Abstract
l-arabitol is an intermediate of the pentose catabolic pathway in fungi but can also be used as a carbon source by many fungi, suggesting the presence of transporters for this polyol. In this study, an l-arabitol transporter, LatA, was identified in Aspergillus niger. Growth and expression profiles as well as sugar consumption analysis indicated that LatA only imports l-arabitol and is regulated by the arabinanolytic transcriptional activator AraR. Moreover, l-arabitol production from wheat bran was increased in a metabolically engineered A. niger mutant by the deletion of latA, indicating its potential for improving l-arabitol-producing cell factories. Phylogenetic analysis showed that homologs of LatA are widely conserved in fungi.
1. Introduction
Polyols (sugar alcohols) have diverse functions in fungi, such as [1] in storage of the reducing power and coenzyme regulation, [2] to adjust the osmotic pressure by acting as compatible solutes, [3] act as endogenous carbohydrate reserves, [4] act as translocatory compounds and [5] as intermediates of major metabolic pathways [1]. Mannitol is the most common polyol found in fungi, while others include l- and d-arabitol, erythritol, d-threitol, xylitol, galactitol, sorbitol and volemitol [2]. Polyols are widely used in food and pharmaceutical industries with several health-related advantages, such as low-calorie, low-glycemic, low-insulinemic, anticarcinogenic and prebiotic properties [3].
l-arabitol occurs intracellularly as an intermediate in the fungal pentose catabolic pathway (PCP) but is rarely found in high amounts in nature [4]. However, it can be industrially produced from plant biomass derived sugars, and it is therefore widely used in the food and pharmaceutical industries as one of 12 building block chemicals (C3–C6 compounds) derived directly from biomass. Improving l-arabitol production is a major target in industrial biotechnology, focusing, in particular, on fermentation optimization and strain screening and development [5].
In the fungal PCP, l-arabinose is reduced to l-arabitol and then converted in two steps to xylitol [6,7]. Studies in A. niger showed that xylitol and l-arabitol can both support growth as a sole carbon source similar to d-xylose and l-arabinose [7,8,9,10,11]. Several mutants of A. niger constructed by metabolic engineering have been shown to accumulate and secrete xylitol and l-arabitol from d-xylose or l-arabinose [10,12]. These results demonstrate that A. niger has the capacity to both take up and secrete these two polyols, indicating the existence of polyol transporters in this fungus.
Reports concerning polyol transporters are limited, but there has been a growing interest in them in recent years. Several polyol transporters have been studied in plants, such as the H+/mannitol transporter in celery and the polyol transporter AtPLT5 from Arabidopsis [13,14]; in red algae, such as transporters from Galdieria sulphuraria [15]; and in bacteria, such as d-arabinitol and ribitol transporters from Klebsiella pneumonia [16]. Some characterized fungal transporters are also able to transport polyols, such as hexose transporters Hxt11, Hxt13, Hxt15, Hxt16 and Hxt17 from Saccharomyces cerevisiae [17]; two l-arabinose transporters Lat1 and Lat2 from Ambrosiozyma monospora [18,19]; and five polyol/H+ symporters Sgl1, Stl1, Syi1, Syl1 and Syl2 from Debaryomyces hansenii [20].
Recently, 86 putative sugar transporter genes were identified in a genome-wide study of the sugar transportome of A. niger [21]. These predicted and 61 characterized fungal sugar transporters were phylogenetically classified to nine clades with diverse functional motifs and possible sugar specificity. Clade I contains pentose and glycerol transporters, such as XAT1 [22], Lat2 [18], Xyp29 [23] and Stl1 [24]. Lat2 has transport activities of l-arabinose, l-arabitol and ribitol in A. monospora grown on l-arabinose instead of d-glucose [18]. In this study, putative l-arabitol transporter encoding genes from A. niger were identified by combining transcriptome and phylogeny analysis. Construction of deletion strains for these genes and subsequent growth profiling revealed that one of them (NRRL3_04757, named latA) encodes an l-arabitol transporter. The application of latA deletion for l-arabitol production from wheat bran and sugar beet pulp by A. niger was also studied. Wheat bran and sugar beet pulp are two feedstock examples that reflect the typical differences between mono- and dicot cell wall composition. While both contain cellulose, wheat bran contains large amounts of arabinoxylan, while sugar beet pulp contains pectin and xyloglucan. These substrates were chosen to evaluate the use of such different feedstocks for l-arabitol production.
2. Materials and Methods
2.1. Strains, Media and Growth Conditions
Escherichia coli DH5α was used for plasmid construction and was grown on Luria-Bertani (LB) medium supplemented with 50 μg/mL ampicillin (Sigma-Aldrich, Zwijndrecht, The Netherlands). A. niger strains used in this study were deposited at the CBS culture collection of Westerdijk Fungal Biodiversity Institute (Utrecht, The Netherlands) with numbers shown in Table 1. The uridine auxotrophic and non-homologous end-joining (NHEJ) deficient A. niger strain N593 Δku70 was used as the reference strain. The mutants were generated using CRISPR/Cas9 genome editing [25]. The primers used for creating two deletion mutations are listed in Table S1. A. niger protoplasting and transformation were performed as described previously [26]. All A. niger strains were grown at 30 °C on Complete Medium (CM) or Minimal Medium (MM) [27] supplemented with required carbon source. For plate cultivations, 1.5% (w/v) agar (Sigma-Aldrich) was added, and 1.22 g/L uridine (Sigma-Aldrich) was supplemented for auxotrophic strains. A total of 1.3 mg/mL 5-fluoroorotic acid (5-FOA) (Sigma-Aldrich) was added in the solid medium for counter selecting colonies containing the pyrG marker gene on ANEp8-Cas9 plasmids.
A. niger strains were grown on CM plates with 1% d-glucose at 30 °C for 5 days. Spores were harvested in ACES buffer (Sigma-Aldrich) and were counted using a haemocytometer (Neubauer, Marienfeld, Germany). Solid MM was used for growth profiles supplemented with 25 mM d-glucose, 25 mM d-xylose (Sigma-Aldrich), 25 mM l-arabinose (Sigma-Aldrich), 25 mM xylitol (Sigma-Aldrich), 25 mM l-arabitol (Sigma-Aldrich), 25 mM d-arabitol (Sigma-Aldrich), 25 mM glycerol (Sigma-Aldrich), 25 mM galactitol (Sigma-Aldrich), 25 mM d-sorbitol (Sigma-Aldrich), 25 mM d-mannitol (Sigma-Aldrich), 25 mM myo-Inositol (Sigma-Aldrich), 25 mM adonitol (ribitol) (Sigma-Aldrich) or 25 mM dl-threitol (Sigma-Aldrich). A total of 200 spores in 5 μL ACES buffer were inoculated on the plates and incubated at 30 °C for up to 12 days.
2.2. Expression Analysis
Expression analysis was performed on previously obtained micro-array data [21,28]. In brief, the strains were pre-cultured for 16 h in CM with 2% fructose (Sigma-Aldrich), after which the mycelium was harvested, washed with MM without carbon sources and aliquots were transferred to MM with different carbon sources, as indicated in Figure 1. After 2 h, mycelium was harvested and used for RNA isolation, which was then used for micro-array analysis. More details on the procedure can be found in [21,28].

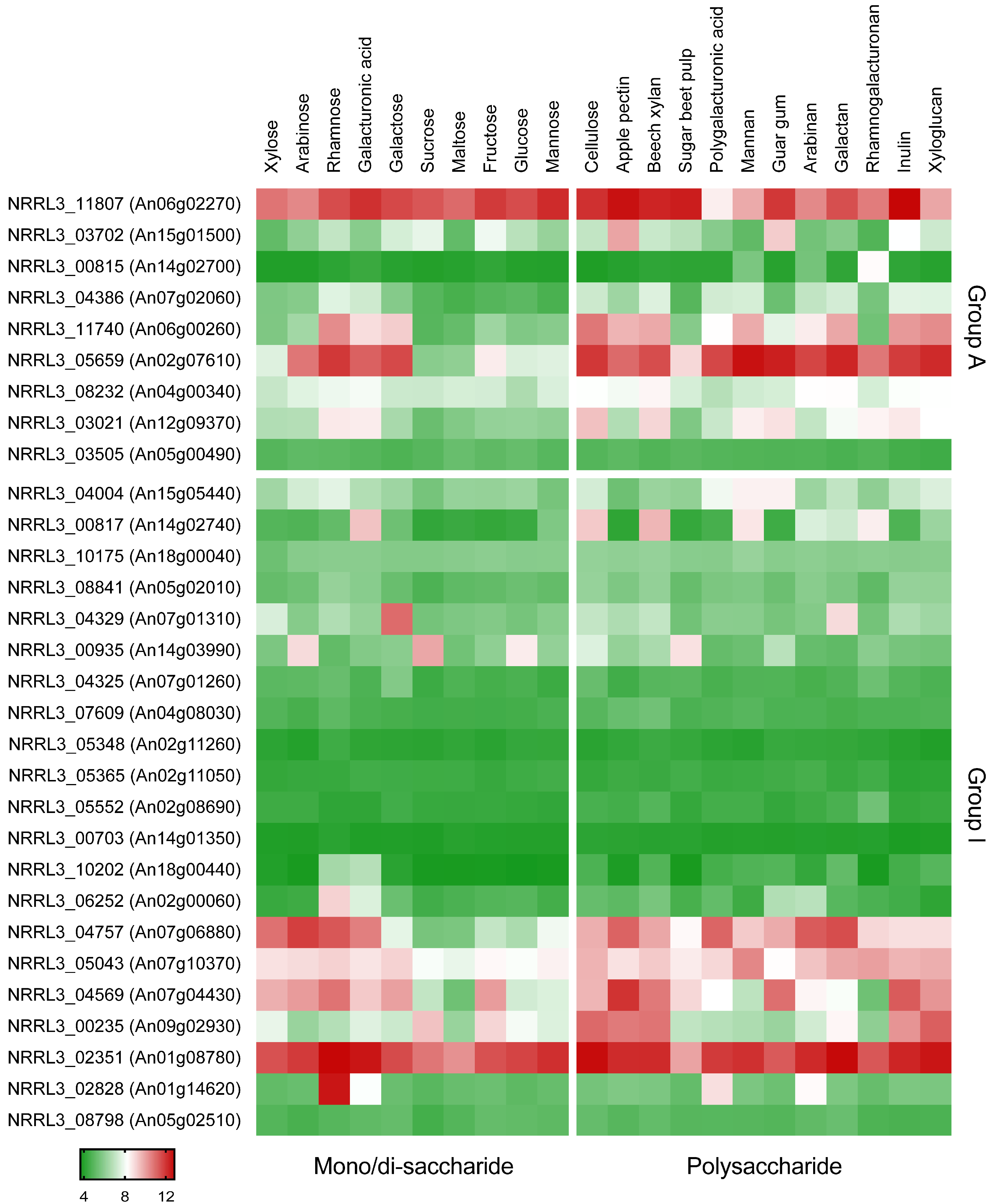
Figure 1.
Expression profiles of putative sugar transporters in A. niger on diverse carbon sources [21]. Protein IDs of A. niger NRRL3 are shown in the figure, and numbers in brackets reflect protein IDs from A. niger CBS 513.88. The color code represents averaged and logged expression values (FPKM + 1) of replicates. The heat map was drawn using GraphPad Prism (https://www.graphpad.com/, accessed on 11 January 2023). Group A and I refer to the phylogenetic grouping of transporters, as described previously [21].

Table 1.
A. niger strains used in this study.
Table 1.
A. niger strains used in this study.
| Strain | CBS Number | Genotype | Reference |
|---|---|---|---|
| N593 Δku70 | CBS 138852 | cspA1, kusA::amdS, pyrG¯ | [29] |
| ΔlatA | CBS 147737 | cspA1, kusA::amdS, pyrG¯, latA¯ | This study |
| Δ05659 | CBS 147735 | cspA1, kusA::amdS, pyrG¯, 05659¯ | This study |
| ΔladAΔxdhAΔsdhA | CBS 144672 | cspA1, kusA::amdS, pyrG¯, ladA¯, xdhA¯, sdhA¯ | [10] |
| ΔladAΔxdhAΔsdhAΔlatA | CBS 149003 | cspA1, kusA::amdS, pyrG¯, ladA¯, xdhA¯, sdhA¯, latA¯ | This study |
2.3. Transfer and Sugar Consumption Experiments
A total of 1 × 106 spores/mL were inoculated to 250 mL CM with 2% d-fructose in 1 L Erlenmeyer flasks for precultures and incubated in rotary shakers (Infors, Basel, Switzerland) at 30 °C and 250 rpm for 16–18 h. The mycelia were harvested by filtration on Miracloth (Sigma-Aldrich) under sterile conditions and washed with MM. Equal amounts of mycelia were transferred to 50 mL MM in 250 mL Erlenmeyer flasks containing 1% wheat bran or 1% sugar beet pulp and were incubated in rotary shakers at 30 °C, 250 rpm. The transfer experiments were performed in triplicate. In total, 2 mL culture liquid was harvested after 0, 4, 8, 24, 32, 48, 56, 72 and 80 h, and supernatants were stored at −20 °C after centrifugation for measurement of extracellular xylitol and l-arabitol concentrations. The same approach was used for sugar consumption experiments, in which 25 mM l-arabitol, 25 mM l-arabinose, 25 mM xylitol, 25 mM d-xylose, 25 mM galactitol, 25 mM d-galactose and a mixture of 25 mM l-arabitol and 25 mM xylitol were used as the substrates. In total, 2 mL culture liquid was harvested after 0, 1, 2, 4, 8 and 24 h to measure concentrations of monosaccharides and polyols that were used as carbon sources in liquid cultures.
2.4. Quantification of Monosaccharides and Polyols
The culture liquid samples were heated at 95 °C for 15 min and centrifuged for 5 min at 14,000 rpm. The supernatants were 10-fold diluted with MilliQ water (Merck, Amsterdam, The Netherlands) prior to analysis of xylitol and l-arabitol by HPLC (Dionex ICS-5000 + system; Thermo Scientific, Nieuwegein, The Netherlands) equipped with CarboPac PA1 column (2 × 250 mm with 2 × 50 mm guard column; Thermo Scientific), as described previously [30]. All selected monosaccharides and polyols as mentioned above with concentrations of 5–250 μM were used as standards for identification and quantitation.
2.5. Phylogenetic Analysis
Homologs of LatA from other fungi were obtained using BLASTP based on the amino acid sequence of A. niger LatA (NRRL3_04757) on the MycoCosm database (https://mycocosm.jgi.doe.gov/mycocosm/home, accessed on 12 February 2022). In this phylogenetic analysis, eight Eurotiomycetes (A. niger NRRL3, Aspergillus nidulans FGSC A4, Aspergillus oryzae RIB40, Penicillium rubens Wisconsin 54-1255, Aspergillus tubingensis v1.0, Aspergillus carbonarius ITEM 5010 v3, Aspergillus fumigatus Af293 and Penicillium subrubescens FBCC1632/CBS 132785), three Sordariomycetes (Neurospora crassa OR74A v2.0, Trichoderma reesei QM6a and Fusarium oxysporum f. sp. Lycopersici 4287 v2), one Leotiomycete (Botrytis cinerea v1.0), two Dothideomycetes (Phaeosphaeria nodorum SN15 v2.0 and Pseudocercospora (Mycosphaerella) fijiensis v2.0) and two Saccharomycetes (Candida albicans SC5314 and Saccharomyces cerevisiae S288C) species were selected, and the best hits were used for construction of a phylogenetic tree. The specific l-arabinose transporter Lat2 from A. monospora was also included in the phylogenetic analysis [19]. All collected sequences were aligned using MAFFT v7.0 (https://www.ebi.ac.uk/Tools/msa/mafft/) [31]. Phylogenetic analysis was computed using the Neighbor joining method with 500 bootstraps of the Molecular Evolutionary Genetics Analysis (MEGA v7.0, https://www.megasoftware.net/) program [32].
3. Results and Discussion
3.1. Identification of Putative l-Arabitol Transporters
In a previous study [21], 86 sugar transporters were proposed in A. niger CBS 513.88 and 30 predicted transporters grouped to Clade A and Clade I mainly containing inositol/fructose and pentose/glycerol transporters, respectively. The gene NRRL3_04757 (An07g06880) is a homolog of Lat2 of A. monospora, which is a characterized l-arabinose transporter that is also able to transport l-arabitol [18,19]. The gene NRRL3_05659 (An02g07610) is the homolog of two polyol/H+ symporters Syl1 and Syl2 from D. hansenii [20]. We assumed that the uptake of l-arabitol would be co-regulated with l-arabinose catabolism since l-arabitol has been shown to be the inducer of AraR, the l-arabinose-related transcriptional activator [9]. As we already had transcriptome data available for a set of monosaccharides [21], we evaluated this data for the expression profiles of sugar transporter genes on different carbon sources (Figure 1). This showed that NRRL3_04757 and NRRL3_05659 were highly expressed on l-arabinose. While the expression of NRRL_04757 was highest on l-arabinose, NRRL_05659 had the highest expression on l-rhamnose. The expression level of NRRL3_04757 was significantly reduced in single deletion mutants of two transcriptional activators AraR and RhaR, respectively, showing that it is regulated by AraR on l-arabinose and by RhaR on l-rhamnose [21]. Since no other sugar transporters had clear expression patterns pointing to putative l-arabitol transporters, the transporters NRRL3_04757 and NRRL3_05659 were selected as putative l-arabitol transporters.
3.1.1. Deletion of latA Resulted in Impaired Growth on l-Arabitol
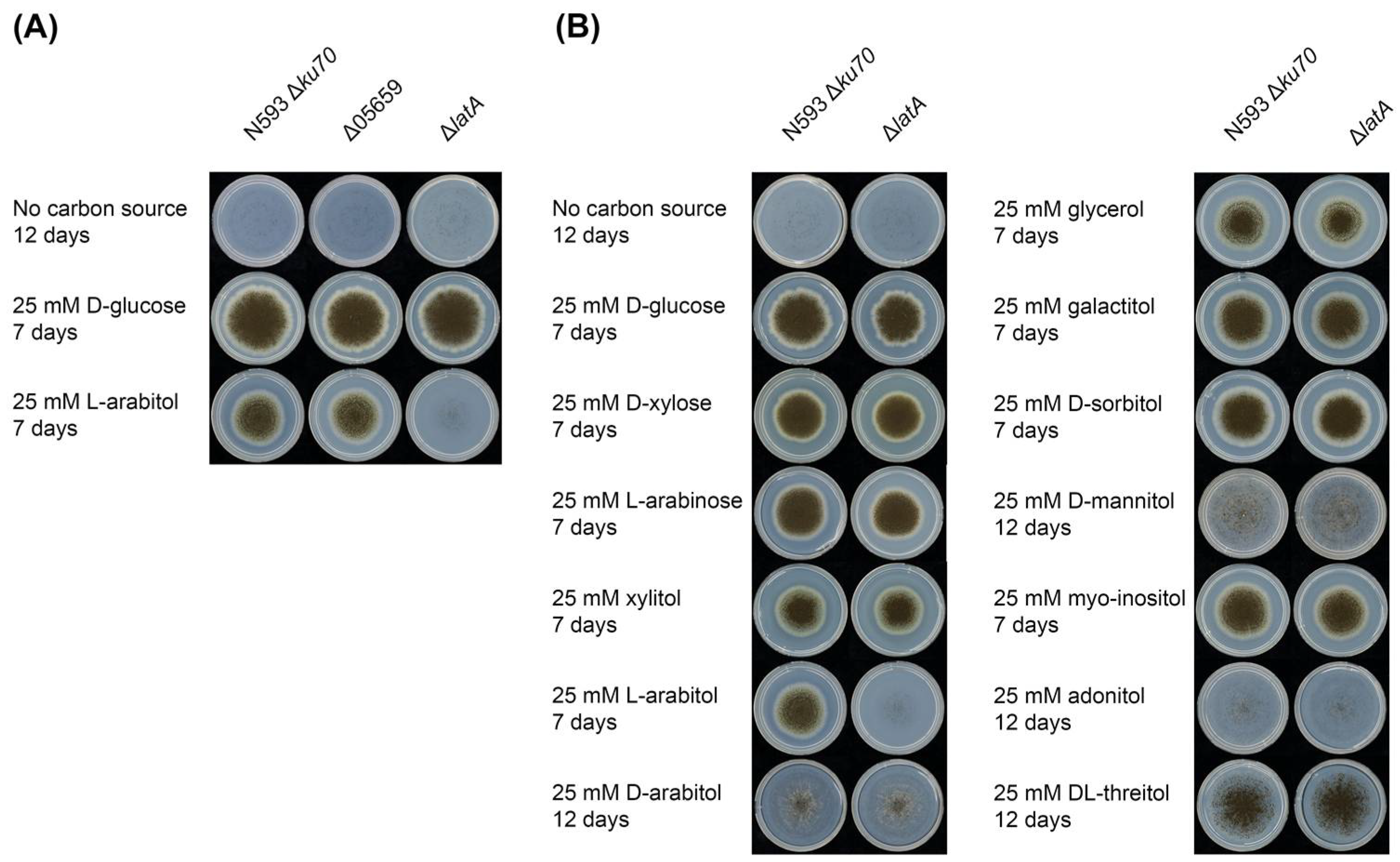
To confirm the function of NRRL3_04757 and NRRL3_05659, single deletion mutants were generated in the A. niger N593 Δku70 reference strain, and their growth on l-arabitol was compared to the reference strain (Figure 2A). The deletion mutant of NRRL3_04757 (ΔlatA) showed impaired growth on l-arabitol compared to the reference strain, indicating that NRRL3_04757 is a main l-arabitol transporter under this condition, and the gene was therefore named latA. The strong phenotype suggests that there is no redundancy in l-arabitol transport in A. niger, which is a clear contrast with the higher number of d-glucose and d-xylose transporters [33,34,35]. A possible explanation for this is that d-glucose and d-xylose are abundantly present in natural carbon sources of A. niger, while l-arabitol is an intermediate of the PCP and, therefore, much less abundant in the natural biotope of this fungus. In contrast, the NRRL3_05659 deletion strain grew identical to the reference strain on l-arabitol (Figure 2A), indicating that NRRL3_05659 is not involved in l-arabitol transport.
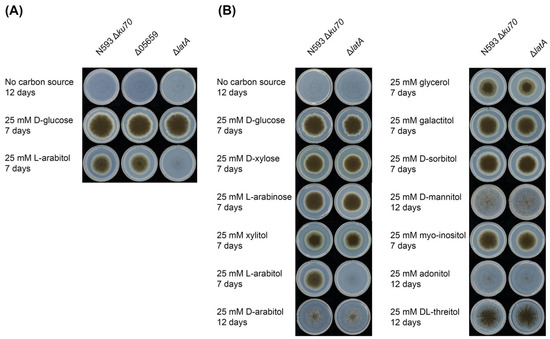
Figure 2.
Phenotypic analysis of mutant and reference strains. (A) Growth profiling of the A. niger reference strain N593 Δku70 and two single deletion mutants Δ05659 and ΔlatA on l-arabitol. (B) Growth profiling of the A. niger reference strain N593 Δku70 and ΔlatA on different sugars and polyols.
The effect of LatA on the growth of A. niger on several pentoses and polyols was also investigated (Figure 2B). No changes in phenotype were observed on any of the other tested compounds, suggesting that LatA is highly specific for l-arabitol. However, we cannot exclude that transport of (some of) these other polyols could be mediated by multiple transporters, affecting a possible phenotype of ΔlatA on these substrates. However, this would mean that LatA has a minor contribution to their transport, as at least reduced growth would otherwise be expected. Production of LatA in a heterologous host (e.g., S. cerevisiae) could shed more light on the range of compounds it is able to transport.
In a previous study [21], LatA (NRRL3_04757, An07g06880) was assigned to Sugar Transporter Group I of A. niger, containing pentose or glycerol transporters. LatA is the closest homolog of Lat2 of A. monospora [21]). Lat2 is capable of transporting l-arabinose [18], but no phenotype on this sugar was observed for the latA deletion strain in our study (Figure 2B). As there may be additional l-arabinose transporters in A. niger, we cannot exclude at this time that LatA may also contribute to l-arabinose transport. The expression data (Figure 1) showed that latA was induced by d-xylose and l-arabinose and regulated by AraR on l-arabinose [21], which could support a role for LatA as a pentose transporter. However, since l-arabitol catabolism is also stimulated by l-arabinose and l-arabitol is an intermediate of this pathway, the expression profile also supports the role as a specific l-arabitol transporter.
3.1.2. Deletion of latA Only Affected l-Arabitol Consumption
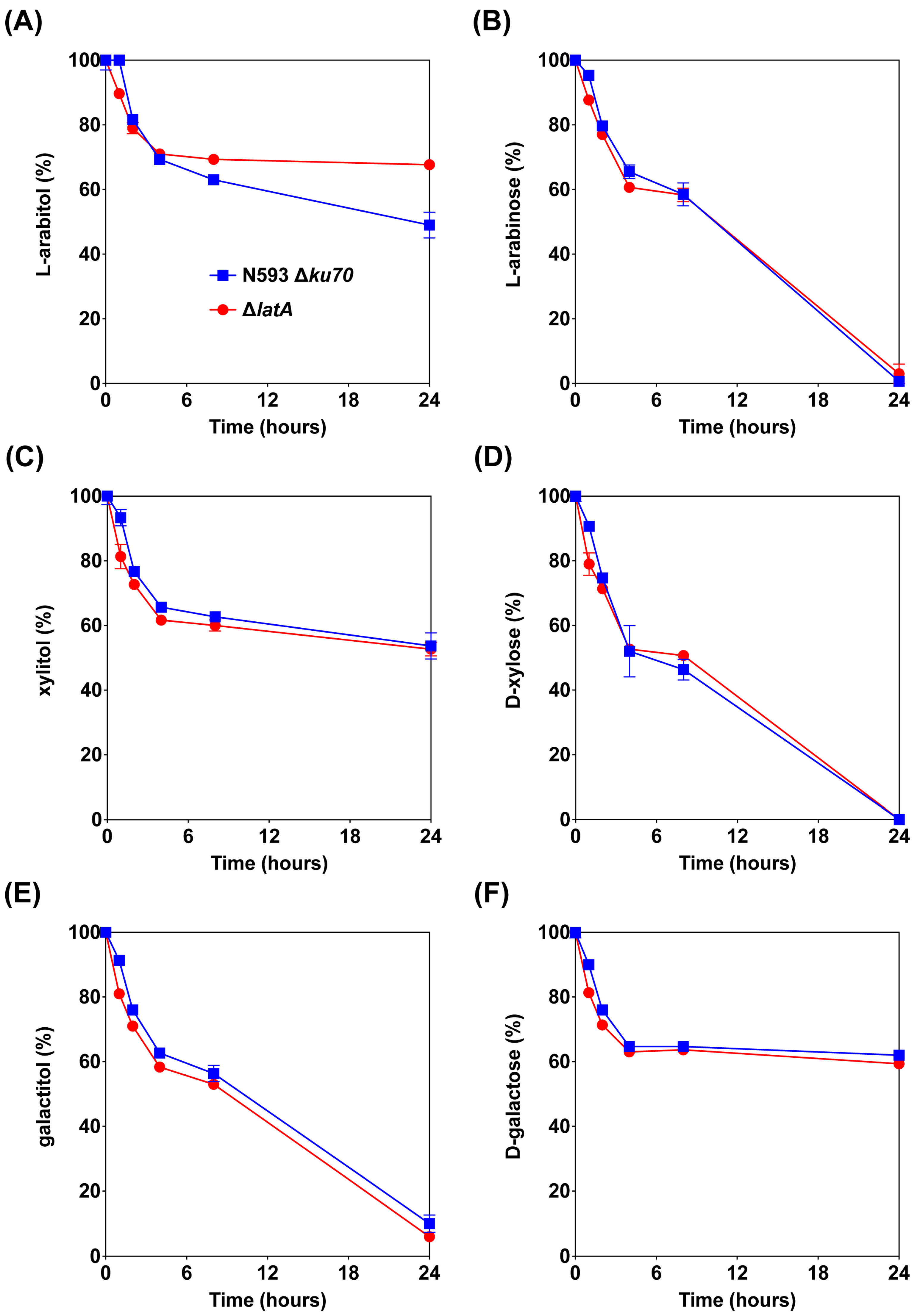
The sugar consumption of the reference strain N593 Δku70 and the mutant ΔlatA was also determined in liquid cultures to study the transport function of LatA on l-arabitol, l-arabinose, xylitol, d-xylose, galactitol and d-galactose (Figure 3). An initial decrease in the extracellular concentrations of all compounds was observed in both strains during the first 4 h of cultivation, which is likely due to absorption to the mycelium. At later time points, the deletion of latA only had an obvious effect on l-arabitol consumption, as this was almost abolished after 4 h of cultivation in the mutant ΔlatA compared to the reference strain (Figure 3A). This suggests that LatA is a highly specific l-arabitol transporter and plays a predominant role in l-arabitol utilization in A. niger under these conditions. This is also consistent with the findings of growth profiling (Figure 2). The deletion of latA did not affect l-arabinose utilization (Figure 3B), so we can exclude a function for LatA in l-arabinose transport.

Figure 3.
Consumption of sugars and polyols by the A. niger reference strain and ΔlatA in liquid cultures supplemented with l-arabitol (A), l-arabinose (B), xylitol (C), d-xylose (D), galactitol (E) and d-galactose (F) as the substrates. The error bars indicate the standard deviation between biological triplicates.
Moreover, the mixture of l-arabitol and xylitol was also used as the substrate to study whether the presence of both compounds affects each other’s uptake in the reference strain and the mutant ΔlatA (Figure S1). After the initial reduction in the first 4 h, no l-arabitol was consumed in ΔlatA, while the presence of xylitol slightly decreased the l-arabitol utilization in the reference strain. The deletion of latA did not affect xylitol utilization on the mixture of l-arabitol and xylitol.
3.1.3. The deletion of latA Positively Affected l-Arabitol Production from Wheat Bran and Sugar Beet Pulp
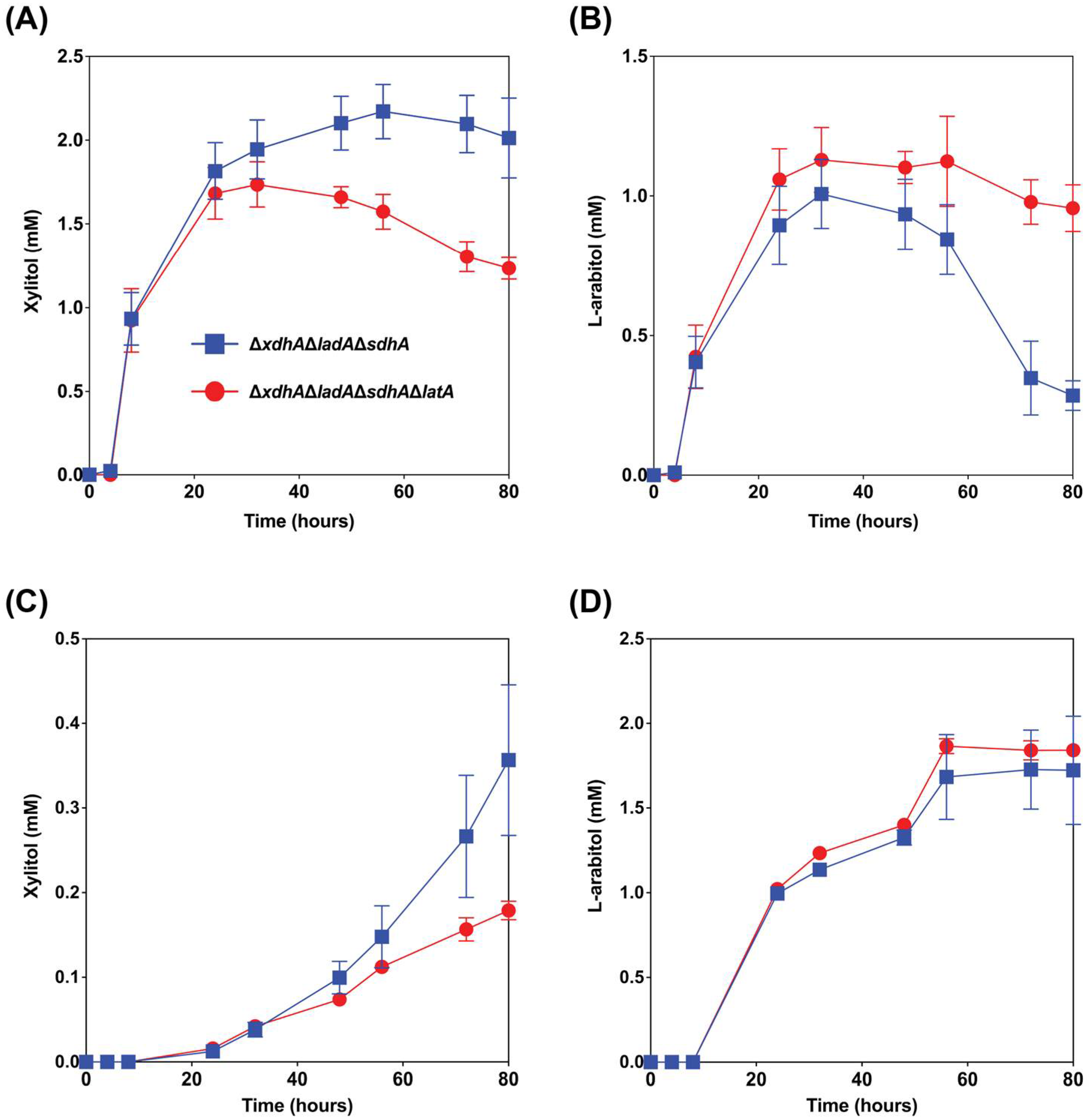
The reference strain N593 Δku70 barely accumulates l-arabitol during growth on wheat bran, as this is metabolized intracellularly [10,12]. In the ΔladAΔxdhAΔsdhA triple mutant, the conversion of l-arabitol to l-xylulose is blocked, and as a result, this mutant accumulates l-arabitol and xylitol during growth on wheat bran and secretes this [10,12]. However, we observed that after some time, l-arabitol is taken up again by the fungus [12]. Deleting latA would potentially prevent re-consumption of l-arabitol by A. niger stimulating l-arabitol production in this metabolically engineered strain. To test this, we deleted latA in the strain ΔladAΔxdhAΔsdhA. The results showed that the deletion of latA in the triple mutant increased the maximum titer of l-arabitol from wheat bran by 12% (Figure 4B). The l-arabitol level decreased in the triple mutant after 32 h of cultivation, most likely by re-consumption, while this was delayed until 56 h when latA was also deleted. It confirmed that deletion of latA can reduce/delay re-consumption l-l-arabitol when other carbon sources are depleted.
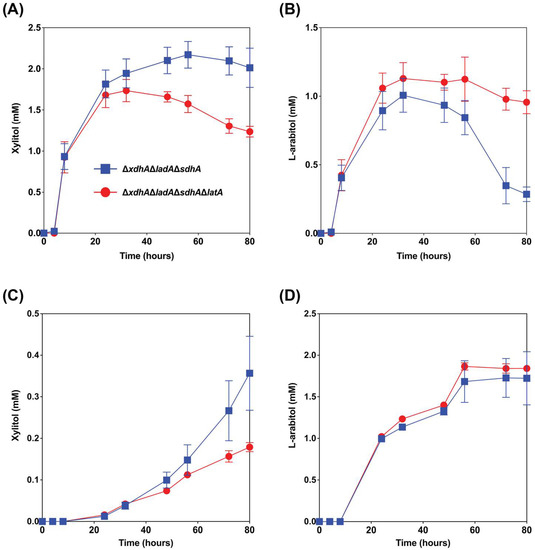
Figure 4.
Extracellular xylitol and l-arabitol concentrations from wheat bran ((A) and (B), respectively) and sugar beet pulp ((C) and (D), respectively) cultures of the A. niger mutant strains ΔladAΔxdhAΔsdhA and ΔladAΔxdhAΔsdhAΔlatA. The error bars indicate the standard deviation between biological triplicates.
In the previous study, l-arabitol production from sugar beet pulp in the triple mutant was up to 2-fold higher than from wheat bran and other studied crude biomass [12]. The proportion of l-arabinose in sugar beet pulp is higher than in wheat bran, and conversely, the content of d-xylose in wheat bran is significantly higher than in sugar beet pulp (Table 2). This difference in composition of these two substrates can explain why the maximum titer of l-arabitol (2.0 mM) from sugar beet pulp was higher than from wheat bran (1.0 mM) in ΔladAΔxdhAΔsdhA strain. While a small increase in l-arabitol titer from sugar beet pulp was observed when latA was also deleted, this is not statistically significant (Figure 4D). The largest effect of the latA deletion was in fact seen on wheat bran, where re-consumption of l-arabitol after 60 h was strongly reduced (Figure 4B).

Table 2.
Composition of wheat bran and sugar beet pulp (mol%) (Adapted from [36]).
Conversely, the extracellular xylitol titer was decreased by almost 50% after 80 h of cultivation when latA was deleted (Figure 4A,C), most likely due to increased re-consumption, possibly because l-arabitol cannot be re-consumed.
Our results demonstrate that the deletion of latA in a xylitol and l-arabitol producing strain has a moderate increase on the l-arabitol concentration, indicating that this gene is a relevant component of a strain engineering approach to generate polyol cell factories.
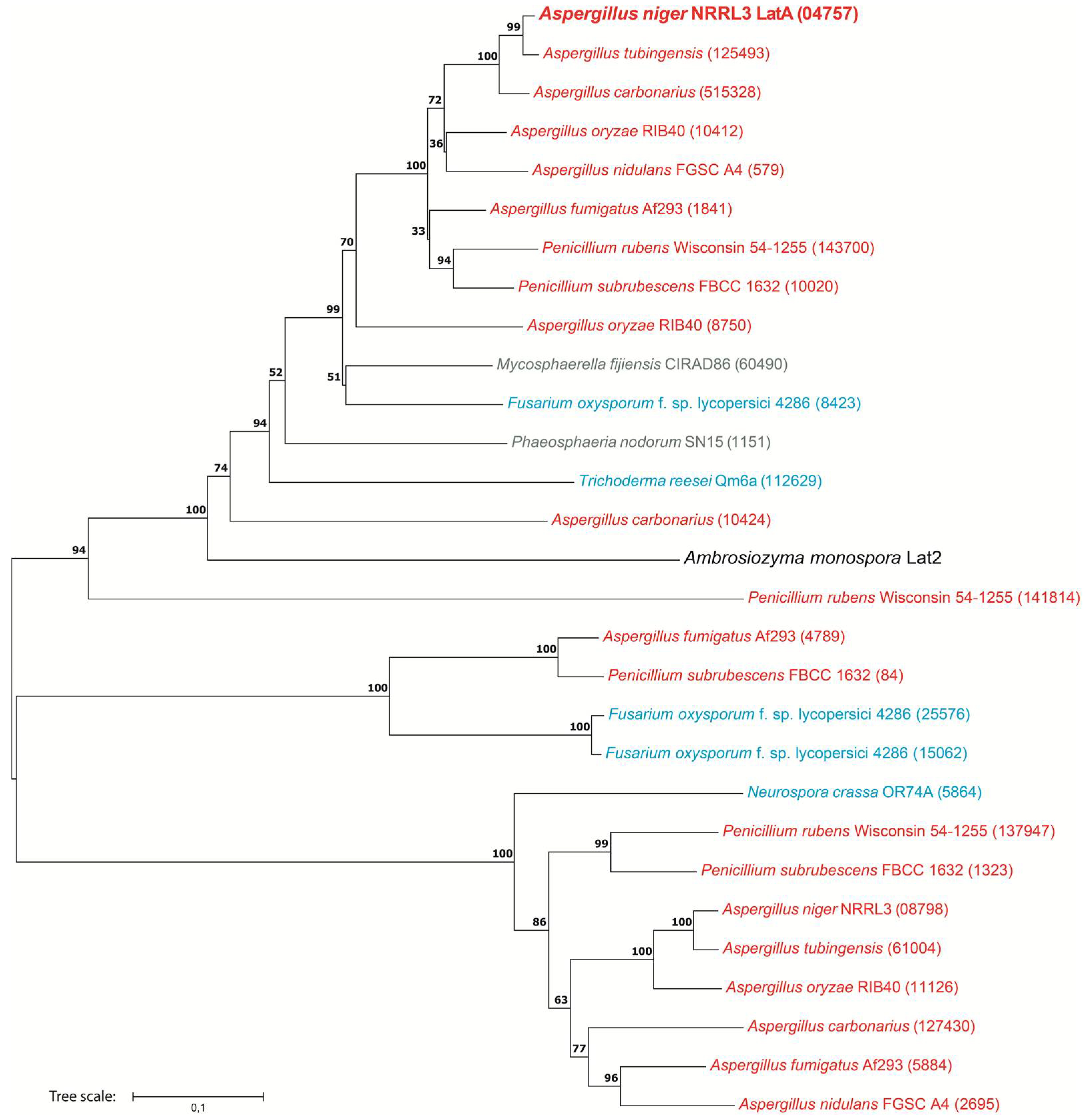
3.1.4. LatA Is Widely Present in Ascomycete Fungi
Homologs of LatA are present in most species selected for our analysis, except B. cinerea, C. albicans and S. cerevisiae (Figure 5), indicating that homologs of LatA are widely present across the phylum Ascomycota. The phylogenetic analysis provided many candidate polyol transporters for further characterization in other fungi. Some species contain multiple candidate l-arabitol transporters, perhaps for enhancing the activity to transport polyols [37]. However, care should be taken in assigning function to these homologs, as already, the functional characterization of Lat2 from A. monospora [19] suggests differences in substrate specificity between these transporters.

Figure 5.
Unrooted phylogenetic tree of homologs of LatA from A. niger. The tree includes a specific l-arabinose transporter Lat2 from A. monospora (shown in larger font and black), the characterized l-arabitol importer LatA in A. niger (shown in larger font and boldface) and homologs of LatA in selected fungi. Bootstrap values are indicated on the nodes, which are supported by the Neighbor joining method. Colors represent the fungal taxonomic groups. Red = Eurotiomycetes, blue = Sordariomycetes, grey = Dothideomycetes. Numbers in brackets reflect the protein ID from JGI MycoCosm [38].
In conclusion, we identified a highly specific l-arabitol transporter in A. niger that contains homologs across Ascomycota and has potential as a target for metabolic engineering of l-arabitol producing fungal cell factories.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom13020188/s1, Table S1: Primers used in this study. Table S2: Composition of wheat bran and sugar beet pulp (mol%). Figure S1: The polyol consumption of the A. niger reference strain N593 Δku70 and the mutant ΔlatA in the liquid culture containing a mixture of l-arabitol and xylitol as the substrate.
Author Contributions
Conceptualization, R.P.d.V.; methodology, R.P.d.V. and J.M.; formal analysis, R.P.d.V. and J.M.; investigation, J.M.; writing—original draft preparation, J.M.; writing—review and editing, R.P.d.V. and M.R.M.; supervision, R.P.d.V. and M.R.M.; project administration, R.P.d.V.; funding acquisition, R.P.d.V., J.M. and M.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chinese Scholarship Council (CSC), grant number CSC201907720027 to J.M. and Novo Nordisk Foundation, grant number NNF21OC0067087 to M.R.M.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jennings, D.B.; Ehrenshaft, M.; Pharr, D.M.; Williamson, J.D. Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proc. Natl. Acad. Sci. USA 1998, 95, 15129–15133. [Google Scholar] [CrossRef]
- Lewis, D.H.; Smith, D.C. Sugar alcohols (polyols) in fungi and green plants. I. Distribution, physiology and metabolism. New Phytol. 1967, 66, 143–184. [Google Scholar] [CrossRef]
- Monedero, V.; Perez-Martinez, G.; Yebra, M.J. Perspectives of engineering lactic acid bacteria for biotechnological polyol production. Appl. Microbiol. Biotechnol. 2010, 86, 1003–1015. [Google Scholar] [CrossRef]
- Mortlock, R.P.; Fossitt, D.D.; Wood, W.A. A basis for utlization of unnatural pentoses and pentitols by Aerobacter aerogenes. Proc. Natl. Acad. Sci. USA 1965, 54, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Kordowska-Wiater, M. Production of arabitol by yeasts: Current status and future prospects. J. Appl. Microbiol. 2015, 119, 303–314. [Google Scholar] [CrossRef] [PubMed]
- de Groot, M.J.L.; van den Dool, C.; Wosten, H.A.B.; Levisson, M.; vanKuyk, P.A.; Ruijter, G.J.G.; de Vries, R.P. Regulation of pentose catabolic pathway genes of Aspergillus niger. Food Technol. Biotechnol. 2007, 45, 134–138. [Google Scholar]
- Mojzita, D.; Penttila, M.; Richard, P. Identification of an l-arabinose reductase gene in Aspergillus niger and its role in l-arabinose catabolism. J. Biol. Chem. 2010, 285, 23622–23628. [Google Scholar] [CrossRef]
- Battaglia, E.; Hansen, S.F.; Leendertse, A.; Madrid, S.; Mulder, H.; Nikolaev, I.; de Vries, R.P. Regulation of pentose utilisation by AraR, but not XlnR, differs in Aspergillus nidulans and Aspergillus niger. Appl. Microbiol. Biotechnol. 2011, 91, 387–397. [Google Scholar] [CrossRef]
- Battaglia, E.; Visser, L.; Nijssen, A.; van Veluw, J.; Wösten, H.A.B.; de Vries, R.P. Analysis of regulation of pentose utilisation in Aspergillus niger reveals evolutionary adaptations in the Eurotiales. Stud. Mycol. 2011, 69, 31–38. [Google Scholar] [CrossRef]
- Chroumpi, T.; Peng, M.; Aguilar-Pontes, M.V.; Muller, A.; Wang, M.; Yan, J.; Lipzen, A.; Ng, V.; Grigoriev, I.V.; Makela, M.R.; et al. Revisiting a ‘simple’ fungal metabolic pathway reveals redundancy, complexity and diversity. Microb. Biotechnol. 2021, 14, 2525–2537. [Google Scholar] [CrossRef]
- Witteveen, C.F.B.; Busink, R.; van de Vondervoort, P.; Dijkema, C.; Swart, K.; Visser, J. l-arabinose and d-xylose catabolism in Aspergillus niger. J. Gen. Microbiol. 1989, 135, 2163–2171. [Google Scholar] [CrossRef][Green Version]
- Meng, J.; Chroumpi, T.; Mäkelä, M.R.; de Vries, R.P. Xylitol production from plant biomass by Aspergillus niger through metabolic engineering. Bioresour. Technol 2021, 344, 126199. [Google Scholar] [CrossRef] [PubMed]
- Noiraud, N.; Maurousset, L.; Lemoine, R. Transport of polyols in higher plants. Plant Physiol. Biochem. 2001, 39, 717–728. [Google Scholar] [CrossRef]
- Reinders, A.; Panshyshyn, J.A.; Ward, J.W. Analysis of transport activity of Arabidopsis sugar alcohol permease homolog AtPLT5. J. Biol. Chem. 2005, 280, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Oesterhelt, C. Structurally reduced monosaccharide transporters in an evolutionarily conserved red alga. Biochem. J. 2007, 406, 325–331. [Google Scholar] [CrossRef]
- Heuel, H.; Shakeri-Garakani, A.; Turgut, S.; Lengeler, J.W. Genes for d-arabinitol and ribitol catabolism from Klebsiella pneumoniae. Microbiology 1998, 144 Pt 6, 1631–1639. [Google Scholar] [CrossRef]
- Jordan, P.; Choe, J.Y.; Boles, E.; Oreb, M. Hxt13, Hxt15, Hxt16 and Hxt17 from Saccharomyces cerevisiae represent a novel type of polyol transporters. Sci. Rep. 2016, 6, 23502. [Google Scholar] [CrossRef]
- Londesborough, J.; Richard, P.; Valkonen, M.; Viljanen, K. Effect of C-terminal Protein tags on pentitol and l-arabinose transport by Ambrosiozyma monospora Lat1 and Lat2 transporters in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2014, 80, 2737–2745. [Google Scholar] [CrossRef]
- Verho, R.; Penttila, M.; Richard, P. Cloning of two genes (LAT1,2) encoding specific l-arabinose transporters of the l-arabinose fermenting yeast Ambrosiozyma monospora. Appl. Biochem. Biotechnol. 2011, 164, 604–611. [Google Scholar] [CrossRef]
- Pereira, I.; Madeira, A.; Prista, C.; Loureiro-Dias, M.C.; Leandro, M.J. Characterization of new polyol/H+ symporters in Debaryomyces hansenii. PLoS ONE 2014, 9, e88180. [Google Scholar] [CrossRef]
- Peng, M.; Aguilar-Pontes, M.V.; de Vries, R.P.; Mäkelä, M.R. In silico analysis of putative sugar transporter genes in Aspergillus niger using phylogeny and comparative transcriptomics. Front. Microbiol. 2018, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, L.; Li, H.; Tian, C.; Ma, Y. Transcriptional comparison of the filamentous fungus Neurospora crassa growing on three major monosaccharides d-glucose, d-xylose and l-arabinose. Biotechnol. Biofuels 2014, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, S.; Zhao, H. Discovery and characterization of novel d-xylose-specific transporters from Neurospora crassa and Pichia stipitis. Mol. Biosyst. 2010, 6, 2150–2156. [Google Scholar] [CrossRef]
- Ferreira, C.; van Voorst, F.; Martins, A.; Neves, L.; Oliveira, R.; Kielland-Brandt, M.C.; Lucas, C.; Brandt, A. A member of the sugar transporter family, Stl1p is the glycerol/H+ symporter in Saccharomyces cerevisiae. Mol. Biol. Cell. 2005, 16, 2068–2076. [Google Scholar] [CrossRef]
- Song, L.; Ouedraogo, J.P.; Kolbusz, M.; Nguyen, T.T.M.; Tsang, A. Efficient genome editing using tRNA promoter-driven CRISPR/Cas9 gRNA in Aspergillus niger. PLoS ONE 2018, 13, e0202868. [Google Scholar] [CrossRef]
- Kowalczyk, J.E.; Lubbers, R.J.M.; Peng, M.; Battaglia, E.; Visser, J.; de Vries, R.P. Combinatorial control of gene expression in Aspergillus niger grown on sugar beet pectin. Sci. Rep. 2017, 7, 12356. [Google Scholar] [CrossRef]
- de Vries, R.; Burgers, K.; van de Vondervoort, P.; Frisvad, J.; Samson, R.; Visser, J. A new black Aspergillus species, A. vadensis, is a promising host for homologous and heterologous protein production. Appl. Environ. Microbiol. 2004, 70, 3954–3959. [Google Scholar] [CrossRef] [PubMed]
- Gruben, B.S.; Mäkelä, M.R.; Kowalczyk, J.E.; Zhou, M.M.; Benoit-Gelber, I.; De Vries, R.P. Expression-based clustering of CAZyme-encoding genes of Aspergillus niger. BMC Genom. 2017, 18, 900. [Google Scholar] [CrossRef]
- Meyer, V.; Arentshorst, M.; El-Ghezal, A.; Drews, A.C.; Kooistra, R.; van den Hondel, C.A.; Ram, A.F. Highly efficient gene targeting in the Aspergillus niger kusA mutant. J. Biotechnol. 2007, 128, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.R.; Bouzid, O.; Robl, D.; Post, H.; Peng, M.; Heck, A.; Altelaar, M.; de Vries, R.P. Cultivation of Podospora anserina on soybean hulls results in an efficient enzyme cocktail for plant biomass hydrolysis. New Biotechnol. 2017, 37, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2015, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Sloothaak, J.; Odoni, D.I.; de Graaff, L.H.; Martins Dos Santos, V.A.P.; Schaap, P.J.; Tamayo-Ramos, J.A. Aspergillus niger membrane-associated proteome analysis for the identification of glucose transporters. Biotechnol. Biofuels 2015, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Sloothaak, J.; Tamayo-Ramos, J.A.; Odoni, D.I.; Laothanachareon, T.; Derntl, C.; Mach-Aigner, A.R.; Martins Dos Santos, V.A.P.; Schaap, P.J. Identification and functional characterization of novel xylose transporters from the cell factories Aspergillus niger and Trichoderma reesei. Biotechnol. Biofuels 2016, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- vanKuyk, P.A.; Diderich, J.A.; MacCabe, A.P.; Hererro, O.; Ruijter, G.J.G.; Visser, J. Aspergillus niger mstA encodes a high-affinity sugar/H+ symporter which is regulated in response to extracellular pH. Biochem. J. 2004, 379, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Ozturkoglu Budak, S.; Zhou, M.; Brouwer, C.; Wiebenga, A.; Benoit, I.; Di Falco, M.; Tsang, A.; de Vries, R.P. A genomic survey of proteases in Aspergilli. BMC Genom. 2014, 15, 523. [Google Scholar] [CrossRef]
- Yoshino, K.; Yamamoto, K.; Hara, K.; Sonoda, M.; Yamamoto, Y.; Sakamoto, K. The conservation of polyol transporter proteins and their involvement in lichenized Ascomycota. Fungal Biol. 2019, 123, 318–329. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Nikitin, R.; Haridas, S.; Kuo, A.; Ohm, R.; Otillar, R.; Riley, R.; Salamov, A.; Zhao, X.; Korzeniewski, F.; et al. MycoCosm portal: Gearing up for 1000 fungal genomes. Nucleic Acids Res. 2014, 42, D699–D704. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).