Abstract
Cardiorenal syndrome (CRS) is a complex, heterogeneous spectrum of symptoms that has kept cardiologists awake for decades. The heart failure (HF) population being burdened with multimorbidity poses diagnostic and therapeutic challenges even for experienced clinicians. Adding deteriorated renal function to the equation, which is one of the strongest predictors of adverse outcome, we measure ourselves against possibly the biggest problem in modern cardiology. With the rapid development of new renal assessment methods, we can treat CRS more effectively than ever. The presented review focuses on explaining the pathophysiology, recent advances and current practices of monitoring renal function in patients with acute CRS. Understanding the dynamic interaction between the heart and the kidney may improve patient care and support the selection of an effective and nephroprotective treatment strategy.
1. Introduction
The interaction between the heart and the kidney is governed by a network of numerous complex and multi-level feedback loops. In recent years, great emphasis has been placed on understanding the spectrum of symptoms called cardiorenal syndrome (CRS) and the pathophysiological phenomena involved, in which failure of either the heart or the kidney results in the injury to the other. In 2010, CRS was classified into five subtypes by Ronco, based on acute or chronic affection and whereby a disorder of the heart induced renal dysfunction or contrariwise [1] (Table 1). The purpose of this review is to present the pathophysiology and clinical significance of acute CRS with an overview and comparison of novel methods of monitoring renal function.

Table 1.
Classification of cardiorenal syndrome.
The classic understanding of CRS was based on the belief that the kidneys are stimulated by lowered renal plasma flow to retain water and sodium in order to improve arterial filling and maintain adequate perfusion to vital organs. Indeed, the basic function of the heart, i.e., providing sufficient blood in- and outflow, is significantly impaired in conditions of compromised systolic and diastolic function of the heart [2].
Initially, it leads to the activation of various hemodynamic and neurohormonal compensatory mechanisms [3,4]. Reduced cardiac output and prerenal hypoperfusion stimulates the sympathetic nervous system (SNS), the renin–angiotensin–aldosterone system (RAAS) and non-osmotic release of vasopressin increasing preload as a consequence of fluid and salt retention [5,6,7]. In addition to the vasoconstrictive response mediated by the SNS, as a result of stimulation of the juxtaglomerular complex in the nephron, the release of renin is increased. This leads to further activation of the RAAS, with a synergistic effect of angiotensin and aldosterone on systemic vascular resistance and sodium reabsorption [8]. In a recent study on 211 patients with acute heart failure, RAAS overactivity was linked to worse renal function, diuretic and natriuretic response and poor outcomes [9]. Increased vasopressin levels result in fluid reabsorption and excretion of more concentrated urine. Moreover, inappropriate stimulation of the RAAS axis results in the formation of reactive oxygen species (ROS), caused by activation of nicotinamide adenine dinucleotide phosphate by angiotensin II. In the setting of developing kidney injury, the homeostasis between nitric oxide (NO) and ROS is shifted toward oxidative stress [10]. Physiologically, NO represents a significant role in paracrine and autocrine signaling as a vasodilatative, natriuretic and vascular endothelial growth factor, as well as an inhibitor of smooth muscle cell proliferation, atherogenesis and platelet aggregation [11]. Disproportionate ROS production accelerates atherosclerosis, which is the most common factor leading to the development of heart failure (HF). A combination of these factors contributes to heart and renal tubular cell fibrosis in the long term.
One of the main determinants of renal dysfunction in CRS is elevated venous pressure [12]. An analysis of studies involving hemodynamic measurements provided evidence for the association of elevated right atrial pressure (RAP) and central venous pressure with deterioration of renal function [13,14].
In patients who presented with acute HF, fluid overload manifesting as pulmonary and systemic congestion is the most prevalent concern, causing effort intolerance and edema [15]. Accompanied by elevated central venous pressure, this ominous condition results in renal venous hypertension, increased renal resistance and decreased renal blood flow [16,17]. In the early phase, efferent arteriolar constriction leads to elevated intraglomerular pressure and therefore preserves the filtration fraction. Only after compensatory capacity is depleted, a decrease in glomerular filtration rate (GFR) can be observed. Increased proximal tubular sodium and water reabsorption eventually results in oliguria and worsening congestion. Recently, an experimental, invasive treatment method based on lowering inferior vena cava pressure through a specially designed Doray catheter has emerged. The first in-human study proved the safety of the method and initial results showed an improved diuretic response, opening the way for further research on its efficacy [18]. In similar fashion, elevated intra-abdominal pressures (IAP) observed in HF may contribute to renal dysfunction by reducing the renal filtration gradient and shunting blood from the kidney, thus starting glomerular necrosis, tubular injury and progressing towards renal insufficiency [19] (Figure 1). Since the population with HF is characterized by advanced age and multimorbidity, as well as that chronic kidney disease (CKD) and HF share similar risk factors, patients newly diagnosed with HF are frequently burdened with CKD. Literature data show that CKD has been observed in more than half of chronic HF patients and is an independent risk factor of mortality [20,21]. Often, it is impossible to determine which condition occurred first.
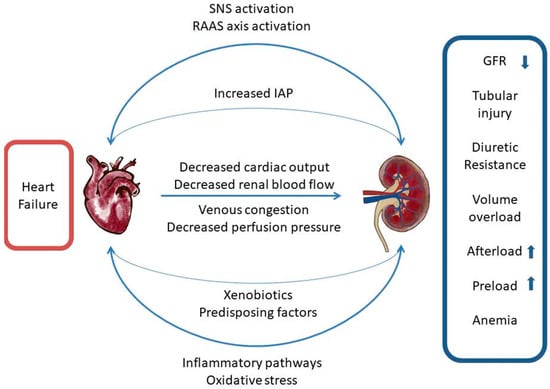
Figure 1.
Cardiorenal network of interactions. SNS—sympathetic nervous system, RAAS—renin-angiotensin-aldosterone system, IAP—intraabdominal pressure, GFR—glomerular filtration rate.
2. Monitoring of the Renal Function
The current European Society of Cardiology (ESC) guidelines recommend only creatinine, urea and natriuresis assessment. There are a number of biomarkers studied for their utility in the evaluation of glomerular and, conventionally omitted from the worsening of renal function (WRF) definition, tubular function. Given the heterogeneous nature of HF (e.g., de novo HF vs. acute decompensated HF (ADHF)), different biomarkers could potentially be applicable in specific clinical situations [22]. Noteworthy, novel, artificial intelligence-based approaches to analyze the nature of WRF in HF have emerged. Clustering, a machine-learning technique that distinguishes smaller subgroups in studied populations, revealed different HF patients phenotypes regarding WRF risk and characteristics [23,24,25].
2.1. Glomerular Filtration
There are numerous definitions of acute kidney injury (Table 2). The traditional approach to monitoring kidney function in HF focused on serial assessments of creatinine levels and estimated glomerular filtration rate (eGFR) calculations. Elevated serum creatinine requires careful evaluation, since it depends on the clinical context and does not always indicate WRF. Creatinine dynamics are characterized by high inertia, meaning that its plasma levels increase when kidney injury is already established. In acute conditions, it can lead to incorrect therapeutic decisions and cessation of therapy [26,27]. Aggressive diuretic treatment, used in ADHF presenting with congestion, can naturally lead to increased creatinine levels by reducing the volume of intravascular fluid and increasing hemoconcentration. Importantly, WRF occurring during decongestive therapy, with achieved target diuresis, natriuresis and clinical improvement is related to a better outcome [28]. This phenomenon has been called pseudo-WRF [29,30]. Moreover, the treatment used for RAAS inhibition may in fact lower baseline glomerular filtration rate (GFR), yet improve long term outcome. In this case, which is particularly true for HF with reduced ejection fraction, benefits outweigh risks of WRF and do not reflect renal injury [31].

Table 2.
Definitions of acute kidney injury.
The gold standard for measuring GFR is using plasma or urinary clearance of an exogenous filtration marker. Traditionally, iothalamate or inulin is used for this purpose, since no renal absorption and secretion occurs [32]. It is a complex procedure that is not routinely performed in everyday clinical practice. Therefore, formulas have been derived to estimate GFR using endogenous molecules and variables such as age, weight, gender and race. A comparative study implicated that none of the creatinine-based methods were accurate in calculating eGFR in HF patients [33]. Depending on patients’ profiles, specific methods of estimating GFR have their advantages and drawbacks. Among commonly used equations, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was proven to be the most accurate in predicting GFR in chronic heart failure, while the Cockcroft–Gault formula has been highlighted to surpass the simplified Modification of Diet in Renal Disease (sMDRD) formula’s ability to predict outcome in heart failure [34,35]. Still, despite the limitations, estimations based on serum creatinine are the most frequently incorporated in clinical practice. Plasma creatinine is a breakdown product of creatine phosphate from muscle and protein metabolism; however, it is subject to error due to dynamic secretion by the renal tubules [36]. Endogenous filtration marker cystatin C (CysC) has now been proposed as a substitute to overcome this issue [37]. CysC is a molecule that functions as a cysteine proteinase inhibitor and is synthetized by nucleated cells at a constant rate. Unlike creatinine, it is freely filtered by the glomeruli and broken down in the process of reabsorption, so its blood level correlates with the glomerular filtration rate. An additional advantage of this measurement is that it is independent of gender, weight, height, muscle mass and age [38]. Nonetheless, both markers can be misinterpreted because their concentrations depend on the individual clinical characteristics of patients. In patients with heart failure, obesity or cachexia may be common factors encountered. Reduced lean muscle mass may lower creatinine levels and lead to overestimation of GFR, while excessive body weight, inflammation and thyroid dysfunction can lead to an increase in cystatin C and underestimation of GFR. [39,40,41]. Creatinine concentration can also vary with diet, particularly protein supply [42]. Given this, assessment should never be made in isolation from the patient’s clinical status. Studies in HF population indicate that CysC might be an accurate tool for prognosis of outcome [43,44].
Another problem of numerous GFR-calculation formulas is their impact on optimizing drug doses. Depending on the study, different GFR cut-off points for safe drug use have been established based on various formulas. In the patients with HF, we most often encounter the problem of existing indications for the use of novel oral anticoagulants. During clinical trials, GFR was mostly estimated based on the Cockcroft–Gault formula, in the setting of stable patients without dynamic changes in renal function. Therefore, eGFR as the sole determinant of renal function is sometimes misleading and requires a broader perspective.
A method that can provide a more accurate assessment of kidney function is the evaluation of renal functional reserve. It is defined as the kidney’s ability to increase glomerular filtration of residual nephrons to maintain GFR under conditions of nephron damage [45]. Physiologically, the kidney can maintain GFR with only 50% of functioning nephrons. Therefore, it can be a precise way to predict the chances for renal recovery in AKI and may provide early detection of renal injury before changes in GFR. There is growing evidence for utility of RFR in risk stratification of developing chronic kidney disease [46]. Yet, the feasibility of this test is limited because it requires complicated protein loading protocols and may be hard to conduct in acute conditions.
2.2. Urine Composition and Biomarkers of Glomerular Integrity
Recently, the focus has been placed on urine composition testing as a material that is simple to collect. The urine analysis provides rapid information on renal function and lacks the inertia that creatinine determinations have. Assessment of electrolyte composition, specifically sodium concentration, is recommended by the current ESC guidelines [47]. It has been proven that spot urinary sodium is not correlated with eGFR, which confirms that it may carry different information that may improve patient profiling [48]. Determination of spot urinary sodium two hours after starting decongestive treatment is recommended to assess the diuretic response [49]. The urinary sodium level in ADHF seems to be the most powerful prognostic tool at admission and during the first days of decongestion [50]. This parameter has gained special importance with reports of better outcome in patients with satisfactory diuretic response and optimal decongestion [51,52,53].
Albuminuria is a simple parameter to assess podocyte integrity and has been used for years in nephrology [54]. When the glomerular membrane becomes damaged, its permeability to large molecules, including various protein fractions, increases [55]. As a result, we can observe a rise in urinary albumin, a parameter conventionally used for monitoring the onset of chronic kidney disease in diabetic patients. Since patients with HF are a group frequently burdened with diabetes or hypertensive nephropathy, microalbuminuria is highly prevalent [56]. It was found to be a powerful independent marker of adverse outcome in chronic HF [57,58].
Urine sediment, being a proven method of diagnosing kidney injury, still remains unexplored in the setting of HF [59]. However, a small study has shown that this simple test can be a helpful tool in predicting AKI [60].
Galectin-3 (gal-3) is a pleiotropic member of the carbohydrate-binding proteins family known as lectins. It exhibits antimicrobial and antifungal activity and takes part in cell adhesion, chemoattraction and cytophysiological processes [61]. It promotes fibrosis, leads to myocardial remodeling and may contribute to heart and kidney failure. Its exact role in the development of HF is still being explored, but several studies have emerged that suggest a link between the aggravated myocardial fibrosis and Gal-3 and stated that it may be a future target for pharmacotherapy [62,63]. The profibrotic properties of gal-3 may be one of the main factors leading to kidney damage [64]. In a study of gal-3 inhibitors conducted on rats with induced hypertension and HF, the efficacy of gal-3 inhibitors in reducing proteinuria and improving their function was proven, resulting in improved renal function [65]. Concerning humans, in a small observation made on 260 patients with chronic heart failure, Gal-3 was linked to 1-year progression of renal dysfunction [66]. Proenkephalin A is an endogenous opioid polypeptide hormone with a cardiodepressive effect, which acts primarily on delta opioid receptors. It is suggested to have a regulatory role in diuresis and natriuresis. A study including 1908 patients indicated its prognostic value for worsening renal function and both in-hospital and 1-year mortality in patients hospitalized for ADHF [67].
2.3. Biomarkers of Tubular Injury
Considering the complex characteristics of renal tubules, it is a challenge to assess their function (Figure 2). This results in a large number of studied biomarkers, which allow early detection of AKI and provide predictive and prognostic value. The assessment of renal tubular function can be a useful tool in differentiating between true and pseudo WRF [68]. Yet, there is no consensus on evaluation of tubular function, and its utility and some assays are used only for research purposes, since they are not available for clinical use.
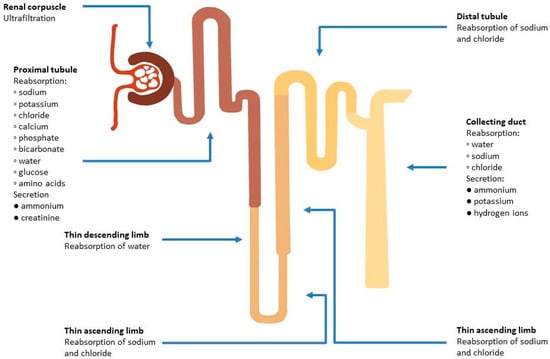
Figure 2.
Functions of different parts of the nephron in maintaining fluid and electrolyte balance.
Neutrophil gelatinase-associated lipocalin (NGAL) is a molecule involved in innate immunity. The primary function of NGAL is in sequestering bacterial siderophores, thus limiting iron usage by bacteria and slowing their growth. It is secreted by neutrophils during bacterial infections and to some extent by organs including the kidney, prostate, gastrointestinal and respiratory tracts [69]. It can be found both in serum and urine and it increases in AKI [70]. There are biased data on the prognostic value of NGAL. Previous observations made on enrolled HF patients suggested an association with WRF and adverse outcome [71,72,73,74,75,76]; however, the largest, multicenter cohort study to date does not support the routine use of serum NGAL for early detection of WRF in AHF treated with diuretic agents. According to the AKINESIS trial, serum NGAL has not been shown to be superior to creatinine in predicting adverse in-hospital outcomes and WRF [77]. On the other hand, studies have implicated that tubular damage might occur in the absence of an increase in serum creatinine, opening up a possibility of early detection of renal injury and timelier deployment of therapy, suggesting that the definition of AKI should be re-assessed [78,79] (Figure 3). A study on urinary NGAL has shown that it can identify high-risk ADHF patients, and predict WRF and early outpatient mortality. Moreover, it has been reported that in a longer, 1-year follow-up period, increased urinary NGAL may predict the risk of HF rehospitalization [80]. It should be noted that NGAL may be overexpressed in conditions of hypertension, hypoxemia, infections, anemia and malignancies, which are greatly prevalent among patients with HF [81,82,83,84,85].
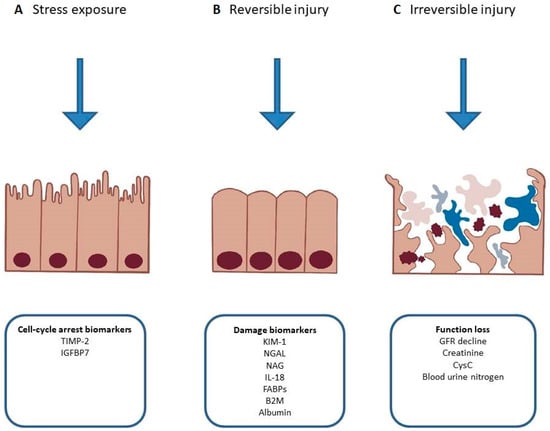
Figure 3.
Phases of acute kidney injury and relevant biomarkers. (A) Release of cell-cycle arrest biomarkers in response to exposure to non-damaging stimuli. (B) Potentially reversible injury resulting in changed metabolism or expression of damage biomarkers. (C) Persistent injury resulting in necrosis, apoptosis and kidney dysfunction. TIMP-2—tissue inhibitor of metalloproteinase 2, IGFBP7—insulin-like growth factor-binding protein 7, KIM-1—kidney injury molecule 1, NGAL—neutrophil gelatinase-associated lipocalin, NAG—N-acetyl-ß-d-glucosaminidase, IL-18—interleukin-18, FABPs—fatty acid-binding proteins, B2M—beta-2 microglobulin, GFR—glomerular filtration rate, CysC—cystatin C.
Kidney Injury Molecule 1 (KIM-1) is a biomarker expressed by the nephron in response to ischemic kidney injury [86]. Urinary KIM-1 has been proven to be a sensitive and specific marker for early AKI and may be useful in differentiating between acute tubular necrosis and other forms of renal injury [87]. Some studies suggest that elevated uKIM1 can indicate tubular damage in states of both acute and chronic heart failure and may be utilized in the prediction of CRS [88,89]. Data on the prognostic value of serum KIM-1 in HF are scarce; however, the study designed for investigation of KIM-1 utility in both chronic and acute HF showed that serum KIM-1 is only moderately associated with clinical outcome [90]. Authors of the Ascend-HF study suggest that pathophysiologic process involved in ADHF, i.e., venous congestion and decreased cardiac output, may affect glomerular filtration and tubular injury and may not be as severe as previously thought [91]. Conclusions from smaller study focused on possible biomarker utility in CRS, stated that uKIM-1 levels were strongly correlated with congestion, serum NT-proBNP, left ventricle dysfunction and NYHA stage and in a 1-year follow-up, KIM-1 was associated with lower overall survival in chronic heart failure [92].
In the same study, similar observations were made for other biomarker, N-acetyl-ß-d-glucosaminidase (NAG) [92]. NAG is a high molecular weight biomarker which originates for proximal tubular cells and serves as a lysosomal enzyme. In addition to the aforementioned study, it is suggested to be significant predictor of all-cause mortality in chronic HF based on 10-year follow-up [93].
Interleukin-18 (IL-18), known as interferon-gamma inducing factor, is a proinflammatory cytokine produced by both hematopoietic and non-hematopoietic cells, but is mainly associated with macrophages [94]. In the kidney injury, it is released from the proximal tubule [95]. A prospective, multicenter study in the setting of ADHF showed that elevated urinary IL-18 was associated with a 3.6-fold risk of AKI compared with lower levels in the adjusted analysis [96].
Angiotensinogen, which belongs to the family of serpin proteins, is a globulin synthesized in the liver. In study mentioned above, it presented the strongest predictor of the progression of AKI and worsening of AKI with death in ADHF patients [96].
Uromodulin or Tamm–Horsfall protein is a kidney specific protein produced by cells of the thick ascending limbs and early distal convoluted tubules, which is found in urine in polymerized and non-polymerized forms under normal circumstances. So far its several important functions have been identified including ion transportation in the ascending limb in nephrons, fluid and electrolyte balance, prevention of kidney stones and innate immunity of the urinary tract [97]. Moreover, mutations in the uromodulin gene (UMOD) cause autosomal dominant tubulointerstitial kidney disease or medullary cystic kidney disease and some variants are associated with the development of hypertension and chronic kidney disease, which proves its important role in the correct development and functioning of the kidney [98,99,100]. LaFavers’ review paper takes a look at new findings on physiological concepts surrounding uromodulin, the separate regulatory pathways for its different forms, and it provides recommendations for reporting uromodulin levels [101]. A study conducted by M. Pruijm et al. confirmed the utility of uromodulin as a biomarker of tubular function, and large study on more than 3000 patients undergoing coronary angiography further proved its value in prognosis of all-cause mortality [102,103]. Another novel biomarker alpha-1 microglobulin (A1M) is a small globular protein synthesized by the liver, which is typically entirely reabsorbed by the renal tubule. In vivo, it has an antioxidant function [104]. The concentration of both of the molecules was observed to rise in the presence of tubular cell damage. Independently from GFR, both low levels of urine uromodulin and high levels of urine A1M may be associated with a greater risk of developing cardiovascular disease and mortality [105].
Tissue inhibitor of metalloproteinases 2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) are cellular stress biomarkers, involved in the cell cycle arrest in the G1 phase. By inhibition of cell division, they prevent the multiplication of damaged DNA in the case of cellular injury. Both are secreted by renal tubules—TIMP2 mainly by distal tubule cells, while IGFBP7 is primarily secreted by proximal tubule cells [106]. TIMP-2 and IGFBP7 activity is increased in harmful conditions such as ischemia, oxidative stress, inflammation and others. Noteworthy, the TIMP2/IGFBP7 ratio is a part of the commercially available NephroCheck Test. In a retrospective analysis, it was shown that it can predict the occurrence of AKI and is more reliable compared to CysC, NGAL, KIM-1, liver fatty acid-binding protein and IL-18. The multicenter, randomized controlled trial has proven that monitoring with these markers and appropriate treatment management can lead to a reduction in AKI [107].
Fatty acid-binding proteins (FABPs) are a family taking part in fatty acid transport and metabolism. With ongoing research, more proteins belonging to this family are being discovered. In this review, two site-specific FABPs will be discussed. Heart FABP, also called FABP-3, mainly presents inside cardiomyocytes, skeletal muscles and kidney together with liver FABP, known under the name of FABP-1, located in the hepatocytes and intestines. Urinary levels of each of them have been proven to rise with ischemic tubular injury and have been associated with an increased risk of AKI development [108,109,110]. In addition, the results of a study highlight the association between elevated heart FABP and the risk of adverse outcome in patients with chronic heart failure [108].
Beta-2 microglobulin (B2M) is a component of major histocompatibility complex class I molecules encoded by the B2M gene and secreted by nucleated cells at a constant rate. Urinary levels of B2M rise in tubular injury due to reduced reabsorption in the proximal tubule. Results from study on rat models showed an early increase in B2M in AKI [111]. Moreover, it is a marker independent of muscle mass, sex, age and race. In comparison to eGFR it appears to have less specificity and better sensitivity for prognosis of deteriorated renal function at one year [112]. Combined evidence presented in a meta-analysis suggested that B2M may be also linked to an increased risk of cardiovascular disease [113].
An overview of characteristics, strengths and limitations of selected tubular injury biomarkers is provided in Table 3.

Table 3.
Characteristics of tubular injury biomarkers.
2.4. Biomarkers of Endothelial Dysfunction
Endothelial dysfunction remains in the spotlight because of its central role in the pathogenesis of cardiovascular and renal diseases and the crosstalk between the heart and the kidney. It is one of the important contributors in the development of CRS, not only leading to the progression of atherosclerotic lesions, but also involved in kidney injury [114]. Various markers of endothelial dysfunction have been identified over the years, including endothelin-1, matrix metalloproteinases, tissue inhibitor of metalloproteinases, angiopoietin-like 2, endoglin, homocysteine or endothelial cell microparticles [115]. While most of them still possess numerous limitations that hinder clinical application, syndecan-1 is a biomarker of endothelial dysfunction that has found particular use in assessing renal function. It is a transmembrane proteoglycan, which reflects endothelial glycocalyx damage. In vivo glycocalyx is an ultra-thin carbohydrate-rich structure which provides protection to the vascular endothelium due to its anticoagulant and antiadhesive properties. In a pioneer study in ADHF patients, the concentration of syndecan-1 at admission was predictive for in-hospital AKI and mortality and was strongly associated with 6-month mortality [116].
2.5. Renal Imaging
We should keep in mind that renal imaging techniques bring a spectrum of possibilities in exploring pathophysiological pathways, and structural and functional alterations, as its capabilities continue to expand. Renal imaging complements laboratory data and may provide early diagnosis of WRF and prognostic information. (Table 4).

Table 4.
Comparison of renal imaging modalities.
2.5.1. Ultrasonography
Renal ultrasonography is a routine examination used in nephrology. The ability to distinguish between the fibrosis and the inflammation and to differentiate between underlying pathophysiologic processes in a standard ultrasound is limited [117]. In addition to the standard structural assessment, Doppler ultrasonography can be used to quantify renal blood flow and hemodynamics. Intrarenal venous Doppler shows the waveform of blood flow in the interlobal veins of the kidney. It is dependent on the RAP and IAP. As the RAP increases, the pattern of the Doppler spectrum changes. In physiological conditions, the waveform is continuous, while in the case of venous congestion, the flow changes to pulsatile and the spectrum splits into biphasic, in which the systolic and diastolic phases can be distinguished. With a further increase in RAP, only monophasic, diastolic flow is observed. Association with RAP was confirmed in patients with HF who underwent right heart catheterization [118]. The abovementioned patterns may be used to evaluate response to diuretic therapy and guide decongestion. An observation made in the comparison study of patients with HF and healthy subjects indicated that venous impedance index, calculated based on intrarenal venous Doppler, was significantly increased in HF and could be restored to normal after loop diuretic administration [119]. Doppler-enhanced venous flow studies reflect renal congestion and might bring important prognostic information and optimize decongestion strategies in patients with chronic HF [120]. The utility of such measurements has not yet been confirmed in large studies, but it is currently a topic of interest to researchers [121,122]. Assessment of the renal resistive index can provide prognostic information in various scenarios. In animal models, contrast-enhanced ultrasonography was proven to be a valuable tool for evaluation of renal perfusion and microvascular function [123]. In the near future, this method may be implemented in daily practice to diagnose and monitor AKI.
2.5.2. Computed Tomography (CT)
CT is a method that allows structural and functional evaluation of the kidney, but has some limitations that preclude its use in serial evaluation. Currently, it is not feasible for serial assessment of the kidney in patients with renal failure due to the risk of inducing post-contrast nephropathy and exposure to high doses of radiation [124].
2.5.3. Magnetic Resonance Imaging (MRI)
MRI compared to CT is an examination that avoids radiation exposure; however, complications associated with gadolinium-based contrast and lower availability should be taken into account [125]. Imaging with this technique uses a strong magnetic field, which can be a relative contraindication for selected HF patients, for instance, those who have a non MRI-friendly cardioverter-defibrillator implanted. MRI may provide various microstructural and functional information about the kidney, depending on the used sequence. In recent years, the literature on the advances in the use of MRI for renal assessment is rapidly expanding. The progress that has been made resulted in the formulation of general principles for the acquisition, processing, and analysis of data to promote standardization and facilitate clinical implementation of further explained techniques [126,127,128,129,130].
T1 and T2 mapping, diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) are sequences used for the evaluation of fibrotic alterations in the kidney. To date, only a few pioneer studies in animals and humans pointed out that functional MRI could be a prognostic tool for AKI and progression of CKD [131,132,133]. This subject still requires further research.
Arterial spin labeling (ASL) is a technique that uses labeled water tracing for regional perfusion mapping. Although it is mostly restricted to research settings, the latest studies have stated that cortical perfusion decreases in CKD proportionally to stages of CKD and correlates to eGFR [134].
Blood oxygen level dependent (BOLD) imaging is a technique, which relies on paramagnetic properties of deoxyhemoglobin and T2-weighted sequence. BOLD allows identification of renal hypoxia and requires no contrast. It remains a diagnostic tool used mainly in scientific research due to difficulties in analyzing real-time estimated oxygen delivery and consumption. The relation between renal T2* and renal tissue pO2, using gold-standard invasive physiological measurements, has been proven to be highly correlated in an animal model [135]. Increasing experimental and clinical data indicate that reduced renal oxygenation and mitochondrial dysfunction are significant contributors to AKI development and CKD progression [136,137]. Moreover, the latest studies showed that monitoring urine oxygenation may be a useful predictor for AKI development in septic and cardiac surgery patients [138,139]. Hence, it is imperative to study the pathophysiology behind this phenomenon further, identify biomarkers of renal hypoxia and improve techniques that will allow for accurate assessment of renal oxygenation. Novel therapies to restore the integrity of oxygen delivery and consumption may improve clinical outcomes. Currently, the activation of hypoxia-inducible factor-1α has been demonstrated to have a beneficial effect on renal function and metabolism [140].
2.5.4. Nuclear Imaging
Renal nuclear imaging is a frequently used modality for renal assessment. Most commonly, it is based on 99mtechnetium bound to non-metabolized molecules with known pharmacokinetics; in this case, dimethylenetriaminepentaacetic (DTPA) and acidmercaptoacetyltriglycine (MAG3) are the tracers of choice for evaluation of GFR and RBF. This method has good availability and reproducibility; however, it is currently not applicable in monitoring renal function due to high radiation doses and poor imaging characteristics [141].
Positron emission tomography (PET) is characterized by higher resolution compared to conventional scintigraphy, and combined with CT may provide additional data on kidney composition and function. At this time, nuclear imaging of the kidney is used mainly for research purposes.
3. Intra-Abdominal Pressure
Increased IAP has received scientific attention since the 19th century [142]. Not long after this phenomenon was described, the first reports appeared regarding its adverse effects on organ function. The accumulation of evidence throughout the last decades has led to a deeper understanding of the underlying pathophysiology and its impact on homeostasis. In 2004, the World Society for Abdominal Compartment Syndrome (WSACS) was established to provide unified definitions, promote research in this field and improve management. According to the created guidelines, the normal IAP in critically ill adults oscillates between 5 mmHg to 7mmHg; intra-abdominal hypertension (IAH) is defined as a sustained or repeated rise in IAP above 12 mmHg. The most ominous condition associated with elevated IAP is an abdominal compartment syndrome, which can be diagnosed with sustained IAP range above 20 mmHg, accompanied by newly diagnosed organ dysfunction or failure [143]. Because of the possible negative impact on every system in the human body and the risk of developing multiple organ failure, such patients are at the highest risk of poor outcomes [144,145]. It has been proven that this phenomenon is widespread in the population of patients hospitalized in intensive care units and represents an independent predictor for mortality and prolonged stay in the hospital. Considering the fact that the kidneys are the most vulnerable to IAP fluctuations, their evaluation is a valuable tool for monitoring in CRS. The WSACS recommends recording IAP by transurethral measurement using a Foley catheter, which is low cost, simple and reliable. This method has been validated in the setting of ADHF [146]. Elevated IAP leads to reduced renal blood flow, renal venous congestion and parenchymal compression, increased pressure in the proximal tubule and reduced renal filtration gradient [11]. As a result, the RAAS is chronically activated and a large decrease in urine sodium concentration may be observed, which contributes to the development of diuretic resistance and further disturbs the fluid balance [49,147]. The effect of IAH highly depends on its severity. With values above 15 mmHg, reduced renal filtration pressure leads to oliguria, while anuria is observed at a pressure of 30 mmHg. Furthermore, an IAP of 20 mmHg causes an increase in renal vascular resistance by 500%, shunting blood from the kidney and resulting in glomerular necrosis and tubular damage [148]. In the population admitted for management of ADHF, a high prevalence of elevated IAP was found, which was strongly correlated with impairment of renal function [149]. In the case of diagnosed IAH, it is recommended to implement an appropriate treatment, perform serial measurements every 4 h and keep a close observation for the occurrence of abdominal compartment syndrome (Figure 4).
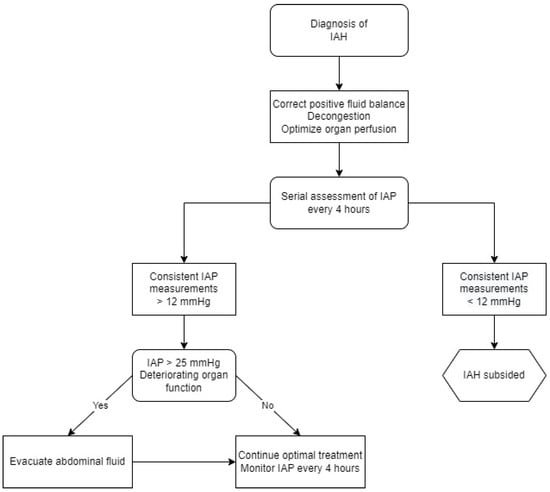
Figure 4.
Management algorithm for intra-abdominal hypertension. IAP—intra-abdominal pressure, IAH—intra-abdominal hypertension.
4. Conclusions
The close physiological relationship of the heart to the kidney puts each HF patient at risk of developing renal failure. The proper phenotyping of patients with cardio-renal syndrome, due to their multimorbidity and heterogeneity, requires an individual approach that often goes beyond guidelines. The presented methods of diagnosing and monitoring renal function provide additional information on the actual state of the kidneys, allowing early detection, characterization of the cause and course of WRF. With the emergence of new studies on novel biomarkers of prognostic potential and the introduction of multimarker testing, we also have the opportunity for better risk stratification and timed interventions. New approaches to renal assessment will certainly be incorporated into management strategies in the near future to improve CRS care.
Author Contributions
Conceptualization, P.Ł., J.B., S.U. and R.Z.; formal analysis, P.Ł.; writing—original draft preparation, P.Ł. and R.Z.; writing—review and editing, P.Ł., J.B., S.U. and R.Z.; visualization, P.Ł.; supervision, R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Guazzi, M.; Testani, J.M.; Borlaug, B.A. Altered Hemodynamics and End-Organ Damage in Heart Failure. Circulation 2020, 142, 98–1012. [Google Scholar] [CrossRef]
- Hsu, S.; Fang, J.C.; Borlaug, B.A. Hemodynamics for the Heart Failure Clinician: A State-of-the-Art Review. J. Card. Fail. 2022, 28, 133–148. [Google Scholar] [CrossRef]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2016, 14, 30–38. [Google Scholar] [CrossRef]
- Jay, C.N.; Barry, L.T.; Maria Teresa, O.; Victoria, G.; Dennis, L.; Gary, F.S.; Ada, S.B.; Thomas, R. The Autonomic Nervous System and Heart Failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef]
- Goldsmith, S.R. The role of vasopressin in congestive heart failure. Clevel. Clin. J. Med. 2006, 73 (Suppl. S3), S19–S23. [Google Scholar] [CrossRef]
- Guzik, M.; Sokolski, M.; Hurkacz, M.; Zdanowicz, A.; Iwanek, G.; Marciniak, D.; Zymliński, R.; Ponikowski, P.; Biegus, J. Serum Osmolarity and Vasopressin Concentration in Acute Heart Failure—Influence on Clinical Course and Outcome. Biomedicines 2022, 10, 2034. [Google Scholar] [CrossRef]
- Fountain, J.H.; Lappin, S.L. Physiology, Renin Angiotensin System; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Biegus, J.; Nawrocka-Millward, S.; Zymliński, R.; Fudim, M.; Testani, J.; Marciniak, D.; Rosiek-Biegus, M.; Ponikowska, B.; Guzik, M.; Garus, M.; et al. Distinct renin/aldosterone activity profiles correlate with renal function, natriuretic response, decongestive ability and prognosis in acute heart failure. Int. J. Cardiol. 2021, 345, 54–60. [Google Scholar] [CrossRef]
- Giam, B.; Kaye, D.M.; Rajapakse, N.W. Role of Renal Oxidative Stress in the Pathogenesis of the Cardiorenal Syndrome. Heart Lung Circ. 2016, 25, 874–880. [Google Scholar] [CrossRef]
- Liu, V.W.T.; Huang, P.L. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008, 77, 19–29. [Google Scholar] [CrossRef]
- Biegus, J.; Ponikowski, P. Cardio-renal syndrome in patients with heart failure: Pathophysiology, epidemiology and clinical significance. Kardiol. Pol. 2011, 69, 1181–1188. [Google Scholar]
- Nohria, A.; Hasselblad, V.; Stebbins, A.; Pauly, D.F.; Fonarow, G.C.; Shah, M.; Yancy, C.W.; Califf, R.M.; Stevenson, L.W.; Hill, J.A. Cardiorenal Interactions: Insights From the ESCAPE Trial. J. Am. Coll. Cardiol. 2008, 51, 1268–1274. [Google Scholar] [CrossRef]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef]
- Zymliński, R.; Biegus, J.; Ponikowski, P. Not all fluid overloads are the same: Some practical considerations for better decongestion. Eur. J. Heart Fail. 2021, 23, 1106–1109. [Google Scholar] [CrossRef]
- Uthoff, H.; Breidthardt, T.; Klima, T.; Aschwanden, M.; Arenja, N.; Socrates, T.; Heinisch, C.; Noveanu, M.; Frischknecht, B.; Baumann, U.; et al. Central venous pressure and impaired renal function in patients with acute heart failure. Eur. J. Heart Fail. 2011, 13, 432–439. [Google Scholar] [CrossRef]
- Redant, S.; Honoré, P.M.; Bels, D. De Fifty Shades of Central Venous Pressure in the Cardiorenal Syndrome. J. Transl. Intern. Med. 2020, 8, 1. [Google Scholar] [CrossRef]
- Zymliński, R.; Dierckx, R.; Biegus, J.; Vanderheyden, M.; Bartunek, J.; Ponikowski, P. Novel IVC Doraya Catheter Provides Congestion Relief in Patients With Acute Heart Failure. Basic Transl. Sci. 2022, 7, 326–327. [Google Scholar] [CrossRef]
- Łagosz, P.; Sokolski, M.; Biegus, J.; Tycinska, A.; Zymlinski, R. Elevated intra-abdominal pressure: A review of current knowledge. World J. Clin. Cases 2022, 10, 3005. [Google Scholar] [CrossRef]
- McCullough, P.A. Cardiorenal syndromes: Pathophysiology to prevention. Int. J. Nephrol. 2010, 2011, 1–10. [Google Scholar] [CrossRef]
- Damman, K.; Valente, M.A.E.; Voors, A.A.; O’Connor, C.M.; Van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef]
- Nawrocka-Millward, S.; Biegus, J.; Hurkacz, M.; Guzik, M.; Rosiek-Biegus, M.; Jankowska, E.A.; Ponikowski, P.; Zymliński, R. Differences in the biomarker profile of de novo acute heart failure versus decompensation of chronic heart failure. Biomolecules 2021, 11, 1701. [Google Scholar] [CrossRef]
- Urban, S.; Błaziak, M.; Jura, M.; Iwanek, G.; Ponikowska, B.; Horudko, J.; Siennicka, A.; Berka, P.; Biegus, J.; Ponikowski, P.; et al. Machine Learning Approach to Understand Worsening Renal Function in Acute Heart Failure. Biomolecules 2022, 12, 1616. [Google Scholar] [CrossRef]
- Yagi, R.; Takei, M.; Kohsaka, S.; Shiraishi, Y.; Ikemura, N.; Shoji, S.; Niimi, N.; Higuchi, S.; Goda, A.; Kohno, T.; et al. Phenomapping in patients experiencing worsening renal function during hospitalization for acute heart failure. ESC Hear Fail. 2021, 8, 5192–5203. [Google Scholar] [CrossRef]
- Zdanowicz, A.; Urban, S.; Ponikowska, B.; Iwanek, G.; Zymliński, R.; Ponikowski, P.; Biegus, J. Novel Biomarkers of Renal Dysfunction and Congestion in Heart Failure. J. Pers. Med. 2022, 12, 898. [Google Scholar] [CrossRef]
- Zannad, F.; Rossignol, P. Cardiorenal Syndrome Revisited. Circulation 2018, 138, 929–944. [Google Scholar] [CrossRef]
- Rossignol, P.; Hernandez, A.F.; Solomon, S.D.; Zannad, F. Heart failure drug treatment. Lancet 2019, 393, 1034–1044. [Google Scholar] [CrossRef]
- Breidthardt, T.; Weidmann, Z.M.; Twerenbold, R.; Gantenbein, C.; Stallone, F.; Rentsch, K.; Rubini Gimenez, M.; Kozhuharov, N.; Sabti, Z.; Breitenbücher, D.; et al. Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur. J. Heart Fail. 2017, 19, 226–236. [Google Scholar] [CrossRef]
- Sokolski, M.; Zymliński, R.; Sokolska, J.M.; Biegus, J.; Banasiak, W.; Ponikowski, P. True worsening renal function identifies patients with acute heart failure with an ominous outcome. Pol. Arch. Intern. Med. 2019, 129, 357–360. [Google Scholar] [CrossRef]
- Ahmad, T.; Jackson, K.; Rao, V.S.; Tang, W.H.W.; Brisco-Bacik, M.A.; Chen, H.H.; Felker, G.M.; Hernandez, A.F.; O’Connor, C.M.; Sabbisetti, V.S.; et al. Worsening Renal Function in Patients With Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated With Tubular Injury. Circulation 2018, 137, 2016–2028. [Google Scholar] [CrossRef]
- Beldhuis, I.E.; Streng, K.W.; Ter Maaten, J.M.; Voors, A.A.; Van Der Meer, P.; Rossignol, P.; McMurray, J.J.V.; Damman, K. Renin-Angiotensin System Inhibition, Worsening Renal Function, and Outcome in Heart Failure Patients With Reduced and Preserved Ejection Fraction: A Meta-Analysis of Published Study Data. Circ. Heart Fail. 2017, 10, e003588. [Google Scholar] [CrossRef]
- Levey, A.S.; Inker, L.A. GFR as the “gold Standard”: Estimated, Measured, and True. Am. J. Kidney Dis. 2016, 67, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.; Viklund, I.; Jonsson, A.; Valham, F.; Bergdahl, E.; Lindmark, K.; Norberg, H. Comparison of creatinine-based methods for estimating glomerular filtration rate in patients with heart failure. ESC Heart Fail. 2020, 7, 1150. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Girerd, N.; Pellicori, P.; Duarte, K.; Girerd, S.; Pfeffer, M.A.; McMurray, J.J.V.; Pitt, B.; Dickstein, K.; Jacobs, L.; et al. Renal function estimation and Cockroft-Gault formulas for predicting cardiovascular mortality in population-based, cardiovascular risk, heart failure and post-myocardial infarction cohorts: The Heart “OMics” in AGEing (HOMAGE) and the high-risk myocardial infarction database initiatives. BMC Med. 2016, 14, 1–14. [Google Scholar] [CrossRef]
- Weidmann, Z.M.; Breidthardt, T.; Twerenbold, R.; Züsli, C.; Nowak, A.; Von Eckardstein, A.; Erne, P.; Rentsch, K.; De Oliveira, M.T.; Gualandro, D.; et al. Prediction of mortality using quantification of renal function in acute heart failure. Int. J. Cardiol. 2015, 201, 650–657. [Google Scholar] [CrossRef]
- Smilde, T.D.J.; Van Veldhuisen, D.J.; Navis, G.; Voors, A.A.; Hillege, H.L. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation 2006, 114, 1572–1580. [Google Scholar] [CrossRef]
- Kar, S.; Paglialunga, S.; Islam, R. Cystatin C Is a More Reliable Biomarker for Determining eGFR to Support Drug Development Studies. J. Clin. Pharmacol. 2018, 58, 1239–1247. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Kwon, C.; Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am. J. Kidney Dis. 2002, 40, 221–226. [Google Scholar] [CrossRef]
- Dedual, M.A.; Wueest, S.; Challa, T.D.; Lucchini, F.C.; Aeppli, T.R.J.; Borsigova, M.; Mauracher, A.A.; Vavassori, S.; Schmid, J.P.; Blüher, M.; et al. Obesity-Induced Increase in Cystatin C Alleviates Tissue Inflammation. Diabetes 2020, 69, 1927–1935. [Google Scholar] [CrossRef]
- Singh, D.; Whooley, M.A.; Ix, J.H.; Ali, S.; Shlipak, M.G. Association of cystatin C and estimated GFR with inflammatory biomarkers: The Heart and Soul Study. Nephrol. Dial. Transplant. 2007, 22, 1087–1092. [Google Scholar] [CrossRef]
- Pricker, M.; Wiesli, P.; Brändle, M.; Schwegler, B.; Schmid, C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003, 63, 1944–1947. [Google Scholar] [CrossRef]
- Pimenta, E.; Jensen, M.; Jung, D.; Schaumann, F.; Boxnick, S.; Truebel, H. Effect of Diet on Serum Creatinine in Healthy Subjects During a Phase I Study. J. Clin. Med. Res. 2016, 8, 836. [Google Scholar] [CrossRef]
- Lassus, J.; Harjola, V.P.; Sund, R.; Siirilä-Waris, K.; Melin, J.; Peuhkurinen, K.; Pulkki, K.; Nieminen, M.S. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur. Heart J. 2007, 28, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, T.; Takeishi, Y.; Niizeki, T.; Takabatake, N.; Okuyama, H.; Fukui, A.; Tachibana, H.; Nozaki, N.; Hirono, O.; Tsunoda, Y.; et al. Cystatin C, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J. Card. Fail. 2005, 11, 595–601. [Google Scholar] [CrossRef]
- Jufar, A.H.; Lankadeva, Y.R.; May, C.N.; Cochrane, A.D.; Bellomo, R.; Evans, R.G. Renal functional reserve: From physiological phenomenon to clinical biomarker and beyond. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 319, R690–R702. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mucino, M.J.; Ronco, C. Renal Functional Reserve and Renal Recovery after Acute Kidney Injury. Nephron. Clin. Pract. 2014, 127, 94–100. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failureDeveloped by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Biegus, J.; Zymliński, R.; Testani, J.; Marciniak, D.; Zdanowicz, A.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Renal profiling based on estimated glomerular filtration rate and spot urine sodium identifies high-risk acute heart failure patients. Eur. J. Heart Fail. 2021, 23, 729–739. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Biegus, J.; Zymliński, R.; Fudim, M.; Testani, J.; Sokolski, M.; Marciniak, D.; Ponikowska, B.; Guzik, M.; Garus, M.; Urban, S.; et al. Spot urine sodium in acute heart failure: Differences in prognostic value on admission and discharge. ESC Heart Fail. 2021, 8, 2597–2602. [Google Scholar] [CrossRef] [PubMed]
- Biegus, J.; Zymliński, R.; Sokolski, M.; Todd, J.; Cotter, G.; Metra, M.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur. J. Heart Fail. 2019, 21, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Ter Maaten, J.M.; Dunning, A.M.; Valente, M.A.E.; Damman, K.; Ezekowitz, J.A.; Califf, R.M.; Starling, R.C.; Van Der Meer, P.; O’Connor, C.M.; Schulte, P.J.; et al. Diuretic response in acute heart failure-an analysis from ASCEND-HF. Am. Heart J. 2015, 170, 313–321.e4. [Google Scholar] [CrossRef]
- Valente, M.A.E.; Voors, A.A.; Damman, K.; Van Veldhuisen, D.J.; Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur. Heart J. 2014, 35, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Redon, J. Measurement of microalbuminuria—What the nephrologist should know. Nephrol. Dial. Transplant. 2006, 21, 573–576. [Google Scholar] [CrossRef]
- Goffredo, G.; Barone, R.; Di Terlizzi, V.; Correale, M.; Brunetti, N.D.; Iacoviello, M. Biomarkers in Cardiorenal Syndrome. J. Clin. Med. 2021, 10, 3433. [Google Scholar] [CrossRef]
- Van De Wal, R.M.A.; Asselbergs, F.W.; Plokker, H.W.T.; Smilde, T.D.J.; Lok, D.; Van Veldhuisen, D.J.; Van Gilst, W.H.; Voors, A.A. High prevalence of microalbuminuria in chronic heart failure patients. J. Card. Fail. 2005, 11, 602–606. [Google Scholar] [CrossRef]
- Masson, S.; Latini, R.; Milani, V.; Moretti, L.; Rossi, M.G.; Carbonieri, E.; Frisinghelli, A.; Minneci, C.; Valisi, M.; Maggioni, A.P.; et al. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: Data from the GISSI-Heart Failure trial. Circ. Heart Fail. 2010, 3, 65–72. [Google Scholar] [CrossRef]
- Jackson, C.E.; Solomon, S.D.; Gerstein, H.C.; Zetterstrand, S.; Olofsson, B.; Michelson, E.L.; Granger, C.B.; Swedberg, K.; Pfeffer, M.A.; Yusuf, S.; et al. Albuminuria in chronic heart failure: Prevalence and prognostic importance. Lancet 2009, 374, 543–550. [Google Scholar] [CrossRef]
- Perazella, M.A.; Coca, S.G.; Kanbay, M.; Brewster, U.C.; Parikh, C.R. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 1615–1619. [Google Scholar] [CrossRef]
- Higuchi, S.; Kabeya, Y.; Matsushita, K.; Yamasaki, S.; Ohnishi, H.; Yoshino, H. Urinary cast is a useful predictor of acute kidney injury in acute heart failure. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Seropian, I.M.; Cerliani, J.P.; Toldo, S.; Van Tassell, B.W.; Ilarregui, J.M.; González, G.E.; Matoso, M.; Salloum, F.N.; Melchior, R.; Gelpi, R.J.; et al. Galectin-1 controls cardiac inflammation and ventricular remodeling during acute myocardial infarction. Am. J. Pathol. 2013, 182, 29–40. [Google Scholar] [CrossRef]
- De Boer, R.A.; Voors, A.A.; Muntendam, P.; Van Gilst, W.H.; Van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817. [Google Scholar] [CrossRef]
- Chen, S.C.; Kuo, P.L. The Role of Galectin-3 in the Kidneys. Int. J. Mol. Sci. 2016, 17, 565. [Google Scholar] [CrossRef] [PubMed]
- Frenay, A.R.S.; Yu, L.; van der Velde, A.R.; Vreeswijk-Baudoin, I.; López-Andrés, N.; van Goor, H.; Silljé, H.H.; Ruifrok, W.P.; de Boer, R.A. Pharmacological inhibition of galectin-3 protects against hypertensive nephropathy. Am. J. Physiol. Renal Physiol. 2015, 308, F500–F509. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, M.; Di Serio, F.; Rizzo, C.; Leone, M.; Grande, D.; Guida, P.; Gioia, M.I.; Parisi, G.; Leopizzi, T.; Caldarola, P.; et al. Association between high Gal-3 serum levels and worsening of renal function in chronic heart failure outpatients. Biomark. Med. 2019, 13, 707–713. [Google Scholar] [CrossRef]
- Ng, L.L.; Squire, I.B.; Jones, D.J.L.; Cao, T.H.; Chan, D.C.S.; Sandhu, J.K.; Quinn, P.A.; Davies, J.E.; Struck, J.; Hartmann, O.; et al. Proenkephalin, Renal Dysfunction, and Prognosis in Patients With Acute Heart Failure: A GREAT Network Study. J. Am. Coll. Cardiol. 2017, 69, 56–69. [Google Scholar] [CrossRef]
- Sokolski, M.; Zymliński, R.; Biegus, J.; Siwołowski, P.; Nawrocka-Millward, S.; Todd, J.; Yerramilli, M.R.; Estis, J.; Jankowska, E.A.; Banasiak, W.; et al. Urinary levels of novel kidney biomarkers and risk of true worsening renal function and mortality in patients with acute heart failure. Eur. J. Heart Fail. 2017, 19, 760–767. [Google Scholar] [CrossRef]
- Buonafine, M.; Martinez-Martinez, E.; Jaisser, F. More than a simple biomarker: The role of NGAL in cardiovascular and renal diseases. Clin. Sci. 2018, 132, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Ott, K.M.; Mori, K.; Jau, Y.L.; Kalandadze, A.; Cohen, D.J.; Devarajan, P.; Barasch, J. Dual action of neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol. 2007, 18, 407–413. [Google Scholar] [CrossRef]
- Bolignano, D.; Basile, G.; Parisi, P.; Coppolino, G.; Nicocia, G.; Buemi, M. Increased Plasma Neutrophil Gelatinase-Associated Lipocalin Levels Predict Mortality in Elderly Patients with Chronic Heart Failure. Rejuvenation Res. 2009, 12, 7–13. [Google Scholar] [CrossRef]
- Aghel, A.; Shrestha, K.; Mullens, W.; Borowski, A.; Tang, W.H.W. Serum Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Predicting Worsening Renal Function in Acute Decompensated Heart Failure. J. Card. Fail. 2010, 16, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Alvelos, M.; Lourenço, P.; Dias, C.; Amorim, M.; Rema, J.; Leite, A.B.; Guimarães, J.T.; Almeida, P.; Bettencourt, P. Prognostic value of neutrophil gelatinase-associated lipocalin in acute heart failure. Int. J. Cardiol. 2013, 165, 51–55. [Google Scholar] [CrossRef]
- Nymo, S.H.; Ueland, T.; Askevold, E.T.; Flo, T.H.; Kjekshus, J.; Hulthe, J.; Wikstrand, J.; McMurray, J.; Van Veldhuisen, D.J.; Gullestad, L.; et al. The association between neutrophil gelatinase-associated lipocalin and clinical outcome in chronic heart failure: Results from CORONA*. J. Intern. Med. 2012, 271, 436–443. [Google Scholar] [CrossRef]
- Maisel, A.S.; Mueller, C.; Fitzgerald, R.; Brikhan, R.; Hiestand, B.C.; Iqbal, N.; Clopton, P.; Van Veldhuisen, D.J. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: The NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur. J. Heart Fail. 2011, 13, 846–851. [Google Scholar] [CrossRef]
- Van Deursen, V.M.; Damman, K.; Voors, A.A.; Van Der Wal, M.H.; Jaarsma, T.; Van Veldhuisen, D.J.; Hillege, H.L. Prognostic value of plasma neutrophil gelatinase-associated lipocalin for mortality in patients with heart failure. Circ. Heart Fail. 2014, 7, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.S.; Wettersten, N.; van Veldhuisen, D.J.; Mueller, C.; Filippatos, G.; Nowak, R.; Hogan, C.; Kontos, M.C.; Cannon, C.M.; Müller, G.A.; et al. Neutrophil Gelatinase-Associated Lipocalin for Acute Kidney Injury During Acute Heart Failure Hospitalizations: The AKINESIS Study. J. Am. Coll. Cardiol. 2016, 68, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; El-Ghoroury, M.; Yamasaki, H. Early Detection of Acute Kidney Injury With Neutrophil Gelatinase-Associated Lipocalin. J. Am. Coll. Cardiol. 2011, 57, 1762–1764. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Horiuchi, Y.U.; Wettersten, N.; van Veldhuisen, D.J.; Mueller, C.; Filippatos, G.; Nowak, R.; Hogan, C.; Kontos, M.C.; Cannon, C.M.; Müeller, G.A.; et al. Potential Utility of Cardiorenal Biomarkers for Prediction and Prognostication of Worsening Renal Function in Acute Heart Failure. J. Card. Fail. 2021, 27, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The Multifaceted Roles of Neutrophil Gelatinase Associated Lipocalin (NGAL) In Inflammation and Cancer. Biochim. Biophys. Acta 2012, 1826, 129. [Google Scholar] [CrossRef]
- Swaminathan, G.; Krishnamurthy, V.K.; Sridhar, S.; Robson, D.C.; Ning, Y.; Grande-Allen, K.J. Hypoxia Stimulates Synthesis of Neutrophil Gelatinase-Associated Lipocalin in Aortic Valve Disease. Front. Cardiovasc. Med. 2019, 6, 156. [Google Scholar] [CrossRef]
- Nasioudis, D.; Witkin, S.S. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med. Microbiol. Immunol. 2015, 204, 471–479. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Lacquaniti, A.; Fazio, M.R.; Bono, C.; Coppolino, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: A new protein enters the scene. Cancer Lett. 2010, 288, 10–16. [Google Scholar] [CrossRef]
- Bolignano, D.; Coppolino, G.; Donato, V.; Lacquaniti, A.; Bono, C.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL): A new piece of the anemia puzzle? Med. Sci. Monit. 2010, 16, 131–135. [Google Scholar]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Damman, K.; Masson, S.; Hillege, H.L.; Voors, A.A.; van Veldhuisen, D.J.; Rossignol, P.; Proietti, G.; Barbuzzi, S.; Nicolosi, G.L.; Tavazzi, L.; et al. Tubular damage and worsening renal function in chronic heart failure. JACC Heart Fail. 2013, 1, 417–424. [Google Scholar] [CrossRef]
- Damman, K.; Van Veldhuisen, D.J.; Navis, G.; Vaidya, V.S.; Smilde, T.D.J.; Westenbrink, B.D.; Bonventre, J.V.; Voors, A.A.; Hillege, H.L. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 2010, 96, 1297–1302. [Google Scholar] [CrossRef]
- Emmens, J.E.; ter Maaten, J.M.; Matsue, Y.; Metra, M.; O’Connor, C.M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Cleland, J.G.; et al. Plasma kidney injury molecule-1 in heart failure: Renal mechanisms and clinical outcome. Eur. J. Heart Fail. 2016, 18, 641–649. [Google Scholar] [CrossRef]
- Grodin, J.L.; Perez, A.L.; Wu, Y.; Hernandez, A.F.; Butler, J.; Metra, M.; Felker, G.M.; Voors, A.A.; McMurray, J.J.; Armstrong, P.W.; et al. Circulating Kidney Injury Molecule-1 Levels in Acute Heart Failure: Insights From the ASCEND-HF Trial (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure). JACC Heart Fail. 2015, 3, 777–785. [Google Scholar] [CrossRef]
- Jungbauer, C.G.; Birner, C.; Jung, B.; Buchner, S.; Lubnow, M.; Von Bary, C.; Endemann, D.; Banas, B.; MacK, M.; Böger, C.A.; et al. Kidney injury molecule-1 and N-acetyl-β-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur. J. Heart Fail. 2011, 13, 1104–1110. [Google Scholar] [CrossRef]
- Strack, C.; Bauer, S.; Hubauer, U.; Ücer, E.; Birner, C.; Luchner, A.; Maier, L.; Jungbauer, C. N-acetyl-ß-D-glucosaminidase is predictive of mortality in chronic heart failure: A 10-year follow-up. Biomark. Med. 2021, 15, 1143–1153. [Google Scholar] [CrossRef]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- Parikh, C.R.; Mishra, J.; Thiessen-Philbrook, H.; Dursun, B.; Ma, Q.; Kelly, C.; Dent, C.; Devarajan, P.; Edelstein, C.L. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006, 70, 199–203. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Lei, Y.; Zha, Y.; Liu, H.; Ma, C.; Tian, J.; Chen, P.; Yang, T.; Hou, F.F. Urinary biomarkers at the time of AKI diagnosis as predictors of progression of AKI among patients with acute cardiorenal syndrome. Clin. J. Am. Soc. Nephrol. 2016, 11, 1536–1544. [Google Scholar] [CrossRef]
- Devuyst, O.; Olinger, E.; Rampoldi, L. Uromodulin: From physiology to rare and complex kidney disorders. Nat. Rev. Nephrol. 2017, 13, 525–544. [Google Scholar] [CrossRef]
- Köttgen, A.; Glazer, N.L.; Dehghan, A.; Hwang, S.J.; Katz, R.; Li, M.; Yang, Q.; Gudnason, V.; Launer, L.J.; Harris, T.B.; et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 2009, 41, 712–717. [Google Scholar] [CrossRef]
- Köttgen, A.; Hwang, S.J.; Larson, M.G.; Van Eyk, J.E.; Fu, Q.; Benjamin, E.J.; Dehghan, A.; Glazer, N.L.; Kao, W.H.L.; Harris, T.B.; et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J. Am. Soc. Nephrol. 2010, 21, 337–344. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Melander, O.; Johnson, T.; Di Blasio, A.M.; Lee, W.K.; Gentilini, D.; Hastie, C.E.; Menni, C.; Monti, M.C.; Delles, C.; et al. Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet. 2010, 6, 1–11. [Google Scholar] [CrossRef]
- LaFavers, K.A.; Micanovic, R.; Sabo, A.R.; Maghak, L.A.; El-Achkar, T.M. Evolving Concepts in Uromodulin Biology, Physiology, and Its Role in Disease: A Tale of Two Forms. Hypertens 2022, 79, 2409–2418. [Google Scholar] [CrossRef]
- Pruijm, M.; Ponte, B.; Ackermann, D.; Paccaud, F.; Guessous, I.; Ehret, G.; Pechére-Bertschi, A.; Vogt, B.; Mohaupt, M.G.; Martin, P.Y.; et al. Associations of Urinary Uromodulin with Clinical Characteristics and Markers of Tubular Function in the General Population. Clin. J. Am. Soc. Nephrol. 2016, 11, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.E.; Kleber, M.E.; Scharnagl, H.; Krämer, B.K.; März, W.; Scherberich, J.E. Serum Uromodulin and Mortality Risk in Patients Undergoing Coronary Angiography. J. Am. Soc. Nephrol. 2017, 28, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Bergwik, J.; Kristiansson, A.; Allhorn, M.; Gram, M.; Åkerström, B. Structure, Functions, and Physiological Roles of the Lipocalin α1-Microglobulin (A1M). Front. Physiol. 2021, 12, 251. [Google Scholar] [CrossRef]
- Garimella, P.S.; Lee, A.K.; Ambrosius, W.T.; Bhatt, U.; Cheung, A.K.; Chonchol, M.; Craven, T.; Hawfield, A.T.; Jotwani, V.; Killeen, A.; et al. Markers of kidney tubule function and risk of cardiovascular disease events and mortality in the SPRINT trial. Eur. Heart J. 2019, 40, 3486–3493. [Google Scholar] [CrossRef]
- Emlet, D.R.; Wen, X.; Kellum, J.A. Comments on the Review ‘Biomarkers in acute kidney injury—Pathophysiological basis and clinical performance’ Acta Physiol 2017, 219, 556–574: An update on kidney localization of IGFBP7 and TIMP2. Acta Physiol. 2018, 222, e12934. [Google Scholar] [CrossRef]
- Zarbock, A.; Küllmar, M.; Ostermann, M.; Lucchese, G.; Baig, K.; Cennamo, A.; Rajani, R.; McCorkell, S.; Arndt, C.; Wulf, H.; et al. Prevention of Cardiac Surgery-Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers: The PrevAKI-Multicenter Randomized Controlled Trial. Anesth. Analg. 2021, 133, 292–302. [Google Scholar] [CrossRef]
- Niizeki, T.; Takeishi, Y.; Arimoto, T.; Nozaki, N.; Hirono, O.; Watanabe, T.; Nitobe, J.; Miyashita, T.; Miyamoto, T.; Koyama, Y.; et al. Persistently increased serum concentration of heart-type fatty acid-binding protein predicts adverse clinical outcomes in patients with chronic heart failure. Circ. J. 2008, 72, 109–114. [Google Scholar] [CrossRef]
- Shirakabe, A.; Kobayashi, N.; Hata, N.; Shinada, T.; Tomita, K.; Tsurumi, M.; Okazaki, H.; Matsushita, M.; Yamamoto, Y.; Yokoyama, S.; et al. The serum heart-type fatty acid-binding protein (HFABP) levels can be used to detect the presence of acute kidney injury on admission in patients admitted to the non-surgical intensive care unit. BMC Cardiovasc. Disord. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Oezkur, M.; Gorski, A.; Peltz, J.; Wagner, M.; Lazariotou, M.; Schimmer, C.; Heuschmann, P.U.; Leyh, R.G. Preoperative serum h-FABP concentration is associated with postoperative incidence of acute kidney injury in patients undergoing cardiac surgery. BMC Cardiovasc. Disord. 2014, 14, 1–6. [Google Scholar] [CrossRef]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.H.; Roumelioti, M.E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef]
- Puthiyottil, D.; Priyamvada, P.S.; Kumar, M.N.; Chellappan, A.; Zachariah, B.; Parameswaran, S. Role of Urinary Beta 2 Microglobulin and Kidney Injury Molecule-1 in Predicting Kidney Function at One Year Following Acute Kidney Injury. Int. J. Nephrol. Renovasc. Dis. 2021, 14, 225. [Google Scholar] [CrossRef]
- Shi, F.; Sun, L.; Kaptoge, S. Association of beta-2-microglobulin and cardiovascular events and mortality: A systematic review and meta-analysis. Atherosclerosis 2021, 320, 70. [Google Scholar] [CrossRef]
- Zhang, J.; Bottiglieri, T.; Mccullough, P.A. The Central Role of Endothelial Dysfunction in Cardiorenal Syndrome. Cardiorenal Med. 2017, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Neves, F.M.; Meneses, G.C.; Sousa, N.E.A.; de Menezes, R.R.P.P.B.; Parahyba, M.C.; Martins, A.M.C.; Libório, A.B. Syndecan-1 in Acute Decompensated Heart Failure—Association With Renal Function and Mortality. Circ. J. 2015, 79, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Grenier, N.; Merville, P.; Combe, C. Radiologic imaging of the renal parenchyma structure and function. Nat. Rev. Nephrol. 2016, 12, 348–359. [Google Scholar] [CrossRef]
- Iida, N.; Seo, Y.; Sai, S.; Machino-Ohtsuka, T.; Yamamoto, M.; Ishizu, T.; Kawakami, Y.; Aonuma, K. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016, 4, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Nijst, P.; Martens, P.; Dupont, M.; Tang, W.H.W.; Mullens, W. Intrarenal Flow Alterations During Transition From Euvolemia to Intravascular Volume Expansion in Heart Failure Patients. JACC Heart Fail. 2017, 5, 672–681. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T. Intrarenal Venous Flow: A Window Into the Congestive Kidney Failure Phenotype of Heart Failure? JACC Heart Fail. 2016, 4, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Puzzovivo, A.; Monitillo, F.; Guida, P.; Leone, M.; Rizzo, C.; Grande, D.; Ciccone, M.M.; Iacoviello, M. Renal Venous Pattern: A New Parameter for Predicting Prognosis in Heart Failure Outpatients. J. Cardiovasc. Dev. Dis. 2018, 5, 52. [Google Scholar] [CrossRef]
- van der Eijk, Y.; Ng, X.Y.; Lee, J.K. Pathophysiology of Cardiorenal Syndrome and Use of Diuretics and Ultrafiltration as Volume Control. Korean Circ. J. 2021, 51, 656. [Google Scholar] [CrossRef]
- Hull, T.D.; Agarwal, A.; Hoyt, K. New Ultrasound Techniques Promise Further Advances in AKI and CKD. J. Am. Soc. Nephrol. 2017, 28, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.M.A.; Mahfouz, A.; Achkar, K.; Rafie, I.M.; Hajar, R. Contrast-induced Nephropathy. Heart Views 2013, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365. [Google Scholar] [CrossRef]
- Dekkers, I.A.; de Boer, A.; Sharma, K.; Cox, E.F.; Lamb, H.J.; Buckley, D.L.; Bane, O.; Morris, D.M.; Prasad, P.V.; Semple, S.I.K.; et al. Consensus-based technical recommendations for clinical translation of renal T1 and T2 mapping MRI. MAGMA 2020, 33, 163–176. [Google Scholar] [CrossRef]
- de Boer, A.; Villa, G.; Bane, O.; Bock, M.; Cox, E.F.; Dekkers, I.A.; Eckerbom, P.; Fernández-Seara, M.A.; Francis, S.T.; Haddock, B.; et al. Consensus-Based Technical Recommendations for Clinical Translation of Renal Phase Contrast MRI. J. Magn. Reson. Imaging 2022, 55, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Nery, F.; Buchanan, C.E.; Harteveld, A.A.; Odudu, A.; Bane, O.; Cox, E.F.; Derlin, K.; Gach, H.M.; Golay, X.; Gutberlet, M.; et al. Consensus-based technical recommendations for clinical translation of renal ASL MRI. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Bane, O.; Mendichovszky, I.A.; Milani, B.; Dekkers, I.A.; Deux, J.F.; Eckerbom, P.; Grenier, N.; Hall, M.E.; Inoue, T.; Laustsen, C.; et al. Consensus-based technical recommendations for clinical translation of renal BOLD MRI. MAGMA 2020, 33, 199–215. [Google Scholar] [CrossRef]
- Mendichovszky, I.; Pullens, P.; Dekkers, I.; Nery, F.; Bane, O.; Pohlmann, A.; de Boer, A.; Ljimani, A.; Odudu, A.; Buchanan, C.; et al. Technical recommendations for clinical translation of renal MRI: A consensus project of the Cooperation in Science and Technology Action PARENCHIMA. MAGMA 2020, 33, 131–140. [Google Scholar] [CrossRef]
- Hueper, K.; Peperhove, M.; Rong, S.; Gerstenberg, J.; Mengel, M.; Meier, M.; Gutberlet, M.; Tewes, S.; Barrmeyer, A.; Chen, R.; et al. T1-mapping for assessment of ischemia-induced acute kidney injury and prediction of chronic kidney disease in mice. Eur. Radiol. 2014, 24, 2252–2260. [Google Scholar] [CrossRef]
- Tewes, S.; Gueler, F.; Chen, R.; Gutberlet, M.; Jang, M.S.; Meier, M.; Mengel, M.; Hartung, D.; Wacker, F.; Rong, S.; et al. Functional MRI for characterization of renal perfusion impairment and edema formation due to acute kidney injury in different mouse strains. PLoS ONE 2017, 12, e0173248. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, I.A.; Paiman, E.H.M.; de Vries, A.P.J.; Lamb, H.J. Reproducibility of native T1 mapping for renal tissue characterization at 3T. J. Magn. Reson. Imaging 2019, 49, 588–596. [Google Scholar] [CrossRef]
- Lu, F.; Yang, J.; Yang, S.; Bernd, K.; Fu, C.; Yang, C.; Xu, H.; Liu, M.; Zhan, S.; Wang, C.; et al. Use of Three-Dimensional Arterial Spin Labeling to Evaluate Renal Perfusion in Patients With Chronic Kidney Disease. J. Magn. Reson. Imaging 2021, 54, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Arakelyan, K.; Hentschel, J.; Cantow, K.; Flemming, B.; Ladwig, M.; Waiczies, S.; Seeliger, E.; Niendorf, T. Detailing the relation between renal T2* and renal tissue pO2 using an integrated approach of parametric magnetic resonance imaging and invasive physiological measurements. Investig. Radiol. 2014, 49, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Bullen, A.; Liu, Z.Z.; Hepokoski, M.; Li, Y.; Singh, P. Renal Oxygenation and Hemodynamics in Kidney Injury. Nephron 2017, 137, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Bullen, A.; Li, Y.; Singh, P. Renal Oxygenation in the Pathophysiology of Chronic Kidney Disease. Front. Physiol. 2017, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Abosamak, M.F.; Lippi, G.; Benoit, S.W.; Henry, B.M.; Shama, A.A.A. Bladder urine oxygen partial pressure monitoring: Could it be a tool for early detection of acute kidney injury? Egypt. J. Anaesth. 2021, 37, 43–49. [Google Scholar] [CrossRef]
- Silverton, N.A.; Lofgren, L.R.; Hall, I.E.; Stoddard, G.J.; Melendez, N.P.; Van Tienderen, M.; Shumway, S.; Stringer, B.J.; Kang, W.S.; Lybbert, C.; et al. Noninvasive Urine Oxygen Monitoring and the Risk of Acute Kidney Injury in Cardiac Surgery. Anesthesiology 2021, 135, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.L.; Pham, H.; Li, Y.; Hall, E.; Perkins, G.A.; Ali, S.S.; Patel, H.H.; Singh, P. Hypoxia-inducible factor-1α activation improves renal oxygenation and mitochondrial function in early chronic kidney disease. Am. J. Physiol.-Ren. Physiol. 2017, 313, F282. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.E.; Riedmüller, K.; Haberkorn, U. Nuclear Medicine Procedures for the Diagnosis of Acute and Chronic Renal Failure. Nephron Clin. Pract. 2006, 103, c77–c84. [Google Scholar] [CrossRef] [PubMed]
- Coombs, H.C. The mechanism of the regulation of intra-abdominal pressure. Am. J. Physiol.-Leg. Content 1922, 61, 159–170. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Cheatham, M.L.; Kirkpatrick, A.; Sugrue, M.; Parr, M.; De Waele, J.; Balogh, Z.; Leppäniemi, A.; Olvera, C.; Ivatury, R.; et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006, 32, 1722–1732. [Google Scholar] [CrossRef]
- Zymliński, R.; Sokolski, M.; Biegus, J.; Siwołowski, P.; Nawrocka-Millward, S.; Sokolska, J.M.; Dudkowiak, M.; Marciniak, D.; Todd, J.; Jankowska, E.A.; et al. Multi-organ dysfunction/injury on admission identifies acute heart failure patients at high risk of poor outcome. Eur. J. Heart Fail. 2019, 21, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Biegus, J.; Demissei, B.; Postmus, D.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Gimpelewicz, C.; Greenberg, B.; Metra, M.; et al. Hepatorenal dysfunction identifies high-risk patients with acute heart failure: Insights from the RELAX-AHF trial. ESC Heart Fail. 2019, 6, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Zymliński, R.; Biegus, J.; Sokolski, M.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Validation of transurethral intra-abdominal pressure measurement in acute heart failure. Pol. Arch. Intern. Med. 2018, 128, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.Q.; Gadiraju, T.V.; Patel, H.; Park, M.; Le Jemtel, T.H.; Jaiswal, A. Intra-abdominal Hypertension: An Important Consideration for Diuretic Resistance in Acute Decompensated Heart Failure. Clin. Cardiol. 2016, 39, 37–40. [Google Scholar] [CrossRef]
- Harman, P.K.; Kron, I.L.; McLachlan, H.D.; Freedlender, A.E.; Nolan, S.P. Elevated intra-abdominal pressure and renal function. Ann. Surg. 1982, 196, 594–597. [Google Scholar] [CrossRef]
- Mullens, W.; Abrahams, Z.; Skouri, H.N.; Francis, G.S.; Taylor, D.O.; Starling, R.C.; Paganini, E.; Tang, W.H.W. Elevated Intra-Abdominal Pressure in Acute Decompensated Heart Failure: A Potential Contributor to Worsening Renal Function? J. Am. Coll. Cardiol. 2008, 51, 300–306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).