Improved Physiochemical Properties of Chitosan@PCL Nerve Conduits by Natural Molecule Crosslinking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nerve Conduits Fabrication
2.3. Nerve Conduits Characterization
2.4. Statistical Analysis
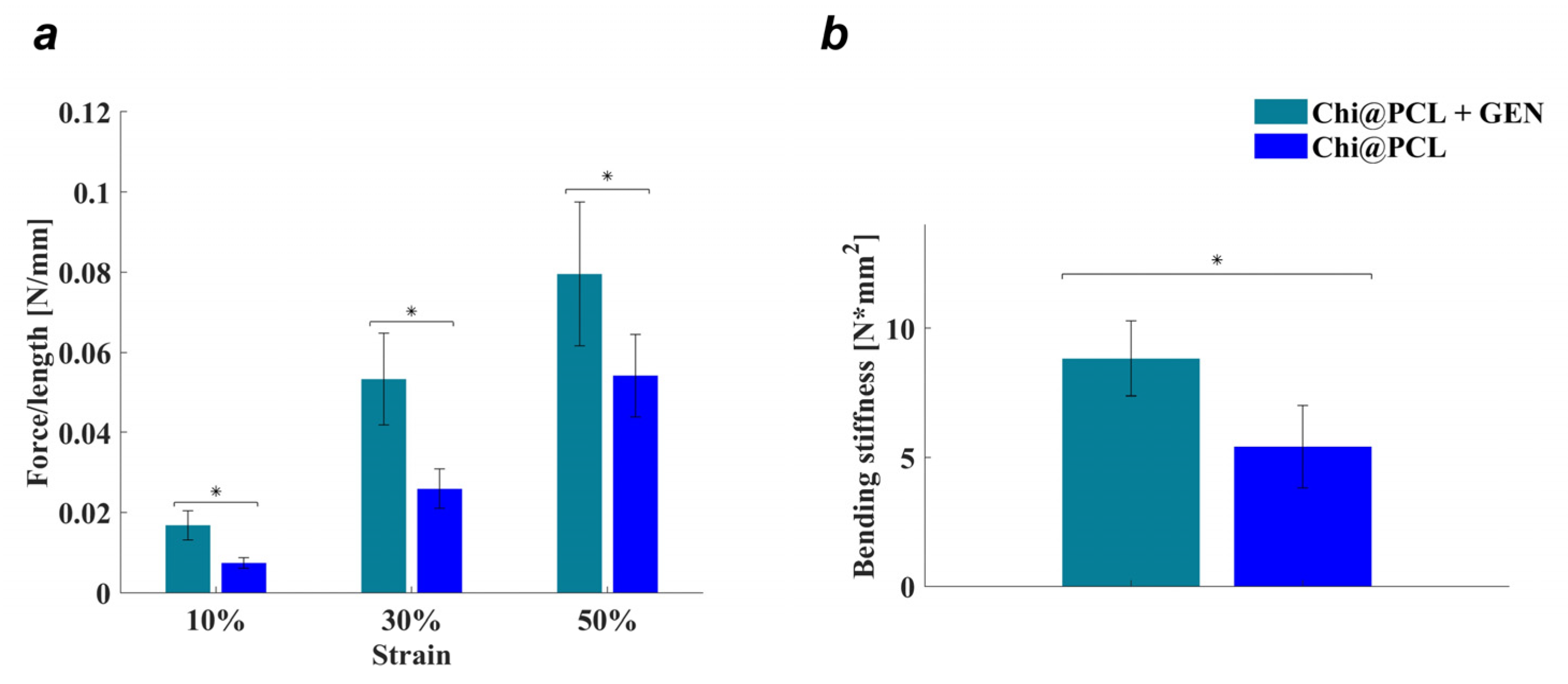
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murphy, R.N.A.; de Schoulepnikoff, C.; Chen, J.H.C.; Columb, M.O.; Bedford, J.; Wong, J.K.; Reid, A.J. The Incidence and Management of Peripheral Nerve Injury in England (2005–2020). J. Plast. Reconstr. Aesthet. Surg. 2023, 80, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.Z.; Mackinnon, S.E. Management of Nerve Gaps: Autografts, Allografts, Nerve Transfers, and End-to-Side Neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.E.; Joosten, E.A.J.; Weis, J.; Brook, G.A. Repairing Injured Peripheral Nerves: Bridging the Gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yao, R.; Zhao, J.; Chen, K.; Duan, L.; Wang, T.; Zhang, S.; Guan, J.; Zheng, Z.; Wang, X.; et al. Implantable Nerve Guidance Conduits: Material Combinations, Multi-Functional Strategies and Advanced Engineering Innovations. Bioact. Mater. 2022, 11, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, M.; Micera, S.; Redolfi Riva, E. Recent Advances in Polymeric Drug Delivery Systems for Peripheral Nerve Regeneration. Pharmaceutics 2023, 15, 640. [Google Scholar] [CrossRef] [PubMed]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of Peripheral Nerve Regeneration: Interactions at the Axon Level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Lin, H.; Yan, Z.; Shi, J.; Fan, C. Functional Nanomaterials in Peripheral Nerve Regeneration: Scaffold Design, Chemical Principles and Microenvironmental Remodeling. Mater. Today 2021, 51, 165–187. [Google Scholar] [CrossRef]
- Redolfi Riva, E.; Micera, S. Progress and Challenges of Implantable Neural Interfaces Based on Nature-Derived Materials. Bioelectron. Med. 2021, 7, 6. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Rudraiah, S.; Arul, M.R.; Bartley, J.M.; Baker, J.T.; Yu, X.; Kumbar, S.G. Biopolymer-Nanotube Nerve Guidance Conduit Drug Delivery for Peripheral Nerve Regeneration: In Vivo Structural and Functional Assessment. Bioact. Mater. 2021, 6, 2881–2893. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, L.; Shi, X.; Xia, B.; Liu, Z.; Zhu, S.; Yang, Y.; Ma, T.; Cheng, P.; Luo, K.; et al. A Compound Scaffold with Uniform Longitudinally Oriented Guidance Cues and a Porous Sheath Promotes Peripheral Nerve Regeneration In Vivo. Acta Biomater. 2018, 68, 223–236. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zheng, Z.; Yan, J.; Zhang, L.; Li, Y.; Zhang, J.; Li, G.; Wang, X.; Kaplan, D. Porous Nerve Guidance Conduits Reinforced with Braided Composite Structures of Silk/Magnesium Filaments for Peripheral Nerve Repair. Acta Biomater. 2021, 134, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Boeckstyns, M.E.H.; Sørensen, A.I.; Viñeta, J.F.; Rosén, B.; Navarro, X.; Archibald, S.J.; Valss-Solé, J.; Moldovan, M.; Krarup, C. Collagen Conduit Versus Microsurgical Neurorrhaphy: 2-Year Follow-Up of a Prospective, Blinded Clinical and Electrophysiological Multicenter Randomized, Controlled Trial. J. Hand Surg. 2013, 38, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, F.; Cobianchi, S.; Geuna, S.; Barwig, C.; Freier, T.; Udina, E.; Navarro, X. Tubulization with Chitosan Guides for the Repair of Long Gap Peripheral Nerve Injury in the Rat. Microsurgery 2015, 35, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Stenberg, L.; Gonzalez-Perez, F.; Wrobel, S.; Ronchi, G.; Udina, E.; Suganuma, S.; Geuna, S.; Navarro, X.; Dahlin, L.B.; et al. Chitosan-Film Enhanced Chitosan Nerve Guides for Long-Distance Regeneration of Peripheral Nerves. Biomaterials 2016, 76, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Haastert-Talini, K.; Geuna, S.; Dahlin, L.B.; Meyer, C.; Stenberg, L.; Freier, T.; Heimann, C.; Barwig, C.; Pinto, L.F.V.; Raimondo, S.; et al. Chitosan Tubes of Varying Degrees of Acetylation for Bridging Peripheral Nerve Defects. Biomaterials 2013, 34, 9886–9904. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.R.; López-Cebral, R.; Silva-Correia, J.; Silva, J.M.; Mano, J.F.; Silva, T.H.; Freier, T.; Reis, R.L.; Oliveira, J.M. Investigation of Cell Adhesion in Chitosan Membranes for Peripheral Nerve Regeneration. Mater. Sci. Eng. C 2017, 71, 1122–1134. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef]
- Ao, Q.; Fung, C.-K.; Yat-Ping Tsui, A.; Cai, S.; Zuo, H.-C.; Chan, Y.-S.; Kwok-Yan Shum, D. The Regeneration of Transected Sciatic Nerves of Adult Rats Using Chitosan Nerve Conduits Seeded with Bone Marrow Stromal Cell-Derived Schwann Cells. Biomaterials 2011, 32, 787–796. [Google Scholar] [CrossRef]
- Redolfi Riva, E.; Micera, S.; Lionetti, V.; Strauss, I. A Guide Channel for Regenerative Nerve Interface Devices 2022. WO2022234401A1. 2004. Available online: https://worldwide.espacenet.com/ (accessed on 16 October 2023).
- Redolfi-Riva, E.; Pérez-Izquierdo, M.; Zinno, C.; Contreras, E.; Rodríguez-Meana, B.; Iberite, F.; Ricotti, L.; Micera, S.; Navarro, X. A Novel 3D-Printed/Porous Conduit with Tunable Properties to Enhance Nerve Regeneration Over the Limiting Gap Length. Adv. Mater. Technol. 2023, 8, 2300136. [Google Scholar] [CrossRef]
- Ramos-de-la-Peña, A.M.; Renard, C.M.G.C.; Montañez, J.; de la Luz Reyes-Vega, M.; Contreras-Esquivel, J.C. A Review through Recovery, Purification and Identification of Genipin. Phytochem. Rev. 2016, 15, 37–49. [Google Scholar] [CrossRef]
- Yao, C.-H.; Liu, B.-S.; Chang, C.-J.; Hsu, S.-H.; Chen, Y.-S. Preparation of Networks of Gelatin and Genipin as Degradable Biomaterials. Mater. Chem. Phys. 2004, 83, 204–208. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of Gelatin Films by Crosslinking with Genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef] [PubMed]
- Nishi, C.; Nakajima, N.; Ikada, Y. In Vitro Evaluation of Cytotoxicity of Diepoxy Compounds Used for Biomaterial Modification. J. Biomed. Mater. Res. 1995, 29, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-W.; Chang, Y.; Chiu, C.-T.; Chen, C.-N.; Liang, H.-C. Crosslinking Characteristics and Mechanical Properties of a Bovine Pericardium Fixed with a Naturally Occurring Crosslinking Agent. J. Biomed. Mater. Res. 1999, 47, 116–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Synthesis and Characterization of Macroporous Chitosan/Calcium Phosphate Composite Scaffolds for Tissue Engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, L.d.Y.; da Conceição, T.F.; Spinelli, A.; Scharnagl, N.; Nunes Pires, A.T. The Influence of the Crosslinking Degree on the Corrosion Protection Properties of Chitosan Coatings in Simulated Body Fluid. Prog. Org. Coat. 2019, 137, 105328. [Google Scholar] [CrossRef]
- Hayat, U.; Raza, A.; Wang, J.-Y. Preparation of Zein/PVP Based Conduits with Tunable Degradation. Polym. Degrad. Stab. 2020, 181, 109303. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef]
- Delmar, K.; Bianco-Peled, H. The Dramatic Effect of Small pH Changes on the Properties of Chitosan Hydrogels Crosslinked with Genipin. Carbohydr. Polym. 2015, 127, 28–37. [Google Scholar] [CrossRef]
- Mi, F.-L.; Shyu, S.-S.; Peng, C.-K. Characterization of Ring-Opening Polymerization of Genipin and pH-Dependent Cross-Linking Reactions between Chitosan and Genipin. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1985–2000. [Google Scholar] [CrossRef]
- Cui, L.; Jia, J.; Guo, Y.; Liu, Y.; Zhu, P. Preparation and Characterization of IPN Hydrogels Composed of Chitosan and Gelatin Cross-Linked by Genipin. Carbohydr. Polym. 2014, 99, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/Chitosan Porous Scaffolds with Improved Biostability for Skin Tissue Engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Moztarzadeh, F.; Aghazadeh Mohandesi, J. Investigating the Effect of Chitosan on Hydrophilicity and Bioactivity of Conductive Electrospun Composite Scaffold for Neural Tissue Engineering. Int. J. Biol. Macromol. 2019, 121, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Moshayedi, S.; Sarpoolaky, H.; Khavandi, A. Fabrication, Swelling Behavior, and Water Absorption Kinetics of Genipin-Crosslinked Gelatin–Chitosan Hydrogels. Polym. Eng. Sci. 2021, 61, 3094–3103. [Google Scholar] [CrossRef]
- Bi, L.; Cao, Z.; Hu, Y.; Song, Y.; Yu, L.; Yang, B.; Mu, J.; Huang, Z.; Han, Y. Effects of Different Cross-Linking Conditions on the Properties of Genipin-Cross-Linked Chitosan/Collagen Scaffolds for Cartilage Tissue Engineering. J. Mater. Sci. Mater. Med. 2011, 22, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Meena, P.; Kakkar, A.; Kumar, M.; Khatri, N.; Nagar, R.K.; Singh, A.; Malhotra, P.; Shukla, M.; Saraswat, S.K.; Srivastava, S.; et al. Advances and Clinical Challenges for Translating Nerve Conduit Technology from Bench to Bed Side for Peripheral Nerve Repair. Cell Tissue Res. 2021, 383, 617–644. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA Approved Guidance Conduits and Wraps for Peripheral Nerve Injury: A Review of Materials and Efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Yannas, I.V.; Hill, B.J. Selection of Biomaterials for Peripheral Nerve Regeneration Using Data from the Nerve Chamber Model. Biomaterials 2004, 25, 1593–1600. [Google Scholar] [CrossRef]
- Lackington, W.A.; Ryan, A.J.; O’Brien, F.J. Advances in Nerve Guidance Conduit-Based Therapeutics for Peripheral Nerve Repair. ACS Biomater. Sci. Eng. 2017, 3, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.; Browne, T. Overcoming Short Gaps in Peripheral Nerve Repair: Conduits and Human Acellular Nerve Allograft. Hand 2014, 9, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Rhodes, D.I.; O’Brien, C.M.; Rodda, A.E.; Cameron, N.R. Nerve Guidance Conduit Development for Primary Treatment of Peripheral Nerve Transection Injuries: A Commercial Perspective. Acta Biomater. 2021, 135, 64–86. [Google Scholar] [CrossRef] [PubMed]
- Redolfi Riva, E.; Desii, A.; Sartini, S.; La Motta, C.; Mazzolai, B.; Mattoli, V. PMMA/Polysaccharides Nanofilm Loaded with Adenosine Deaminase Inhibitor for Targeted Anti-Inflammatory Drug Delivery. Langmuir 2013, 29, 13190–13197. [Google Scholar] [CrossRef] [PubMed]
- Redolfi Riva, E.; Desii, A.; Sinibaldi, E.; Ciofani, G.; Piazza, V.; Mazzolai, B.; Mattoli, V. Gold Nanoshell/Polysaccharide Nanofilm for Controlled Laser-Assisted Tissue Thermal Ablation. ACS Nano 2014, 8, 5552–5563. [Google Scholar] [CrossRef] [PubMed]
- Redolfi Riva, E.; D’Alessio, A.; Micera, S. Polysaccharide Layer-by-Layer Coating for Polyimide-Based Neural Interfaces. Micromachines 2022, 13, 692. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.T.; Di Lisa, D.; Massobrio, P.; Colistra, N.; Pesce, M.; Catelani, T.; Dellacasa, E.; Raiteri, R.; Martinoia, S.; Pastorino, L. Soft Chitosan Microbeads Scaffold for 3D Functional Neuronal Networks. Biomaterials 2018, 156, 159–171. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Neuroprotective Properties of Chitosan and Its Derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef]
- Hao, C.; Wang, W.; Wang, S.; Zhang, L.; Guo, Y. An Overview of the Protective Effects of Chitosan and Acetylated Chitosan Oligosaccharides against Neuronal Disorders. Mar. Drugs 2017, 15, 89. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Zhao, W.; Zhang, L.; Wang, C.; Jiang, M.; Gu, X.; Yang, Y. Porous Chitosan Scaffolds with Surface Micropatterning and Inner Porosity and Their Effects on Schwann Cells. Biomaterials 2014, 35, 8503–8513. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, P.; Yang, Y.; Wang, X.; Gu, X. The Interaction of Schwann Cells with Chitosan Membranes and Fibers In Vitro. Biomaterials 2004, 25, 4273–4278. [Google Scholar] [CrossRef]
- Fereshteh, Z. 7—Freeze-Drying Technologies for 3D Scaffold Engineering. In Functional 3D Tissue Engineering Scaffolds; Deng, Y., Kuiper, J., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 151–174. ISBN 978-0-08-100979-6. [Google Scholar]
- Reys, L.L.; Silva, S.S.; Pirraco, R.P.; Marques, A.P.; Mano, J.F.; Silva, T.H.; Reis, R.L. Influence of Freezing Temperature and Deacetylation Degree on the Performance of Freeze-Dried Chitosan Scaffolds towards Cartilage Tissue Engineering. Eur. Polym. J. 2017, 95, 232–240. [Google Scholar] [CrossRef]
- Williams, L.R.; Longo, F.M.; Powell, H.C.; Lundborg, G.; Varon, S. Spatial-Temporal Progress of Peripheral Nerve Regeneration within a Silicone Chamber: Parameters for a Bioassay. J. Comp. Neurol. 1983, 218, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Qian, Y.; Wang, H.; Fan, L.; Huang, C.; Yin, A.; Mo, X. Genipin-Crosslinked Silk Fibroin/Hydroxybutyl Chitosan Nanofibrous Scaffolds for Tissue-Engineering Application. J. Biomed. Mater. Res. Part A 2010, 95A, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-P.; Wang, Y.-J.; Ren, L.; Wu, G.; Caridade, S.G.; Fan, J.-B.; Wang, L.-Y.; Ji, P.-H.; Oliveira, J.M.; Oliveira, J.T.; et al. Genipin-Cross-Linked Collagen/Chitosan Biomimetic Scaffolds for Articular Cartilage Tissue Engineering Applications. J. Biomed. Mater. Res. Part A 2010, 95A, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.-J.; Lim, K.-H.; Jung, H.-J.; Park, E.-H. Anti-Inflammatory Evaluation of Gardenia Extract, Geniposide and Genipin. J. Ethnopharmacol. 2006, 103, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.N.; Choi, Y.-S.; Jung, H.-J.; Park, G.H.; Park, J.-M.; Moon, S.-K.; Cho, K.-H.; Kang, C.; Kang, I.; Oh, M.S.; et al. Genipin Inhibits the Inflammatory Response of Rat Brain Microglial Cells. Int. Immunopharmacol. 2010, 10, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Chiba, K.; Mohri, T.; Hatanaka, H. Cyclic GMP-Dependent Neurite Outgrowth by Genipin and Nerve Growth Factor in PC12h Cells. Eur. J. Pharmacol. 2004, 488, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Chiba, K. Genipin Exhibits Neurotrophic Effects through a Common Signaling Pathway in Nitric Oxide Synthase-Expressing Cells. Eur. J. Pharmacol. 2008, 581, 255–261. [Google Scholar] [CrossRef]
- Hughes, R.H.; Silva, V.A.; Ahmed, I.; Shreiber, D.I.; Morrison, B. Neuroprotection by Genipin against Reactive Oxygen and Reactive Nitrogen Species-Mediated Injury in Organotypic Hippocampal Slice Cultures. Brain Res. 2014, 1543, 308–314. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, W.; He, J.; Zhao, Y.; Ding, F.; Gu, X. Nerve Conduits Based on Immobilization of Nerve Growth Factor onto Modified Chitosan by Using Genipin as a Crosslinking Agent. Eur. J. Pharm. Biopharm. 2011, 79, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kočí, Z.; Sridharan, R.; Hibbitts, A.J.; Kneafsey, S.L.; Kearney, C.J.; O’Brien, F.J. The Use of Genipin as an Effective, Biocompatible, Anti-Inflammatory Cross-Linking Method for Nerve Guidance Conduits. Adv. Biosyst. 2020, 4, 1900212. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Tonda-Turo, C. Trends in the Design of Nerve Guidance Channels in Peripheral Nerve Tissue Engineering. Prog. Neurobiol. 2015, 131, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yang, Y.; Bao, Z.; Midgley, A.C.; Li, F.; Dai, S.; Yang, Z.; Wang, J.; Liu, L.; Li, W.; et al. Micro-Nanofiber Composite Biomimetic Conduits Promote Long-Gap Peripheral Nerve Regeneration in Canine Models. Bioact. Mater. 2023, 30, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Lin, Z.; Liu, Y.; Wang, Z.; Lin, Y.; Gong, C.; Ruan, R.; Zhang, J.; Yang, H. Functionalization Strategies of Electrospun Nanofibrous Scaffolds for Nerve Tissue Engineering. Smart Mater. Med. 2021, 2, 260–279. [Google Scholar] [CrossRef]
- Udina, E.; Voda, J.; Gold, B.G.; Navarro, X. Comparative Dose-Dependence Study of FK506 on Transected Mouse Sciatic Nerve Repaired by Allograft or Xenograft. J. Peripher. Nerv. Syst. 2003, 8, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Reay, S.L.; Jackson, E.L.; Ferreira, A.M.; Hilkens, C.M.U.; Novakovic, K. In Vitro Evaluation of the Biodegradability of Chitosan–Genipin Hydrogels. Mater. Adv. 2022, 3, 7946–7959. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, J.; Luo, Y.; Zhou, T.; Wu, H. Preparation and Degradation of Chitosan-Poly(p-Dioxanone)/Silk Fibroin Porous Conduits. Polym. Degrad. Stab. 2015, 119, 46–55. [Google Scholar] [CrossRef]
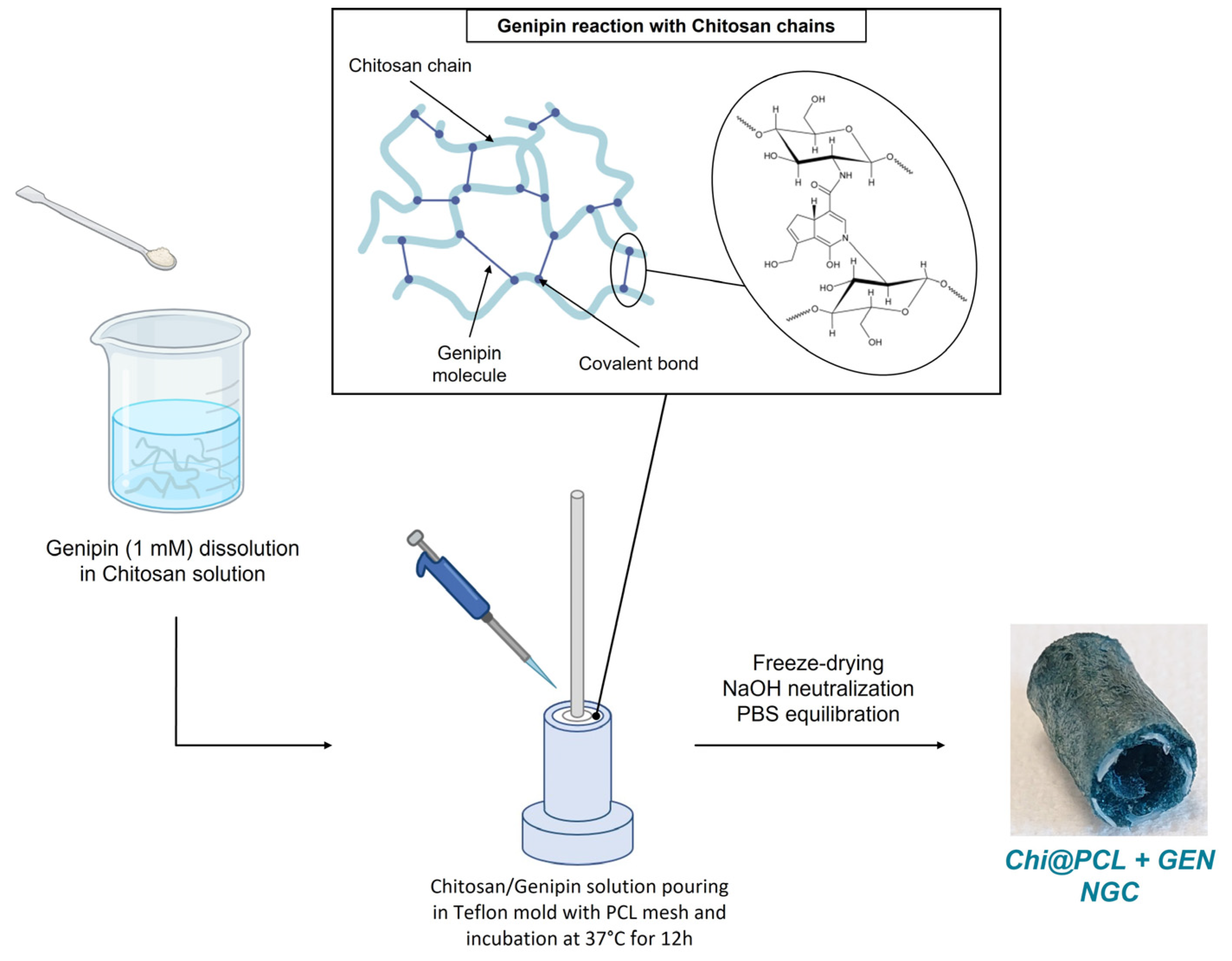
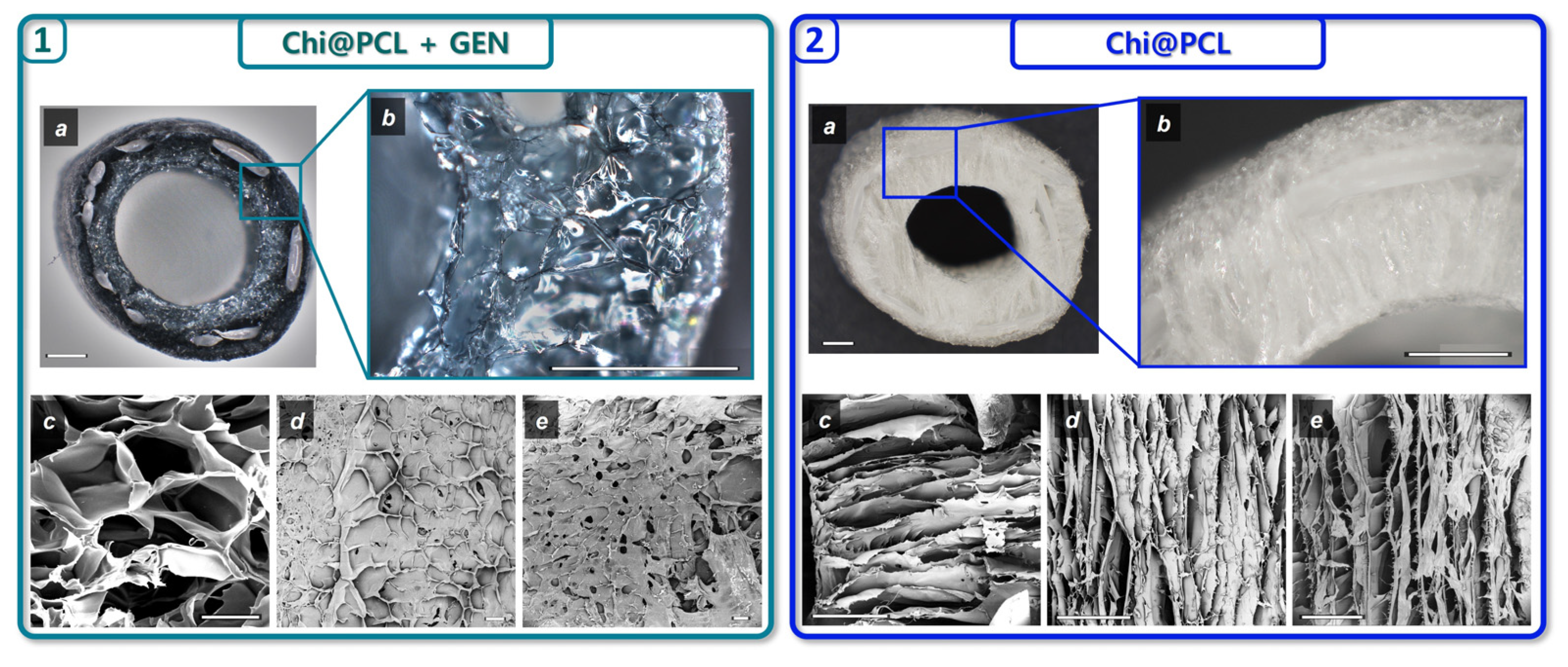

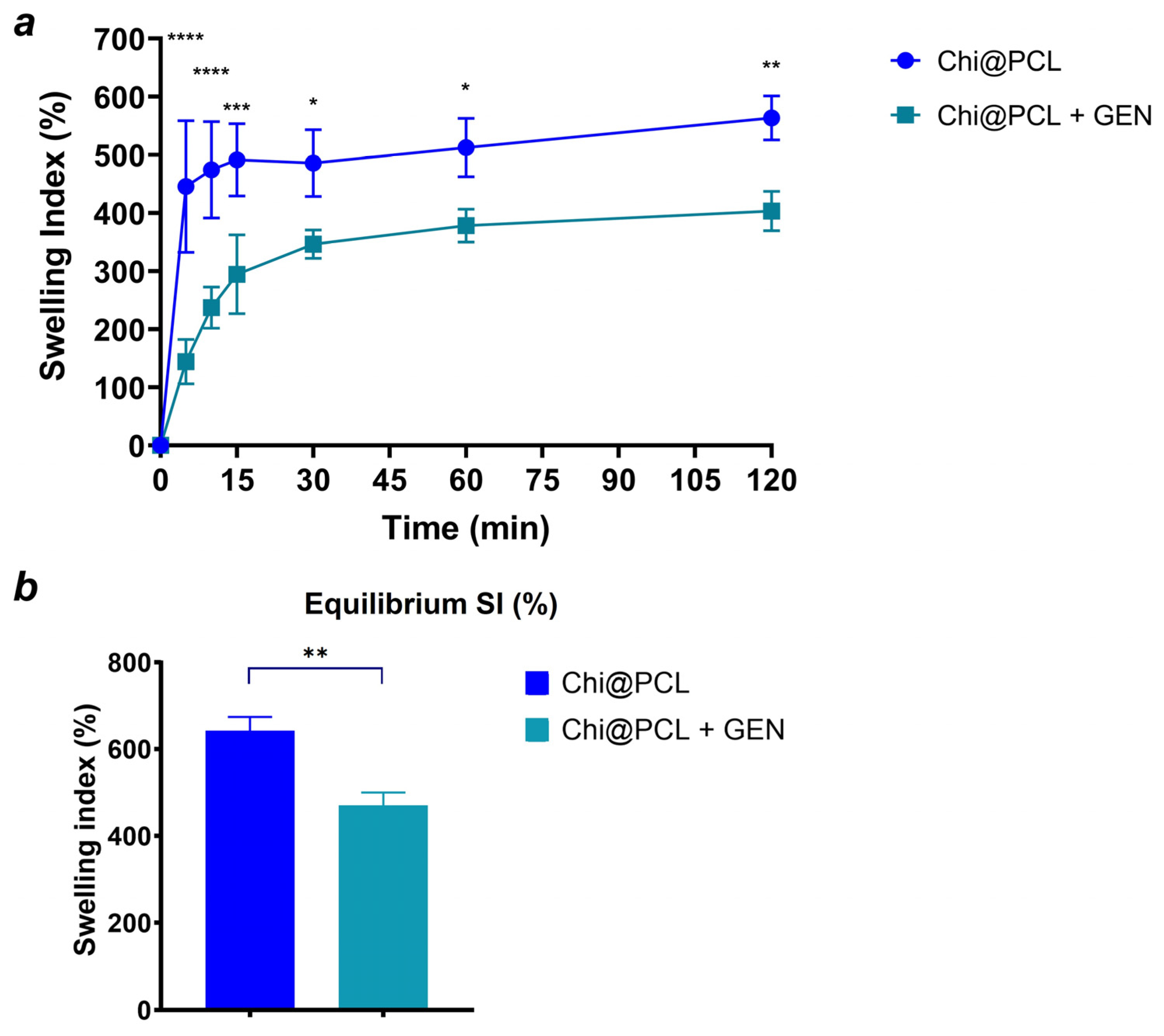
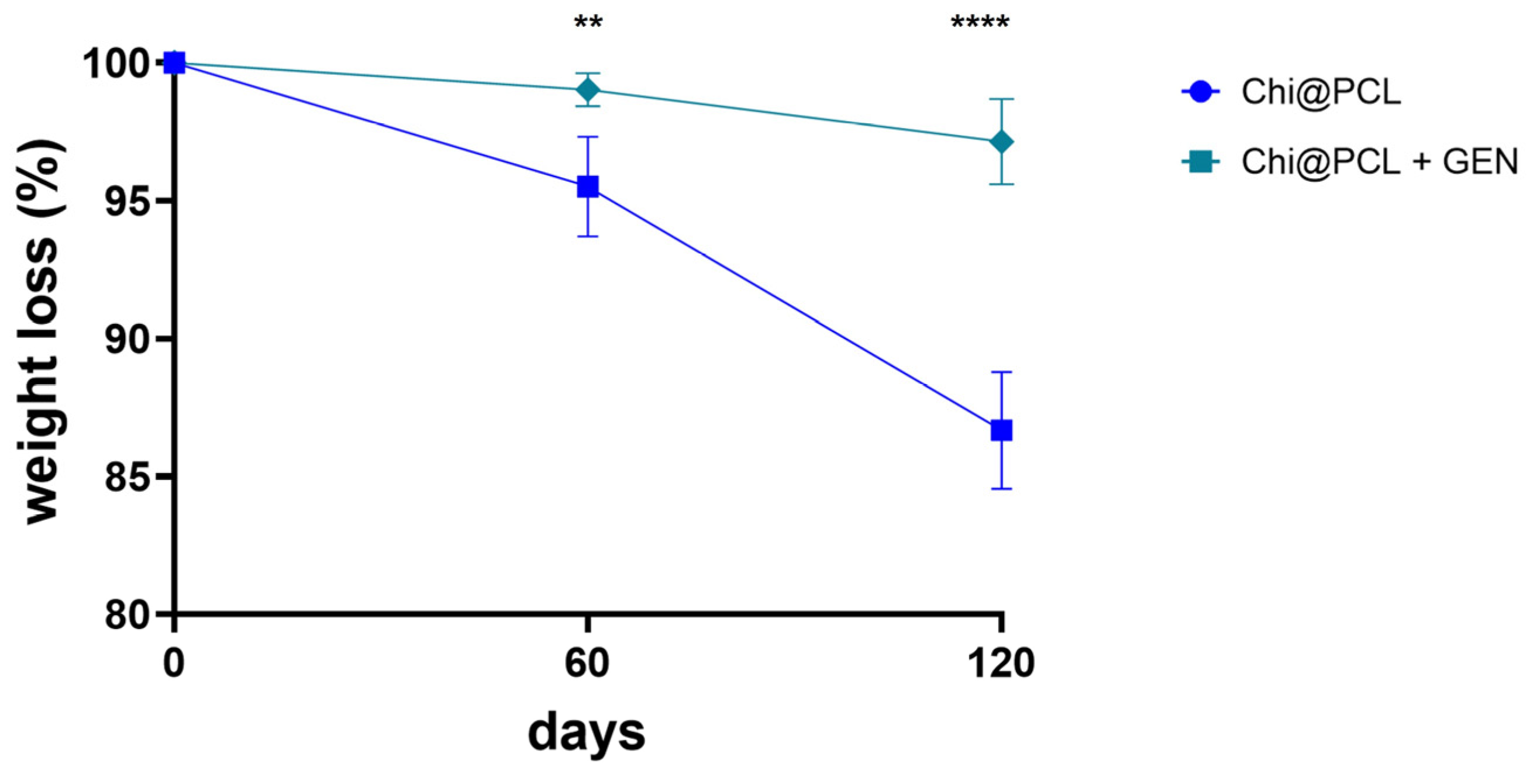
| Genipin (mM) | Porosity (%) | Eq SI (%) | Wall Thickness, Dry (mm) | Internal Diameter, Dry (mm) | Wall Thickness, Wet (mm) | Internal Diameter, Wet (mm) | |
|---|---|---|---|---|---|---|---|
| Chi@PCL + GEN | 1.1 | 76.18 ± 1.13 | 470.3 ± 29.7 | 1.29 ± 0.26 | 3.89 ± 0.25 | 1.45 ± 0.15 | 3.2 ± 0.2 |
| Chi@PCL | 0 | 91.25 ± 2.26 | 642.3 ± 31.6 | 1.7 ± 0.14 | 3.81 ± 0.3 | 1.87 ± 0.12 | 3.48 ± 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchini, M.; Zinno, C.; Micera, S.; Redolfi Riva, E. Improved Physiochemical Properties of Chitosan@PCL Nerve Conduits by Natural Molecule Crosslinking. Biomolecules 2023, 13, 1712. https://doi.org/10.3390/biom13121712
Bianchini M, Zinno C, Micera S, Redolfi Riva E. Improved Physiochemical Properties of Chitosan@PCL Nerve Conduits by Natural Molecule Crosslinking. Biomolecules. 2023; 13(12):1712. https://doi.org/10.3390/biom13121712
Chicago/Turabian StyleBianchini, Marta, Ciro Zinno, Silvestro Micera, and Eugenio Redolfi Riva. 2023. "Improved Physiochemical Properties of Chitosan@PCL Nerve Conduits by Natural Molecule Crosslinking" Biomolecules 13, no. 12: 1712. https://doi.org/10.3390/biom13121712
APA StyleBianchini, M., Zinno, C., Micera, S., & Redolfi Riva, E. (2023). Improved Physiochemical Properties of Chitosan@PCL Nerve Conduits by Natural Molecule Crosslinking. Biomolecules, 13(12), 1712. https://doi.org/10.3390/biom13121712






