1. Introduction
The increase in life expectancy in Western countries has resulted in a higher incidence of bone and cartilage pathologies. Consequently, the demand for, and optimization of orthopedic implants, has grown [
1]. For many years, traditional implants have been metallic, usually composed of titanium or titanium alloys due to their resistance to oxidation, excellent mechanical properties, and biocompatibility [
2]. The misalignment between the elastic modulus of these metals and that of the cortical bone, together with the release of metal ions from the implant surface, can create associated problems and, in some cases, implant failure. Additionally, polymeric materials are usually not able to withstand repetitive loads without incurring plastic deformations [
3]. An exception to this is represented by poly (ether ether ketone) (PEEK). PEEK is a semi-crystalline polyaromatic linear polymer with (–C
6H
4–O–C
6H
4–O–C
6H
4–CO–)
n chemical formula, and it possesses incredible mechanical properties. Young’s modulus of PEEK is around 3.6 GPa, similar to the value of cortical bone. It is much lower than any metallic material employed in the common bone implant (110–180 GPa) [
4,
5]. For this reason, by the late 1990s, PEEK was proposed as an alternative to the metal components of orthopedic implants. More specifically, it is used for femoral prostheses and hip joints [
6]. Currently, even dentists are looking at PEEK with increasing interest considering its exceptional mechanical and aesthetic properties [
7].
PEEK Young’s modulus is manageable through additive inclusion: for example, by adding carbon reinforcement in a PEEK matrix, its modulus increases up to 18 GPa, which is superimposable to the values of the cortical bone (18.4–20.7 GPa) [
8]. Therefore, PEEK implants decrease the effect of stress shielding compared to traditional metal implants. PEEK is a thermoplastic polymer, and it melts at a relatively high temperature (343 °C) compared to most of this class of polymers [
8,
9]. In the range of its melting temperature, it can be processed using 3D printing. The possibility of employing PEEK polymer in the 3D printing process could increase scientific interest and its future potential [
9,
10]. Additionally, PEEK has interesting biological properties: it is highly resistant to thermal degradation, to the attack by organic and aqueous environments, and also to biodegradation [
11]. Moreover, it is insoluble at room temperature in all conventional solvents, and its thermal properties make it stable in the human body. Therefore, PEEK implants are biocompatible but biologically inert [
12]: their applications cause only mild inflammatory responses, and even their debris (that might be produced after a long period of implantation) may give rise to normal reactions of the biological tissue to a foreign body as the worst scenario [
13]. Despite being a non-toxic, non-mutagenic, and non-inflammatory material, PEEK remains a bio-inert material. Furthermore, this polymer has a hydrophobic surface, which neither allows protein absorption nor promotes cell adhesion [
14].
The growth of new bone tissue in direct apposition with the implant is desirable for all prostheses but becomes a necessity when the osseointegration process is critical, i.e., for elderly patients or patients suffering from multiple pathologies. With this in mind, modification of the implant surface to enhance the adhesion and proliferation of osteoblasts is a favorable approach. Taking this into consideration, enhancing the bioactivity of PEEK presents a desirable challenge to increase the potential benefit from the wide advantages of this material [
15].
To date, a number of physical methods for PEEK surface modification have been proposed, such as thermal spraying, pulsed laser, ion sputtering, sandblasting, and electrochemical methods, which require a lot of optimization work and complex equipment. Our strategy concerns the chemical functionalization of PEEK surfaces with bioactive motifs. Previous studies have used the reduction of the carbonyl groups of PEEK to alcohol groups (PEEK-OH). PEEK-OH is then used to obtain isocyanate groups or amino groups on the surface [
16,
17,
18] to be reacted with signal sequences for cell adhesion or proliferation.
In our grafting strategy, the carbonyl groups already present on the PEEK surface react with an amino-oxy group ad hoc inserted in peptide sequence: the method allows the covalent anchoring of bioactive peptides in a single-step without the use of condensing agents or organic solvents [
19,
20]. This study reports the chemical, physicochemical, mechanical, and biological characterization of PEEK surfaces functionalized with two adhesive peptides: a nonapeptide of human vitronectin (HVP) capable of promoting an osteoblast adhesion mechanism mediated by cell surface glycosaminoglycans [
21] and its retro-inversed dimer (D2HVP) which has enhanced biological activity with respect to the first peptide but which is also stabilized against enzymatic degradation [
22].
2. Materials and Methods
2.1. Materials
Acetic acid, acetone, acetonitrile, Fmoc protected Amino acids, Bis-Boc-aminooxy acetic acid (Aoa), Dichloromethane (DCM), N,N-Diisopropylethylamine (DIPEA), N,N-dimethylformamide (DMF), Ethyl cyano(hydroxyimino)acetate (Oxyma Pure), Methanol, Rink-Amide MBHA resin, Triethylamine (TEA), Triethylsilane (TES), 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluoro phosphate (HBTU) were purchased from Merck Millipore (Burlington, MA, USA). Diethyl ether, Trifluoroacetin acid (TFA) were purchased from Biosolve (Valkenswaard, Holland). 2-Proponal for HPLC (aldehyde and ketone free) was purchased from PanReac AppliChem (Darmstadt, Germany). Ethanol was purchased from Carlo Erba (Milan, Italy). N-Methyl-2-pyrrolidone (NMP) was purchased from Iris Biotech GmbH (Marktredwitz, Germany).
2.2. Synthesis of the Peptide Aoa-x-HVP
Aoa-x-HVP (sequence: H-Aoa-(7-aminoheptanoic acid)-Phe-Arg-His-Arg-Asn-Arg-Lys-Gly-Tyr-NH2) was synthesized using the Solid Phase Peptide technique (SPPS) on Tink amide MBHA resin by a Syro I synthesizer (Multisyntech, Witten, Germany). All the amino acids were protected with a Fmoc group on the N-termini and with a Pbf, Trt, Boc, or tBu group on the side chain. Every amino acid was introduced through a double coupling with five equivalents of each amino acid, five equivalents of activating agent HBTU/Oxima Pure, and ten equivalents of DIPEA with respect to the resin reactive groups used for each coupling of 45 min. Aoa was added to the sequence through a double coupling with five equivalents of each amino acid, five equivalents of activating agent HATU/Oxima Pure, and ten equivalents of collidine with respect to the resin reactive groups were used for each coupling of 45 min.
After the addition of Aoa, the peptide was cleaved from the resin and deprotected from Bis-Boc of Aoa and the side-chain protecting groups using 4.75 mL TFA, 0.125 mL TES, and 0.125 H2O for 1.5 h at room temperature.
The resin was filtered off, and the solution was concentrated and added with cold diethyl ether. The product was precipitated and filtered. The identity of the peptide was determined by mass spectrometry (theoretical mass = 1432.587 Da; experimental mass = 1431.8156 Da; ESI-TOF, Mariner System 5220, Applied Biosystem, PerkinElmer, Foser City, CA, USA). The peptide Aoa-x-HVP was purified by RP-HPLC (purity grade 98.74%) and characterized by analytical RP-HPLC (condition: Jupiter C18 column [5 μm, 300 Å, 4.6 × 205 mm], eluent A: 0.05% TFA in H2O; eluent B: 0.05% TFA in 40% isopropanol and 60% MilliQ water; gradient: from 20 to 50% B in 30 min, flow rate: 0.5 mL/min; detector at 214 nm. tR = 13.215 min).
2.3. Synthesis of the Peptide Aoa-x-F-HVP
Aoa-x-F-HVP (sequence: H-Aoa-(7-aminoheptanoic acid)-(p-F)Phe-Arg-His-Arg-Asn-Arg-Lys-Gly-Tyr-NH2, where (p-F)Phe indicates a para-Fluoro Phenylalanine residue) was synthesized using the Solid Phase Peptide technique (SPPS) on Tink amide MBHA resin by a Syro I synthesizer (Multisyntech, Witten, Germany). All the amino acids were protected with a Fmoc group on the N-termini and with a Pbf, Trt, Boc, or tBu group on the side chain. Every amino acid was introduced through a double coupling with five equivalents of each amino acid, five equivalents of activating agent HBTU/Oxima Pure, and ten equivalents of DIPEA with respect to the resin reactive groups used for each coupling of 45 min. Aoa was added to the sequence through a double coupling with five equivalents of each amino acid, five equivalents of activating agent HATU/Oxima Pure, and ten equivalents of collidine with respect to the resin reactive groups were used for each coupling of 45 min.
After the addition of Aoa, the peptide was cleaved from the resin and deprotected from Bis-Boc of Aoa and the side-chain protecting groups as reported for Aoa-x-HVP.
The resin was filtered off, and the solution was concentrated and added with cold diethyl ether. The product was precipitated and filtered. The identity of the peptide was determined by mass spectrometry (theoretical mass = 1450.58 Da; experimental mass = 1450.32 Da; SCIEX TOF-TOF 4800 instrument). The peptide Aoa-x-F-HVP was purified by RP-HPLC (purity grade 99.36%) and characterized by analytical RP-HPLC (condition: Jupiter C18 column [5 μm, 300 Å, 4.6 × 205 mm], eluent A: 0.05% TFA in H2O; eluent B: 0.05% TFA in 40% isopropanol and 60% MilliQ water; gradient: from 20 to 50% B in 30 min, flow rate: 0.5 mL/min; detector at 214 nm. tR = 14.688 min).
2.4. Synthesis of the Peptide Aoa-x-D2HVP
Aoa-x-D2HVP (sequence: H-Aoa-(7-aminoheptanoic acid)-(DTyr-Gly-DLys-DArg-DAsn-DArg-DHis-DArg-DPhe)2-NH2) was synthesized using the Solid Phase Peptide technique (SPPS) on Rink amide MBHA resin by a Syro I synthesizer (Multisyntech, Witten, Germany). All the amino acids are of D- type, and they were protected with a Fmoc group on the N-termini and with a Pbf, Trt, Boc, or tBu group on the side chain. Every amino acid was introduced through a single coupling with five equivalents of each amino acid, five equivalents of activating agent HATU/Oxima Pure, and ten equivalents of DIPEA with respect to the resin reactive groups used for each coupling of 45 min. Aoa was added to the sequence through a double coupling with five equivalents of each amino acid, five equivalents of activating agent HATU/Oxima Pure, and ten equivalents of collidine with respect to the resin reactive groups were used for each coupling of 45 min.
After the addition of Aoa, the peptide was cleaved from the resin and deprotected from Bis-Boc of Aoa and the side-chain protecting groups as reported for Aoa-x-HVP.
The resin was filtered off, and the solution was concentrated and added with cold diethyl ether. The product was precipitated and filtered. The identity of the peptide was determined by mass spectrometry (theoretical mass = 2647.984 Da; experimental mass = 2647.02 Da; SCIEX TOF-TOF 4800 instrument). The peptide was purified by RP-HPLC (purity grade 95%) and characterized by analytical RP-HPLC (condition: Vydac C18 column [5 μm, 300 Å, 4.6 × 250 mm], eluent A: 0.05% TFA in H2O; eluent B: 0.05% TFA in 40% isopropanol and 60% MilliQ water; gradient: from 5 to 45% B in 40 min, flow rate: 0.5 mL/min; detector at 214 nm. tR = 32.510 min).
2.5. 3D-Printing of PEEK Scaffold
AutoDesk Fusion 360 (San Rafael, CA, USA) computer-aided-design (CAD) software was used to design PEEK disks 10 mm in diameter and 4 mm in height and exported in an stl format into Simplify 3D software (Cincinnati, OH, USA). The files were then sliced to generate printer-ready G-code and printed using an Apium P155 Peek filament printer (Apium Additive Technologies GmBh, Willy, Karlsruhe, Germany). Peek filament (Vitrex Rotherham, Rotherham, UK) 1.75 mm in diameter was extruded through a 0.4 mm diameter nozzle at a temperature of 485 °C onto a print bed with a temperature of 130 °C to create the PEEK disks with a 100% infill at a print speed of 33.3 mm/s. The resulting 3D-printed PEEK disks possess a flat surface and a patterned one.
2.6. PEEK Surface Functionalization
PEEK scaffolds were functionalized with all the peptides. The linkages between the amino-oxy group on the N-terminus of the peptide chain and ketonic groups on the PEEK surface are realized by means of chemoselective ligation. Each peptide was dissolved in 40 mM monobasic sodium phosphate at pH 6 and left to react with PEEK samples for 24 h at room temperature. Peptide concentrations used were 10−5, 10−4, and 10−3 M. After that, the disks were rinsed three times with buffer and three times with MilliQ water.
2.7. X-ray Photoelectron Spectroscopy (XPS) Measurements
XPS analysis was performed with a homemade instrument, consisting of preparation and analysis of UHV chambers separated by a gate valve. The analysis chamber is equipped with a six-degree-of-freedom manipulator and a 150 mm mean radius hemispherical electron analyzer with a five-lens output system combined with a 16-channel detector giving a total instrument resolution of 1.0 eV as measured at the Ag 3d 5/2 core level. Samples were introduced in the preparation chamber and left outgassing overnight at a base pressure of about 10
−8 Torr before introduction in the analysis chamber. The typical vacuum pressure in the analysis chamber during measurements was in the 10
−9–10
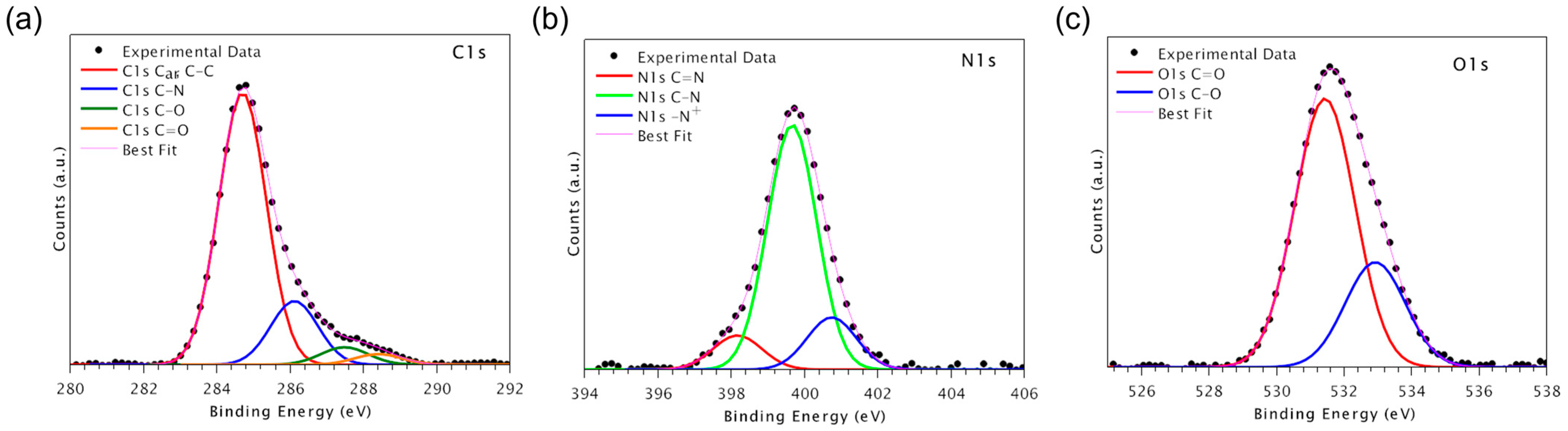
−10 Torr range. The used X-ray radiation is a non-monochromatic Mg Kα(1253.6 eV). The spectra were energy referenced to the C 1s signal of aromatic and aliphatic C atoms having a binding energy BE = 284.7 eV. Curve-fitting analysis of the C1s, N1s, O1s, and F1s spectra was performed using Gaussian profiles as fitting functions after subtraction of a Shirley-type background. Atomic ratio values were calculated from peak intensities. The surface atomic concentrations were determined from photoelectron peak areas using the atomic sensitivity factors reported by Scofield [
23]. When several different species were identified in a spectrum, the same FWHM value was set for all individual photoemission bands. XPS analyses were performed on flat PEEK surfaces.
2.8. Atomic Force Microscopy (AFM) Analysis
AFM analyses were carried out on different functionalized PEEK disks at room temperature in order to evaluate the effects of the peptides on the mechanical properties of interfaces separately. The Young modulus was calculated from the collected force–distance curves measured with the NTEGRA AFM (NT-MDT, Moscow, Russia). Stiff single-crystal silicon cantilevers with a symmetric tip shape were used (model Tap300Al-G, BudgetSensors, Bulgaria: nom. Frequency 300 kHz, nom. Spring constant 40 Nm
−1, tip radius < 10 nm). AFM measurements were made in triplicate. The probe was characterized by measuring the cantilever spring constant by the Sader method [
24]. Each probe was calibrated by performing a force curve on a hard-cleaned substrate (<100> silicon wafer), where no indentation occurred. Probe calibration was needed to calculate their sensitivity and the spring constant. The Young modulus was obtained from the experimental force–distance curves by the Derjaguin–Müller–Toporov (DMT) model [
25] using the following equation:
where
F is the applied force;
Fad is the adhesion force;
Es is Young’s modulus;
νs is the Poisson’s ratio for the sample;
R is the radius of the spherical indenter; and
δ is the elastic indentation depth. Each surface was indented in different areas, and about three hundred curves were acquired. The tip-sample approaching rate was set to 0.3 µms
−1 for all force curves. Young’s modulus was calculated by fitting experimental curves with the DMT model in the elastic region. Elastic modulus distribution was assessed with OriginPro 8.5 software.
For adhesion force measurements, Au-coated silicon nitride tips were used. The spring constants of the cantilevers used were in the range of 0.003−0.13 N/m. Individual spring constants were calibrated using the Sader method. The adhesion force is taken as the pull-off force required to separate the AFM tip from the surface. The surfaces were probed in different areas, and about three hundred approach-retract cycles were acquired for each system. The tip-sample approaching velocity was set to 0.3 μs
−1 for all force curves. The adhesion force between the tip and surface can be calculated from the deflection distance of the cantilever and the cantilever spring constant by applying Hook’s law:
where
F is the force (nN),
k is the spring constant of the cantilever, and Δ
L is the deflection distance (nm). The cantilever spring constants were equal to 0.05 N/m for PEEK-HVP and 0.08 N/m for PEEK-D2HVP.
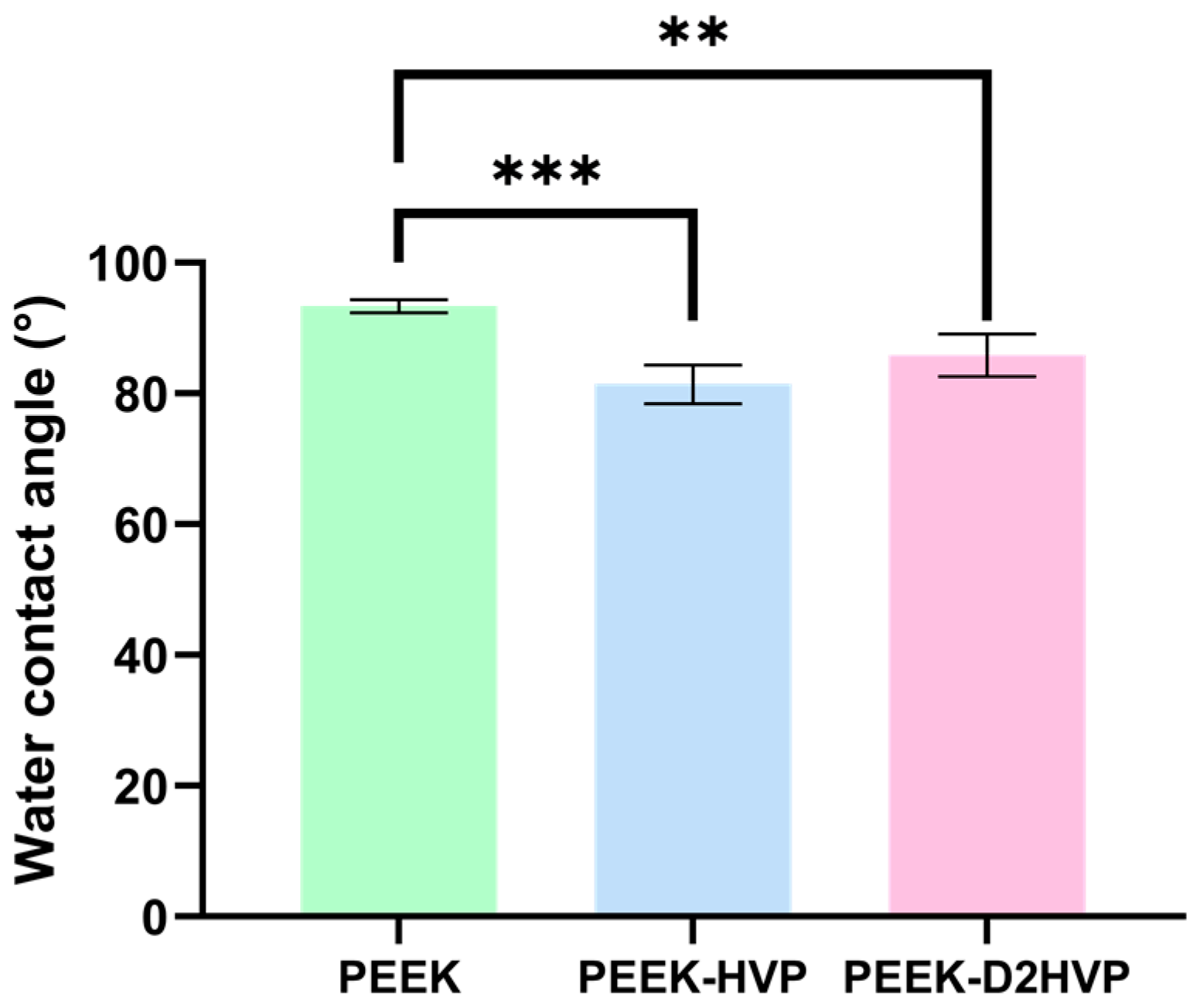
2.9. Water Contact Angle (WCA) Analysis
The surface wettability was traced by measuring the static water contact angle (WCA). An OCA30 instrument (Dataphysics) was used at 25 °C and 65% relative humidity. Probe liquid drops of 2 µL of volume were dispended on different zones of each sample surface, and by digital image analysis, the static contact angle, as the tangent to the drop at the point of contact with the surface, both on the right and on the left side, has been acquired. At least three measurements were made for each sample and then averaged. WCA analyses were performed on flat PEEK surfaces.
2.10. Biological Assays
All biological studies were performed on the patterned surface of the 3D-printed PEEK disks.
2.10.1. Cell Culture
Human osteoblasts (HOB) were used to evaluate the functionalized PEEK disks. Prior to seeding, PEEK specimens were sterilized in 70% ethanol for 24 h and then washed with PBS. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10 mL of 1 M HEPES, 5 mL of 200 mM L-glutamine, 5 mL of 100× penicillin/streptomycin, 50 mL of Fetal Calf Serum (FCS), 5 mL of minimal essential medium and 0.075 g of solid ascorbate acid. HOB cells were microseeded onto the surfaces of the disks at a density of 1 × 105 cells per disc. Seeded samples were incubated at 37 °C and 5% CO2 for the time duration required or all tests performed. For gene expression evaluation, a density of 2 × 105 HOB cells/per disc was used. Culture media was changed every three days for optimal growth conditions.
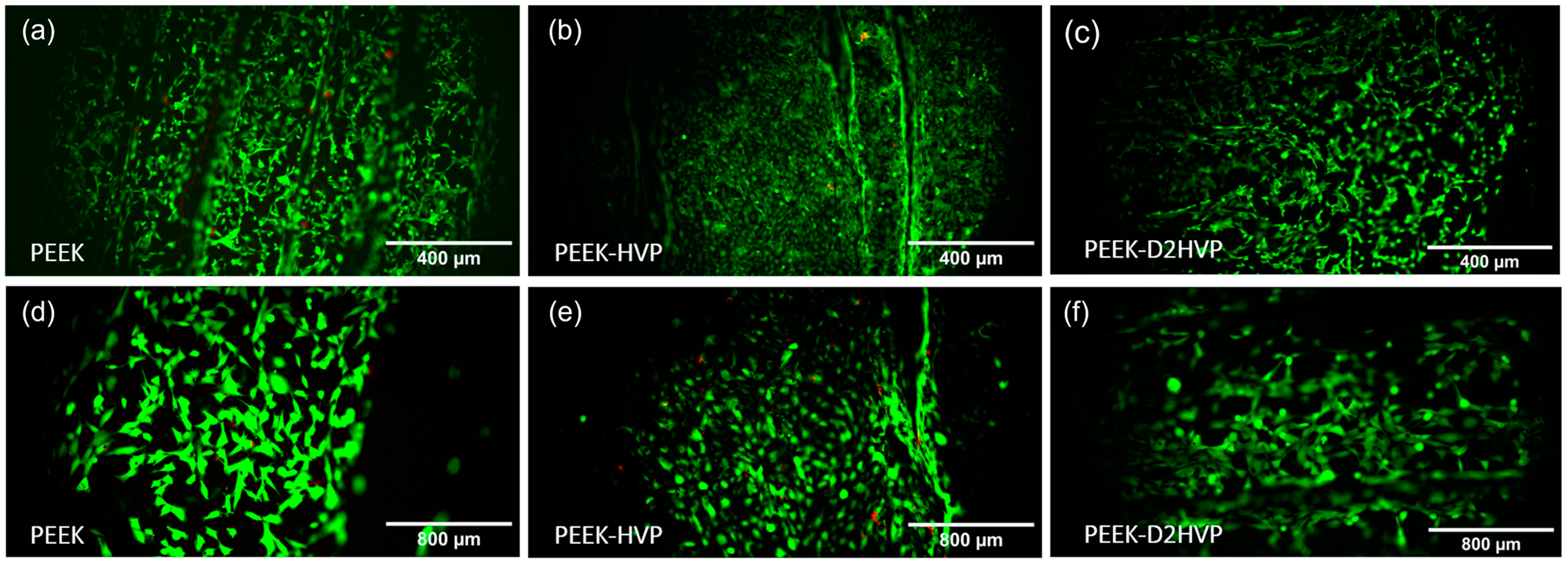
2.10.2. Live and Dead
Cell viability was assessed using a LIVE/DEADTM Viability/Cytotoxicity Kit (Thermo Fisher Scientific, Waltham, MA, USA) after 48 h of cell culture. Two PBS washings were performed prior to exposure to live/dead stains. Images were captured with an Olympus IX51 fluorescent microscope. ImageJ software was used to display and merge images of living cells (green fluorescence at 530 nm) and dead cells (red fluorescence at 600 nm) of the same location into one image.
2.10.3. Scanning Electron Microscopy (SEM) Analysis
The morphology of HOB cells on the 3D-printed PEEK structures was observed using SEM (Zeiss Gemini SEM 360, Jena, Germany) at a voltage of 10kV. Forty-eight hours after seeding, samples were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer for at least 24 h at 4 °C to preserve the structure of cells. Following this, the glutaraldehyde was removed, and the samples were rinsed with 0.1 M sodium cacodylate buffer solution before treatment with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1 h. The osmium tetroxide was then removed, and samples were further rinsed with 0.1 M sodium cacodylate buffer. 1% tannic acid in 0.1 M sodium cacodylate buffer was used to treat the samples for 1 h. Following this, the samples were dehydrated in a series of aqueous ethanol solutions (20%, 30%, 40%, 50%, 60%, 70%, 90%, 96%, and 100%). For each concentration, the samples were treated twice, and each treatment was 5 min. After dehydration, the samples were air-dried and sputter coated with gold prior to observation under SEM.
2.10.4. Alizarin Red
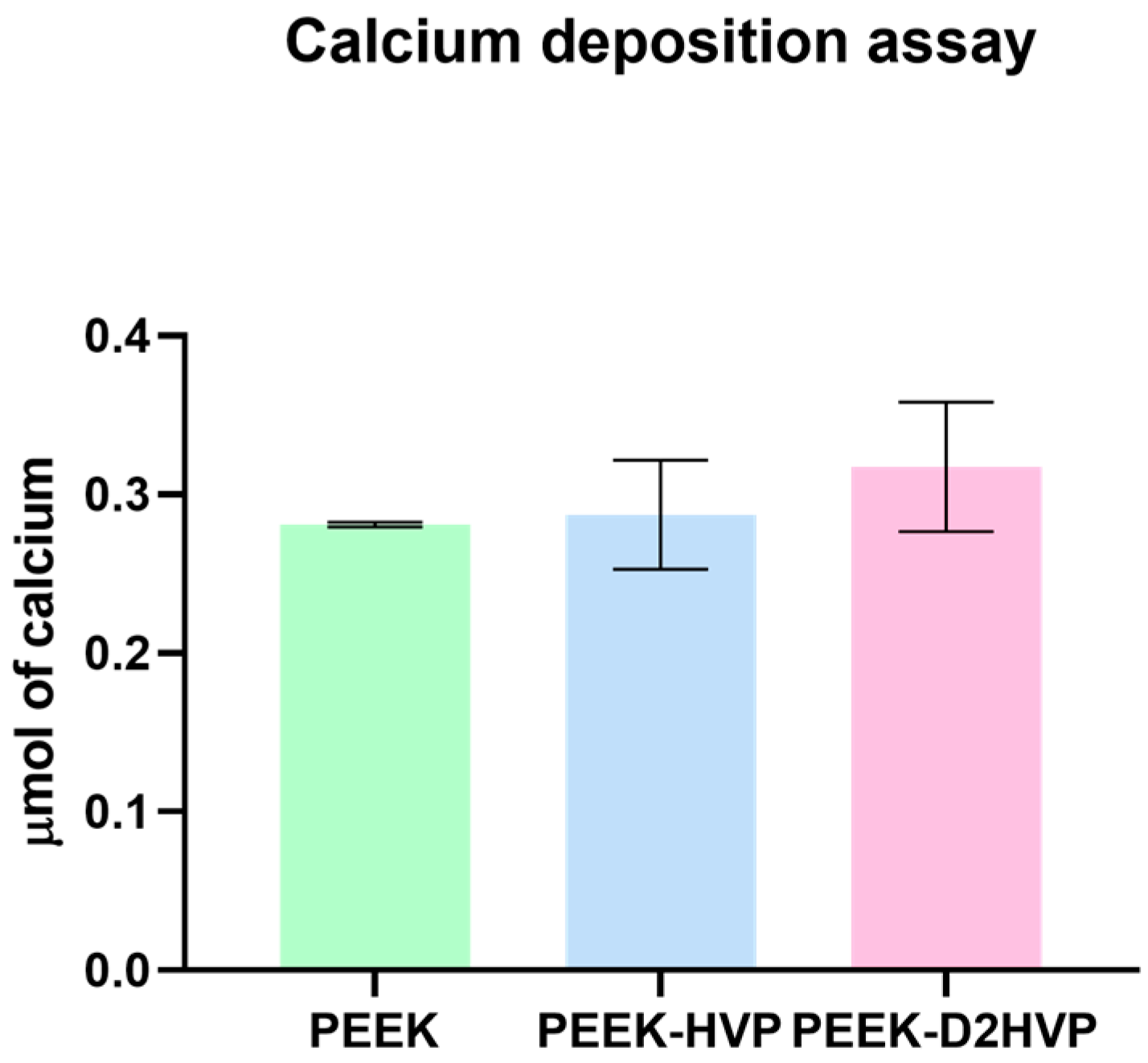
Calcium deposition was evaluated by Alizarin Red S staining on HOB cells. Alizarin red S is an anthraquinone dye that reacts with calcium and forms an Alizarin Red S/calcium complex that is birefringent. HOB cells were cultured for 21 days, and the disks were then washed in PBS, fixed with 10% formal saline for 15 min, and washed with deionized water three times. 2% Alizarin red S solution was added to the specimens for 15 min, followed by five washes in 50% ethanol. For qualitative assessment, images of the stained scaffolds were taken. A quantitative assessment was conducted using Lee et al. protocol [
26]. The bound stain taken up by the cells on the disks was released using 1 mL of 10% cetylpyridium chloride (CPC) solution added to each well overnight (24 well plates). Aliquots (100 μL) of the supernatant were removed and read using a microplate reader (Opsys MRTM 96-well microplate reader, Dynex Technologies, Chantilly, VA, USA) at 570 nm. A standard curve, using known concentrations of alizarin red S in CPC solution, was used for the quantification. Cell mineralization results were expressed as μmol of calcium per well as 1 mole of Alizarin red S binds to 2 mol of calcium in an Alizarin red S-calcium complex [
27].
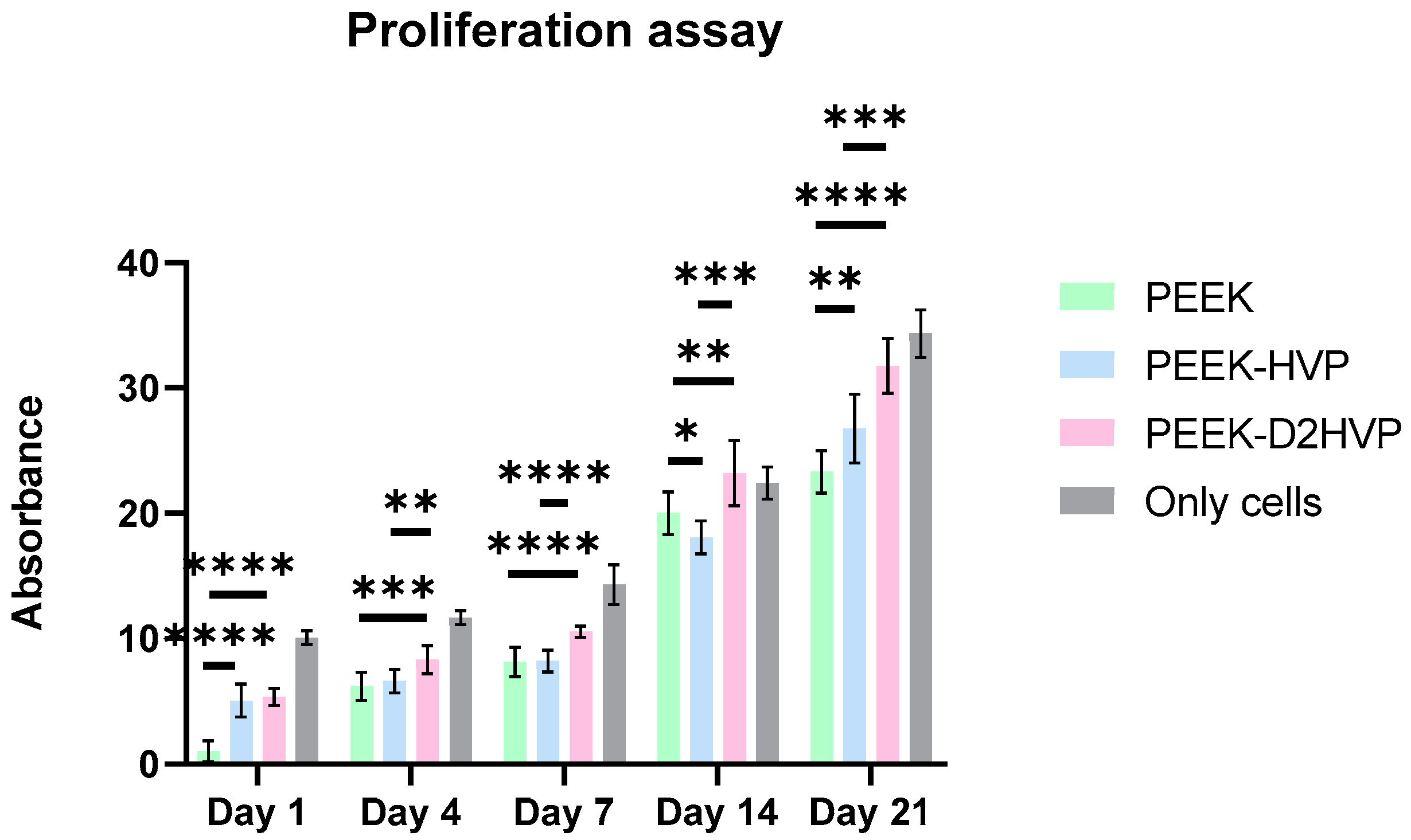
2.10.5. Proliferation Assay
The proliferation of osteoblasts cultured on functionalized and non-functionalized PEEK disks was assessed at 1, 4, 7, 14, or 21 days using the AlamarBlueTM kit. For each sample, three replicates were measured with a microplate reader (Opsys MRTM 96-well microplate reader, Dynex Technologies, Chantilly, VA, USA), which measured the fluorescence values at 570 nm test wavelength and 630 nm reference wavelength.
2.10.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Specific mRNA transcript levels coding human SRY-Box transcription factor 9 (Sox9), Runt-related transcription factor 2 (Runx2), Alkaline Phosphatase (Alp), and Osteocalcin (Oct) were quantified in osteoblast cells cultured for 24 h on functionalized and non-functionalized PEEK. At the end of incubation, the total RNA was extracted using the SV Total RNA Isolation System kit (Promega, Milan, Italy). Contaminating DNA was removed by DNase I digestion (Omega Bio-Tek, Norcross, GA, USA). cDNA synthesis and subsequent polymerization were performed in one step using the iTaq Universal SYBR Green One-Step Kit (Bio-Rad, Hercules, CA, USA). The reaction mixture contained a 200 nM forward primer, 200 nM reverse primer, iTaq universal SyBR Green reaction mix, iScript reverse transcriptase, and a 200 ng total RNA. Real-time PCR was performed using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Milan, Italy). Data were quantified by the ∆∆CT method using human Gapdh as the reference gene. Target and reference genes were amplified with efficiencies near 100%. The oligonucleotides used for PCR are listed in
Table 1.
2.10.7. Statistical Analysis
All assays were performed at least in triplicate, and all values are reported as mean ± standard deviation.
Data were analyzed and processed with Prism (GraphPad Software 9). Alizarin Red S results were compared by using a one-way analysis of variance (ANOVA) test. Tukey’s multiple comparisons test was carried out to confront every condition. AlamarBlueTM and gene expression results were compared with two-way ANOVA tests, followed by Tukey’s post-hoc test.
The significance level applied was 5%.
4. Discussion
We investigated the effect of functionalizing PEEK to determine whether the performance could be enhanced. Preliminarily, experiments were performed to ascertain whether the use of increasing concentrations of peptides led to different surface peptide densities. For this purpose, an analog of the HVP peptide was prepared by substitution of Phe → (p-F) Phe. The presence of fluorine as a marker allowed for easier XPS analysis. From the XPS data, it can be deduced that the increase in the concentration of peptide in solution leads to an increased surface peptide density, as evidenced by the enhancement of the F/C ratio when passing from the operating concentration of 10−5 M up to the concentration 10−3 M. Further investigations examined cell proliferation on variable surface peptide densities. The data obtained indicated that a concentration of 10−4 M HVP functionalization solution was able to promote the proliferation of human osteoblast cells at all times investigated.
The advantage of the anchoring method proposed via oxime is that it is extremely simple and absolutely free of unwanted reactions or side effects (persistence of condensing agents or traces of organic solvents). We had evidence of the chemistry solidity of this anchoring strategy in chemoselective conjugations where the conjugate identity determination was possible by MALDI-TOF spectrometry [
30]. Furthermore, the anchoring strategy via imine was performed in the functionalization of Mn-containing Bioactive Glass-Ceramics with a BMP-2 fragment. In this case, the band of C-N stretching mode of the secondary amine formed upon the reduction of the Schiff’s base was detected [
31]. Compared to the Schiff base anchoring already proposed by Becker M. et al. [
32] for PEEK functionalization, the anchoring via oxime does not use an easily reversible reaction, so it does not require the reduction of the Schiff base to amine. The dynamic co-assembly, in fact [
33], is not functional to obtain biomaterials that encourage cell adhesion as it is the stable anchoring of the adhesive sequence to the material that mediates cell adhesion. In contrast, the release of the adhesive sequence from the surface of the material can induce adhesion inhibition as this, as a soluble molecule, blocks cell integrins [
34].
The enrichment of the surface with adhesive peptides was ascertained with XPS analysis. From the N/C ratios, it is clear how the two peptides reacted differently: considering that D2HVP has a double sequence then HVP (double nitrogen content), it can be estimated that the surface concentration of D2HVP is about 1/3 compared to that obtained using the peptide HVP.
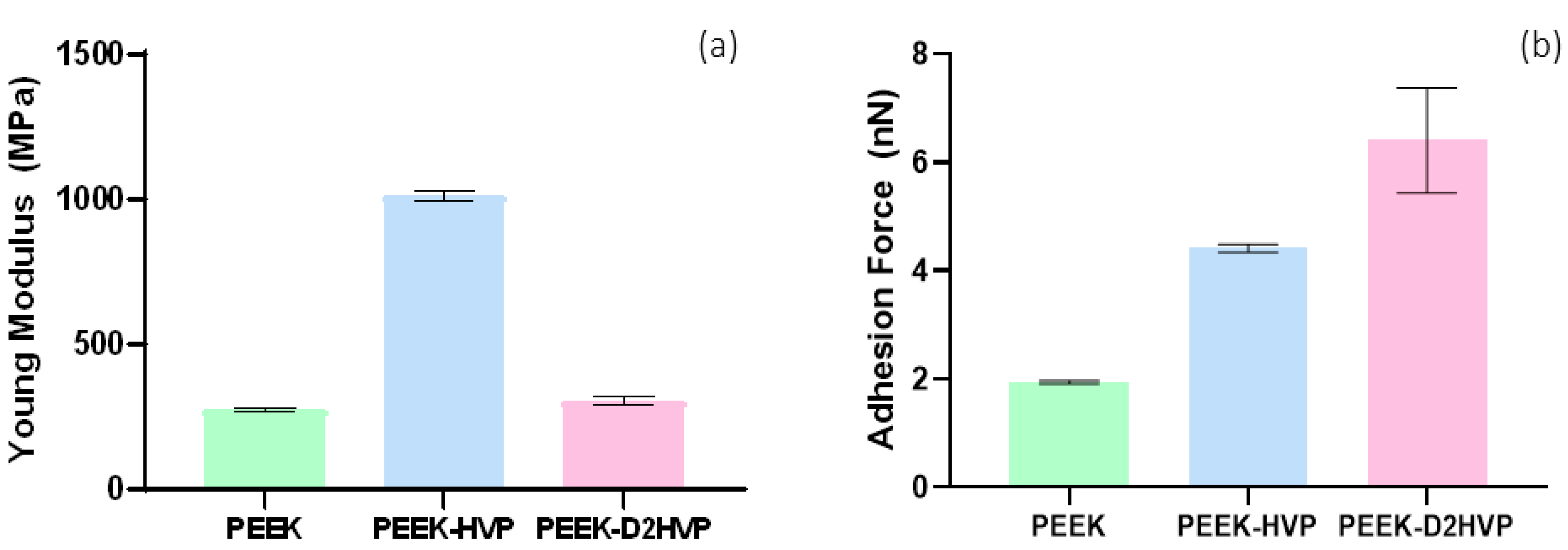
The presence of peptides modified the surface properties of the material, making PEEK more hydrophilic and, therefore, more suitable for interaction with cells. The evaluation of Young’s modulus by AFM showed that functionalization of the surface increases its stiffness. The result is particularly relevant following the functionalization with the shorter HVP peptide. The hypothesis is that this smaller peptide compared to D2HVP, is able to fill the gaps between the surface polymer chains while its retro-inverted dimer does so only in part due to the greater steric hindrance. Both peptides increased the adhesion forces with the AFM cantilever compared to the control. In particular, the presence of the HVP peptide doubles the adhesive force with the cantilever tip compared to PEEK alone, while the D2HVP peptide tripled the adhesion force compared to the control. This may be due to the longer sequence of the retro-inverted dimer compared to HVP.
The Live and Dead staining at 48 h showed a large number of viable cells attached to all the surfaces, and only a few red dead cells were visible on the HVP sample but were absent on the D2HVP sample. The elongated morphology, index of spreading, and, therefore, adhesion were observed on the control PEEK, and the PEEK was functionalized with D2HVP. Cells on PEEK-HVP appeared more rounded, indicating more metabolically active cells.
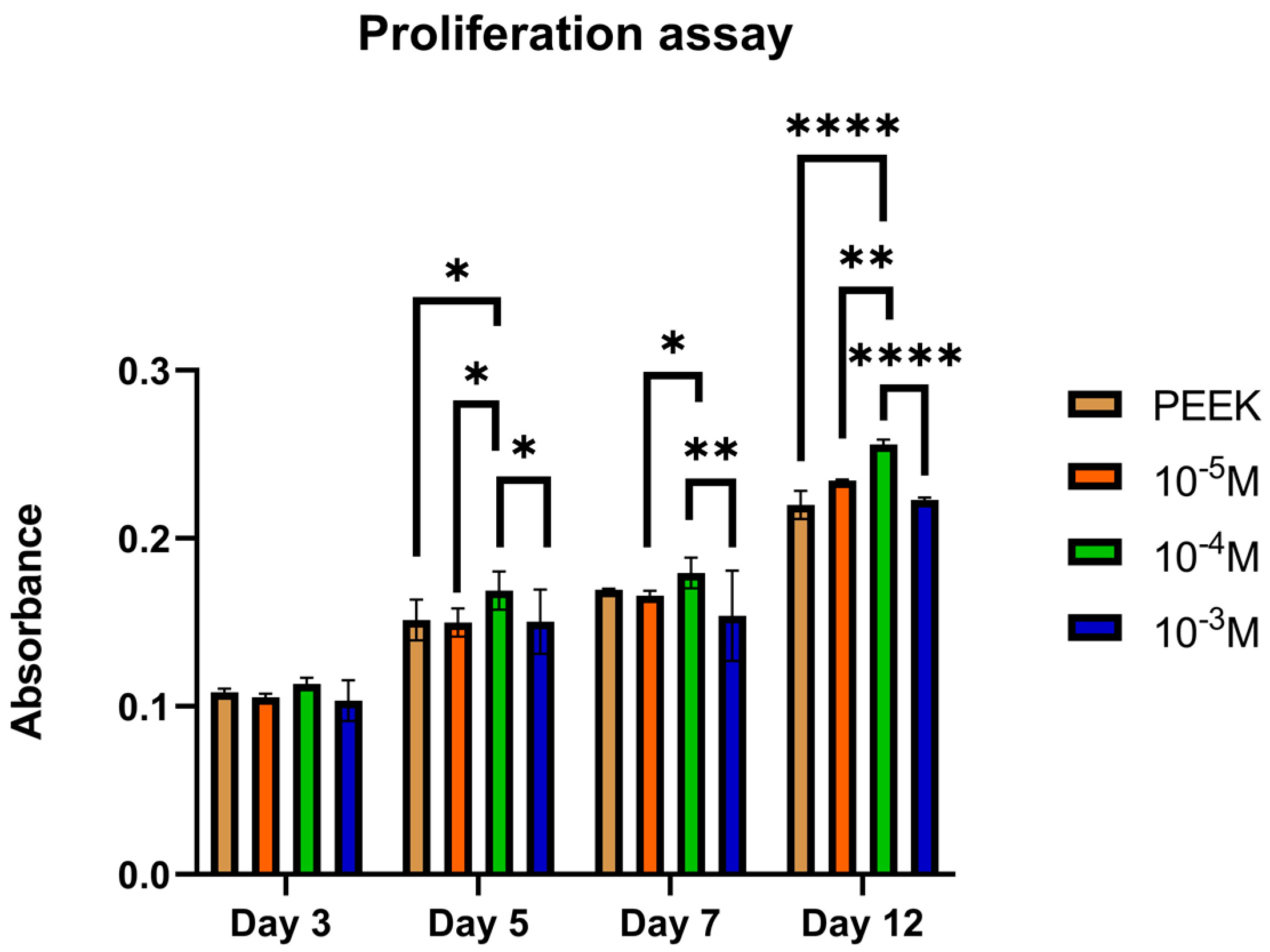
Quantification of the cells at different time points was assessed with the AlamarBlue
TM test. On day one, an increase in the number of adhered cells was observed on both functionalized surfaces when compared to the control by about 300% in accordance with the adhesive nature of the sequences [
22]. At the subsequent time points (4, 7, and 14 days), the surface functionalized with the D2HVP peptide induced a greater proliferation than the control. At 21 days post-seeding, both functionalized surfaces had a significantly higher number of cells than the control. This confirms that f, the D2HVP peptide, resulted in an increase in proliferation rate. The PEEK functionalization with the D2HVP peptide produced an enhanced proliferation rate at all time points investigated. The best biological response was observed on samples with D2HVP. This could be attributable to the greater stability of the sequence against serum proteases [
22].
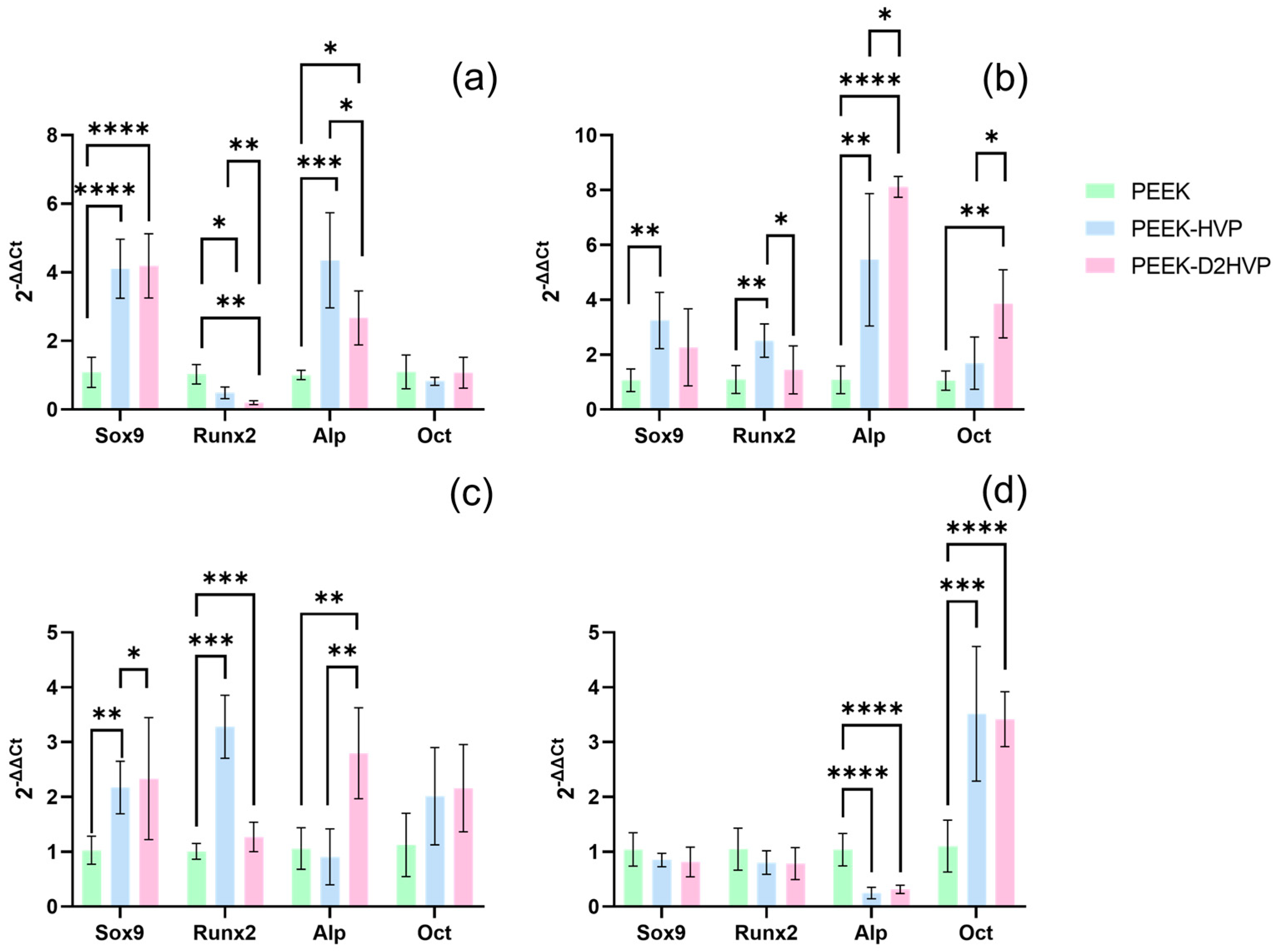
The calcium ion deposition was greater on the D2HVP peptide, indicating an increase in mineralization with respect to its monomer and control: the difference, however, was not significant. Furthermore, HVP and D2HVP functionalization significantly enhanced the gene expression of Sox9 at one day from cell seeding, and although it induced a down-regulation of Runx2 at one day, this is inverted at days 7 and 14, suggesting a positive trend over time (for HVP only). Expression of Sox9 has been observed in in vitro osteogenic differentiation of human Mesenchymal Stem Cells (hMSCs) [
35]. However, the enhanced expression seen in this study could be indicative of a possible role for Sox9 in mature osteoblasts in direct osteogenesis, supported by overexpression of ALP at early stages and of Oct at later stages in functionalized samples; this interesting observation warrants further investigation.
Scaffolds play a significant role in cell adherence, a major contributing factor to subsequent morphology, proliferation, and maturation of cells. The presence of bioactive sequences on the PEEK surface allowed the design of an engineered surface able to promote the proliferation and differentiation of human osteoblasts. Results have shown an increase in adherence, proliferation, and maturation of osteoblasts. To further examine and elucidate the mechanistic actions of these peptides, future studies will explore their role in the induction of stem cells towards the osteogenic lineage and examine regulatory signaling pathways in vitro.