Using ΔK280 TauRD Folding Reporter Cells to Screen TRKB Agonists as Alzheimer’s Disease Treatment Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cell Culture and Cell Viability Assay
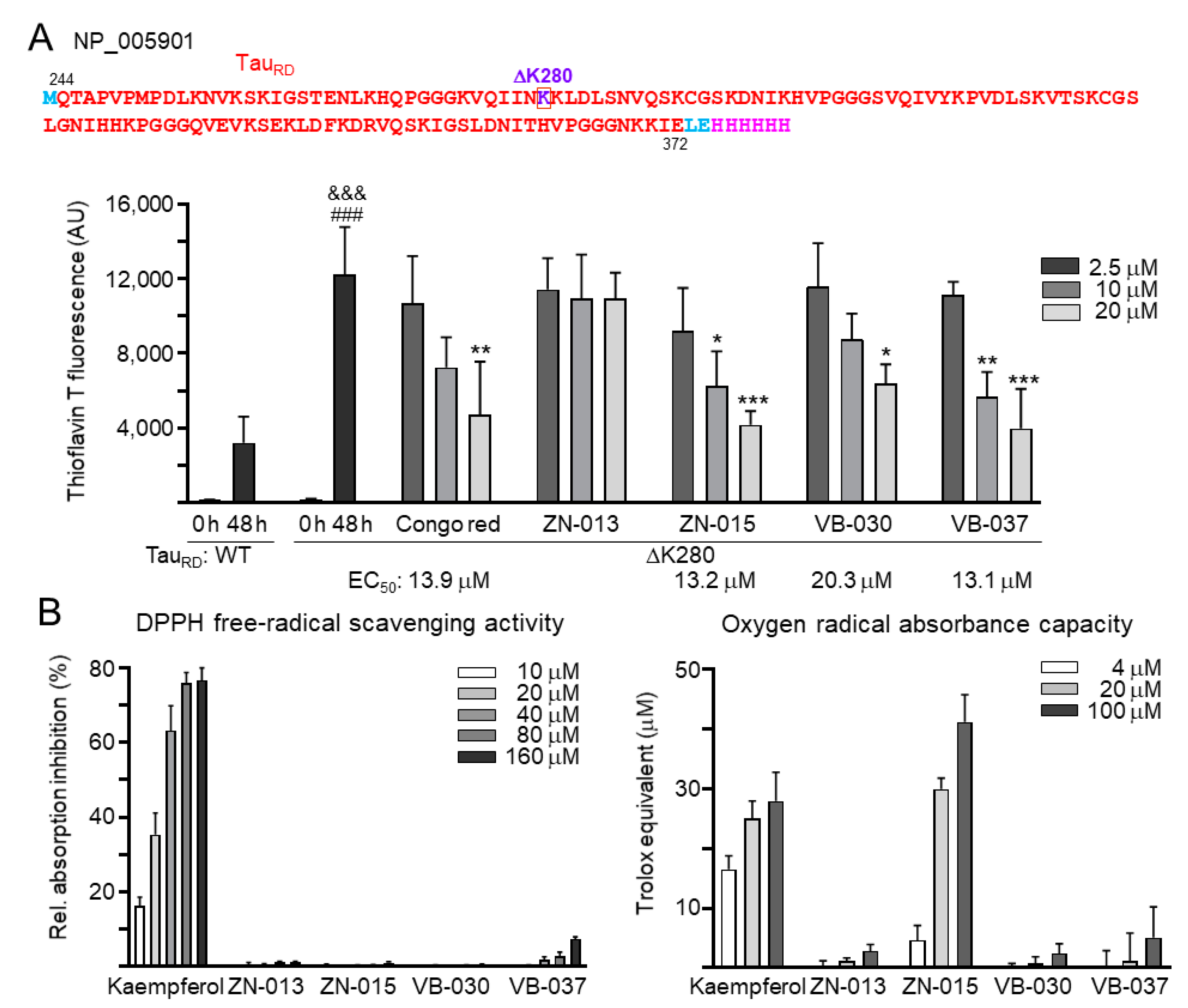
2.3. Antioxidant Assays
2.4. Biochemical Fluorescence-Based TauRD Aggregation Assay
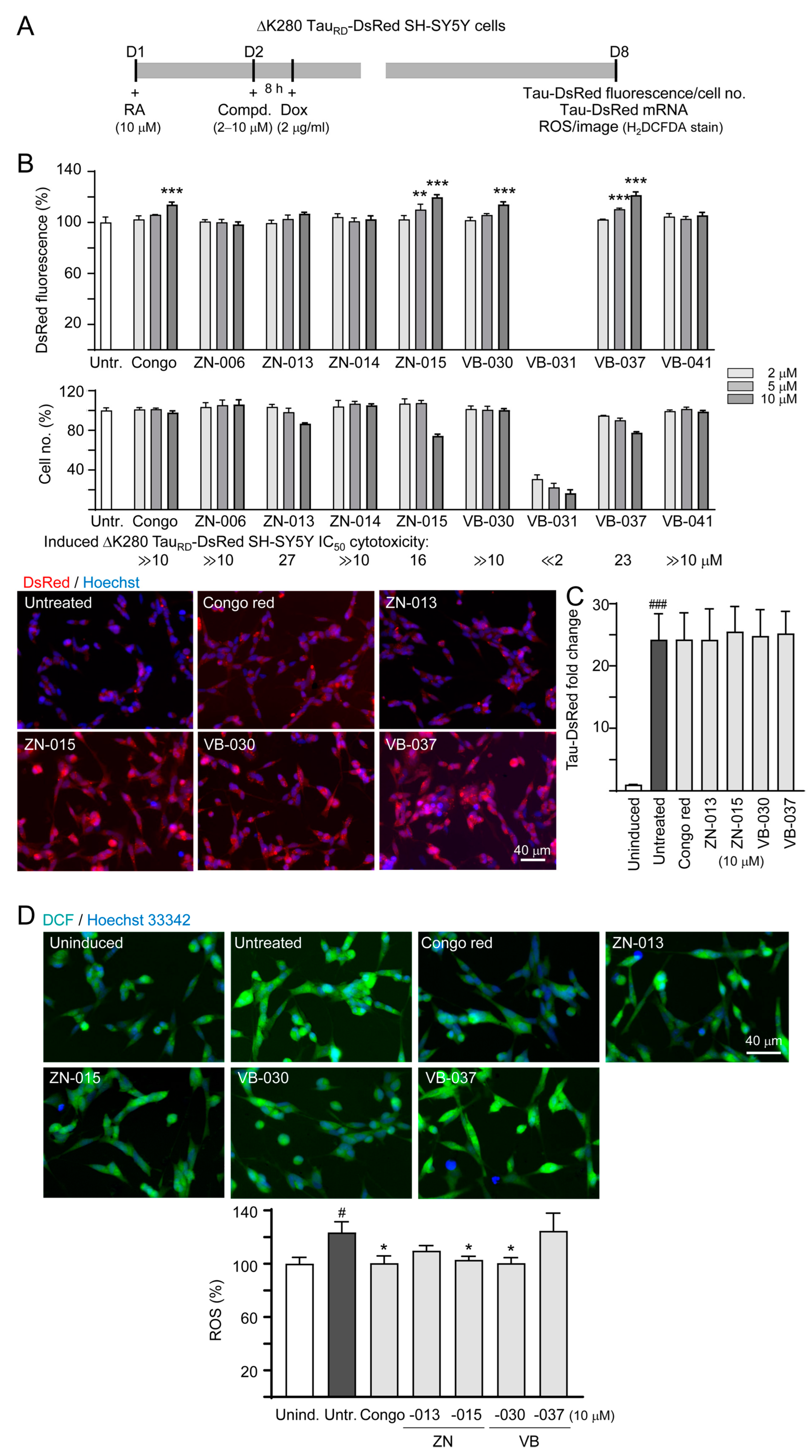
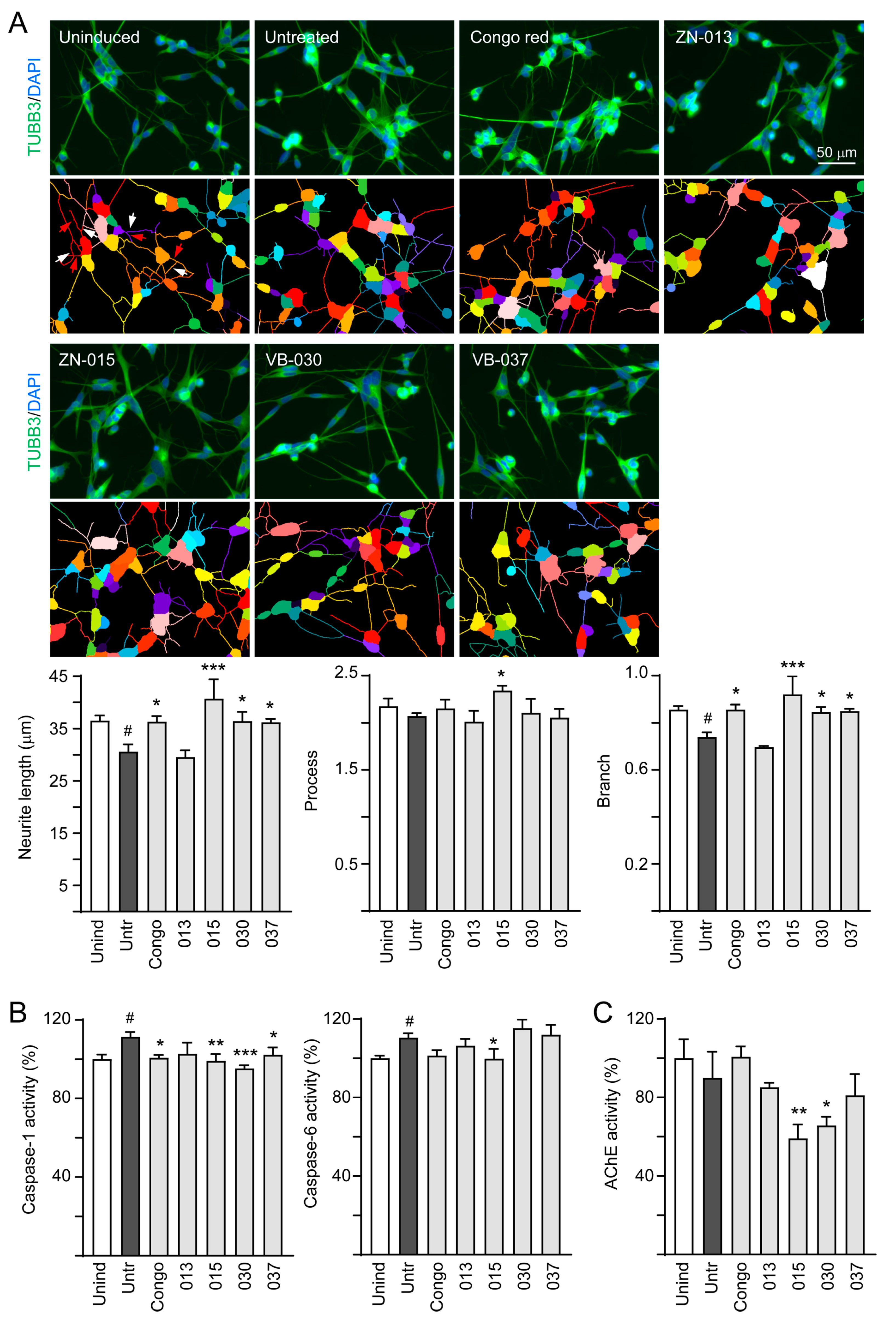
2.5. High-Content Analysis of Cellular ΔK280 TauRD-DsRed Aggregation, Reactive Oxygen Species (ROS) and Neurite Outgrowth
2.6. Real-Time PCR Analysis
2.7. Caspase-1/6 and Acetylcholinesterase (AChE) Activity Assays
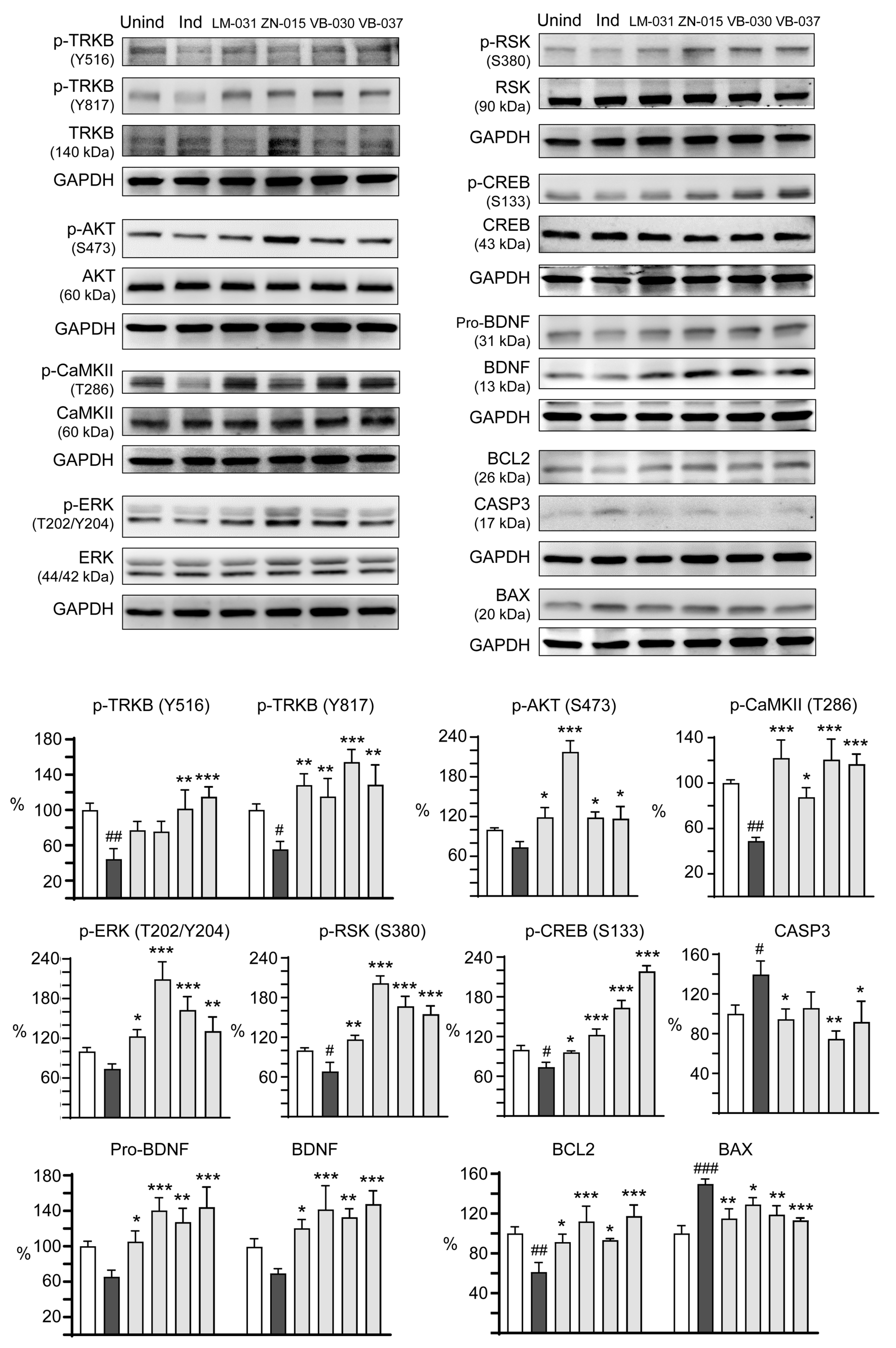
2.8. Western Blot Analysis
2.9. RNA Interference
2.10. Tryptophan Fluorescence Quenching Assay
2.11. Statistical Analysis
3. Results
3.1. Cytotoxicity of Heterocyclic ZN/VB Compounds
3.2. Screening Compounds Promoting ΔK280 TauRD-DsRed Folding and Reducing Cellular Oxidative Stress
3.3. Chemical Chaperone and Antioxidant Activities of ZN/VB Compounds
3.4. Neuroprotective Effects of ZN/VB Compounds
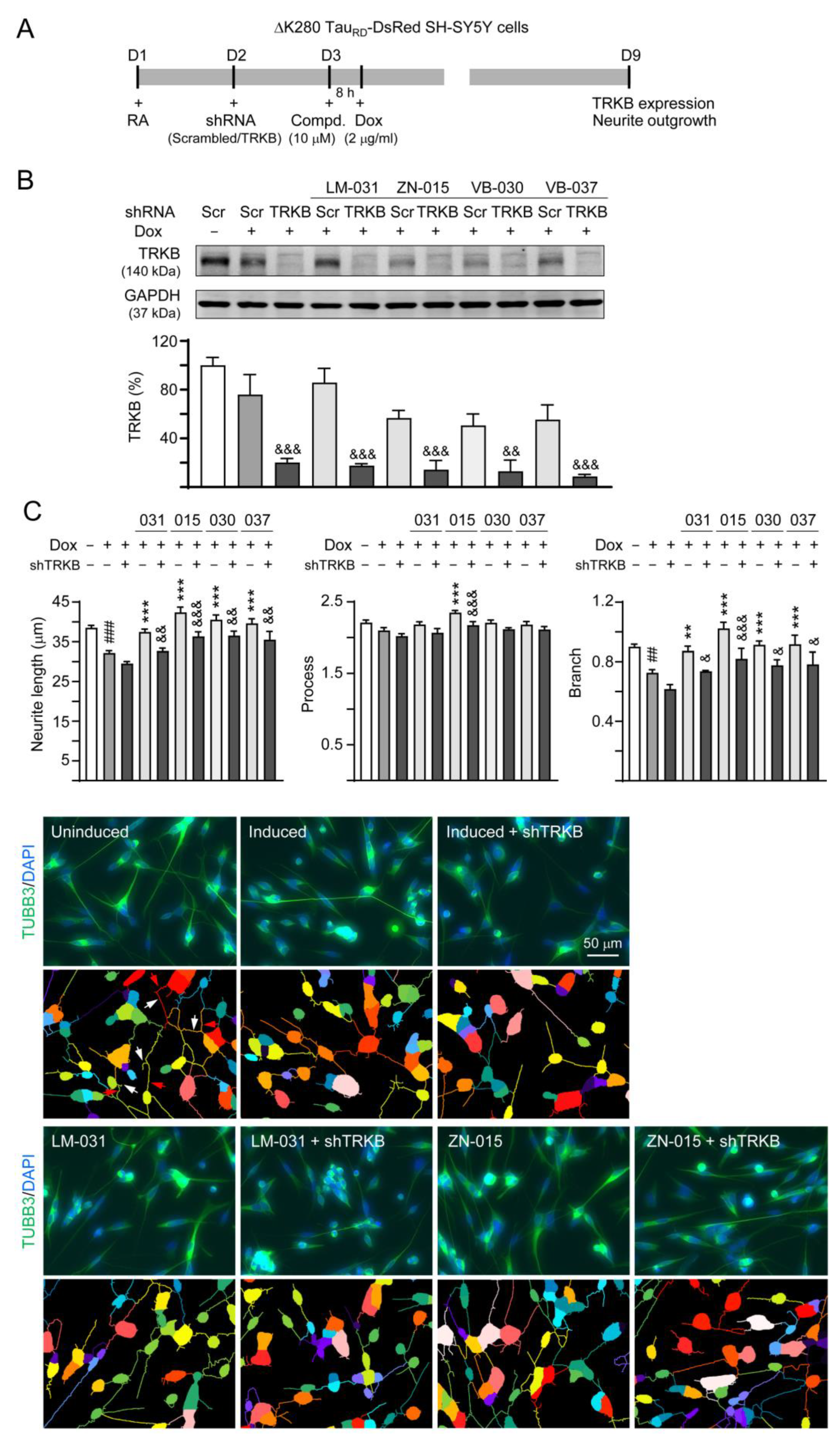
3.5. Targets of ZN/VB Compounds on TRKB Pathway
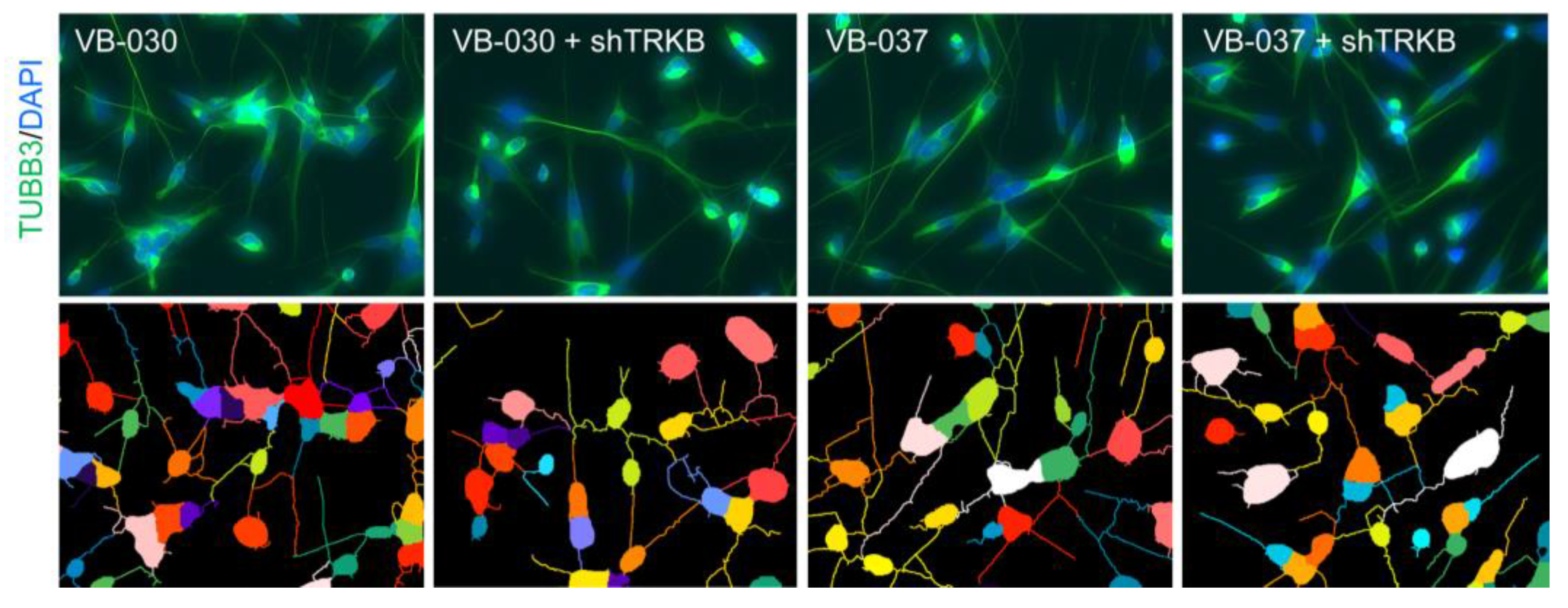
3.6. Effects of TRKB Knockdown on Neurite Outgrowth
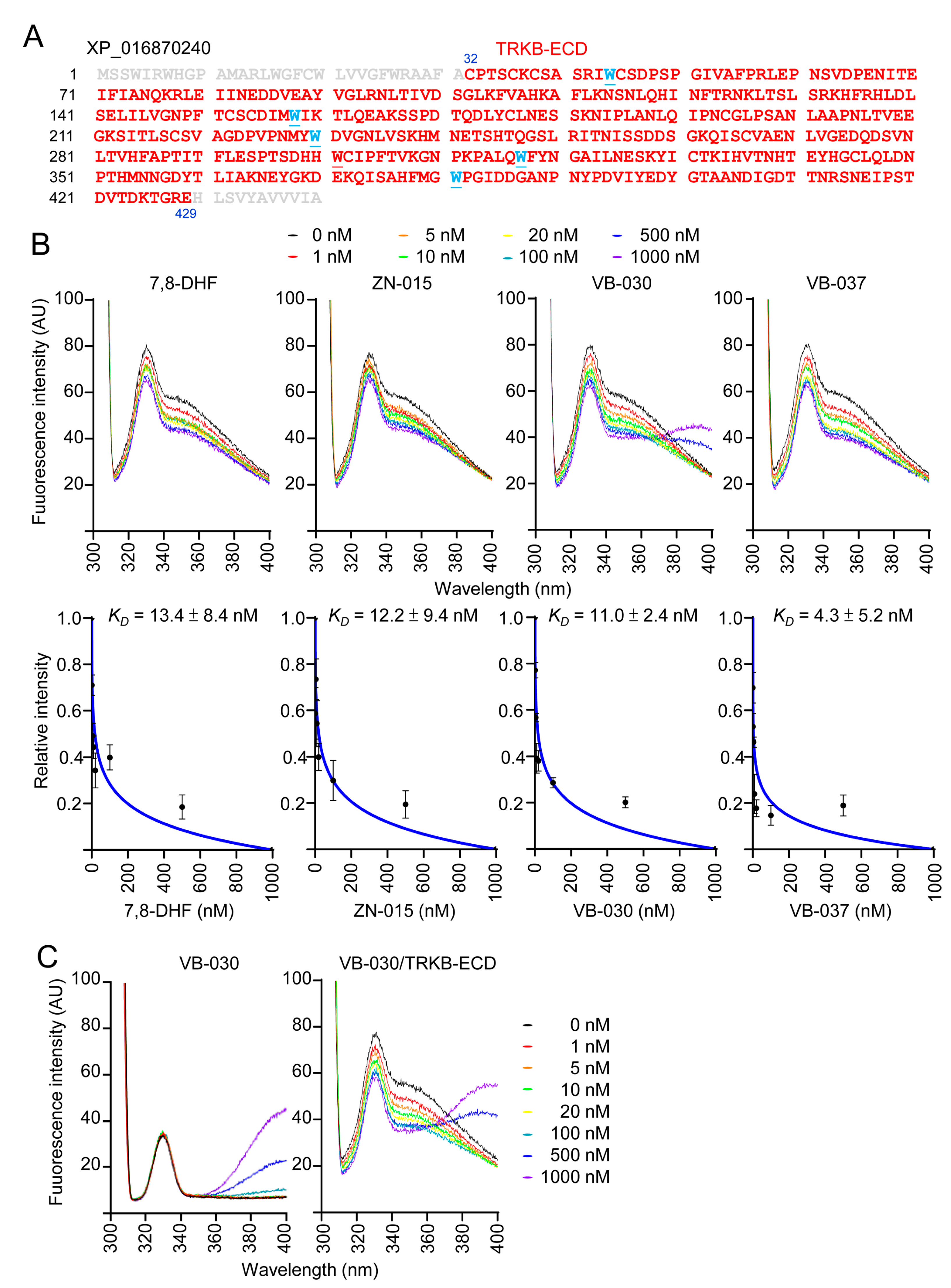
3.7. Binding Affinity of ZN-015, VB-030 and VB-037 with TRKB-ECD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, K.; Alonso Adel, C.; Chen, S.; Chohan, M.O.; El-Akkad, E.; Gong, C.X.; Khatoon, S.; Li, B.; Liu, F.; Rahman, A.; et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta 2005, 1739, 198–210. [Google Scholar] [CrossRef]
- Drubin, D.G.; Kirschner, M.W. Tau protein function in living cells. J. Cell Biol. 1986, 103, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Terwel, D.; Dewachter, I.; Van Leuven, F. Axonal transport, tau protein, and neurodegeneration in Alzheimer’s disease. Neuromol. Med. 2002, 2, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, I.; Poorkaj, P.; Hong, M.; Nochlin, D.; Lee, V.M.; Bird, T.D.; Schellenberg, G.D. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc. Natl. Acad. Sci. USA 1999, 96, 5598–5603. [Google Scholar] [CrossRef]
- Momeni, P.; Pittman, A.; Lashley, T.; Vandrovcova, J.; Malzer, E.; Luk, C.; Hulette, C.; Lees, A.; Revesz, T.; Hardy, J.; et al. Clinical and pathological features of an Alzheimer’s disease patient with the MAPT ∆K280 mutation. Neurobiol. Aging 2009, 30, 388–393. [Google Scholar] [CrossRef]
- Chang, K.H.; Chen, I.C.; Lin, H.Y.; Chen, H.C.; Lin, C.H.; Lin, T.H.; Weng, Y.-T.; Chao, C.-Y.; Wu, Y.-R.; Chen, C.; et al. The aqueous extract of Glycyrrhiza inflata can upregulate unfolded protein response-mediated chaperones to reduce tau misfolding in cell models of Alzheimer’s disease. Drug Des. Devel. Ther. 2016, 10, 885–896. [Google Scholar] [CrossRef]
- Lin, T.H.; Chang, K.H.; Chiu, Y.J.; Weng, Z.K.; Sun, Y.C.; Lin, W.; Lee-Chen, G.-J.; Chen, C.-M. Neuroprotective action of coumarin derivatives through activation of TRKB-CREB-BDNF pathway and reduction of caspase activity in neuronal cells expressing pro-aggregated Tau protein. Int. J. Mol. Sci. 2022, 23, 12734. [Google Scholar] [CrossRef]
- Rosa, E.; Mahendram, S.; Ke, Y.D.; Ittner, L.M.; Ginsberg, S.D.; Fahnestock, M. Tau downregulates BDNF expression in animal and cellular models of Alzheimer’s disease. Neurobiol. Aging 2016, 48, 135–142. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J. Neurochem. 2005, 93, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.S.; Hains, J.M.; Armanini, M.; Laramee, G.R.; Johnson, S.A.; Winslow, J.W. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 1991, 7, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, F.; Masuyama, N.; Gotoh, Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J. Biol. Chem. 2002, 277, 14040–14047. [Google Scholar] [CrossRef]
- Hauge, C.; Frödin, M. RSK and MSK in MAP kinase signaling. J. Cell Sci. 2006, 119, 3021–3023. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, A.; Sakaya, H.; Kisukeda, T.; Fushiki, H.; Tsuda, M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J. Biol. Chem. 2002, 277, 35920–35931. [Google Scholar] [CrossRef]
- Riccio, A.; Ahn, S.; Davenport, C.M.; Blendy, J.A.; Ginty, D.D. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 1999, 286, 2358–2361. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G. CREB: A multifaceted target for Alzheimer’s disease. Curr. Alzheimer Res. 2020, 17, 1280–1293. [Google Scholar] [CrossRef]
- Tao, X.; Finkbeiner, S.; Arnold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Lin, T.H.; Chen, C.M.; Lin, C.H.; Teng, Y.S.; Lin, C.Y.; Sun, Y.-C.; Hsieh-Li, H.M.; Su, M.-T.; Lee-Chen, G.-J.; et al. Novel synthetic coumarin-chalcone derivative (E)-3-(3-(4-(dimethylamino)phenyl)acryloyl)-4-hydroxy-2H-chromen-2-one activates CREB-mediated neuroprotection in Aβ and tau cell models of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2021, 2021, 3058861. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Hung, H.C.; Chen, S.H.; Gean, P.W. Social interaction rescues memory deficit in an animal model of Alzheimer’s disease by increasing BDNF-dependent hippocampal neurogenesis. J. Neurosci. 2014, 34, 16207–16219. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.H.; Merrill, D.; Coppola, G.; Tsukada, S.; Schroeder, B.E.; Shaked, G.M.; Wang, L.; Blesch, A.; Kim, A.; Conner, J.M.; et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 2009, 15, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Bubols, G.B.; da Rocha Vianna, D.; Medina-Remon, A.; von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Garcia, S. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Dawood, K.M. An update on benzofuran inhibitors: A patent review. Expert Opin. Ther. Pat. 2019, 29, 841–870. [Google Scholar] [CrossRef]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Gao, L.; Tian, M.; Zhao, H.Y.; Xu, Q.Q.; Huang, Y.M.; Si, Q.C.; Tian, Q.; Wu, Q.-M.; Hu, X.-M.; Sun, L.-B.; et al. TrkB activation by 7, 8-dihydroxyflavone increases synapse AMPA subunits and ameliorates spatial memory deficits in a mouse model of Alzheimer’s disease. J. Neurochem. 2016, 136, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Schroeder, J.P.; Chan, C.B.; Song, M.; Yu, S.P.; Weinshenker, D.; Ye, K. 7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2014, 39, 638–650. [Google Scholar] [CrossRef]
- Huang, C.C.; Chang, K.H.; Chiu, Y.J.; Chen, Y.R.; Lung, T.H.; Hsieh-Li, H.M.; Su, M.-T.; Sun, Y.-C.; Chen, C.-M.; Lin, W.; et al. Multi-target effects of novel synthetic coumarin derivatives protecting Aβ-GFP SH-SY5Y cells against Aβ toxicity. Cells 2021, 10, 3095. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hsieh, Y.S.; Sun, Y.C.; Huang, W.H.; Chen, S.L.; Weng, Z.K.; Lin, T.-H.; Wu, Y.-R.; Chang, K.-H.; Huang, H.-J.; et al. Virtual screening and testing of GSK-3 inhibitors using human SH-SY5Y cells expressing Tau folding reporter and mouse hippocampal primary culture under Tau cytotoxicity. Biomol. Ther. 2022. [CrossRef]
- Lee, S.Y.; Chiu, Y.J.; Yang, S.M.; Chen, C.M.; Huang, C.C.; Lee-Chen, G.J.; Lin, W.; Chang, K. Novel synthetic chalcone-coumarin hybrid for Aβ aggregation reduction, antioxidation, and neuroprotection. CNS Neurosci. Ther. 2018, 24, 1286–1298. [Google Scholar] [CrossRef]
- Sharma, O.P.; and Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4926. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Chiu, Y.J.; Lin, C.H.; Lin, C.Y.; Chao, C.Y.; Chen, Y.C.; Yang, S.; Lin, W.; Hsieh-Li, H.M.; Wu, Y.; et al. Exploration of multi-target effects of 3-benzoyl-5-hydroxychromen-2-one in Alzheimer’s disease cell and mouse models. Aging Cell 2020, 19, e13169. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.N.; Lin, T.H.; Teng, Y.S.; Sun, Y.C.; Chang, K.H.; Lin, C.Y.; Hsieh-Li, H.M.; Su, M.-T.; Chen, C.-M.; Lee-Chen, G.-J. Flavones 7,8-DHF, quercetin, and apigenin against Tau toxicity via activation of TRKB Signaling in ΔK280 TauRD-DsRed SH-SY5Y Cells. Front. Aging Neurosci. 2021, 13, 758895. [Google Scholar] [CrossRef]
- Liu, X.; Obianyo, O.; Chan, C.B.; Huang, J.; Xue, S.; Yang, J.J.; Zeng, F.; Goodman, M.; Ye, K. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TrkB receptor. J. Biol. Chem. 2014, 289, 27571–27584. [Google Scholar] [CrossRef]
- Gendron, T.F.; Petrucelli, L. The role of tau in neurodegeneration. Mol. Neurodegener. 2009, 4, 13. [Google Scholar] [CrossRef]
- Sivananthan, S.N.; Lee, A.W.; Goodyer, C.G.; LeBlanc, A.C. Familial amyloid precursor protein mutants cause caspase-6-dependent but amyloid beta-peptide independent neuronal degeneration in primary human neuron cultures. Cell Death Dis. 2010, 1, e100. [Google Scholar] [CrossRef]
- Guo, H.; Pétrin, D.; Zhang, Z.; Bergeron, C.; Goodyer, C.G.; LeBlanc, A.C. Caspase-1 activation of caspase-6 in human apoptotic neurons. Cell Death Differ. 2006, 13, 285–292. [Google Scholar] [CrossRef]
- Mathew, A.; Balaji E, V.; Pai, S.R.K.; Kishore, A.; Pai, V.; Pemmireddy, R.; Chandrashekar, K.S. Current drug targets in Alzheimer’s associated memory impairment: A comprehensive review. CNS Neurol. Disord. Drug Targets 2023, 22, 255–275. [Google Scholar] [CrossRef]
- Jang, S.W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef]
- Qian, W.; Liu, F. Regulation of alternative splicing of tau exon 10. Neurosci. Bull. 2014, 30, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Yen, C.Y.; Chen, W.L.; Lin, C.H.; Wu, Y.R.; Chang, K.H.; Lee-Chen, G.-J. Pathomechanism characterization and potential therapeutics identification for Parkinson’s disease targeting neuroinflammation. Int. J. Mol. Sci. 2021, 22, 1062. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Renis, M.; Calderone, A.; Russo, A.; Barcellona, M.L.; Rizza, V. Stress proteins and SH-groups in oxidant-induced cell damage after acute ethanol administration in rat. Free Radic. Biol. Med. 1996, 20, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Kozumbo, W.J. The hormetic dose-response mechanism: Nrf2 activation. Pharmacol. Res. 2021, 167, 105526. [Google Scholar] [CrossRef]
- Devi, L.; Ohno, M. TrkB reduction exacerbates Alzheimer’s disease-like signaling aberrations and memory deficits without affecting β-amyloidosis in 5XFAD mice. Transl. Psychiatry 2015, 5, e562. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Razani, E.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Zoghi, A.; Shanaki-Bavarsad, M.; Bashash, D. The PI3K/Akt signaling axis in Alzheimer’s disease: A valuable target to stimulate or suppress? Cell Stress Chaperones 2021, 26, 871–887. [Google Scholar] [CrossRef]
- Sun, X.Y.; Tuo, Q.Z.; Liuyang, Z.Y.; Xie, A.J.; Feng, X.L.; Yan, X.; Qiu, M.; Li, S.; Wang, X.-L.; Cao, F.-Y.; et al. Extrasynaptic NMDA receptor-induced tau overexpression mediates neuronal death through suppressing survival signaling ERK phosphorylation. Cell Death Dis. 2016, 7, e2449. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.J. Effect of obesity on cognitive impairment in vascular dementia rat model via BDNF-ERK-CREB pathway. Biol. Res. Nurs. 2021, 23, 248–257. [Google Scholar] [CrossRef]
- Yu, X.W.; Oh, M.M.; Disterhoft, J.F. CREB, cellular excitability, and cognition: Implications for aging. Behav. Brain Res. 2017, 322, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Miller, S.; Yasuda, M.; Coats, J.K.; Jones, Y.; Martone, M.E.; Mayford, M. Disruption of dendritic translation of CaMKIIα impairs stabilization of synaptic plasticity and memory consolidation. Neuron 2002, 36, 507–519. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Ye, Z.; Huang, J.; He, F.; Xiao, W.; Hu, X.; Luo, Z. CaMKII-mediated CREB phosphorylation is involved in Ca2+-induced BDNF mRNA transcription and neurite outgrowth promoted by electrical stimulation. PLoS ONE 2016, 11, e0162784. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Jung, I.H.; Yi, J.H.; Kim, J.H.; Park, J.H.; Lee, S.; Jung, J.W.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. The seed of Zizyphus jujuba var. spinosa attenuates Alzheimer’s disease-associated hippocampal synaptic deficits through BDNF/TrkB signaling. Biol. Pharm. Bull. 2017, 40, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ahn, E.H.; Liu, X.; Wang, Z.H.; Luo, S.; Liao, J.; Ye, K. Optimized TrkB agonist ameliorates Alzheimer’s disease pathologies and improves cognitive functions via inhibiting δ-secretase. ACS Chem. Neurosci. 2021, 12, 2448–2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, H.; Xu, Y.; Hao, R.; Zhang, W.; Liu, H.; Huang, Y.; Guo, W.; Lu, B. Therapeutic potential of a TrkB agonistic antibody for Alzheimer’s disease. Theranostics 2020, 10, 6854–6874. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, Z.-K.; Lin, T.-H.; Chang, K.-H.; Chiu, Y.-J.; Lin, C.-H.; Tseng, P.-H.; Sun, Y.-C.; Lin, W.; Lee-Chen, G.-J.; Chen, C.-M. Using ΔK280 TauRD Folding Reporter Cells to Screen TRKB Agonists as Alzheimer’s Disease Treatment Strategy. Biomolecules 2023, 13, 219. https://doi.org/10.3390/biom13020219
Weng Z-K, Lin T-H, Chang K-H, Chiu Y-J, Lin C-H, Tseng P-H, Sun Y-C, Lin W, Lee-Chen G-J, Chen C-M. Using ΔK280 TauRD Folding Reporter Cells to Screen TRKB Agonists as Alzheimer’s Disease Treatment Strategy. Biomolecules. 2023; 13(2):219. https://doi.org/10.3390/biom13020219
Chicago/Turabian StyleWeng, Zheng-Kui, Te-Hsien Lin, Kuo-Hsuan Chang, Ya-Jen Chiu, Chih-Hsin Lin, Pei-Hsuan Tseng, Ying-Chieh Sun, Wenwei Lin, Guey-Jen Lee-Chen, and Chiung-Mei Chen. 2023. "Using ΔK280 TauRD Folding Reporter Cells to Screen TRKB Agonists as Alzheimer’s Disease Treatment Strategy" Biomolecules 13, no. 2: 219. https://doi.org/10.3390/biom13020219
APA StyleWeng, Z.-K., Lin, T.-H., Chang, K.-H., Chiu, Y.-J., Lin, C.-H., Tseng, P.-H., Sun, Y.-C., Lin, W., Lee-Chen, G.-J., & Chen, C.-M. (2023). Using ΔK280 TauRD Folding Reporter Cells to Screen TRKB Agonists as Alzheimer’s Disease Treatment Strategy. Biomolecules, 13(2), 219. https://doi.org/10.3390/biom13020219





