Inoculation of A. thaliana Plants and Pharmacological Treatment
2.4. Quantification of Plant Hormones
2.5. Quantitative Real-Time PCR
2.6. Measurement of Photosynthetic Activity
3. Results and Discussion
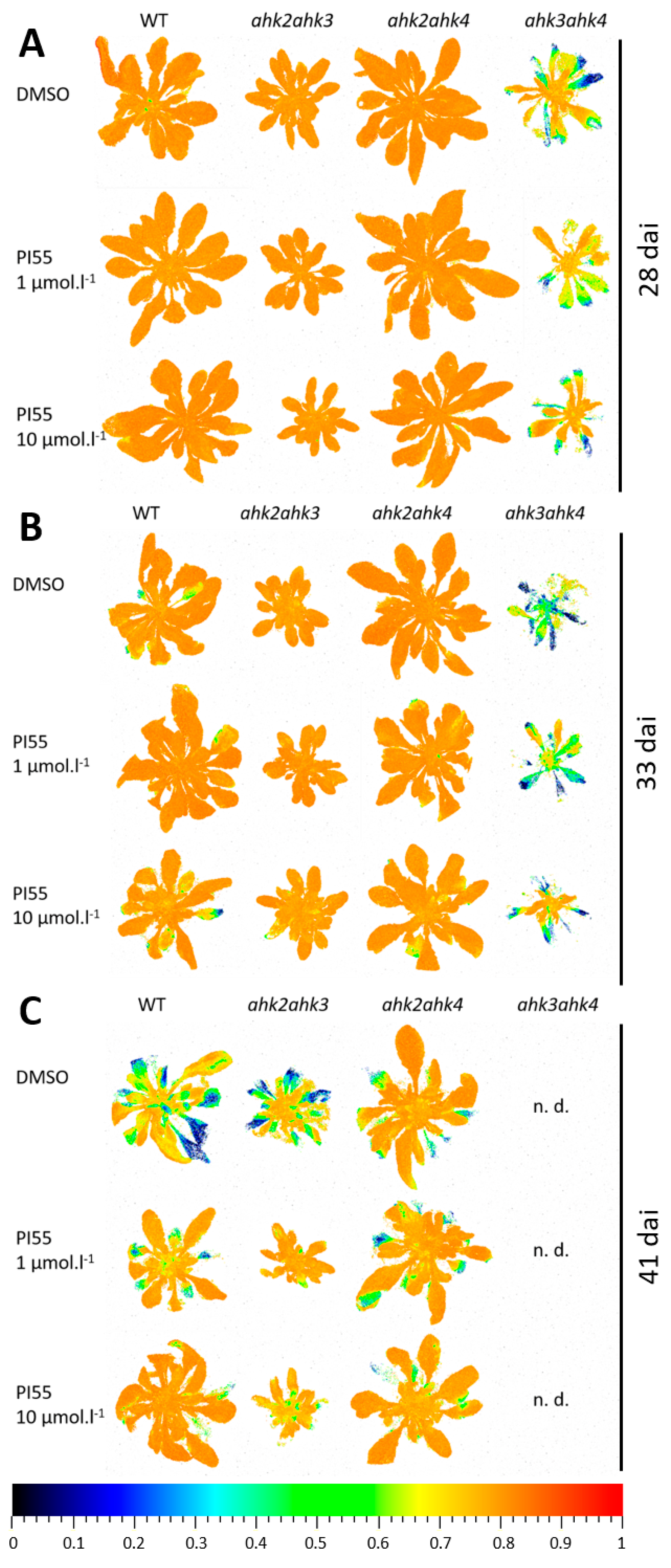
3.1. Phenotypic Characterization of Clubroot-Infected Cytokinin Receptor Mutant Lines
3.2. Photosynthetic Performance in Infected Plants and Effects of PI-55 Treatment
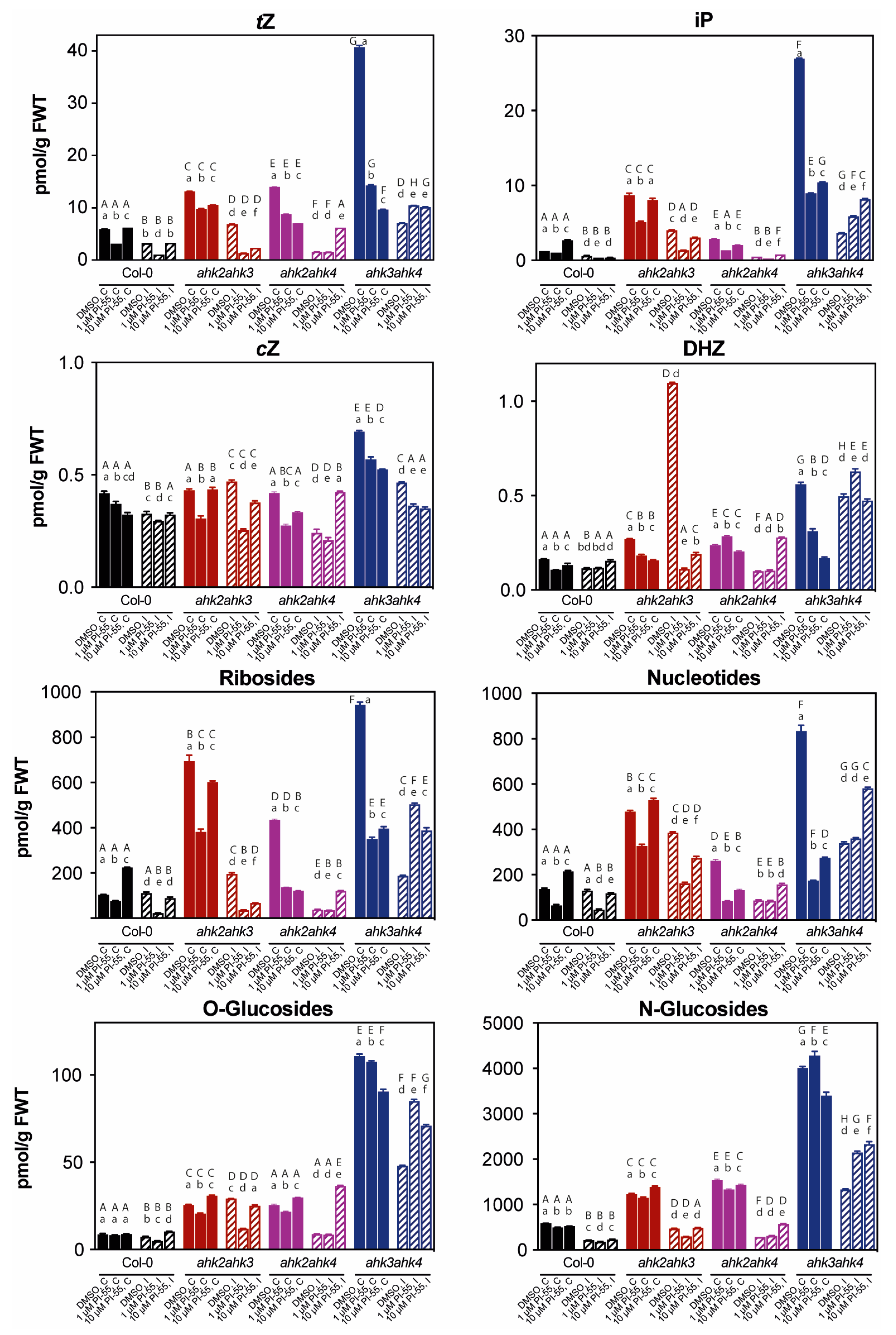
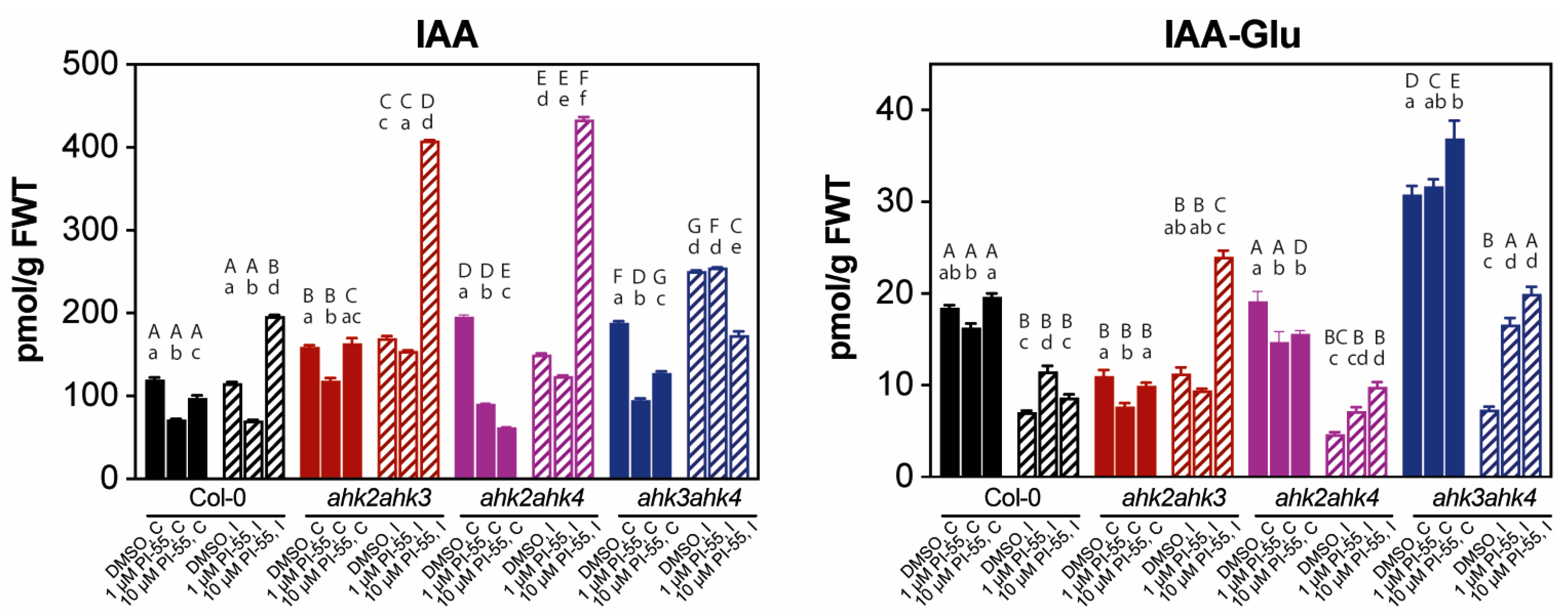
3.3. The Hormonal Content in Infected Galls and Effects of PI-55 Treatment
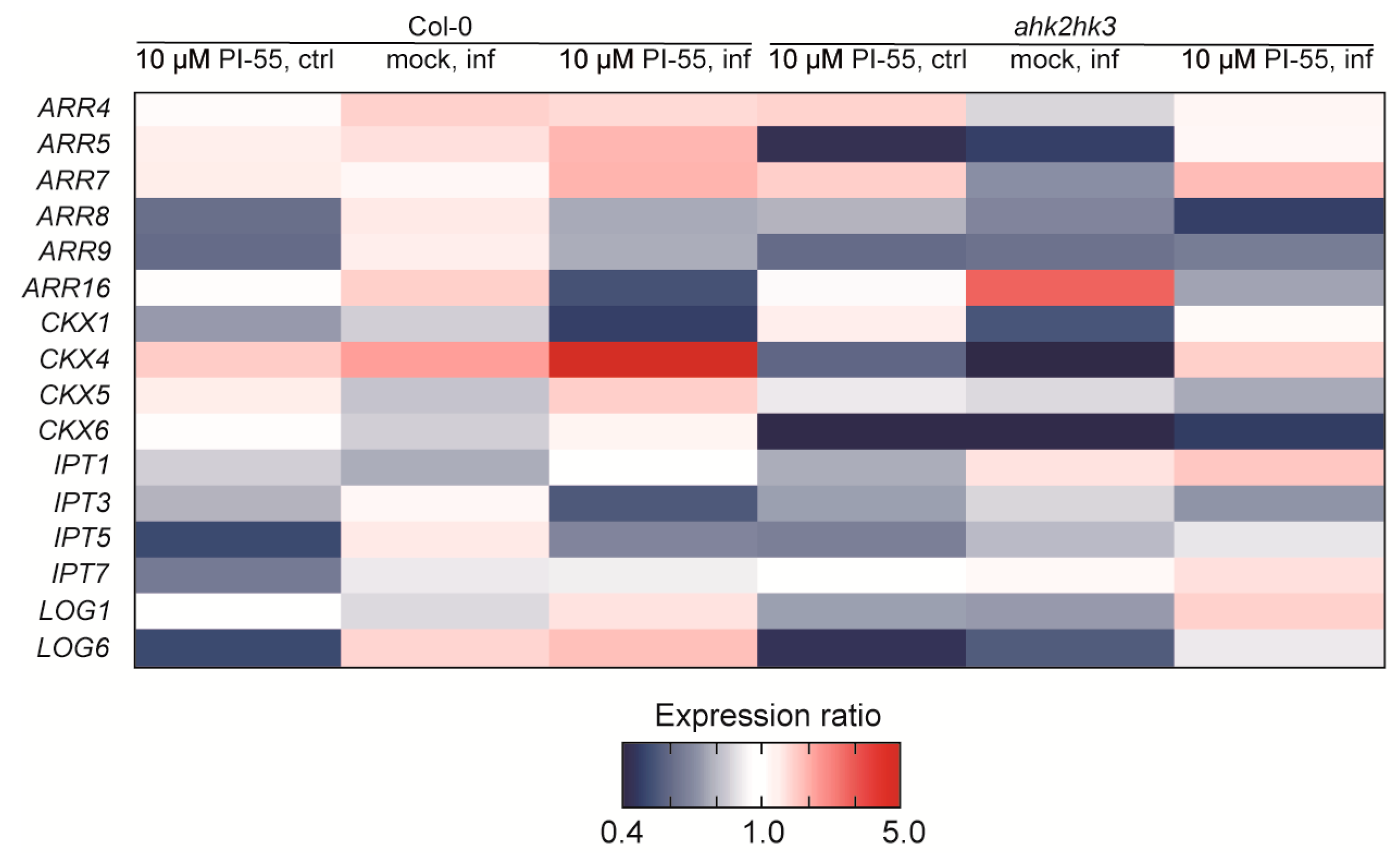
3.4. Expression Analysis of Cytokinin-Related Genes in Clubroot-Infected Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, G.R. The Occurrence and Economic Impact of Plasmodiophora brassicae and Clubroot Disease. J. Plant Growth Regul. 2009, 28, 194–202. [Google Scholar] [CrossRef]
- Ciaghi, S.; Schwelm, A.; Neuhauser, S. Transcriptomic response in symptomless roots of clubroot infected kohlrabi (Brassica oleracea var. gongylodes) mirrors resistant plants. BMC Plant Biol. 2019, 19, 288. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.; Trost, B.; Waldner, M.; Nayidu, N.; Tu, J.; Kusalik, A.J.; Todd, C.D.; Wei, Y.; Bonham-Smith, P.C. Transcriptome analysis of response to Plasmodiophora brassicae infection in the Arabidopsis shoot and root. BMC Genom. 2018, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, S.A.; Strelkov, S.E.; Links, M.G.; Clarke, W.E.; Robinson, S.J.; Djavaheri, M.; Malinowski, R.; Haddadi, P.; Kagale, S.; Parkin, I.A.P.; et al. The compact genome of the plant pathogen Plasmodiophora brassicae is adapted to intracellular interactions with host Brassica spp. BMC Genom. 2016, 17, 272. [Google Scholar] [CrossRef]
- Schwelm, A.; Fogelqvist, J.; Knaust, A.; Jülke, S.; Lilja, T.; Rosso, G.B.; Karlsson, M.; Shevchenko, A.; Dhandapani, V.; Choi, S.R.; et al. The Plasmodiophora brassicae genome reveals insights in its life cycle and ancestry of chitin synthases. Sci. Rep. 2015, 5, 11153. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.A.; Schwelm, A.; Zamani-Noor, N.; Salih, R.; Vañó, M.S.; Wu, J.; García, M.G.; Heick, T.M.; Luo, C.; Prakash, P.; et al. The clubroot pathogen Plasmodiophora brassicae: A profile update. Mol. Plant Pathol. 2022, 24, 89–106. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Jülke, S.; Geiß, K.; Richter, F.; Mithöfer, A.; Šola, I.; Rusak, G.; Keenan, S.; Bulman, S. A novel methyltransferase from the intracellular pathogen Plasmodiophora brassicae methylates salicylic acid. Mol. Plant Pathol. 2015, 16, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, K.; Asano, T. Life Cycle of Plasmodiophora brassicae. J. Plant Growth Regul. 2009, 28, 203–211. [Google Scholar] [CrossRef]
- Aist, J.R.; Williams, P.H. The cytology and kinetics of cabbage root hair penetration by Plasmodiophora brassicae. Can. J. Bot. 1971, 49, 2023–2034. [Google Scholar] [CrossRef]
- Malinowski, R.; Truman, W.; Blicharz, S. Genius Architect or Clever Thief—How Plasmodiophora brassicae Reprograms Host Development to Establish a Pathogen-Oriented Physiological Sink. Mol. Plant Microbe Interact. 2019, 32, 1259–1266. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; Zhao, Y.; Xie, Z.; Hossain, M.R.; Yang, S.; Shi, G.; Lv, Y.; Wang, Z.; Tian, B.; et al. Root Transcriptome and Metabolome Profiling Reveal Key Phytohormone-Related Genes and Pathways Involved Clubroot Resistance in Brassica rapa L. Front. Plant Sci. 2021, 12, 759623. [Google Scholar] [CrossRef] [PubMed]
- Devos, S.; Laukens, K.; Deckers, P.; Van Der Straeten, D.; Beeckman, T.; Inzé, D.; Van Onckelen, H.; Witters, E.; Prinsen, E. A Hormone and Proteome Approach to Picturing the Initial Metabolic Events During Plasmodiophora brassicae Infection on Arabidopsis. Mol. Plant Microbe Interact. 2006, 19, 1431–1443. [Google Scholar] [CrossRef]
- Siemens, J.; Keller, I.; Sarx, J.; Kunz, S.; Schuller, A.; Nagel, W.; Schmülling, T.; Parniske, M.; Ludwig-Müller, J. Transcriptome Analysis of Arabidopsis Clubroots Indicate a Key Role for Cytokinins in Disease Development. Mol. Plant Microbe Interact. 2006, 19, 480–494. [Google Scholar] [CrossRef]
- Boivin, S.; Fonouni-Farde, C.; Frugier, F. How Auxin and Cytokinin Phytohormones Modulate Root Microbe Interactions. Front. Plant Sci. 2016, 7, 1240. [Google Scholar] [CrossRef]
- Malinowski, R.; Smith, J.A.; Fleming, A.J.; Scholes, J.D.; Rolfe, S.A. Gall formation in clubroot-infected Arabidopsis results from an increase in existing meristematic activities of the host but is not essential for the completion of the pathogen life cycle. Plant J. 2012, 71, 226–238. [Google Scholar] [CrossRef]
- Malinowski, R.; Novak, O.; Borhan, M.H.; Spíchal, L.; Strnad, M.; Rolfe, S.A. The role of cytokinins in clubroot disease. Eur. J. Plant Pathol. 2016, 145, 543–557. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. Book 2014, 12, e0168. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choi, D.; Lee, S.; Ryu, C.-M.; Hwang, I. Cytokinins and plant immunity: Old foes or new friends? Trends Plant Sci. 2011, 16, 388–394. [Google Scholar] [CrossRef]
- Bryksová, M.; Dabravolski, S.; Kučerová, Z.; Kokáš, F.Z.; Špundová, M.; Plíhalová, L.; Takáč, T.; Grúz, J.; Hudeček, M.; Hloušková, V.; et al. Aromatic Cytokinin Arabinosides Promote PAMP-like Responses and Positively Regulate Leaf Longevity. ACS Chem. Biol. 2020, 15, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Mok, D.W.; Mok, M.C. Cytokinin metabolism and action. Annu. Rev. Plant Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Kiba, T.; Sakakibara, H. Metabolism and Long-distance Translocation of Cytokinins. J. Integr. Plant Biol. 2010, 52, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, J.M.; Armstrong, D.J. Regulation of Cytokinin Oxidase Activity in Callus Tissues of Phaseolus vulgaris L. cv Great Northern. Plant Physiol. 1986, 80, 493–499. [Google Scholar] [CrossRef]
- Kakimoto, T. Identification of Plant Cytokinin Biosynthetic Enzymes as Dimethylallyl Diphosphate:ATP/ADP Isopentenyltransferases. Plant Cell Physiol. 2001, 42, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Higuchi, M.; Hashimoto, Y.; Seki, M.; Kobayashi, M.; Kato, T.; Tabata, S.; Shinozaki, K.; Kakimoto, T. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 2001, 409, 1060–1063. [Google Scholar] [CrossRef]
- Ueguchi, C.; Sato, S.; Kato, T.; Tabata, S. The AHK4 Gene Involved in the Cytokinin-Signaling Pathway as a Direct Receptor Molecule in Arabidopsis thaliana. Plant Cell Physiol. 2001, 42, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Ueguchi, C.; Koizumi, H.; Suzuki, T.; Mizuno, T. Novel family of sensor histidine kinase genes in Arabidopsis thaliana. Plant Cell Physiol. 2001, 42, 231–235. [Google Scholar] [CrossRef]
- Suzuki, T.; Miwa, K.; Ishikawa, K.; Yamada, H.; Aiba, H.; Mizuno, T. The Arabidopsis Sensor His-kinase, AHK4, Can Respond to Cytokinins. Plant Cell Physiol. 2001, 42, 107–113. [Google Scholar] [CrossRef]
- Yamada, H.; Suzuki, T.; Terada, K.; Takei, K.; Ishikawa, K.; Miwa, K.; Yamashino, T.; Mizuno, T. The Arabidopsis AHK4 Histidine Kinase is a Cytokinin-Binding Receptor that Transduces Cytokinin Signals Across the Membrane. Plant Cell Physiol. 2001, 42, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Riefler, M.; Novak, O.; Strnad, M.; Schmülling, T. ArabidopsisCytokinin Receptor Mutants Reveal Functions in Shoot Growth, Leaf Senescence, Seed Size, Germination, Root Development, and Cytokinin Metabolism. Plant Cell 2006, 18, 40–54. [Google Scholar] [CrossRef]
- Spíchal, L.; Werner, T.; Popa, I.; Riefler, M.; Schmülling, T.; Strnad, M. The purine derivative PI-55 blocks cytokinin action via receptor inhibition. FEBS J. 2009, 276, 244–253. [Google Scholar] [CrossRef]
- Hoyerova, K.; Gaudinova, A.; Malbeck, J.; Dobrev, P.; Kocabek, T.; Solcova, B.; Trávníčková, A.; Kaminek, M. Efficiency of different methods of extraction and purification of cytokinins. Phytochemistry 2006, 67, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Svačinová, J.; Novák, O.; Plačková, L.; Lenobel, R.; Holík, J.; Strnad, M.; Doležal, K. A new approach for cytokinin isolation from Arabidopsis tissues using miniaturized purification: Pipette tip solid-phase extraction. Plant Methods 2012, 8, 17. [Google Scholar] [CrossRef]
- Novák, O.; Hauserová, E.; Amakorová, P.; Doležal, K.; Strnad, M. Cytokinin profiling in plant tissues using ultra-performance liquid chromatography–electrospray tandem mass spectrometry. Phytochemistry 2008, 69, 2214–2224. [Google Scholar] [CrossRef]
- Pěnčík, A.; Rolčík, J.; Novák, O.; Magnus, V.; Barták, P.; Buchtík, R.; Salopek-Sondi, B.; Strnad, M. Isolation of novel indole-3-acetic acid conjugates by immunoaffinity extraction. Talanta 2009, 80, 651–655. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Grsic-Rausch, S.; Kobelt, P.; Siemens, J.M.; Bischoff, M.; Ludwig-Müller, J. Expression and Localization of Nitrilase during Symptom Development of the Clubroot Disease in Arabidopsis. Plant Physiol. 2000, 122, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Devos, S.; Vissenberg, K.; Verbelen, J.; Prinsen, E. Infection of Chinese cabbage by Plasmodiophora brassicae leads to a stimulation of plant growth: Impacts on cell wall metabolism and hormone balance. New Phytol. 2005, 166, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, K.; Tarkowski, P.; Matsumoto-Kitano, M.; Kato, T.; Sato, S.; Tarkowska, D.; Tabata, S.; Sandberg, G.; Kakimoto, T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar] [CrossRef]
- Bielach, A.; Podlešáková, K.; Marhavý, P.; Duclercq, J.; Cuesta, C.; Müller, B.; Grunewald, W.; Tarkowski, P.; Benková, E. Spatiotemporal Regulation of Lateral Root Organogenesis in Arabidopsis by Cytokinin. Plant Cell 2012, 24, 3967–3981. [Google Scholar] [CrossRef]
- Ando, S.; Asano, T.; Tsushima, S.; Kamachi, S.; Hagio, T.; Tabei, Y. Changes in gene expression of putative isopentenyltransferase during clubroot development in Chinese cabbage (Brassica rapa L.). Physiol. Mol. Plant Pathol. 2005, 67, 59–67. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bíbová, J.; Kábrtová, V.; Večeřová, V.; Kučerová, Z.; Hudeček, M.; Plačková, L.; Novák, O.; Strnad, M.; Plíhal, O. The Role of a Cytokinin Antagonist in the Progression of Clubroot Disease. Biomolecules 2023, 13, 299. https://doi.org/10.3390/biom13020299
Bíbová J, Kábrtová V, Večeřová V, Kučerová Z, Hudeček M, Plačková L, Novák O, Strnad M, Plíhal O. The Role of a Cytokinin Antagonist in the Progression of Clubroot Disease. Biomolecules. 2023; 13(2):299. https://doi.org/10.3390/biom13020299
Chicago/Turabian StyleBíbová, Jana, Veronika Kábrtová, Veronika Večeřová, Zuzana Kučerová, Martin Hudeček, Lenka Plačková, Ondřej Novák, Miroslav Strnad, and Ondřej Plíhal. 2023. "The Role of a Cytokinin Antagonist in the Progression of Clubroot Disease" Biomolecules 13, no. 2: 299. https://doi.org/10.3390/biom13020299
APA StyleBíbová, J., Kábrtová, V., Večeřová, V., Kučerová, Z., Hudeček, M., Plačková, L., Novák, O., Strnad, M., & Plíhal, O. (2023). The Role of a Cytokinin Antagonist in the Progression of Clubroot Disease. Biomolecules, 13(2), 299. https://doi.org/10.3390/biom13020299







