Abstract
OA is a common and debilitating condition that restricts mobility and diminishes the quality of life. Recent work indicates that the generation of adenosine at the cell surface is an important mediator of chondrocyte homeostasis, and topical application of adenosine in a slow-release form (liposomes) can halt the progression of OA and diminish the pain associated with OA. Here, we review the evidence indicating that adenosine, acting at A2A receptors, plays a critical role in endogenous and exogenous treatment and reversal of OA.
1. Osteoarthritis
Osteoarthritis (OA), a form of degenerative arthritis, is the most common type of arthritis. OA affects as many as 54 million people in the United States and 528 million people worldwide (http://ghdx.healthdata.org/gbd-results-tool accessed on 15 October 2023). The main symptoms of OA include pain, stiffness, and swelling of the affected joint, leading to increasing disability. Age, female gender, prior trauma to the affected joint, anatomic factors, and genetics may all contribute to the development of OA. Inflammation, osteophytes, bone changes, and loss of cartilage are all present in the joints of people with OA [1].
A leading cause of disability worldwide, the disease drives millions to seek therapeutic interventions. However, at present, there is no definitive medical treatment to prevent or reverse the progression of OA. The injection of corticosteroids and hyaluronic acid provides temporary relief without altering the course of the disease, and the number of joint replacements has increased dramatically and is expected to continue to increase [1].
The pathogenesis and treatment of OA have been studied in a variety of rodent models. As in humans, trauma may lead to the development of OA, and there are a number of post-traumatic OA models in which the affected joint is surgically disrupted. Age and degenerative models of OA are also used to study the treatment and pathogenesis of OA (obesity and intra-articular injection of monosodium iodoacetate) [2]. Various gene knockouts also develop spontaneous OA. All of these models have their flaws and advantages, and since no new therapies have been introduced based on the use of these models, it is difficult to determine the most useful model of OA. As discussed below, adenosine and adenosine receptor agonists have been studied in mice, rats, and dogs using spontaneous gene knockout), post-traumatic, and degenerative OA models [3,4].
2. Purine Metabolism, Receptors, and OA
The central player in OA is the chondrocyte. Chondrocytes respond to excess mechanical loading by releasing inflammatory mediators and proteolytic enzymes causing further cartilage damage [5]. In 2002, Tesch and colleagues reported that equine chondrocytes respond to adenosine A2 receptor stimulation and, in subsequent experiments, reported that the treatment of cartilage explants with adenosine deaminase led to a spontaneous expression of proteases associated with cartilage degeneration and the development of OA [6,7]. The interpretation of these results is complicated since cellular levels of adenosine deaminase in equine tissues approached those reported for children with adenosine deaminase deficiency and adenosine deaminase activity is undetectable in equine plasma [8,9,10,11]. The minimal adenosine deaminase levels in equine chondrocytes and plasma suggests that chondrocytes and cartilage might be disproportionately affected by endogenous adenosine levels.
In subsequent studies, it was reported that patients with a hereditary absence of CD73 activity with, presumably, lower adenosine levels at the cell surface lead to the premature development of osteoarthritis [12,13]. Mice lacking CD73 activity also develop spontaneous OA [4]. Similarly, mice lacking A2A receptors [4] and A3 receptors [14] develop spontaneous OA. Moreover, the intra-articular administration of a selective A2A agonist [3,4] and the administration of a selective A3 receptor agonist prevent the development of OA in murine models [14,15,16]. The anti-inflammatory effects of A3 receptors likely mediate the effects of the agent used on the development of OA [4]. Similarly, the deletion of A2A receptors leads, in mice, to the spontaneous development of OA [4], as does the deletion of CD73, in both mice and humans [4], but the effects of loss of A2A receptors also involve changes in chondrocyte physiology [3,4,17,18]. Moreover, with age and inflammation, chondrocytes have a reduced capacity to synthesize and maintain adenosine triphosphate (ATP). The levels of ATP and adenosine, its metabolite, fall after treatment of mouse chondrocytes and rat tibia explants with interleukin-1β (IL-1β), an inflammatory mediator that contributes to OA [4]. However, restoring the levels of adenosine with the intra-articular (IA) injection of long-acting adenosine with liposomal adenosine or adenosine-functionalized polymeric nanoparticles prevents the development of OA in a rat model of post-traumatic OA [19] and reverses cartilage damage in both a rat model of post-traumatic OA and an obesity-induced model of OA in mice via interaction with A2A receptors [3,4]. Furthermore, the IA administration of liposomal adenosine in dogs with OA, after medial meniscus release, led to a steady decrease in pain, with a corresponding gain in function for up to 6mo after treatment. Moreover, injections improved comfortable range of motion (CROM) to near-normal values at 6 mo. Compared to the placebo, the treatment also improved radiographic markers, and MRIs showed a significant slowing in OA progression, including improvement in cartilage morphology, synovitis, and osteophyte occurrence [20].
3. The Mechanisms by Which Adenosine Mitigates OA
Extracellular adenosine derives mainly from the hydrolysis of ATP (primarily, but not exclusively, by the ectoenzymes CD39 and CD73) and mediates its effects via the activation of G-protein-coupled receptors (A1, A2A, A2B and A3). However, the chondroprotective effects of long-acting adenosine are exclusively mediated via A2A receptors [4]. A2A stimulation mediates its effects on chondrocytes, cartilage, and OA via multiple mechanisms. The deletion of A2A leads to the differential expression of genes associated with senescence, aging, and inflammation in neonatal murine chondrocytes [21]. In addition, A2A stimulation resensitizes TGF-β receptors, which, in OA, do not transmit signals in chondrocytes [3], promotes mitophagy, increases mitochondrial function (and ATP production [18]), and stimulates autophagy via a FoxO-dependent mechanism [22]. When studied in vitro, A2A stimulation prevents both spontaneous and oxidant-mediated cellular senescence in chondrocytic cells, diminishes the nuclear localization of the senescence markers/mediators p16 and p21, reduces the cellular expression of full-length p53, and increases the expression of the D133p53 splice variant of p53, the expression of which has potent anti-senescent effects [23]. Moreover, as noted above, in chondrocytes from A2A-deficient mice, there is an increased expression of genes associated with cellular senescence, downregulation of autophagy, enhanced expression of inflammatory genes, increased expression of genes coding for proteases that digest the matrix, and diminished matrix protein gene expression [21]. These findings are consistent with the hypothesis that autocrine activation of A2A is critical for the maintenance of chondrocyte and cartilage homeostasis (Figure 1).
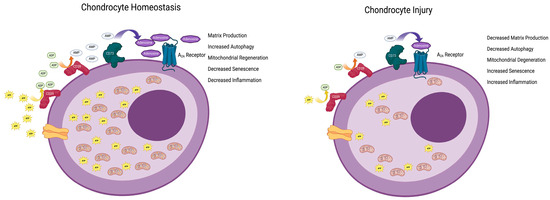
Figure 1.
Endogenous adenosine maintains chondrocyte homeostasis. ATP is transported into the extracellular space where it is sequentially dephosphorylated via the cell surface enzymes CD39 and CD73 (Ectonucleoside triphosphate diphosphohydrolase 1 and 5-nucleotidase, respectively) to adenosine. Adenosine stimulates the A2A adenosine receptor to diminish cellular aging and apoptosis and increase autophagy, mitophagy, and inflammation in chondrocytes, thereby promoting homeostasis. Following injury, there is less ATP transported into the extracellular space, leading to diminished adenosine concentrations resulting in diminished autophagy, mitophagy, and inflammation with increased senescence and apoptosis. Figure created with BioRender.com.
In contrast to the reported beneficial effects of adenosine on cartilage and chondrocytes, Mistry and colleagues reported that the treatment of susceptible mice with an adenosine deaminase inhibitor (thereby inducing an increase in adenosine levels) led to injury and death to chondrocytes with development of OA and in vitro studies demonstrated that this effect required adenosine uptake [24]. In studies of the mechanism of action by which shockwave therapy ameliorates OA, Tan and colleagues found that the A2B receptor expression is increased and that A2B receptor stimulation reduces chondrocyte differentiation in human mesenchymal stem cells [25]). These studies further suggested that the A2B receptor-mediated effects on chondrogenesis were linked to IL-6 expression and signaling. It should be noted that children lacking adenosine deaminase activity (with a marked increase in extracellular levels) do not develop OA but have short growth plates with few proliferating chondrocytes and chondrocyte necrosis [26]. Thus, extreme adenosine levels, such as those encountered in adenosine deaminase-deficient children, in the setting of chondrocyte injury or extracorporeal shockwave therapy may lead to diminished chondrogenesis via the stimulation of A2B receptors.
Further confirmation of the OA-protective role of adenosine and its receptors in cartilage is provided in a recent observational study in which patients treated with ticagrelor, a P2Y12 antagonist that also blocks adenosine uptake and increases local adenosine levels, were less likely to develop OA than patients treated with clopidogrel, a P2Y12 antagonist that does not inhibit adenosine uptake [27], a finding consistent with the role of adenosine in maintaining chondrocyte homeostasis. Other studies have suggested that increased ingestion of caffeine, a common constituent of food that is a non-selective adenosine receptor antagonist, is a risk factor for the development of OA (recently reviewed in [28]). Istradefylline is a selective A2A receptor antagonist used to treat Parkinson’s Disease and, based on the discussion above, would be expected to promote the development of OA. Insufficient data regarding the long-term effects of this drug, e.g., OA, have not been fully established.
4. Conclusions
The findings reviewed here strongly support the hypothesis that adenosine, acting at A2A and A3 receptors, plays a central role in maintaining chondrocyte homeostasis. Moreover, these studies suggest that adenosine receptors may be a new therapeutic target in the treatment of OA.
Author Contributions
B.N.C. and S.R.A. carried out the literature review, conceptualization, and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Work supported by the NYU-HHC Clinical Translational Science Institute (CTSI) and the NIH (UL1TR000038).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
B.N.C. and S.R.A. have patents covering the use of adenosine receptor agonists for the treatment of OA and the formulation of adenosine-containing liposomes. Both B.N.C. and S.R.A. have equity in Regenosine, a company with a goal to develop adenosine liposomes as a treatment for OA in humans, and Vetosine, a company with a goal to develop adenosine liposomes for the treatment of OA for the veterinary market.
References
- Duruoz, M.T. Exploring osteoarthritis: Unraveling challenges, innovations, and hope for a better future. Best. Pract. Res. Clin. Rheumatol. 2023, 101872. [Google Scholar] [CrossRef]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: The good, the bad and the promising. Osteoarthr. Cartil. 2019, 27, 230–239. [Google Scholar] [CrossRef]
- Corciulo, C.; Castro, C.M.; Coughlin, T.; Jacob, S.; Li, Z.; Fenyo, D.; Rifkin, D.B.; Kennedy, O.D.; Cronstein, B.N. Intraarticular injection of liposomal adenosine reduces cartilage damage in established murine and rat models of osteoarthritis. Sci. Rep. 2020, 10, 13477. [Google Scholar] [CrossRef] [PubMed]
- Corciulo, C.; Lendhey, M.; Wilder, T.; Schoen, H.; Cornelissen, A.S.; Chang, G.; Kennedy, O.D.; Cronstein, B.N. Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression. Nat. Commun. 2017, 8, 15019. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, M.; Berenbaum, F.; Houard, X. Why subchondral bone in osteoarthritis? The importance of the cartilage bone interface in osteoarthritis. Osteoporos. Int. 2012, 23 (Suppl. S8), S841–S846. [Google Scholar] [CrossRef]
- Tesch, A.M.; MacDonald, M.H.; Kollias-Baker, C.; Benton, H.P. Endogenously produced adenosine regulates articular cartilage matrix homeostasis: Enzymatic depletion of adenosine stimulates matrix degradation. Osteoarthr. Cartil. 2004, 12, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Tesch, A.M.; MacDonald, M.H.; Kollias-Baker, C.; Benton, H.P. Chondrocytes respond to adenosine via A(2)receptors and activity is potentiated by an adenosine deaminase inhibitor and a phosphodiesterase inhibitor. Osteoarthr. Cartil. 2002, 10, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.J.; Oosterhof, A.; Veerkamp, J.H. Purine metabolism in splenocytes and thymocytes of various mammalian species. Adv. Exp. Med. Biol. 1984, 165 Pt B, 107–110. [Google Scholar] [CrossRef]
- Peters, G.J.; Oosterhof, A.; Veerkamp, J.H. Metabolism of purine nucleosides and phosphoribosylpyrophosphate in thymocytes and splenocytes of various mammalian species. Comp. Biochem. Physiol. B 1982, 73, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.J.; Oosterhof, A.; Veerkamp, J.H. Adenosine and deoxyadenosine metabolism in mammalian lymphocytes. Int. J. Biochem. 1981, 13, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Kolb, E.; Siebert, P.; Dittrich, H. Adenosine deaminase activity in blood and tissues of horses of the Rassen Haflinger and Thuringer Kaltblut breeds. Dtsch. Tierarztl. Wochenschr. 1995, 102, 405–407. [Google Scholar]
- Ichikawa, N.; Taniguchi, A.; Kaneko, H.; Kawamoto, M.; Sekita, C.; Nakajima, A.; Yamanaka, H. Arterial Calcification Due to Deficiency of CD73 (ACDC) As One of Rheumatic Diseases Associated with Periarticular Calcification. J. Clin. Rheumatol. 2015, 21, 216–220. [Google Scholar] [CrossRef]
- St Hilaire, C.; Ziegler, S.G.; Markello, T.C.; Brusco, A.; Groden, C.; Gill, F.; Carlson-Donohoe, H.; Lederman, R.J.; Chen, M.Y.; Yang, D.; et al. NT5E mutations and arterial calcifications. N. Engl. J. Med. 2011, 364, 432–442. [Google Scholar] [CrossRef]
- Shkhyan, R.; Lee, S.; Gullo, F.; Li, L.; Peleli, M.; Carlstrom, M.; Chagin, A.S.; Banks, N.W.; Limfat, S.; Liu, N.Q.; et al. Genetic ablation of adenosine receptor A3 results in articular cartilage degeneration. J. Mol. Med. 2018, 96, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bai, H.; Jiao, G.; Wang, X.; Zhang, Z.; Song, X.; Ma, T.; Li, T.; Gao, L. CF101 alleviates OA progression and inhibits the inflammatory process via the AMP/ATP/AMPK/mTOR axis. Bone 2022, 155, 116264. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yehuda, S.; Rath-Wolfson, L.; Del Valle, L.; Ochaion, A.; Cohen, S.; Patoka, R.; Zozulya, G.; Barer, F.; Atar, E.; Pina-Oviedo, S.; et al. Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum. 2009, 60, 3061–3071. [Google Scholar] [CrossRef]
- Friedman, B.; Corciulo, C.; Castro-Rivera, C.; Cronstein, B. Adenosine A2A Receptor Signaling Activates FoxO1 and FoxO3 and Promotes Cartilage Autophagy [abstract]. Arthritis Rheumatol. 2019, 71. Available online: https://acrabstracts.org/abstract/adenosine-a2a-receptor-signaling-activates-foxo1-and-foxo3-and-promotes-cartilage-autophagy/ (accessed on 15 October 2023).
- Castro, C.M.; Corciulo, C.; Solesio, M.E.; Liang, F.; Pavlov, E.V.; Cronstein, B.N. Adenosine A2A receptor (A2AR) stimulation enhances mitochondrial metabolism and mitigates reactive oxygen species-mediated mitochondrial injury. FASEB J. 2020, 34, 5027–5045. [Google Scholar] [CrossRef]
- Liu, X.; Corciulo, C.; Arabagian, S.; Ulman, A.; Cronstein, B.N. Adenosine-Functionalized Biodegradable PLA-b-PEG Nanoparticles Ameliorate Osteoarthritis in Rats. Sci. Rep. 2019, 9, 7430. [Google Scholar] [CrossRef]
- Li, D.; Cronstein, B.N.; Cook, J.; Bozynski, C.; Angle, S. Intra-articular injections of two liposomal adenosine formulations provide significant pain relief and gain in function, improve comfortable range of motion and slowed radiologic progression in a preclinical canine model of osteoarthritis. Ann. Rheum. Dis. 2023, 82, 133. [Google Scholar]
- Castro, C.M.; Corciulo, C.; Friedman, B.; Li, Z.; Jacob, S.; Fenyo, D.; Cronstein, B.N. Adenosine A2A receptor null chondrocyte transcriptome resembles that of human osteoarthritic chondrocytes. Purinergic Signal. 2021, 17, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.; Corciulo, C.; Castro, C.M.; Cronstein, B.N. Adenosine A2A receptor signaling promotes FoxO associated autophagy in chondrocytes. Sci. Rep. 2021, 11, 968. [Google Scholar] [CrossRef]
- Friedman, B.; Larranaga-Vera, A.; Castro, C.M.; Corciulo, C.; Rabbani, P.; Cronstein, B.N. Adenosine A2A receptor activation reduces chondrocyte senescence. FASEB J. 2023, 37, e22838. [Google Scholar] [CrossRef] [PubMed]
- Mistry, D.; Chambers, M.G.; Mason, R.M. The role of adenosine in chondrocyte death in murine osteoarthritis and in a murine chondrocyte cell line. Osteoarthr. Cartil. 2006, 14, 486–495. [Google Scholar] [CrossRef]
- Liao, C.D.; Huang, Y.Y.; Chen, H.C.; Liou, T.H.; Lin, C.L.; Huang, S.W. Relative Effect of Extracorporeal Shockwave Therapy Alone or in Combination with Noninjective Treatments on Pain and Physical Function in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials. Biomedicines 2022, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Ratech, H.; Greco, M.A.; Gallo, G.; Rimoin, D.L.; Kamino, H.; Hirschhorn, R. Pathologic findings in adenosine deaminase-deficient severe combined immunodeficiency. I. Kidney, adrenal, and chondro-osseous tissue alterations. Am. J. Pathol. 1985, 120, 157–169. [Google Scholar] [PubMed]
- Baker, M.C.; Weng, Y.; Robinson, W.H.; Ahuja, N.; Rohatgi, N. Reduction in Osteoarthritis Risk After Treatment With Ticagrelor Compared to Clopidogrel: A Propensity Score-Matching Analysis. Arthritis Rheumatol. 2020, 72, 1829–1835. [Google Scholar] [CrossRef]
- Guillan-Fresco, M.; Franco-Trepat, E.; Alonso-Perez, A.; Jorge-Mora, A.; Lopez-Fagundez, M.; Pazos-Perez, A.; Gualillo, O.; Gomez, R. Caffeine, a Risk Factor for Osteoarthritis and Longitudinal Bone Growth Inhibition. J. Clin. Med. 2020, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).