Abstract
The malignancy of glioblastoma (GBM), the most aggressive type of human brain tumor, strongly correlates with the presence of hypoxic areas within the tumor mass. Oxygen levels have been shown to control several critical aspects of tumor aggressiveness, such as migration/invasion and cell death resistance, but the underlying mechanisms are still unclear. GBM cells express abundant K+ and Cl− channels, whose activity supports cell volume and membrane potential changes, critical for cell proliferation, migration and death. Volume-regulated anion channels (VRAC), which mediate the swelling-activated Cl− current, and the large-conductance Ca2+-activated K+ channels (BK) are both functionally upregulated in GBM cells, where they control different aspects underlying GBM malignancy/aggressiveness. The functional expression/activity of both VRAC and BK channels are under the control of the oxygen levels, and these regulations are involved in the hypoxia-induced GBM cell aggressiveness. The present review will provide a comprehensive overview of the literature supporting the role of these two channels in the hypoxia-mediated GBM malignancy, suggesting them as potential therapeutic targets in the treatment of GBM.
1. Glioblastoma
Glioblastoma (GBM) is the most common and aggressive type of brain tumor. It has been classified by the World Health Organization (WHO) as a grade IV astrocytoma among the four grades (I–IV) of greater progressive malignancy, defined according to clinical and histopathological criteria, such as excessive hypercellularity, endothelial cell hypertrophy, microvascular hyperplasia and necrotic areas. GBM is not curable and patients’ median survival, applying the best standard care possible, is around 15 months from diagnosis [1,2]. The current standard treatment includes surgical tumor resection based on pre-operative magnetic resonance images (if available), followed by radiotherapy and chemotherapy (with temozolomide) [3,4,5]. Full surgical resection of the tumor, on which most hopes for solid tumors are focused, is never feasible or complete for GBM due to its high invasiveness. As result, although surgery can significantly increase patient survival, it is never resolving and eventually tumor progression continues and patient death occurs. The high migratory and invasive potential, which is responsible for the widespread infiltration of tumor cells into the healthy brain parenchyma, and the formation of new foci are therefore the main problems in GBM tumors.
GBM cells invade following different routes, mainly through the brain parenchyma, white matter tracts and blood vessels [6]. Using the magnetic resonance imaging of GBM patients, Esmaeili and coworkers found a preferential direction of GBM cell migration along the white matter fibers [7], confirming earlier studies from Sontheimer’s laboratory [8]. A later study, carried out by combining organotypic brain slices and live imaging to trace the dynamics of GBM cells’ invasion, found that GBM cells migrate preferentially along blood vessels [9]. In any case, cell invasion outside the central mass is the major feature of GBM and the reason for the limited beneficial effects of tumor surgery and the fatal outcome of the disease.
2. Hypoxia and GBM Aggressiveness
Extensive tissue hypoxia is one of the main features of GBM and a major determinant of the tumor’s aggressive phenotype. Hypoxia in GBM is the result of the rapid proliferation and expansion of the tumor mass, which the vascular system, although strongly stimulated to grow, is unable to reach, making the tumor microenvironment heavily hypoxic [10]. The hypoxic condition stimulates a variety of biological responses to counteract this adverse situation, starting with the stabilization of the hypoxia-inducible transcription factor 1 (HIF-1), which activates the expression of several hypoxia response genes [11]. These primarily include vascular endothelial growth factor, which stimulates the proliferation of endothelial cells and the formation of new blood vessels to supply the growing tumor mass with adequate blood and oxygen [12]. However, this neovascularization develops aberrantly, producing large, tortuous and malfunctioning new vessels that are highly prone to vascular thrombosis and vessel occlusion, which in turn leads to severe hypoxia and acidosis [13]. These conditions, which the tumor cells cannot tolerate for long, eventually lead to massive cell death and the formation of the necrotic areas (foci) observed histologically, which are one of the most distinctive features of GBM (Figure 1) [14].
Surviving tumor cells escape the heavily hypoxic regions, heading for more oxygen-richer areas near functioning blood vessels, where they form dense cell fronts called pseudopalisades, assemblages of elongated tumor cells stacked in rows on the periphery of the necrotic regions formed around the occluded vessels [15]. This view is based on the observation that pseudopalisading cells are much less proliferative and more prone to apoptosis than adjacent tumor cells, ruling out the possibility that these dense cell fronts do not result from increased clonal expansion or resistance to apoptosis [16,17,18]. It is this cell front that mainly spreads into and invades healthy brain tissue, making the surgical resection of the tumor problematic and ultimately being the principal cause of death in GBM patients [19].
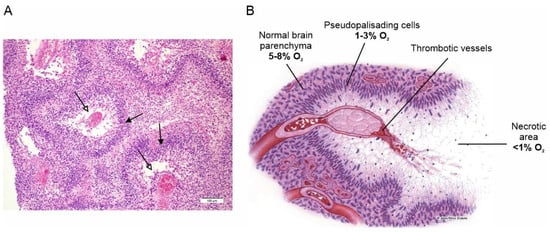
Figure 1.
Necrotic areas and pseudopalisades, the distinctive histopathological features of GBM. (A) Classical histologic findings of GBM showing prominent pseudopalisading necrosis (empty arrows). Indented area of necrosis with pseudopalisade formation characterized by accumulation of escaping tumor cells (filled arrows). Adapted with permission from Ref. [20]. © 2015 Lim et al. (B) Schematic representation of pseudopalisade formation in GBM. Vaso-occlusion/collapse and intravascular thrombosis lead to tissue hypoxia in the perivascular region. Hypoxic tumor cells then migrate away along cellular processes, leaving initially a fibrillar center. Tumor cells that do not migrate become hypoxic and undergo apoptosis or necrosis, eventually leaving a central necrotic zone. Adapted with permission from Ref. [21]. © 2004 USCAP, Inc.
Besides increasing their migratory ability to survive the adverse hypoxic conditions, GBM cells implement several other changes, such as (i) switching from aerobic to anaerobic metabolism [22,23]; (ii) promoting the selection and maintenance of the more aggressive glioblastoma stem cells [24,25,26]; (iii) upregulating a second form of HIF, HIF-2, which increases erythropoietin production in the kidney and liver, resulting in increased hemoglobin synthesis [27] and endowing GBM cells with properties that help them to evade apoptotic death [28].
Among the many effects induced by hypoxia, two—cell migration/invasion and resistance to cell death—are strongly correlated with cell volume regulation [29,30,31,32,33], a complex process under the strict control of transporters and ion channels localized on the plasma membrane [34]. These observations would suggest the possible involvement of volume-regulated ion channels in the hypoxia-induced aggressiveness of tumor cells.
3. Volume-Regulated Ion Channels in GBM
The volume-regulated anion channel (VRAC) is widely expressed in vertebrate cells, including GBM tumors, where it mediates the swelling-activated Cl− current (ICl,swell) required for cell volume regulation [35,36,37,38,39]. Electrophysiological studies characterized the VRAC currents more than 30 years ago [40,41], but the molecular identity of the channel has been a source of uncertainty and controversy for decades [42]. In 2014, two laboratories, independently, reported that VRAC is a heteromeric channel encoded by different members of the Lrrc8 gene family [43,44].
As the name implies, VRAC is sensitive to volume changes, so routinely VRAC is activated by an increase in cell volume, generally obtained by exposing the cell to hypotonic solutions. Other distinguishing features of VRAC are its significant permeability to many anionic species (SCN−, I−, NO3−, Br−, Cl−, HCO3−, F−, glycine) and the generation of outward rectifying currents. VRAC has also been found to pass large molecules, such as taurine and glutamate, and several anticancer drugs, such as cisplatin and carboplatin, which makes this channel the main one responsible for cancer drug resistance [45,46].
In GBM cells, when electrophysiological recordings are performed in the presence of Ca2+-activated K+ channel inhibitors, the application of a hypotonic solution leads to the activation of a current with a reversal potential very close to the Cl− equilibrium and completely blocked by VRAC-mediated ICl,swell current antagonists such as NPPB, DIDS and DCPIB [39,47,48]. The slow inactivation of the hypotonic-induced VRAC-mediated ICl,swell at high voltages is another typical feature of the current [49]. We also investigated in detail the signal transduction pathway that leads to swelling-induced VRAC activation. Our data suggest that cell swelling activates a volume-sensitive PLC and increases DAG levels. DAG is then phosphorylated by DGK to phosphatidic acid, which is known to activate the Rac1 G protein. Activated Rac1 will then induce the polymerization of cortical actin, which is the final step needed for the activation of the VRAC channel [39]. Notably, this transduction pathway is very different from that proposed for astrocytes, which represent the normal cells from which GBM cells originate [50,51,52].
In addition to responding to osmotic shock, VRAC is modulated by severe hypoxia or elevated serum content, both of which are typical of the tumor environment and known to favor glioma malignancy [47]. Other intracellular signals, such as ATP, cytoplasmic Ca2+, reactive oxygen species, G-proteins and phosphorylation, have been reported to activate VRAC. None, however, has shown its efficacy across different cell types to gain general significance.
Regarding the role of VRAC in GBM malignancy, Sontheimer and coworkers suggested that the infiltration of GBM cells into the healthy brain parenchyma requires major changes in cell shape and volume and that Cl– currents are crucial in this process [53,54]. Later studies added new roles for VRAC in GBM malignancy, such as in cell proliferation, escape from cell death and multidrug resistance [48,49,55,56,57]. However, these roles must be considered with caution because, prior to the molecular identification of VRAC in 2014, recorded currents were assigned to VRAC mainly based on studies using pharmacological blockers that were not very specific to VRAC, such as DIDS and NPPB [58,59,60]. Unfortunately, these uncertainties precluded a thorough investigation of its pathophysiological roles in GBM and the underlying mechanisms for many years. Indeed, some discrepancies regarding the role of VRAC in cell proliferation and migration have been reported in the literature. In a recent study, Liu and Stauber demonstrated that the genetic ablation of the LRRC8A subunit, which is essential for the establishment of a functional VRAC, affected neither proliferation nor migration in two well-established GBM cell lines, concluding that VRAC is not essential for either process [61]. By contrast, though, Serpe and colleagues showed that the reduction of both the LRRC8A and LRRC8C subunits, induced by treating GL261 murine GBM cells with astrocyte-derived exosomes, significantly hampered both migration and invasion [62]. The reasons for the discrepancies have not yet been fully clarified.
Another important process in which VRAC plays a central role is observed when GBM cells are placed in a hypotonic solution. After the immediate swelling of the cell due to water rebalancing caused by the osmotic gradient across the membrane, the cell slowly tries to re-establish its original cell volume, a process called regulatory volume decrease (RVD). The mechanism, now clearly established, is that VRAC channels, activated by membrane stretching and the PLC-DAG-phospatidic acid pathway, together with activated K+ channels, extrude KCl salt. The osmotic imbalance created is followed by the efflux of water, which restores the volume of the swollen cell to its original size. This process is often used experimentally to verify the involvement of specific ion channels in the regulation of the cell volume [63]. Recently, we confirmed the role of VRAC channels in the RVD process in a murine GBM cell line using DCPIB, a selective VRAC inhibitor [62].
The Ca2+-Activated K+ Channels
Another extensively studied channel family, firmly associated with cell volume control and GBM migration and invasion, is that of the Ca2+-activated K+ (KCa) channels, which includes the large-conductance (~250 pS) (KCa1.1, or BK), the intermediate (20–80 pS; KCa3.1 or IK) and the small-conductance (12–20 pS; KCa2.1–2.3 or SK 1–3) channels [64]. A common and distinctive property of this channel family is their activation by intracellular Ca2+, although with mechanisms and sensitivities that are considerably different. All types of KCa channels are expressed in GBM cells; however, only BK and IK channels have emerged as the KCa channels certainly associated with GBM malignancy, being involved in cell migration and invasion, cell proliferation and escape from cell death [53,65,66,67].
The BK channel belongs to the voltage-gated K+ channel family, having the typical structure of four α subunits forming the channel, each composed of six transmembrane segments (S1–S6) plus an extra one, the S0, with charged S4 segments that confer upon the channel the sensitivity to voltage [68,69]. Its Ca2+ sensitivity is instead imparted by intrinsic Ca2+ binding sites (collectively called the Ca2+ bowl) in their carboxyl terminal tail [70]. The Ca2+ sensitivity of the channel is modest, with a Kd ~3 µM at 0 mV [71]. As result, co-localization with Ca2+-permeable channels is needed to provide local Ca2+ sufficient to activate the channels [72]. Given the double sensitivity to both Ca2+ and voltage, the BK channel is activated cooperatively by a cytoplasmic Ca2+ increase and membrane depolarization [73,74,75,76]. Given their very high conductance, the activation of BK channels leads to the massive efflux of K+ ions, which hyperpolarizes the cell membrane but may also serve other purposes.
By combining biochemical and pharmacological approaches, Sontheimer and coworkers found that BK channels were highly overexpressed in several GBM cell lines (D54-MG, U251-MG, STTG-1), as well as in primary cultures of glioma cells established from patient biopsies [77,78]. Moreover, when analyzing biopsies from patients with WHO grade I to IV gliomas, it was found that BK channel expression correlated with the tumor grade [79]. Several other studies have shown that BK channels are overexpressed in malignant glioma cells [65,80,81,82].
BK channels were suggested very early and repeatedly to be involved in GBM migration and invasion. Evidence was obtained by testing the selective BK channel blocker iberiotoxin on GBM transwell migration [53] or on migration induced by different agents that increase cytoplasmic Ca2+, such as acetylcholine [83], phloretin [84] and menthol [85]. In all these cases, the pharmacological inhibition of BK channels affected cell migration. Ionizing radiation (IR) has also been shown to enhance Ca2+ signaling and stimulate cell migration of GBM cells [86,87,88]. Huber and coworkers first showed, in an in vitro study, that IR-induced BK channel activation is a key event in IR-induced GBM migration, and that the BK channel blocker paxilline suppresses it [86]. These results were later confirmed by the same laboratory in vivo [87]. Similar results were reported for another KCa channel by Limatola and coworkers. They demonstrated that high-dose radiation of patient-derived GBM cells increased the functional expression of IK channels, which correlated with the pro-invasive phenotype of tumor cells induced by IR [88].
The IK channel is the other member of the KCa channel family that has been most investigated in the context of GBM invasion [66,67,89]. The expression of IK currents and IK channel transcripts on established GBM cell lines (GL15 and U251) was first reported by our laboratory [90]. IK channel transcripts (but not the current) were also found by Sontheimer’s group in the U251 and D54-MG GBM cell lines [78]. The IK channel was later found to be overexpressed in ~30% of glioma patient biopsies, where it correlated with poor patient survival [67].
We also demonstrated the involvement of IK channels in cell migration in human GBM cell lines and in freshly dissociated tissues from GBM patients. Evidence for major IK channel involvement in human GBM cell migration and invasion was later provided in vivo in severe combined immunodeficiency (SCID) mice xenografted with GBM GL15 cells [91]. Namely, IK channel inhibition by shRNA or specific blocker TRAM-34 markedly reduced the tumor-infiltrated area (especially along the white matter tracts) and the antero-rostral spreading of GBM cells in the brain parenchyma [91]. These results were later replicated by Sontheimer’s laboratory both in vitro and ex vivo, using patient-derived glioma tissues xenografted in the flanks of nude mice, as well as U251 GBM cell lines [67]. IK channels also promote GBM cell migration by inducing or modulating cytoplasmic Ca2+ signals in the form of Ca2+ oscillations [92,93,94]. Altogether, these results confirm the crucial role of IK channels in brain tumor cell migration and invasion.
We recently reported that both BK and IK currents were significantly activated by exposure to a 30% hypotonic solution [95]. Since both K+ channels are activated by Ca2+ and since cell swelling increases Ca2+ in many cell types, we tested whether Ca2+ influx was needed for the hypotonic-induced BK and IK channel activation. We found that the activation of both channels was prevented by incubating the cells in extracellular solutions with nominally zero Ca2+. In addition, the hypotonic-induced activation of both BK and IK currents could also be prevented by the unspecific inhibitor of mechanosensitive channels, gadolinium (Gd3+). This suggests that, following cell swelling, Ca2+ influx through mechanosensitive channels mediates the activation of both K+ channels [95]. Ca2+-permeable mechanosensitive channels (MSCs) expressed in eukaryotic cells were found to have a single-channel conductance of 20–40 pS and to be inhibited by Gd3+, by the spider toxin GsMTx4 and by streptomycin [96,97,98]. In 2010, two channels, Piezo1 and Piezo2, were identified as the long-sought structural counterparts of the non-selective cationic MSC current found in many cells [99]. Indeed, Piezo1 expression in heterologous cell types was found to replicate the gating properties of the endogenous, ubiquitous non-selective cation channels in patches, producing elementary currents of 20–50 pS with rapid voltage-dependent inactivation, and inhibited by GsMTx4 [100,101]. The Piezo1 channel is a very large protein of over 2500 amino acids and was initially thought to be formed by tetramers [99]. However, the recently obtained cryo-EM structure of Piezo1 clearly shows that the channel is a trimer [102]. Structure–function studies suggest that Piezo1 opening occurs upon the application of a wide range of mechanical forces on the plasma membrane, including membrane stretching [99,103]. However, Piezo1 has highly selective activators, such as yoda1 and jedi2, that can open the channel in the absence of mechanical stimuli [104,105].
Notably, it has been recently found that Piezo1 channels are highly expressed in gliomas and their expression correlates with the tumor grade and malignancy [106,107,108]. In addition, a recent study reported that the yoda1-mediated activation of Piezo1 in isotonic conditions reduced GBM cells’ resistance to TNF-α-related apoptosis-inducing ligand (TRAIL), significantly increasing TRAIL-mediated apoptosis in two different GBM cell lines [109].
In accordance with these results, in GBM cells we found that IK and BK channels could also be activated under isotonic conditions by the selective Piezo1 agonist yoda1. We also reported that, in the simultaneous presence of DCPIB, TRAM-34 and paxilline, both yoda1 and hypotonic solutions activated a strongly outward-rectifying, cation-unselective current resembling the current observed by others after the exogenous expression of Piezo1 [95,110].
In addition to responding to the increase in cell volume, IK and BK channels also participate in the regulation of the GBM cell volume, as assessed using the RVD paradigm. More specifically, in U87-MG GBM cells, the exposure of cells to a 30% hypotonic solution induced rapid swelling followed by the slow return of the original cell volume. Notably, both paxilline and TRAM-34, selective inhibitors of the BK and IK channels, respectively, significantly hamper the RVD process (Figure 2A,B) [95]. All these findings are synthetized in the mechanistic model of RVD shown in Figure 2C, illustrating how the selective blockade of each of the ion channels mentioned above (i.e., VRAC, IK, and BK) is able to significantly inhibit the RVD process (Figure 2A,B). The hypotonic-induced cell swelling causes an increase in the plasma membrane tension/stretching, which represents the stimulus for the opening of mechanosensitive Ca2+-permeable channels, such as Piezo1. The resulting increment in the intracellular Ca2+ concentration, due to Piezo1-mediated Ca2+ entry, triggers the activation of both KCa channels. VRAC channels, which in GBM cells respond to the hypotonic shock in a Ca2+-independent manner [39], open instead via the activation of the PLC/DAG/Rac1 pathway. However, we cannot rule out that the opening of Piezo1 may also modulate the VRAC-mediated ICl,swell, as we reported in HEK293 cells [111]. In any case, the net efflux of KCl creates the osmotic gradient necessary for the loss of water and the consequent restoration of the original cell volume (Figure 2C).
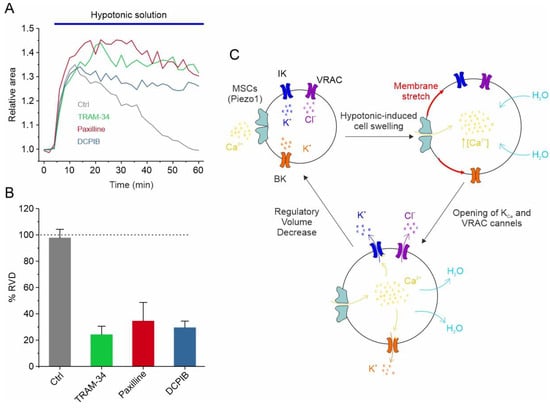
Figure 2.
VRAC, IK and BK channels regulate RVD GBM cells. (A) Time course of RVD, as evaluated from changes in the relative cell area during application of 30% hypotonic solution (blue bar), in control conditions (grey line) and when either VRAC (blue line), IK (green line) or BK (red line) channel was pharmacologically blocked by 10 μM DCPIB, 3 μM TRAM-34 and 1 μM paxilline, respectively. (B) Bar plot showing the %RVD assessed in the different experimental conditions. (C) Possible mechanisms underlying the hypotonic-induced cell swelling and the activation of the RVD process. Adapted with permission from Ref. [95]. © 2023 The Authors. Journal of Cellular Physiology published by Wiley Periodicals LLC.
4. Ion Channels in GBM Cell Migration and Death
The basic mechanism underlying the migration of GBM cells, indeed of cells in general, can be described as the cyclic succession of two distinct processes: the protrusion of the cell front, due to actin polymerization with the formation of pseudopods, and the retraction of the rear cell body, due to forces produced by actomyosin contraction. Both processes, to develop properly, must be accompanied by the local remodeling of the cell volume and shape [112,113,114], a notion confirmed by live imaging studies in glioma cells [8]. These major remodeling of the cell shape and volume are made possible by the complex interplay of ion channels and transporters outlined below [49,67,114,115,116].
At the leading edge of GBM cells, the activation of the Na+/K+/2Cl− cotransporter (NKCC1), which brings one Na+, one K+ and two Cl– ions inside the cell, accompanied by the iso-osmotic influx of water, leads to an increase in cell volume [117]. For the cell to move forward, the protrusion of the cell front must be followed by the retraction of the rear end, which, as shown in Figure 3, results in a significant decrease in cell volume by the extrusion of ions (i.e., K+ and Cl−) and osmotic water [112,113,114]. More precisely, the retraction of the trailing edge begins with the activation of MSCs and VRAC, which occurs in response to the stretching of the membrane caused by the NKCC1-dependent increase in cell volume. The activation of the MSCs and the consequent Ca2+ influx activate the KCa channels and the efflux of K+ ions that, in combination with the efflux of Cl− through the VRAC and osmotic water, results in the reduction of cell volume required for the retraction of the rear end (Figure 3).
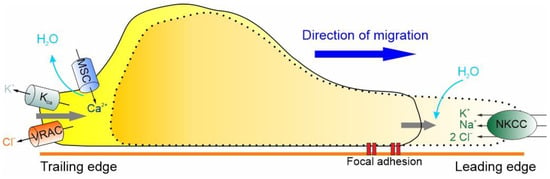
Figure 3.
The basic hydrodynamic model of GBM cell migration. GBM cells express ion channels and transporters that underlie shape and volume changes, steps required for cell migration. These include, in the leading edge, NKCC1, and, in the trailing edge, VRAC, KCa and the MSC channels. The migration cycle includes the uptake of Na+, K+ and Cl− ions al the leading edge, which attracts osmotic water, resulting in a volume increase and leading edge protrusion. This stretches the membrane and activates both MSC and VRAC at the trailing edge. The resulting Ca2+ influx activates the KCa channels, determining in this way the efflux of K+ and Cl− ions, followed by osmotic water, and a decrease in volume at the trailing edge, promoting its retraction.
These hydrodynamic events, involving the concerted transmembrane flow of ions and osmotic water and consequent changes in shape and volume, help GBM cells to invade the brain parenchyma. It is important to emphasize, however, that they only have a permissive or facilitatory role in the process, with no implications for tumor initiation [112].
Volume changes induced by ion channels are also crucial for cell death. In GBM, KCa channels have been implicated in the so-called apoptotic volume decrease (AVD), a mechanism whereby the cell reduces its volume before apoptosis (Figure 4, left branch). Using a grade IV human GBM cell line, Sontheimer and coworkers examined the contribution of KCa channels to AVD after the addition of either staurosporine or TRAIL to activate the intrinsic or extrinsic pathway of apoptosis, respectively [118]. The use of specific KCa channel inhibitors revealed that staurosporine-induced AVD was dependent on K+ efflux through IK channels, while TRAIL-induced AVD was mediated by BK channels. Conversely, an increase in cell volume, called necrotic volume increase (NVI), is observed during necrosis (Figure 4, right branch). This is mainly caused by the influx of NaCl, due to the reduced energy supply and activity of the Na+/K+ pump, followed by the osmotically driven entry of water. This second type of death is particularly interesting in this context, since GBM cells need to develop strategies to resist hypoxic-induced necrosis.
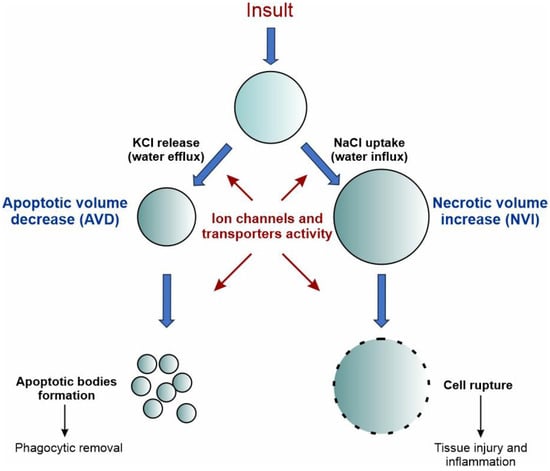
Figure 4.
Schematic representation of the role of ion channels in the regulation of cell volume during cell death. Upon persistent death insult, cell shrinkage and cell swelling are hallmarks of the early phases of apoptotic and necrotic cell death, respectively. The early-phase shrinkage of apoptotic cells is termed apoptotic volume decrease (AVD) and is mediated by Cl− and K+ channels and the net efflux of KCl, which promotes the osmotic loss of water from the cell. The early-phase swelling of necrotic cells is termed necrotic volume increase (NVI) and is mainly mediated by the transport of Na+ and Cl− ions inside the cell, with the resulting influx of water from the extracellular environment. Inspired by [119].
5. Hypoxic Modulation of Volume-Regulated Ion Channels in GBM
We have seen that the activity of the volume-regulated channels expressed in GBM cells can control at least two of the functional processes also regulated by hypoxia, namely cell migration/invasion and cell death. It is therefore possible that hypoxia regulates these processes by modulating the activity of the same ion channels. Below, we show evidence for such hypoxia-induced modulation of GBM ion channels.
5.1. VRAC Modulation by Hypoxia
It has long been known that hypoxia induces cell swelling in various cellular models [120,121,122], which led us to postulate that VRAC could be activated by hypoxic stimuli. Electrophysiological experiments in a voltage-clamp configuration, designed to specifically study VRAC activation, show that the application of hypoxic solutions (pO2 = 1–3%) activates a current with biophysical (i.e., mild outward rectification and time- and voltage-dependent inactivation) and pharmacological (i.e., DCPIB sensitivity) properties congruent with VRAC. The demonstration that the hypoxia-induced increase in cell volume is a crucial step in VRAC activation is further supported by the observation that the application of a 30% hypertonic solution, which induces cell shrinkage, dramatically inhibits the hypoxia-activated VRAC current [47].
Consistent with the fact that GBM cells in vivo experience persistent hypoxia, their chronic (24 h) exposure to a hypoxic condition, known to induce long-term HIF-1-dependent responses to hypoxia, strongly decreases the VRAC current elicited by acute application of the hypoxic solution, as well as the fraction of hypoxic-responsive cells [47]. The reduction in the VRAC current is arguably the consequence of the decreased expression of VRAC channels, as suggested by the even greater reduction in the current when probed with an extracellular hypotonic solution. Interestingly, the downregulation of VRAC channel expression induced by chronic hypoxia significantly affects the ability of GBM cells to recover the original cell volume, further indicating the central role of VRAC in the RVD process [47].
5.2. Hypoxia-Induced VRAC-Mediated Volume Decrease Counteracts Necrotic Cell Death
Necrotic or unprogrammed cell death is caused by excessive chemical or physical stress, including severe oxygen deficiency, which leads to an uncontrolled increase in volume and eventually to the rupture of the cell membrane [123,124]. The activation of VRAC in this context can therefore counteract or limit the cell volume increase, thereby promoting the survival of GBM cells that experience hypoxic conditions immediately after the formation of the thrombotic vessel. This function would be lost or attenuated upon chronic exposure to hypoxic conditions, due to the long-term downregulation of VRAC. We can therefore hypothesize that VRAC activation represents a pro-survival factor for GBM cells acutely exposed to hypoxia, increasing their likelihood of remaining alive until they acquire the promigratory phenotype that will help them to escape towards more oxygenated regions of the brain.
We tested this rationale, illustrated in Figure 5A, in a study published in 2017, in which we showed that the hypoxic activation of VRAC currents opposes both hypoxia-induced cell swelling (assessed by the RVD protocol) and necrotic cell death (by the lactate dehydrogenase assay, LDH) [47]. As reported in Figure 5B, hypoxia induces initial transient cell swelling, followed by the essentially complete recovery of the original cell volume (in the continuous presence of hypoxic solution). Repeating the experiment with the only difference of adding the VRAC inhibitor DIDS to the hypoxic solution, to appreciate the role of VRAC in the process, the rate of cell volume recovery was found to be much slower and never complete (Figure 5B). Interestingly, the inhibition of VRAC upon chronic hypoxia significantly increases the number of necrotic cells (Figure 5C), consistent with the notion that increased VRAC activity makes tumor cells more resistant to necrotic death under hypoxic conditions.
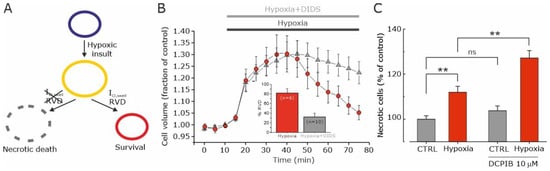
Figure 5.
Hypoxia induces VRAC-mediated volume decrease that prevents necrotic cell death. (A) Scheme illustrating the postulated role of the hypoxia-activated VRAC current in GBM cell survival in hypoxia-induced necrotic cell death. Following a hypoxic insult, cells undergo an increase in cell volume, which is followed by the activation of the VRAC-mediated ICl,swell current and the consequent RVD. In the absence of VRAC-mediated ICl,swell current activation, there is no RVD and cell swelling persists for a longer period, leading to necrotic cell death. (B) Time course of the relative cell area in U87-MG cells exposed to a hypoxic solution (red circles) and to a hypoxic solution containing the VRAC channel blocker DIDS (grey triangles). Inset: Bar plot showing the % RVD at 60 min in U87-MG cells exposed to hypoxic solution with and without DIDS. (C) Number of necrotic cells (percent of control), measured with the LDH assay, after a hypoxic insult, in absence and presence of the VRAC channel inhibitor DCPIB (10 μM). The hypoxic insult consisted of U87-MG cells’ re-suspension in a medium previously conditioned in a hypoxic chamber. ** p < 0.01; ns = not statistically significant. Adapted with permission from Ref. [47]. © 2016 Wiley Periodicals, Inc.
5.3. Hypoxia Enhances Activation of BK Channel and Promotes Migration of GBM Cells
Another key channel regulated by hypoxia and involved in GBM malignancy is the BK channel [82,125,126]. The acute application of hypoxic solutions to U87-MG cells with varying internal Ca2+ concentrations (from nominally zero to 300 nM Ca2+) had no effect on the activation of the BK currents, suggesting that acute hypoxia has no effect on the combined voltage/Ca2+ gating mechanism of BK channels [125]. Conversely, chronic treatment (24 h) increases significantly the BK current, as assessed by stimulating the cells with depolarizing stimuli (Figure 6A, inset). However, the increased current does not correlate with increased BK channel expression [125] but is rather the result of a marked shift in the half-activation voltage of the hypoxia-conditioned cells towards more hyperpolarized potentials (Figure 6A, main), suggesting that the hypoxic condition significantly affects the gating properties of the BK channel.
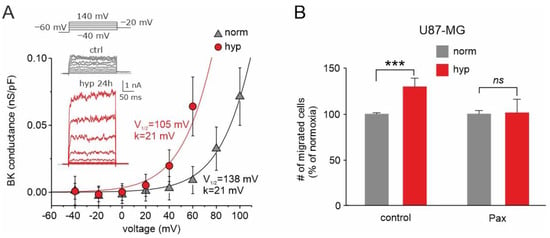
Figure 6.
Effects of chronic hypoxia treatment on both BK current and U87-MG cells’ migration. (A) Mean BK channel conductance vs. voltage relationships assessed from cells grown either in normoxia or hypoxia. The BK channel conductance was obtained by subtracting the leak currents from the total currents and dividing the resulting pure BK currents for the driving force under our recording conditions. The solid lines represent the best fit of the experimental data with Boltzmann relationships having identical maximal conductance (0.5 nS/pF) and voltage steepness (21 mV), but variable half-activation voltage (105 and 138 mV in hypoxia and normoxia, respectively). Inset: Representative BK currents from control cells, held in normoxia (upper, grey traces), and cells held for 24 h in hypoxia (bottom, red traces). Stimulation protocol consisted of depolarizing voltage steps from −40 to +140 mV, from a holding potential of −60 mV. (B) Transwell migration assay showing the percent of migrated U87-MG cells in normoxia (grey bars) compared to hypoxia (red bars), tested under control conditions and in presence of paxilline. This assay highlights a markedly different effect of hypoxia in the presence and absence of paxilline. *** p < 0.001; ns = not statistically significant. Adapted with permission from Ref. [125]. © 2018 Wiley Periodicals, Inc.
Consistent with the role played by BK channels in cell migration and invasion and considering the striking effects of chronic hypoxia on BK currents, the BK current enhancement induced by hypoxia could increase the migration/invasion ability of these cells, in line with its significant role in these processes. We tested this view by using the three-dimensional transwell migration assay. The results obtained suggest that the increased activation of the BK current by chronic hypoxia is instrumental in increasing the invasive activity of GBM cells and consequently the malignancy of the tumor (Figure 6B). These results are fully congruent with our previous wound healing assay, which showed that the hypoxia-induced migration enhancement is significantly reduced in the presence of the BK channel inhibitor paxilline [125]. The results obtained suggest that the increased activation of the BK current by chronic hypoxia is instrumental in increasing the invasive activity of GBM cells and consequently the malignancy of the tumor.
6. Conclusions
The data currently available in the literature show the crucial role played by VRAC and BK channels in the formation and organization of the pseudopalisading necrosis and the increased aggressiveness of GBM (Figure 7). According to current views, immediately after vessel occlusion, the resulting hypoxia brings most tumor cells to death, forming the necrotic regions of the tumor mass. Instead, in a certain percentage of cells, VRAC activation is able to oppose the necrotic volume increase and allow survival long enough to acquire a more aggressive and migratory phenotype. Among the features acquired after a sufficiently long time spent in hypoxia is the increased activity of BK channels, which would increase the ability of GBM cells to migrate towards more oxygenated regions and lead to the formation of the characteristic pseudopalisades.
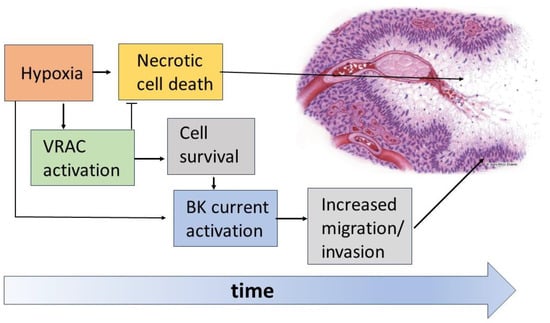
Figure 7.
Schematic of the hypoxia-induced events in the organization of pseudopalisades. The hypoxic conditions promote massive cell death with the formation of necrotic areas. However, the hypoxic insult is also the stimulus for the activation of VRAC and BK channels in GBM cells. The enhanced activity of VRAC would help cells to face these challenging conditions, thus rendering GBM cells less prone to necrosis. On the other hand, survived GBM cells use the enhanced activity of BK channels to increase their migratory/invasive potential, thus escaping the hypoxic/necrotic areas.
7. Future Perspectives
Although the role of IK channels in the invasiveness and migration of tumor cells and in the malignancy of GBM has been largely demonstrated, no data are currently available in the literature on the regulation of this channel by hypoxia. Given the relevant role it plays in GBM malignancy, it is possible that the IK channel, like VRAC and BK channels, is also modulated by the hypoxic insult, possibly through different pathways, and exerts important roles under the hypoxic conditions of GBM. We therefore plan to address these aspects in future studies. Besides the IK channel, another important channel should be investigated for its response to hypoxia, as well as for its role in GBM malignancy: the Piezo1 channel. First, because it is highly expressed in GBM, and its expression correlates with the grade of GBM malignancy. Second, because Piezo1 has been shown to contributes to the regulation of cell volume (and migration) through the modulation of VRAC in HEK293 cells [111], and of both IK and BK channels in U87-MG cells [95].
Finally, our data point to inhibitors of BK, IK and VRAC channels as possible adjuvants in the therapy of this lethal tumor. This prospect of an ion channel-based adjuvant GBM therapy could easily be tested, given the following observations. First, FDA-approved neuroleptics such as haloperidol or chlorpromazine, which inhibit BK channels, are currently undergoing a phase I dose escalation study investigating the repurposing of chlorpromazine adjuvant to radio-chemotherapy with temozolomide for the treatment of newly diagnosed glioblastoma (ClinicalTrials.gov identifier number NCT05190315) [127]. Second, the IK channel inhibitor Senicapoc was well tolerated by patients in clinical trials studying the effect of pharmacological IK channel targeting on sickle cell crisis [128].
Author Contributions
Conceptualization, A.M. and L.C.; writing-original draft preparation, A.M., F.F. and L.C.; writing-review and editing, A.M., L.S., F.F. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Abbreviations
AVD, apoptotic volume decrease; BK, large-conductance Ca2+-activated K+ channel; GBM, glioblastoma; HIF, hypoxia-inducible transcription factor; ICl,swell, swelling-activated Cl− current; KCa, Ca2+-activated K+ channel; IK, intermediate-conductance Ca2+-activated K+ channel; MSCs, non-selective Ca2+-permeable mechanosensitive channels; NVI, necrotic volume increase; RVD, regulatory volume decrease; SK, small-conductance Ca2+-activated K+ channel; TRAIL, TNF-α-related apoptosis-inducing ligand; VRAC, volume-regulated anion channel.
References
- Davis, M.E. Epidemiology and Overview of Gliomas. Semin. Oncol. Nurs. 2018, 34, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.G.; Freels, S.; Grutsch, J.; Barlas, S.; Brem, S. Survival Rates in Patients with Primary Malignant Brain Tumors Stratified by Patient Age and Tumor Histological Type: An Analysis Based on Surveillance, Epidemiology, and End Results (SEER) Data, 1973–1991. J. Neurosurg. 1998, 88, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with Temozolomide for Patients with Newly Diagnosed, EGFRvIII-Expressing Glioblastoma (ACT IV): A Randomised, Double-Blind, International Phase 3 Trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Hara, A.; Kanayama, T.; Noguchi, K.; Niwa, A.; Miyai, M.; Kawaguchi, M.; Ishida, K.; Hatano, Y.; Niwa, M.; Tomita, H. Treatment Strategies Based on Histological Targets against Invasive and Resistant Glioblastoma. J. Oncol. 2019, 2019, 2964783. [Google Scholar] [CrossRef]
- Esmaeili, M.; Stensjøen, A.L.; Berntsen, E.M.; Solheim, O.; Reinertsen, I. The Direction of Tumour Growth in Glioblastoma Patients. Sci. Rep. 2018, 8, 1199. [Google Scholar] [CrossRef] [PubMed]
- Cuddapah, V.A.; Robel, S.; Watkins, S.; Sontheimer, H. A Neurocentric Perspective on Glioma Invasion. Nat. Rev. Neurosci. 2014, 15, 455–465. [Google Scholar] [CrossRef]
- Liu, C.J.; Shamsan, G.A.; Akkin, T.; Odde, D.J. Glioma Cell Migration Dynamics in Brain Tissue Assessed by Multimodal Optical Imaging. Biophys. J. 2019, 117, 1179–1188. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.K. Current Understanding of Hypoxia in Glioblastoma Multiforme and Its Response to Immunotherapy. Cancers 2022, 14, 1176. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Hill, R.; Pilkington, G.J.; Madureira, P.A. The Role of Hypoxia in Glioblastoma Invasion. Cells 2017, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor 1 Is a Basic-Helix-Loop-Helix-PAS Heterodimer Regulated by Cellular O2 Tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Bullitt, E.; Zeng, D.; Gerig, G.; Aylward, S.; Joshi, S.; Smith, J.K.; Lin, W.; Ewend, M.G. Vessel Tortuosity and Brain Tumor Malignancy: A Blinded Study. Acad. Radiol. 2005, 12, 1232–1240. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, I.K.; Han, S.; Park, I.; Kim, C.; Bae, J.; Oh, S.J.; Lee, S.; Kim, J.H.; Woo, D.C.; et al. Normalization of Tumor Vessels by Tie2 Activation and Ang2 Inhibition Enhances Drug Delivery and Produces a Favorable Tumor Microenvironment. Cancer Cell 2016, 30, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Wippold, F.J.; Lämmle, M.; Anatelli, F.; Lennerz, J.; Perry, A. Neuropathology for the Neuroradiologist: Palisades and Pseudopalisades. Am. J. Neuroradiol. 2006, 27, 2037–2041. [Google Scholar]
- Brat, D.J.; Castellano-Sanchez, A.A.; Hunter, S.B.; Pecot, M.; Cohen, C.; Hammond, E.H.; Devi, S.N.; Kaur, B.; Van Meir, E.G. Pseudopalisades in Glioblastoma Are Hypoxic, Express Extracellular Matrix Proteases, and Are Formed by an Actively Migrating Cell Population. Cancer Res. 2004, 64, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Cavalla, P.; Migheli, A.; Chiò, A.; Giordana, M.T.; Marino, S.; Attanasio, A. Apoptosis and Cell Proliferation in Human Neuroepithelial Tumors. Neurosci. Lett. 1995, 195, 81–84. [Google Scholar] [CrossRef]
- Tachibana, O.; Lampe, J.; Kleihues, P.; Ohgaki, H. Preferential Expression of Fas/APO1 (CD95) and Apoptotic Cell Death in Perinecrotic Cells of Glioblastoma Multiforme. Acta Neuropathol. 1996, 92, 431–434. [Google Scholar] [CrossRef]
- Brat, D.J.; Van Meir, E.G. Glomeruloid Microvascular Proliferation Orchestrated by VPF/VEGF: A New World of Angiogenesis Research. Am. J. Pathol. 2001, 158, 789–796. [Google Scholar] [CrossRef]
- Lim, S.M.; Choi, J.; Chang, J.H.; Sohn, J.; Jacobson, K.; Policht, F.; Schulz, J.; Cho, B.C.; Kim, S.H. Lack of ROS1 Gene Rearrangement in Glioblastoma Multiforme. PLoS ONE 2015, 10, e0137678. [Google Scholar] [CrossRef]
- Brat, D.J.; Van Meir, E.G. Vaso-Occlusive and Prothrombotic Mechanisms Associated with Tumor Hypoxia, Necrosis, and Accelerated Growth in Glioblastoma. Lab. Investig. 2004, 84, 397–405. [Google Scholar] [CrossRef]
- Dang, C.V.; Semenza, G.L. Oncogenic Alterations of Metabolism. Trends Biochem. Sci. 1999, 24, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s Contributions to Current Concepts of Cancer Metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Soeda, A.; Park, M.; Lee, D.; Mintz, A.; Androutsellis-Theotokis, A.; McKay, R.D.; Engh, J.; Iwama, T.; Kunisada, T.; Kassam, A.B.; et al. Hypoxia Promotes Expansion of the CD133-Positive Glioma Stem Cells through Activation of HIF-1α. Oncogene 2009, 28, 3949–3959. [Google Scholar] [CrossRef] [PubMed]
- Seidel, S.; Garvalov, B.K.; Wirta, V.; Von Stechow, L.; Schänzer, A.; Meletis, K.; Wolter, M.; Sommerlad, D.; Henze, A.T.; Nistér, M.; et al. A Hypoxic Niche Regulates Glioblastoma Stem Cells through Hypoxia Inducible Factor 2α. Brain 2010, 133, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Gaelzer, M.M.; dos Santos, M.S.; Coelho, B.P.; de Quadros, A.H.; Simão, F.; Usach, V.; Guma, F.C.R.; Setton-Avruj, P.; Lenz, G.; Salbego, C.G. Hypoxic and Reoxygenated Microenvironment: Stemness and Differentiation State in Glioblastoma. Mol. Neurobiol. 2017, 54, 6261–6272. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Roccaro, A.M.; Crivellato, E.; Presta, M. Erythropoietin as an Angiogenic Factor. Eur. J. Clin. Investig. 2003, 33, 891–896. [Google Scholar] [CrossRef]
- Ge, X.; Pan, M.H.; Wang, L.; Li, W.; Jiang, C.; He, J.; Abouzid, K.; Liu, L.Z.; Shi, Z.; Jiang, B.H. Hypoxia-Mediated Mitochondria Apoptosis Inhibition Induces Temozolomide Treatment Resistance through MiR-26a/Bad/Bax Axis. Cell Death Dis. 2018, 9, 1128. [Google Scholar] [CrossRef]
- Haas, B.R.; Sontheimer, H. Inhibition of the Sodium-Potassium-Chloride Cotransporter Isoform-1 Reduces Glioma Invasion. Cancer Res. 2010, 70, 5597–5606. [Google Scholar] [CrossRef]
- Haas, B.R.; Cuddapah, V.A.; Watkins, S.; Rohn, K.J.; Dy, T.E.; Sontheimer, H. With-No-Lysine Kinase 3 (WNK3) Stimulates Glioma Invasion by Regulating Cell Volume. Am. J. Physiol. Cell Physiol. 2011, 301, C1150–C1160. [Google Scholar] [CrossRef]
- Kang, S.S.; Han, K.S.; Ku, B.M.; Lee, Y.K.; Hong, J.; Shin, H.Y.; Almonte, A.G.; Woo, D.H.; Brat, D.J.; Hwang, E.M.; et al. Caffeine-Mediated Inhibition of Calcium Release Channel Inositol 1,4,5-Trisphosphate Receptor Subtype 3 Blocks Glioblastoma Invasion and Extends Survival. Cancer Res. 2010, 70, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Mcferrin, M.B.; Sontheimer, H. A Role for Ion Channels in Glioma Cell Invasion. Neuron Glia Biol. 2006, 2, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Sforna, L.; Cenciarini, M.; Belia, S.; D’Adamo, M.C.; Pessia, M.; Franciolini, F.; Catacuzzeno, L. The Role of Ion Channels in the Hypoxia-Induced Aggressiveness of Glioblastoma. Front. Cell Neurosci. 2015, 8, 467. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Sforna, L.; Esposito, V.; Limatola, C.; Franciolini, F. Ion Channels in Glioma Malignancy. Rev. Physiol. Biochem. Pharmacol. 2021, 181, 223–267. [Google Scholar] [CrossRef] [PubMed]
- Strange, K.; Emma, F.; Jackson, P.S. Cellular and Molecular Physiology of Volume-Sensitive Anion Channels. Am. J. Physiol. 1996, 270, C711–C730. [Google Scholar] [CrossRef]
- Voets, T.; Droogmans, G.; Raskin, G.; Eggermont, J.; Nilius, B. Reduced Intracellular Ionic Strength as the Initial Trigger for Activation of Endothelial Volume-Regulated Anion Channels. Proc. Natl. Acad. Sci. USA 1999, 96, 5298–5303. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of Cell Volume Regulation in Vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Sontheimer, H. Ion Channels and Transporters [Corrected] in Cancer. 2. Ion Channels and the Control of Cancer Cell Migration. Am. J. Physiol. Cell Physiol. 2011, 301, C541–C549. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Michelucci, A.; Sforna, L.; Aiello, F.; Sciaccaluga, M.; Fioretti, B.; Castigli, E.; Franciolini, F. Identification of Key Signaling Molecules Involved in the Activation of the Swelling-Activated Chloride Current in Human Glioblastoma Cells. J. Membr. Biol. 2014, 247, 45–55. [Google Scholar] [CrossRef]
- Cahalan, M.D.; Lewis, R.S. Role of Potassium and Chloride Channels in Volume Regulation by T Lymphocytes. Soc. Gen. Physiol. Ser. 1988, 43, 281–301. [Google Scholar]
- Hazama, A.; Okada, Y. Ca2+ Sensitivity of Volume-Regulatory K+ and Cl− Channels in Cultured Human Epithelial Cells. J. Physiol. 1988, 402, 687–702. [Google Scholar] [CrossRef]
- Strange, K.; Yamada, T.; Denton, J.S. A 30-Year Journey from Volume-Regulated Anion Currents to Molecular Structure of the LRRC8 Channel. J. Gen. Physiol. 2019, 151, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Dubin, A.E.; Mathur, J.; Tu, B.; Reddy, K.; Miraglia, L.J.; Reinhardt, J.; Orth, A.P.; Patapoutian, A. SWELL1, a Plasma Membrane Protein, Is an Essential Component of Volume-Regulated Anion Channel. Cell 2014, 157, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Voss, F.K.; Ullrich, F.; Münch, J.; Lazarow, K.; Lutte, D.; Mah, N.; Andrade-Navarro, M.A.; Von Kries, J.P.; Stauber, T.; Jentsch, T.J. Identification of LRRC8 Heteromers as an Essential Component of the Volume-Regulated Anion Channel VRAC. Science 2014, 344, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Kimelberg, H.K.; Goderie, S.K.; Higman, S.; Pang, S.; Waniewski, R.A. Swelling-Induced Release of Glutamate, Aspartate, and Taurine from Astrocyte Cultures. J. Neurosci. 1990, 10, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Planells-Cases, R.; Lutter, D.; Guyader, C.; Gerhards, N.M.; Ullrich, F.; Elger, D.A.; Kucukosmanoglu, A.; Xu, G.; Voss, F.K.; Reincke, S.M.; et al. Subunit Composition of VRAC Channels Determines Substrate Specificity and Cellular Resistance to Pt-Based Anti-Cancer Drugs. EMBO J. 2015, 34, 2993–3008. [Google Scholar] [CrossRef] [PubMed]
- Sforna, L.; Cenciarini, M.; Belia, S.; Michelucci, A.; Pessia, M.; Franciolini, F.; Catacuzzeno, L. Hypoxia Modulates the Swelling-Activated Cl Current in Human Glioblastoma Cells: Role in Volume Regulation and Cell Survival. J. Cell Physiol. 2017, 232, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Chen, W.; Zhong, X.; Rutka, J.T.; Feng, Z.P.; Sun, H.S. Swelling-Induced Chloride Current in Glioblastoma Proliferation, Migration, and Invasion. J. Cell Physiol. 2018, 233, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Caramia, M.; Sforna, L.; Franciolini, F.; Catacuzzeno, L. The Volume-Regulated Anion Channel in Glioblastoma. Cancers 2019, 11, 307. [Google Scholar] [CrossRef]
- Mola, M.G.; Saracino, E.; Formaggio, F.; Amerotti, A.G.; Barile, B.; Posati, T.; Cibelli, A.; Frigeri, A.; Palazzo, C.; Zamboni, R.; et al. Cell Volume Regulation Mechanisms in Differentiated Astrocytes. Cell Physiol. Biochem. 2021, 55, 196–212. [Google Scholar] [CrossRef]
- Brignone, M.S.; Lanciotti, A.; Michelucci, A.; Mallozzi, C.; Camerini, S.; Catacuzzeno, L.; Sforna, L.; Caramia, M.; D’Adamo, M.C.; Ceccarini, M.; et al. The CaMKII/MLC1 Axis Confers Ca2+-Dependence to Volume-Regulated Anion Channels (VRAC) in Astrocytes. Cells 2022, 11, 2656. [Google Scholar] [CrossRef] [PubMed]
- Elorza-Vidal, X.; Sirisi, S.; Gaitán-Peñas, H.; Pérez-Rius, C.; Alonso-Gardón, M.; Armand-Ugón, M.; Lanciotti, A.; Brignone, M.S.; Prat, E.; Nunes, V.; et al. GlialCAM/MLC1 Modulates LRRC8/VRAC Currents in an Indirect Manner: Implications for Megalencephalic Leukoencephalopathy. Neurobiol. Dis. 2018, 119, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Manning, T.J.; Sontheimer, H. Modulation of Glioma Cell Migration and Invasion Using Cl(−) and K(+) Ion Channel Blockers. J. Neurosci. 1999, 19, 5942–5954. [Google Scholar] [CrossRef] [PubMed]
- Ransom, C.B.; O’Neal, J.T.; Sontheimer, H. Volume-Activated Chloride Currents Contribute to the Resting Conductance and Invasive Migration of Human Glioma Cells. J. Neurosci. 2001, 21, 7674–7683. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, B.; Dąbrowska, A.; Saczko, J.; Kulbacka, J. The Role of Chloride Channels in the Multidrug Resistance. Membranes 2021, 12, 38. [Google Scholar] [CrossRef]
- Rubino, S.; Bach, M.D.; Schober, A.L.; Lambert, I.H.; Mongin, A.A. Downregulation of Leucine-Rich Repeat-Containing 8A Limits Proliferation and Increases Sensitivity of Glioblastoma to Temozolomide and Carmustine. Front. Oncol. 2018, 8, 142. [Google Scholar] [CrossRef]
- Xu, R.; Wang, X.; Shi, C. Volume-Regulated Anion Channel as a Novel Cancer Therapeutic Target. Int. J. Biol. Macromol. 2020, 159, 570–576. [Google Scholar] [CrossRef]
- Bowens, N.H.; Dohare, P.; Kuo, Y.H.; Mongin, A.A. DCPIB, the Proposed Selective Blocker of Volume-Regulated Anion Channels, Inhibits Several Glutamate Transport Pathways in Glial Cells. Mol. Pharmacol. 2013, 83, 22–32. [Google Scholar] [CrossRef]
- Minieri, L.; Pivonkova, H.; Caprini, M.; Harantova, L.; Anderova, M.; Ferroni, S. The Inhibitor of Volume-Regulated Anion Channels DCPIB Activates TREK Potassium Channels in Cultured Astrocytes. Br. J. Pharmacol. 2013, 168, 1240–1254. [Google Scholar] [CrossRef]
- Fujii, T.; Takahashi, Y.; Takeshima, H.; Saitoh, C.; Shimizu, T.; Takeguchi, N.; Sakai, H. Inhibition of Gastric H+,K+-ATPase by 4-(2-Butyl-6,7-Dichloro-2-Cyclopentylindan-1-on-5-Yl)Oxybutyric Acid (DCPIB), an Inhibitor of Volume-Regulated Anion Channel. Eur. J. Pharmacol. 2015, 765, 34–41. [Google Scholar] [CrossRef]
- Liu, T.; Stauber, T. The Volume-Regulated Anion Channel LRRC8/VRAC Is Dispensable for Cell Proliferation and Migration. Int. J. Mol. Sci. 2019, 20, 2663. [Google Scholar] [CrossRef]
- Serpe, C.; Michelucci, A.; Monaco, L.; Rinaldi, A.; De Luca, M.; Familiari, P.; Relucenti, M.; Di Pietro, E.; Di Castro, M.A.; D’agnano, I.; et al. Astrocytes-Derived Small Extracellular Vesicles Hinder Glioma Growth. Biomedicines 2022, 10, 2952. [Google Scholar] [CrossRef]
- Ernest, N.J.; Weaver, A.K.; Van Duyn, L.B.; Sontheimer, H.W. Relative Contribution of Chloride Channels and Transporters to Regulatory Volume Decrease in Human Glioma Cells. Am. J. Physiol. Cell Physiol. 2005, 288, C1451–C1460. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Oberhauser, A.; Labarca, P.; Alvarez, O. Varieties of Calcium-Activated Potassium Channels. Annu. Rev. Physiol. 1989, 51, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.K.; Liu, X.; Sontheimer, H. Role for Calcium-Activated Potassium Channels (BK) in Growth Control of Human Malignant Glioma Cells. J. Neurosci. Res. 2004, 78, 224–234. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Turner, K.L.; Seifert, S.; Sontheimer, H. Bradykinin-Induced Chemotaxis of Human Gliomas Requires the Activation of KCa3.1 and ClC-3. J. Neurosci. 2013, 33, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.L.; Honasoge, A.; Robert, S.M.; Mcferrin, M.M.; Sontheimer, H. A Proinvasive Role for the Ca(2+) -Activated K(+) Channel KCa3.1 in Malignant Glioma. Glia 2014, 62, 971–981. [Google Scholar] [CrossRef]
- Adelman, J.P.; Shen, K.Z.; Kavanaugh, M.P.; Warren, R.A.; Wu, Y.N.; Lagrutta, A.; Bond, C.T.; Alan North, R. Calcium-Activated Potassium Channels Expressed from Cloned Complementary DNAs. Neuron 1992, 9, 209–216. [Google Scholar] [CrossRef]
- Aldrich, R.; Chandy, K.G.; Grissmer, S.; Gutman, G.A.; Kaczmarek, L.K.; Wei, A.D.; Wulff, H. Calcium- and Sodium-Activated Potassium Channels (KCa, KNa) in GtoPdb v.2023.1. IUPHAR/BPS Guide Pharmacol. CITE 2023, 2023, 1–16. [Google Scholar] [CrossRef]
- Xia, X.M.; Zeng, X.; Lingle, C.J. Multiple Regulatory Sites in Large-Conductance Calcium-Activated Potassium Channels. Nature 2002, 418, 880–884. [Google Scholar] [CrossRef]
- Sweet, T.B.; Cox, D.H. Measurements of the BKCa Channel’s High-Affinity Ca2+ Binding Constants: Effects of Membrane Voltage. J. Gen. Physiol. 2008, 132, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Fakler, B.; Adelman, J.P. Control of K(Ca) Channels by Calcium Nano/Microdomains. Neuron 2008, 59, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, B.S.; Magleby, K.L. Voltage and Ca2+ Activation of Single Large-Conductance Ca2+-Activated K+ Channels Described by a Two-Tiered Allosteric Gating Mechanism. J. Gen. Physiol. 2000, 116, 75–99. [Google Scholar] [CrossRef]
- Horrigan, F.T.; Aldrich, R.W. Coupling between Voltage Sensor Activation, Ca2+ Binding and Channel Opening in Large Conductance (BK) Potassium Channels. J. Gen. Physiol. 2002, 120, 267–305. [Google Scholar] [CrossRef] [PubMed]
- Franciolini, F.; Hogg, R.; Catacuzzeno, L.; Petris, A.; Trequattrini, C.; Adams, D.J. Large-Conductance Calcium-Activated Potassium Channels in Neonatal Rat Intracardiac Ganglion Neurons. Pflügers Arch. 2001, 441, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.A.; Catacuzzeno, L.; Trequattrini, C.; Petris, A.; Franciolini, F. Verapamil Block of Large-Conductance Ca-Activated K Channels in Rat Aortic Myocytes. J. Membr. Biol. 2001, 179, 103–111. [Google Scholar] [CrossRef]
- Ransom, C.B.; Sontheimer, H. BK Channels in Human Glioma Cells. J. Neurophysiol. 2001, 85, 790–803. [Google Scholar] [CrossRef]
- Weaver, A.K.; Bomben, V.C.; Sontheimer, H. Expression and Function of Calcium-Activated Potassium Channels in Human Glioma Cells. Glia 2006, 54, 223–233. [Google Scholar] [CrossRef]
- Liu, X.; Chang, Y.; Reinhart, P.H.; Sontheimer, H. Cloning and Characterization of Glioma BK, a Novel BK Channel Isoform Highly Expressed in Human Glioma Cells. J. Neurosci. 2002, 22, 1840–1849. [Google Scholar] [CrossRef]
- Abdullaev, I.F.; Rudkouskaya, A.; Mongin, A.A.; Kuo, Y.H. Calcium-Activated Potassium Channels BK and IK1 Are Functionally Expressed in Human Gliomas but Do Not Regulate Cell Proliferation. PLoS ONE 2010, 5, e12304. [Google Scholar] [CrossRef]
- Debska, G.; Kicinska, A.; Dobrucki, J.; Dworakowska, B.; Nurowska, E.; Skalska, J.; Dolowy, K.; Szewczyk, A. Large-Conductance K+ Channel Openers NS1619 and NS004 as Inhibitors of Mitochondrial Function in Glioma Cells. Biochem. Pharmacol. 2003, 65, 1827–1834. [Google Scholar] [CrossRef]
- Rosa, P.; Sforna, L.; Carlomagno, S.; Mangino, G.; Miscusi, M.; Pessia, M.; Franciolini, F.; Calogero, A.; Catacuzzeno, L. Overexpression of Large-Conductance Calcium-Activated Potassium Channels in Human Glioblastoma Stem-Like Cells and Their Role in Cell Migration. J. Cell Physiol. 2017, 232, 2478–2488. [Google Scholar] [CrossRef]
- Bordey, A.; Sontheimer, H.; Trouslard, J. Muscarinic Activation of BK Channels Induces Membrane Oscillations in Glioma Cells and Leads to Inhibition of Cell Migration. J. Membr. Biol. 2000, 176, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kraft, R.; Krause, P.; Jung, S.; Basrai, D.; Liebmann, L.; Bolz, J.; Patt, S. BK Channel Openers Inhibit Migration of Human Glioma Cells. Pflügers Arch. 2003, 446, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Wondergem, R.; Bartley, J.W. Menthol Increases Human Glioblastoma Intracellular Ca2+, BK Channel Activity and Cell Migration. J. Biomed. Sci. 2009, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Steinle, M.; Palme, D.; Misovic, M.; Rudner, J.; Dittmann, K.; Lukowski, R.; Ruth, P.; Huber, S.M. Ionizing Radiation Induces Migration of Glioblastoma Cells by Activating BK K(+) Channels. Radiother. Oncol. 2011, 101, 122–126. [Google Scholar] [CrossRef]
- Edalat, L.; Stegen, B.; Klumpp, L.; Haehl, E.; Schilbach, K.; Lukowski, R.; Kühnle, M.; Bernhardt, G.; Buschauer, A.; Zips, D.; et al. BK K+ Channel Blockade Inhibits Radiation-Induced Migration/Brain Infiltration of Glioblastoma Cells. Oncotarget 2016, 7, 14259–14278. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, G.; Monaco, L.; Catacuzzeno, L.; Antonangeli, F.; Santoro, A.; Esposito, V.; Franciolini, F.; Wulff, H.; Limatola, C. Radiation Increases Functional KCa3.1 Expression and Invasiveness in Glioblastoma. Cancers 2019, 11, 279. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Franciolini, F. Editorial: The Role of Ca2+-Activated K+ Channels of Intermediate Conductance in Glioblastoma Malignancy. Curr. Neuropharmacol. 2018, 16, 607. [Google Scholar] [CrossRef]
- Fioretti, B.; Catacuzzeno, L.; Sforna, L.; Aiello, F.; Pagani, F.; Ragozzino, D.; Castigli, E.; Franciolini, F. Histamine Hyperpolarizes Human Glioblastoma Cells by Activating the Intermediate-Conductance Ca2+-Activated K+ Channel. Am. J. Physiol. Cell Physiol. 2009, 297, C102–C110. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Catalano, M.; Sciaccaluga, M.; Chece, G.; Cipriani, R.; Rosito, M.; Grimaldi, A.; Lauro, C.; Cantore, G.; Santoro, A.; et al. KCa3.1 Channels Are Involved in the Infiltrative Behavior of Glioblastoma in Vivo. Cell Death Dis. 2013, 4, e773. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Franciolini, F. Role of KCa3.1 Channels in Modulating Ca2+ Oscillations during Glioblastoma Cell Migration and Invasion. Int. J. Mol. Sci. 2018, 19, 2970. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Fioretti, B.; Franciolini, F. Expression and Role of the Intermediate-Conductance Calcium-Activated Potassium Channel KCa3.1 in Glioblastoma. J. Signal. Transduct. 2012, 2012, 421564. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, B.; Franciolini, F.; Catacuzzeno, L. A Model of Intracellular Ca2+ Oscillations Based on the Activity of the Intermediate-Conductance Ca2+-Activated K+ Channels. Biophys. Chem. 2005, 113, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Sforna, L.; Di Battista, A.; Franciolini, F.; Catacuzzeno, L. Ca2+-Activated K+ Channels Regulate Cell Volume in Human Glioblastoma Cells. J. Cell Physiol. 2023, 238, 2120–2134. [Google Scholar] [CrossRef] [PubMed]
- Suchyna, T.M.; Johnson, J.H.; Hamer, K.; Leykam, J.F.; Gage, D.A.; Clemo, H.F.; Baumgarten, C.M.; Sachs, F. Identification of a Peptide Toxin from Grammostola Spatulata Spider Venom That Blocks Cation-Selective Stretch-Activated Channels. J. Gen. Physiol. 2000, 115, 583–598. [Google Scholar] [CrossRef]
- Shen, M.R.; Chou, C.Y.; Chiu, W.T. Streptomycin and Its Analogues Are Potent Inhibitors of the Hypotonicity-Induced Ca2+ Entry and Cl− Channel Activity. FEBS Lett. 2003, 554, 494–500. [Google Scholar] [CrossRef]
- Ermakov, Y.A.; Kamaraju, K.; Sengupta, K.; Sukharev, S. Gadolinium Ions Block Mechanosensitive Channels by Altering the Packing and Lateral Pressure of Anionic Lipids. Biophys. J. 2010, 98, 1018–1027. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Bae, C.; Sachs, F.; Gottlieb, P.A. The Mechanosensitive Ion Channel Piezo1 Is Inhibited by the Peptide GsMTx4. Biochemistry 2011, 50, 6295–6300. [Google Scholar] [CrossRef]
- Alcaino, C.; Knutson, K.; Gottlieb, P.A.; Farrugia, G.; Beyder, A. Mechanosensitive Ion Channel Piezo2 Is Inhibited by D-GsMTx4. Channels 2017, 11, 245–253. [Google Scholar] [CrossRef]
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the Mammalian Mechanosensitive Piezo1 Channel. Nature 2015, 527, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical Activation of the Mechanotransduction Channel Piezo1. eLife 2015, 4, e07369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A Lever-like Transduction Pathway for Long-Distance Chemical- and Mechano-Gating of the Mechanosensitive Piezo1 Channel. Nat. Commun. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.K.; Hu, M.; Draghici, D.; et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron 2018, 100, 799–815.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, X.; van Wijnbergen, J.W.M.; Yuan, L.; Liu, Y.; Zhang, C.; Jia, W. Identification of PIEZO1 as a Potential Prognostic Marker in Gliomas. Sci. Rep. 2020, 10, 16121. [Google Scholar] [CrossRef]
- Qu, S.; Li, S.; Hu, Z. Upregulation of Piezo1 Is a Novel Prognostic Indicator in Glioma Patients. Cancer Manag. Res. 2020, 12, 3527–3536. [Google Scholar] [CrossRef]
- Knoblauch, S.V.; Desai, S.H.; Dombroski, J.A.; Sarna, N.S.; Hope, J.M.; King, M.R. Chemical Activation and Mechanical Sensitization of Piezo1 Enhance TRAIL-Mediated Apoptosis in Glioblastoma Cells. ACS Omega 2023, 8, 16975–16986. [Google Scholar] [CrossRef]
- Moroni, M.; Servin-Vences, M.R.; Fleischer, R.; Sánchez-Carranza, O.; Lewin, G.R. Voltage Gating of Mechanosensitive PIEZO Channels. Nat. Commun. 2018, 9, 1096. [Google Scholar] [CrossRef]
- Sforna, L.; Michelucci, A.; Morena, F.; Argentati, C.; Franciolini, F.; Vassalli, M.; Martino, S.; Catacuzzeno, L. Piezo1 Controls Cell Volume and Migration by Modulating Swelling-Activated Chloride Current through Ca2+ Influx. J. Cell Physiol. 2022, 237, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.; Sontheimer, H. Hydrodynamic Cellular Volume Changes Enable Glioma Cell Invasion. J. Neurosci. 2011, 31, 17250–17259. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Nechyporuk-Zloy, V.; Fabian, A.; Stock, C. Cells Move When Ions and Water Flow. Pflügers Arch. 2007, 453, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of Ion Channels and Transporters in Cell Migration. Physiol. Rev. 2012, 92, 1865–1913. [Google Scholar] [CrossRef] [PubMed]
- Simon, O.J.; Müntefering, T.; Grauer, O.M.; Meuth, S.G. The Role of Ion Channels in Malignant Brain Tumors. J. Neurooncol. 2015, 125, 225–235. [Google Scholar] [CrossRef]
- Schwab, A.; Stock, C. Ion Channels and Transporters in Tumour Cell Migration and Invasion. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130102. [Google Scholar] [CrossRef]
- Schiapparelli, P.; Guerrero-Cazares, H.; Magaña-Maldonado, R.; Hamilla, S.M.; Ganaha, S.; Goulin Lippi Fernandes, E.; Huang, C.H.; Aranda-Espinoza, H.; Devreotes, P.; Quinones-Hinojosa, A. NKCC1 Regulates Migration Ability of Glioblastoma Cells by Modulation of Actin Dynamics and Interacting with Cofilin. EBioMedicine 2017, 21, 94–103. [Google Scholar] [CrossRef]
- McFerrin, M.B.; Turner, K.L.; Cuddapah, V.A.; Sontheimer, H. Differential Role of IK and BK Potassium Channels as Mediators of Intrinsic and Extrinsic Apoptotic Cell Death. Am. J. Physiol. Cell Physiol. 2012, 303, C1070–C1078. [Google Scholar] [CrossRef]
- Okada, Y. Ion Channel Roles in Cell Death Induction. J. Membr. Biol. 2006, 209, 1–2. [Google Scholar] [CrossRef]
- Kreisman, N.R.; LaManna, J.C. Rapid and Slow Swelling during Hypoxia in the CA1 Region of Rat Hippocampal Slices. J. Neurophysiol. 1999, 82, 320–329. [Google Scholar] [CrossRef]
- Barros, R.C.H.; Zimmer, M.E.; Branco, L.G.S.; Milsom, W.K. Hypoxic Metabolic Response of the Golden-Mantled Ground Squirrel. J. Appl. Physiol. 2001, 91, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Pasantes-Morales, H.; Vázquez-Juárez, E. Transporters and Channels in Cytotoxic Astrocyte Swelling. Neurochem. Res. 2012, 37, 2379–2387. [Google Scholar] [CrossRef]
- Steinbach, J.P.; Wolburg, H.; Klumpp, A.; Probst, H.; Weller, M. Hypoxia-Induced Cell Death in Human Malignant Glioma Cells: Energy Deprivation Promotes Decoupling of Mitochondrial Cytochrome c Release from Caspase Processing and Necrotic Cell Death. Cell Death Differ. 2003, 10, 823–832. [Google Scholar] [CrossRef]
- Lenihan, C.R.; Taylor, C.T. The Impact of Hypoxia on Cell Death Pathways. Biochem. Soc. Trans. 2013, 41, 657–663. [Google Scholar] [CrossRef]
- Rosa, P.; Catacuzzeno, L.; Sforna, L.; Mangino, G.; Carlomagno, S.; Mincione, G.; Petrozza, V.; Ragona, G.; Franciolini, F.; Calogero, A. BK Channels Blockage Inhibits Hypoxia-Induced Migration and Chemoresistance to Cisplatin in Human Glioblastoma Cells. J. Cell Physiol. 2018, 233, 6866–6877. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Caramia, M.; Sforna, L.; Belia, S.; Guglielmi, L.; D’Adamo, M.C.; Pessia, M.; Franciolini, F. Reconciling the Discrepancies on the Involvement of Large-Conductance Ca(2+)-Activated K Channels in Glioblastoma Cell Migration. Front. Cell Neurosci. 2015, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; McKenna, F.; Rowe, I.C.M.; Ashford, M.L.J. The Effects of Neuroleptic and Tricyclic Compounds on BKCa Channel Activity in Rat Isolated Cortical Neurones. Br. J. Pharmacol. 1997, 121, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Ataga, K.I.; Smith, W.R.; De Castro, L.M.; Swerdlow, P.; Saunthararajah, Y.; Castro, O.; Vichinsky, E.; Kutlar, A.; Orringer, E.P.; Rigdon, G.C.; et al. Efficacy and Safety of the Gardos Channel Blocker, Senicapoc (ICA-17043), in Patients with Sickle Cell Anemia. Blood 2008, 111, 3991–3997. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).