Critical Roles of METTL3 in Translation Regulation of Cancer
Abstract
1. Introduction
2. Aberrant Translation in Cancer
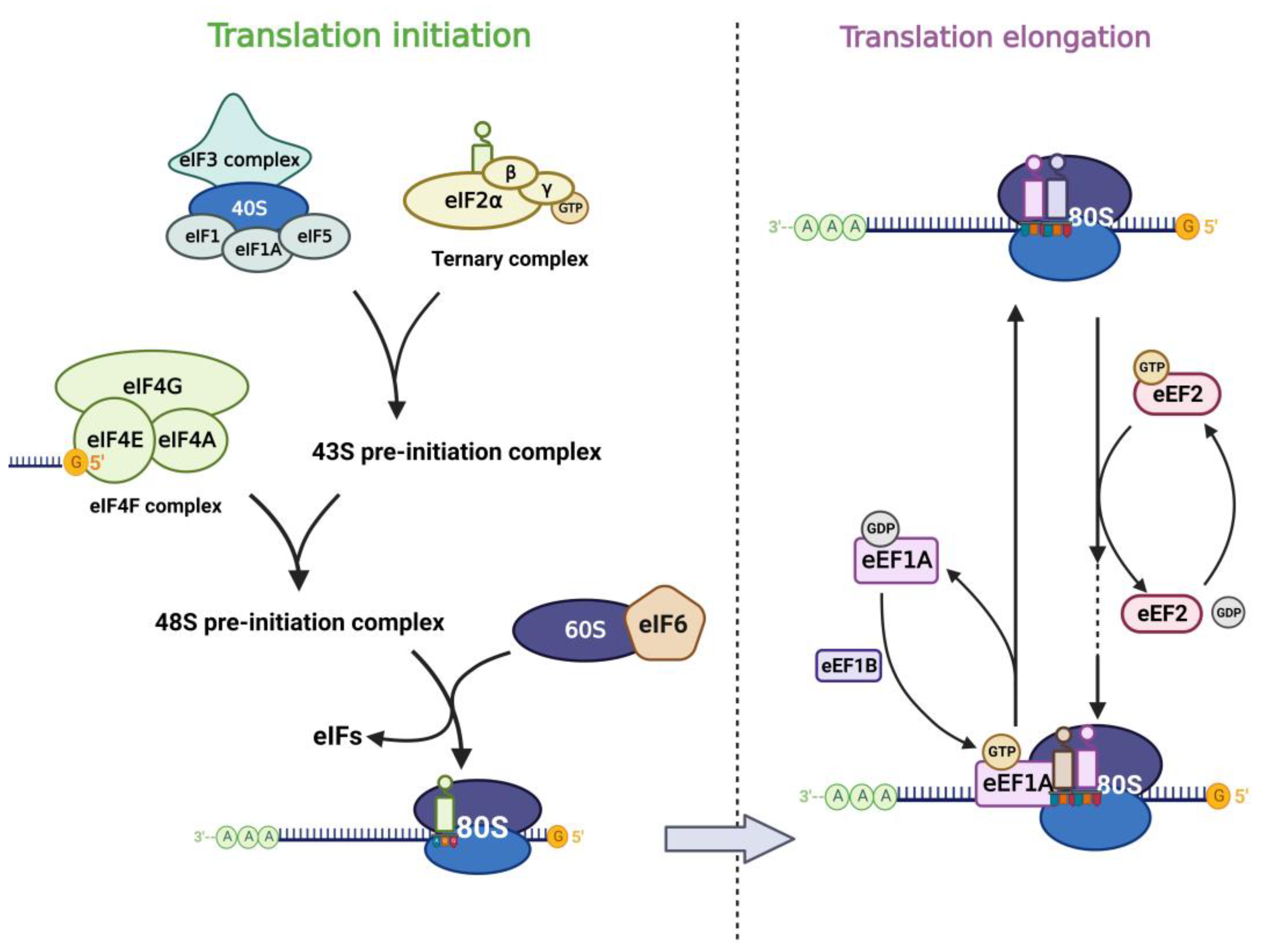
2.1. Translation Factors in Eukaryotic Translation
2.2. Dysregulation of Translation Factors in Cancer
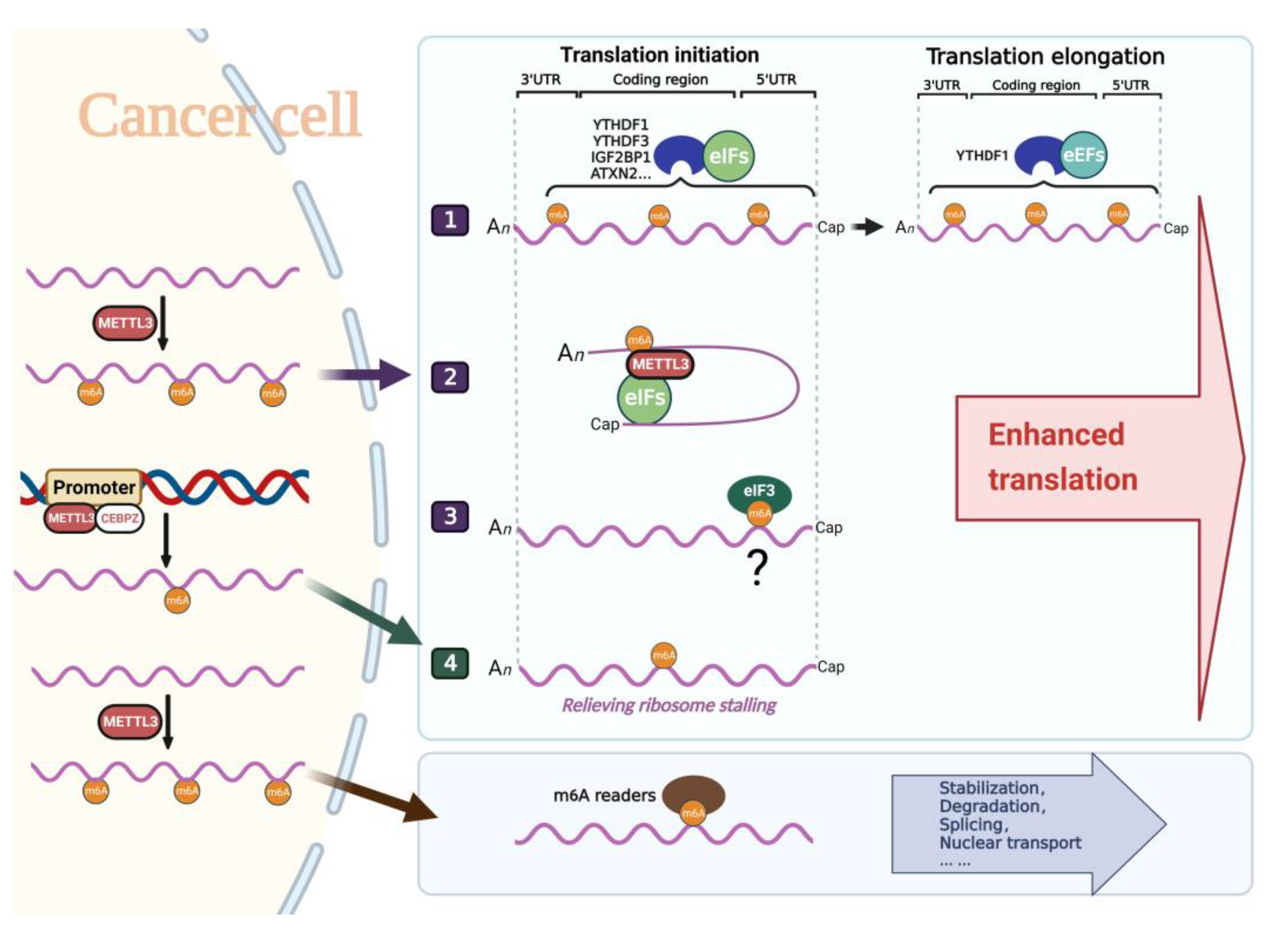
3. METTL3 Functions as a Translation Regulator in Cancer
3.1. METTL3 Mediates the Binding of Other m6A Reader Proteins to Promote Translation through m6A Modification
3.2. METTL3 Enters the Cytoplasm to Facilitate the Translation Process
3.3. Promoter-Bound METTL3 Enhances Translation
3.4. Other Possible Pathways
- (1)
- METTL3 modifies m6A in target mRNAs and then recruits canonical translation machinery through classical or novel m6A readers YTHDF1/YTHDF3/IGF2BP1/ATXN2 to promote translation.
- (2)
- After m6A modification of target mRNAs, METTL3 directly binds m6A sites in the cytoplasm and recruits canonical translation machinery to promote translation.
- (3)
- METTL3 interacts with CEBPZ to bind the promoter of target genes and enhances the translation of the associated mRNAs by relieving ribosomal arrest through m6A modification.
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desrosiers, R.; Friderici, K.; Rottman, F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA 1974, 71, 3971–3975. [Google Scholar] [CrossRef] [PubMed]
- Bokar, J.A.; Shambaugh, M.E.; Polayes, D.; Matera, A.G.; Rottman, F.M. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 1997, 3, 1233–1247. [Google Scholar] [PubMed]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Grozhik, A.; Olarerin-George, A.; Meydan, C. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods 2015, 12, 767–772. [Google Scholar] [CrossRef]
- Perry, R.P.; Scherrer, K. The methylated constituents of globin mRNA. FEBS Lett. 1975, 57, 73–78. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.S.; Roundtree, I.A. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, K.; Song, B.; Ma, J.; Wu, X.; Xu, Q.; Wei, Z.; Su, J.; Liu, G.; Rong, R.; et al. m6A-Atlas: A comprehensive knowledgebase for unraveling the N6-methyladenosine (m6A) epitranscriptome. Nucleic Acids Res. 2021, 49, D134–D143. [Google Scholar] [CrossRef]
- Geula, S.; Moshitch-Moshkovitz, S.; Dominissini, D.; Mansour, A.A.; Kol, N.; Salmon-Divon, M.; Hershkovitz, V.; Peer, E.; Mor, N.; Manor, Y.S.; et al. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 2015, 347, 1002–1006. [Google Scholar] [CrossRef]
- Yoon, K.-J.; Ringeling, F.R.; Vissers, C.; Jacob, F.; Pokrass, M.; Jimenez-Cyrus, D.; Su, Y.; Kim, N.-S.; Zhu, Y.; Zheng, L.; et al. Temporal Control of Mammalian Cortical Neurogenesis by m6A Methylation. Cell 2017, 171, 877–889.e17. [Google Scholar]
- Livneh, I.; Moshitch-Moshkovitz, S.; Amariglio, N.; Rechavi, G.; Dominissini, D. The m 6 A epitranscriptome: Transcriptome plasticity in brain development and function. Nat. Rev. Neurosci. 2020, 21, 36–51. [Google Scholar] [PubMed]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar]
- Deng, L.J.; Deng, W.Q.; Fan, S.R.; Chen, M.F.; Qi, M.; Lyu, W.Y.; Qi, Q.; Tiwari, A.K.; Chen, J.X.; Zhang, D.M.; et al. m6A modification: Recent advances, anticancer targeted drug discovery and beyond. Mol. Cancer 2022, 21, 52. [Google Scholar] [PubMed]
- Oerum, S.; Meynier, V.; Catala, M.; Tisné, C. A comprehensive review of m6A/m6Am RNA methyltransferase structures. Nucleic Acids Res. 2021, 49, 7239–7255. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Sheng, L.; Gao, Q.; Xiong, Q.; Zhang, H.; Wu, M.; Liang, Y.; Zhu, F.; Zhang, Y.; Zhang, X.; et al. The m6A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF-κB/MYC signaling network. Oncogene 2019, 38, 3667–3680. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Sun, G.; Wu, Q.; Ma, J.; Zhang, X.; Huang, N.; Bian, Z.; Gu, S.; Xu, M.; et al. m6A mRNA methylation regulates CTNNB1 to promote the proliferation of hepatoblastoma. Mol. Cancer 2019, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shao, F.; Yang, X.; Wang, J.; Zhu, R.; Yang, Y.; Zhao, G.; Guo, D.; Sun, Y.; Wang, J.; et al. METTL3 promotes tumour development by decreasing APC expression mediated by APC mRNA N6-methyladenosine-dependent YTHDF binding. Nat. Commun. 2021, 12, 3803. [Google Scholar] [CrossRef]
- Cui, Q.; Shi, H.; Ye, P.; Li, L.; Qu, Q.; Sun, G.; Sun, G.; Lu, Z.; Huang, Y.; Yang, C.-G.; et al. m(6)A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep. 2017, 18, 2622–2634. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, N.; Zhang, Y.; Zhang, X.; Xu, L.; Che, Y.; Qiao, T.; Bin Wu, B.; Zhou, Y.; Jiang, J.; et al. METTL3-mediated m(6)A mRNA modification of FBXW7 suppresses lung adenocarcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 90. [Google Scholar] [CrossRef]
- Buttgereit, F.; Brand, M.D. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 1995, 312, 163–167. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Robichaud, N.; Hulea, L.; Sonenberg, N.; Pelletier, J.; Topisirovic, I. Targeting the translation machinery in cancer. Nat. Rev. Drug Discov. 2015, 14, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Colina, R.; Costa-Mattioli, M.; Dowling, R.J.O.; Jaramillo, M.; Tai, L.-H.; Breitbach, C.J.; Martineau, Y.; Larsson, O.; Rong, L.; Svitkin, Y.V.; et al. Translational control of the innate immune response through IRF-7. Nature 2008, 452, 323–328. [Google Scholar] [CrossRef]
- Larsson, O.; Perlman, D.M.; Fan, D.; Reilly, C.S.; Peterson, M.; Dahlgren, C.; Liang, Z.; Li, S.; Polunovsky, V.A.; Wahlestedt, C.; et al. Apoptosis resistance downstream of eIF4E: Posttranscriptional activation of an anti-apoptotic transcript carrying a consensus hairpin structure. Nucleic Acids Res. 2006, 34, 4375–4386. [Google Scholar] [CrossRef]
- Ruggero, D. Translational control in cancer etiology. Cold Spring Harb. Perspect. Biol. 2013, 5, a012336. [Google Scholar] [CrossRef]
- Rubio, A.; Garland, G.D.; Sfakianos, A. Aberrant protein synthesis and cancer development: The role of canonical eukaryotic initiation, elongation and termination factors in tumorigenesis. Semin. Cancer Biol. 2022, 86, 151–165. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, A.; Graff, J.R. eIF-4E expression and its role in malignancies and metastases. Oncogene 2004, 23, 3189–3199. [Google Scholar] [CrossRef]
- Feoktistova, K.; Tuvshintogs, E.; Do, A.; Fraser, C.S. Human eIF4E promotes mRNA restructuring by stimulating eIF4A helicase activity. Proc. Natl. Acad. Sci. USA 2013, 110, 13339–13344. [Google Scholar] [CrossRef]
- Merrick, W.C.; Pavitt, G.D. Protein Synthesis Initiation in Eukaryotic Cells. Cold Spring Harb. Perspect. Biol. 2018, 10, a033092. [Google Scholar] [CrossRef]
- Kozak, M. New ways of initiating translation in eukaryotes? Mol. Cell Biol. 2001, 21, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, U.; Powell, P.; Goss, D.J. Eukaryotic initiation factor (eIF) 3 mediates Barley Yellow Dwarf Viral mRNA 3’-5’ UTR interactions and 40S ribosomal subunit binding to facilitate cap-independent translation. Nucleic Acids Res. 2019, 47, 6225–6235. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G. The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 2014, 83, 779–812. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fuller, T.J.; Mahe, M.; Walters, B.; Abbadi, D.; Pérez-Baos, S. Translation Regulation by eIF2α Phosphorylation and mTORC1 Signaling Pathways in Non-Communicable Diseases (NCDs). Int. J. Mol. Sci. 2020, 21, 5301. [Google Scholar] [CrossRef] [PubMed]
- Sanvito, F.; Piatti, S.; Villa, A.; Bossi, M.; Lucchini, G.; Marchisio, P.C.; Biffo, S. The beta4 integrin interactor p27(BBP/eIF6) is an essential nuclear matrix protein involved in 60S ribosomal subunit assembly. J. Cell Biol. 1999, 144, 823–837. [Google Scholar] [CrossRef]
- Basu, U.; Si, K.; Warner, J.R.; Maitra, U. The Saccharomyces cerevisiae TIF6 gene encoding translation initiation factor 6 is required for 60S ribosomal subunit biogenesis. Mol. Cell Biol. 2001, 21, 1453–1462. [Google Scholar] [CrossRef]
- Gandin, V.; Miluzio, A.; Barbieri, A.M.; Beugnet, A.; Kiyokawa, H.; Marchisio, P.C.; Biffo, S. Eukaryotic initiation factor 6 is rate-limiting in translation, growth and transformation. Nature 2008, 455, 684–688. [Google Scholar] [CrossRef]
- Miluzio, A.; Beugnet, A.; Volta, V.; Biffo, S. Eukaryotic initiation factor 6 mediates a continuum between 60S ribosome biogenesis and translation. EMBO Rep. 2009, 10, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Proud, C.G. Regulation and roles of elongation factor 2 kinase. Biochem. Soc. Trans. 2015, 43, 328–332. [Google Scholar] [CrossRef]
- Robichaud, N.; Sonenberg, N.; Ruggero, D.; Schneider, R.J. Translational Control in Cancer. Cold Spring Harb. Perspect. Biol. 2019, 11, a032896. [Google Scholar] [CrossRef]
- Kevil, C.G.; De Benedetti, A.; Payne, D.K.; Coe, L.L.; Laroux, F.S.; Alexander, J.S. Translational regulation of vascular permeability factor by eukaryotic initiation factor 4E: Implications for tumor angiogenesis. Int. J. Cancer 1996, 65, 785–790. [Google Scholar] [CrossRef]
- Nathan, C.-A.; Carter, P.; Liu, L.; Li, B.D.; Abreo, F.; Tudor, A.; Zimmer, S.G.; De Benedetti, A. Elevated expression of eIF4E and FGF-2 isoforms during vascularization of breast carcinomas. Oncogene 1997, 15, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, F.; Bänziger, R.; Burger, M.M. Cloning of a novel protein overexpressed in human mammary carcinoma. Cancer Res. 1997, 57, 988–994. [Google Scholar] [PubMed]
- Dong, Z.; Zhang, J.T. EIF3 p170, a mediator of mimosine effect on protein synthesis and cell cycle progression. Mol. Biol. Cell 2003, 14, 3942–3951. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, G.L.; Hershey, J.W. Malignant transformation by the eukaryotic translation initiation factor 3 subunit p48 (eIF3e). FEBS Lett. 2002, 514, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Nupponen, N.N.; Porkka, K.; Kakkola, L.; Tanner, M.; Persson, K.; Borg, A.; Isola, J.; Visakorpi, T. Amplification and overexpression of p40 subunit of eukaryotic translation initiation factor 3 in breast and prostate cancer. Am. J. Pathol. 1999, 154, 1777–1783. [Google Scholar] [CrossRef]
- Saramäki, O.; Willi, N.; Bratt, O.; Gasser, T.C.; Koivisto, P.; Nupponen, N.N.; Bubendorf, L.; Visakorpi, T. Amplification of EIF3S3 gene is associated with advanced stage in prostate cancer. Am. J. Pathol. 2001, 159, 2089–2094. [Google Scholar] [CrossRef]
- Golob-Schwarzl, N.; Puchas, P.; Gogg-Kamerer, M.; Weichert, W.; Göppert, B.; Haybaeck, J. New Pancreatic Cancer Biomarkers eIF1, eIF2D, eIF3C and eIF6 Play a Major Role in Translational Control in Ductal Adenocarcinoma. Anticancer Res. 2020, 40, 3109–3118. [Google Scholar] [CrossRef]
- Sehrawat, U.; Koning, F.; Ashkenazi, S.; Stelzer, G.; Leshkowitz, D.; Dikstein, R. Cancer-Associated Eukaryotic Translation Initiation Factor 1A Mutants Impair Rps3 and Rps10 Binding and Enhance Scanning of Cell Cycle Genes. Mol. Cell Biol. 2019, 39, e00441-18. [Google Scholar] [CrossRef] [PubMed]
- García-Jiménez, C.; Goding, C.R. Starvation and Pseudo-Starvation as Drivers of Cancer Metastasis through Translation Reprogramming. Cell Metab. 2019, 29, 254–267. [Google Scholar] [CrossRef]
- Li, Z.; Ge, Y.; Dong, J.; Wang, H.; Zhao, T.; Wang, X.; Liu, J.; Gao, S.; Shi, L.; Yang, S.; et al. BZW1 Facilitates Glycolysis and Promotes Tumor Growth in Pancreatic Ductal Adenocarcinoma Through Potentiating eIF2α Phosphorylation. Gastroenterology 2022, 162, 1256–1271.e14. [Google Scholar] [CrossRef]
- Golob-Schwarzl, N.; Krassnig, S.; Toeglhofer, A.M.; Park, Y.N.; Gogg-Kamerer, M.; Vierlinger, K.; Schröder, F.; Rhee, H.; Schicho, R.; Fickert, P.; et al. New liver cancer biomarkers: PI3K/AKT/mTOR pathway members and eukaryotic translation initiation factors. Eur. J. Cancer 2017, 83, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, S.; Wang, X.; Zheng, X.; Chen, Y.; Shen, H. eIF6 promotes the malignant progression of human hepatocellular carcinoma via the mTOR signaling pathway. J. Transl. Med. 2021, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Scagliola, A.; Miluzio, A.; Mori, G.; Ricciardi, S.; Oliveto, S.; Manfrini, N.; Biffo, S. Inhibition of eIF6 Activity Reduces Hepatocellular Carcinoma Growth: An In Vivo and In Vitro Study. Int. J. Mol. Sci. 2022, 23, 7720. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yu, X.; Xie, L.; Wang, P.; Li, T.; Xiao, Y.; Zhou, J.; Peng, S.; Huang, J.; Luo, Y.; et al. eIF6 Promotes Colorectal Cancer Proliferation and Invasion by Regulating AKT-Related Signaling Pathways. J. Biomed. Nanotechnol. 2019, 15, 1556–1567. [Google Scholar] [CrossRef]
- Golob-Schwarzl, N.; Wodlej, C.; Kleinegger, F.; Gogg-Kamerer, M.; Birkl-Toeglhofer, A.M.; Petzold, J.; Aigelsreiter, A.; Thalhammer, M.; Park, Y.N.; Haybaeck, J. Eukaryotic translation initiation factor 6 overexpression plays a major role in the translational control of gallbladder cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 2699–2711. [Google Scholar] [CrossRef]
- Scagliola, A.; Miluzio, A.; Ventura, G.; Oliveto, S.; Cordiglieri, C. Targeting of eIF6-driven translation induces a metabolic rewiring that reduces NAFLD and the consequent evolution to hepatocellular carcinoma. Nat. Commun. 2021, 12, 4878. [Google Scholar] [CrossRef]
- Abbas, W.; Kumar, A.; Herbein, G. The eEF1A Proteins: At the Crossroads of Oncogenesis, Apoptosis, and Viral Infections. Front. Oncol. 2015, 7, 75. [Google Scholar] [CrossRef]
- Lee, S.; Francoeur, A.; Liu, E.W.S. Tissue-specific expression in mammalian brain, heart, and muscle of S1, a member of the elongation factor-1 alpha gene family. J. Biol. Chem. 1992, 267, 24064–24068. [Google Scholar] [CrossRef]
- Tomlinson, V.A.; Newbery, H.J.; Wray, N.R.; Jackson, J.; Larionov, A.; Miller, W.R.; Dixon, J.M. Translation elongation factor eEF1A2 is a potential oncoprotein that is overexpressed in two-thirds of breast tumours. BMC Cancer 2005, 12, 113. [Google Scholar] [CrossRef]
- Liu, R.; Proud, C.G. Eukaryotic elongation factor 2 kinase as a drug target in cancer, and in cardiovascular and neurodegenerative diseases. Acta Pharmacol. Sin. 2016, 37, 285–294. [Google Scholar] [CrossRef]
- Castello, A.; Fischer, B.; Eichelbaum, K.; Horos, R.; Beckmann, B.M.; Strein, C.; Davey, N.E.; Humphreys, D.T.; Preiss, T.; Steinmetz, L.M.; et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 2012, 8, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Lin, S.; Zhang, W.; Liu, Q.; Wang, L.; Ramirez-Moya, J.; Du, P.; Kim, W.; Tang, S.; Sliz, P.; et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature 2018, 561, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Jaffrey, S.R. Rethinking m6A Readers, Writers, and Erasers. Annu. Rev. Cell Dev. Biol. 2017, 33, 319–342. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, X.; Lu, Z.; Zhao, B.S.; Ma, H.; Hsu, P.J.; Liu, C.; He, C. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017, 27, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Feng, L.; Li, J.; Dai, D.; Zhu, L.; Wang, C.; Li, J.; Li, L.; Zhou, Q.; Shi, R.; et al. β-catenin represses miR455-3p to stimulate m6A modification of HSF1 mRNA and promote its translation in colorectal cancer. Mol. Cancer 2020, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Eckert, M.A.; Harada, B.T.; Liu, S.-M.; Lu, Z.; Yu, K.; Tienda, S.M.; Chryplewicz, A.; Zhu, A.C.; Yang, Y.; et al. m6A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat. Cell Biol. 2018, 20, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, X.; Yang, P.; Zhang, X.; Peng, Y.; Li, D.; Yu, Y.; Wu, Y.; Wang, Y.; Zhang, J.; et al. RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat. Commun. 2021, 12, 1394. [Google Scholar] [CrossRef]
- Huo, F.-C.; Zhu, Z.-M.; Zhu, W.-T.; Du, Q.-Y.; Liang, J.; Mou, J. METTL3-mediated m6A methylation of SPHK2 promotes gastric cancer progression by targeting KLF2. Oncogene 2021, 40, 2968–2981. [Google Scholar] [CrossRef]
- Ma, L.; Xue, X.; Zhang, X.; Yu, K.; Xu, X.; Tian, X.; Miao, Y.; Meng, F.; Liu, X.; Guo, S.; et al. The essential roles of m6A RNA modification to stimulate ENO1-dependent glycolysis and tumorigenesis in lung adenocarcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 36. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, D.; Yan, C.; Su, H. METTL3 promotes lung adenocarcinoma tumor growth and inhibits ferroptosis by stabilizing SLC7A11 m 6 A modification. Cancer Cell Int. 2022, 22, 11. [Google Scholar] [CrossRef]
- Jia, R.; Chai, P.; Wang, S.; Sun, B. m6A modification suppresses ocular melanoma through modulating HINT2 mRNA translation. Mol. Cancer 2019, 18, 161. [Google Scholar] [CrossRef]
- Lin, X.; Chai, G.; Wu, Y.; Li, J.; Chen, F.; Liu, J.; Luo, G.; Tauler, J.; Du, J.; Lin, S.; et al. RNA m6A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat. Commun. 2019, 10, 2065. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhang, Q.; Chen, M.; Li, B.; Wang, N.; Li, C.; Gao, Z.; Zhang, D.; Yang, L.; Xu, Z.; et al. N6-methyladenosine modification regulates imatinib resistance of gastrointestinal stromal tumor by enhancing the expression of multidrug transporter MRP1. Cancer Lett. 2022, 530, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Y.; Li, J. N6-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat. Commun. 2020, 11, 2578. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Z.; Guan, T. N6-methyladenosine regulates mRNA stability and translation efficiency of KRT7 to promote breast cancer lung metastasis. Cancer Res. 2021, 81, 2847–2860. [Google Scholar] [CrossRef]
- Jin, D.; Guo, J.; Wu, Y.; Du, J.; Yang, L.; Wang, X.; Di, W.; Hu, B.; An, J.; Kong, L.; et al. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J. Hematol. Oncol. 2019, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ying, X.; Que, B. N6-methyladenosine modification of ITGA6mRNA promotes the development and progression of bladder cancer. EBioMedicine 2019, 47, 195–207. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Li, Q.; Liang, J.; He, Q. METTL3 Promotes Tumorigenesis and Metastasis through BMI1 m6A Methylation in Oral Squamous Cell Carcinoma. Mol. Ther. 2020, 28, 2177–2190. [Google Scholar] [CrossRef]
- Li, R.; Zeng, L.; Zhao, H.; Deng, J.; Pan, L.; Zhang, S.; Wu, G.; Ye, Y.; Zhang, J.; Su, J.; et al. ATXN2-mediated translation of TNFR1 promotes esophageal squamous cell carcinoma via m(6)A-dependent manner. Mol. Ther. 2022, 30, 1089–1103. [Google Scholar] [CrossRef]
- Lin, S.; Choe, J.; Du, P.; Triboulet, R.; Gregory, R.I. The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol. Cell 2016, 62, 335–345. [Google Scholar] [CrossRef]
- Hua, W.; Zhao, Y.; Jin, X.; Yu, D.; He, J.; Xie, D.; Duan, P. METTL3 promotes ovarian carcinoma growth and invasion through the regulation of AXL translation and epithelial to mesenchymal transition. Gynecol. Oncol. 2018, 151, 356–365. [Google Scholar] [CrossRef]
- Ianniello, Z.; Sorci, M.; Ginistrelli, L.C.; Iaiza, A.; Marchioni, M.; Tito, C.; Capuano, E.; Masciarelli, S.; Ottone, T.; Attrotto, C.; et al. New insight into the catalytic -dependent and -independent roles of METTL3 in sustaining aberrant translation in chronic myeloid leukemia. Cell Death Dis. 2021, 12, 870. [Google Scholar] [CrossRef]
- Yang, F.; Jin, H.; Que, B.; Chao, Y.; Zhang, H.; Ying, X.; Zhou, Z.; Yuan, Z.; Su, J.; Wu, B.; et al. Dynamic m6A mRNA methylation reveals the role of METTL3-m6A-CDCP1 signaling axis in chemical carcinogenesis. Oncogene 2019, 38, 4755–4772. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Jiang, X.; Zhang, H.; Liu, B. Programmable N6-methyladenosine modification of CDCP1 mRNA by RCas9-methyltransferase like 3 conjugates promotes bladder cancer development. Mol. Cancer 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Huo, Y.; Pi, J.; Gao, Y.; Rao, S.; He, M.; Wei, Q.; Song, P.; Chen, Y.; Lu, D.; et al. METTL3 preferentially enhances non-m(6)A translation of epigenetic factors and promotes tumourigenesis. Nat. Cell Biol. 2022, 24, 1278–1290. [Google Scholar] [CrossRef]
- Barbieri, I.; Tzelepis, K.; Pandolfini, L.; Shi, J.; Millán-Zambrano, G.; Robson, S.C.; Aspris, D.; Migliori, V.; Bannister, A.J.; Han, N.; et al. Promoter-bound METTL3 maintains myeloid leukaemia by m6A-dependent translation control. Nature 2017, 552, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.-B.; Jaffrey, S.R. 5’ UTR m(6)A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, J.; Gao, X.; Zhang, X.; Jaffrey, S.R.; Qian, S.-B. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature 2015, 526, 591–594. [Google Scholar] [CrossRef]
- Moerke, N.J.; Aktas, H.; Chen, H.; Cantel, S.; Reibarkh, M.Y.; Fahmy, A.; Gross, J.D.; Degterev, A.; Yuan, J.; Chorev, M.; et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell 2007, 128, 257–267. [Google Scholar] [CrossRef]
- Bordeleau, M.-E.; Mori, A.; Oberer, M.; Lindqvist, L.; Chard, L.S.; Higa, T.; Belsham, G.J.; Wagner, G.; Tanaka, J.; Pelletier, J. Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat. Chem. Biol. 2006, 2, 213–220. [Google Scholar] [CrossRef]
- Pesce, E.; Miluzio, A.; Turcano, L.; Minici, C.; Cirino, D.; Calamita, P.; Manfrini, N.; Oliveto, S.; Ricciardi, S.; Grifantini, R.; et al. Discovery and Preliminary Characterization of Translational Modulators that Impair the Binding of eIF6 to 60S Ribosomal Subunits. Cells 2020, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Yankova, E.; Blackaby, W.; Albertella, M. Small molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 2021, 593, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yuan, Y.; Xu, L.; Zhao, F.; Wang, W.; Xu, Y.; Tian, X. Discovery of METTL3 Small Molecule Inhibitors by Virtual Screening of Natural Products. Front. Pharmacol. 2022, 13, 878135. [Google Scholar] [CrossRef] [PubMed]
- Moroz-Omori, E.V.; Huang, D.; Bedi, R.K.; Cheriyamkunnel, S.J.; Bochenkova, E.; Dolbois, A.; Rzeczkowski, M.D.; Li, Y.; Wiedmer, L.; Caflisch, A. METTL3 Inhibitors for Epitranscriptomic Modulation of Cellular Processes. ChemMedChem 2021, 16, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, N. Eltrombopag as an Allosteric Inhibitor of the METTL3-14 Complex Affecting the m(6)A Methylation of RNA in Acute Myeloid Leukemia Cells. Pharmaceuticals 2022, 15, 440. [Google Scholar] [CrossRef]
- Cook, D.; Brown, D.; Alexander, R.; March, R.; Morgan, P.; Satterthwaite, G.; Pangalos, M.N. Lessons learned from the fate of AstraZeneca’s drug pipeline: A five-dimensional framework. Nat. Rev. Drug Discov. 2014, 13, 419–431. [Google Scholar] [CrossRef]
| PMID | Cancer Types | Reader Proteins * | Targets | Function | m6A Sites | RBPs of Canonical Translational Machinery |
|---|---|---|---|---|---|---|
| 31061416 | Liver cancer | YTHDF1 | SNAI1 | Epithelial-mesenchymal transition | CDS | eEF2 |
| 35032557 | Gastrointestinal stromal tumor | YTHDF1 | MRP1 | Drug resistance | 5′UTR | eEF1 |
| 32444598 | Cervical and liver cancer | YTHDF1 | PDK4 | Glycolysis | 5′UTR | eEF2 |
| 33795252 | Breast cancer | YTHDF1 | KRT7 | Metastasis | CDS | eEF1 |
| 33654093 | Melanoma | YTHDF1 | SPRED2 | Tumor growth and metastasis | CDS, 3′UTR | |
| 30154548 | Endometrial cancer | YTHDF1 | PHLPP2 | Proliferation and tumorigenicity | ||
| 33758320 | Gastric cancer | YTHDF1 | SPHK2 | Progression | eIF3a | |
| 35078505 | Lung adenocarcinoma | YTHDF1 | ENO1 | Glycolysis and tumorigenesis | eIF3e | |
| 31722709 | Ocular melanoma | YTHDF1 | HINT2 | Progression | 3’UTR | |
| 34996469 | Lung adenocarcinoma | YTHDF1 | SLC7A11 | Progression | ||
| 33618740 | NSCLC | YTHDF1, YTHDF3 | YAP | Drug resistance and metastasis | 3’UTR | eIF3b |
| 31409574 | Bladder cancer | YTHDF1, YTHDF3 | ITGA6 | Progression | 3’UTR | |
| 32621798 | Oral squamous cell carcinoma | YTHDF1, IGF2BP1 | BMI1 | Tumorigenesis and metastasis | 3’UTR | |
| 32838807 | Colorectal cancer | METTL3, YTHDF1 | HSF1 | Progression | 3’UTR | |
| 30796352 | Bladder cancer | METTL3, YTHDF1 | CDCP1 | Chemical carcinogenesis | 3’UTR | |
| 27117702 | Lung cancer | METTL3 | EGFR, TAZ | Progression | 3’UTR | eIF4E, eIF3 |
| 30232453 | Lung cancer | METTL3 | BRD4 | Tumorigenesis | 3’UTR | eIF3h, eIF4E |
| 34561421 | Chronic myeloid leukemia | METTL3 | PES1 | Proliferation and drug resistance | 3’UTR | |
| 30249526 | Ovarian carcinoma | METTL3 | AXL | Epithelial-mesenchymal transition | ||
| 34995801 | Esophageal cancer | ATXN2 | TNFR1 | Progression and tumorigenesis | 3’UTR | |
| 29186125 | Acute myeloid leukemia | SP1,SP2 | Proliferation | CDS | ||
| 34631715 | Kidney cancer | ABCD1 | Progression | 5’UTR | ||
| 33676554 | Lung adenocarcinoma | FBXW7 | Apoptosis and proliferation | CDS | ||
| 34530048 | Melanoma | EGFR | Drug resistance | 3’UTR | ||
| 33217448 | Colorectal cancer | GLUT1 | Progression | 3’UTR | ||
| 28920958 | Acute myeloid leukemia | c-MYC, BCL-2, PTEN | Proliferation | 3’UTR | ||
| 33267838 | Bladder cancer | CDCP1 | Progression | 3’UTR | ||
| 31454538 | Breast cancer | BCL-2 | Progression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, W.; Xiao, H.; Mei, P.; Chen, J.; Wang, Y.; Zhao, R.; Liao, Y. Critical Roles of METTL3 in Translation Regulation of Cancer. Biomolecules 2023, 13, 243. https://doi.org/10.3390/biom13020243
Meng W, Xiao H, Mei P, Chen J, Wang Y, Zhao R, Liao Y. Critical Roles of METTL3 in Translation Regulation of Cancer. Biomolecules. 2023; 13(2):243. https://doi.org/10.3390/biom13020243
Chicago/Turabian StyleMeng, Wangyang, Han Xiao, Peiyuan Mei, Jiaping Chen, Yangwei Wang, Rong Zhao, and Yongde Liao. 2023. "Critical Roles of METTL3 in Translation Regulation of Cancer" Biomolecules 13, no. 2: 243. https://doi.org/10.3390/biom13020243
APA StyleMeng, W., Xiao, H., Mei, P., Chen, J., Wang, Y., Zhao, R., & Liao, Y. (2023). Critical Roles of METTL3 in Translation Regulation of Cancer. Biomolecules, 13(2), 243. https://doi.org/10.3390/biom13020243







