The Roles of WNT Signaling Pathways in Skin Development and Mechanical-Stretch-Induced Skin Regeneration
Abstract
:1. Introduction
2. Overview of WNT Signaling Pathways
2.1. WNT Signaling Pathways
2.2. Regulation of the WNT Signaling Pathway
2.2.1. DKK
2.2.2. SFRPs
2.2.3. APCDD1
3. The Role of WNT Signaling in Skin Development
3.1. Epidermal Development
3.2. Dermal Development
3.3. Development of Skin Appendages
3.3.1. HF Formation
3.3.2. Development of SGs and SwGs
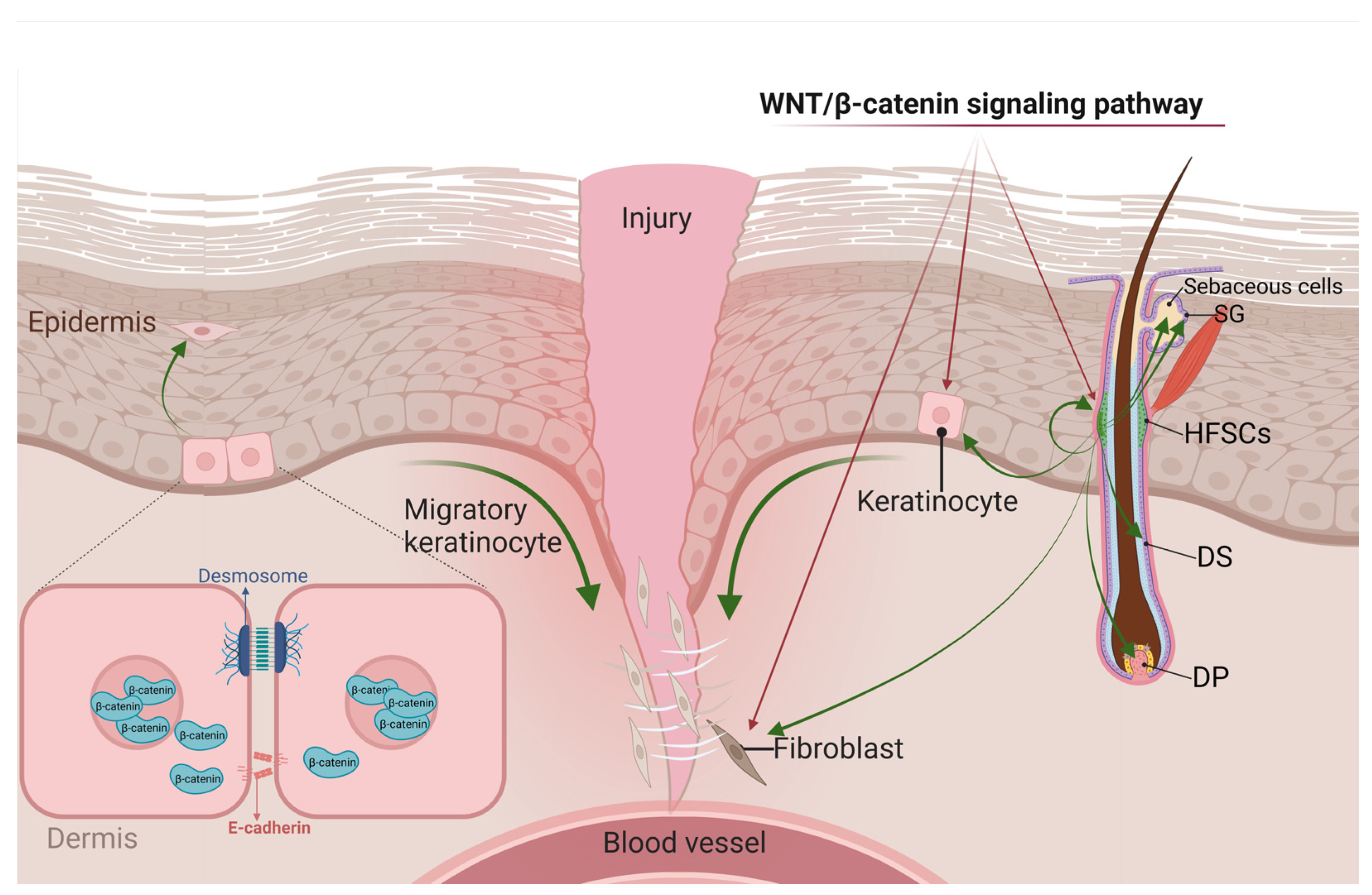
4. The Role of WNT Signaling in Skin Wound Repair
4.1. WNT Signaling and Inflammatory Responses
4.2. WNT Signaling in the Proliferative Stage of Wound Healing
4.2.1. WNT Signals in KCs
4.2.2. WNT Signals in HFSCs
4.2.3. WNT Signals in Fibroblasts
4.3. WNT Signaling in Remodeling
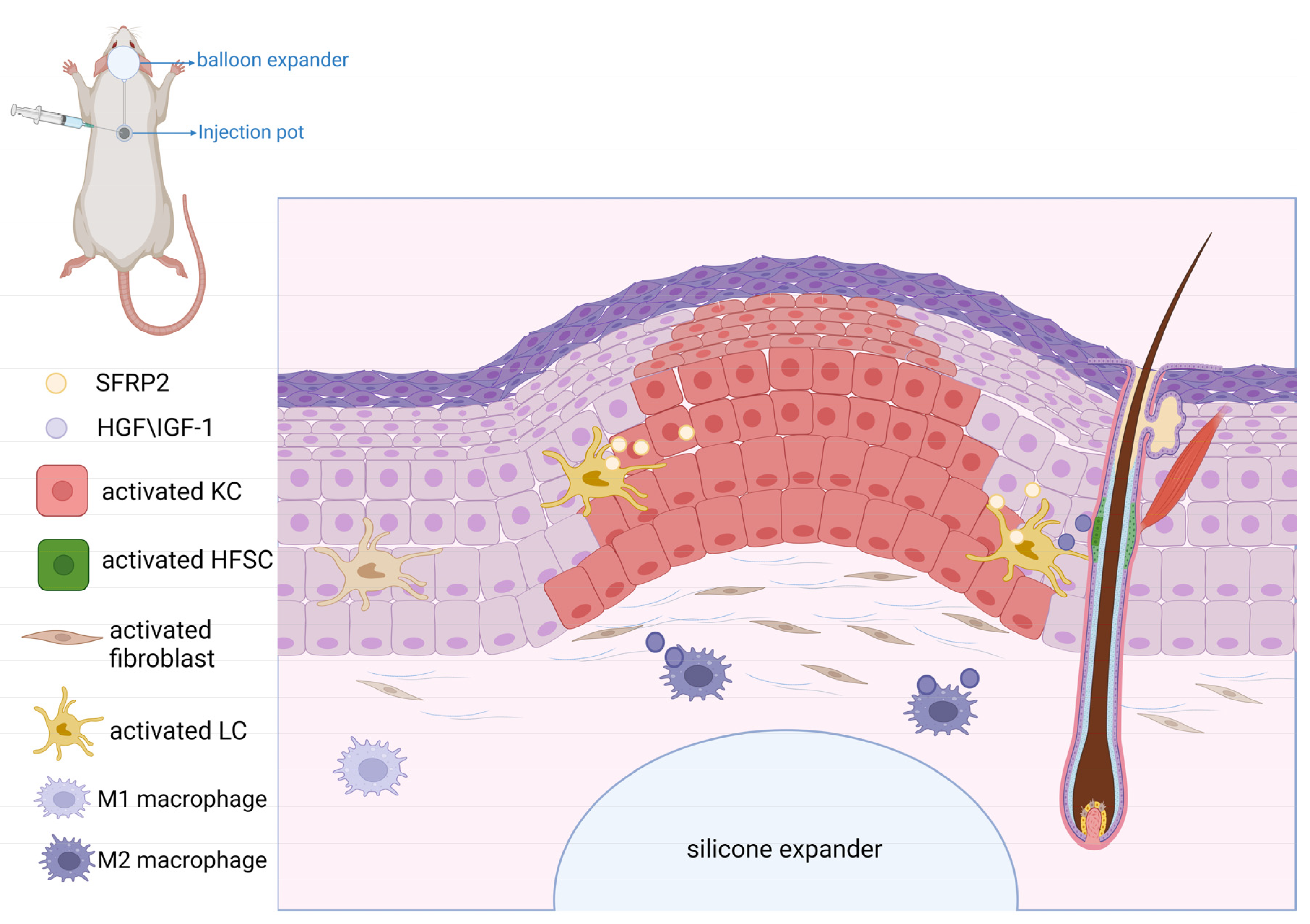
5. Role of WNT Signaling in Mechanical-Stretch-Induced Skin Regeneration during Tissue Expansion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef]
- Veeman, M.T.; Axelrod, J.D.; Moon, R.T. A Second Canon.Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell 2003, 5, 367–377. [Google Scholar] [CrossRef]
- Croce, J.C.; McClay, D.R. Evolution of the Wnt pathways. Methods Mol. Biol. 2008, 469, 3–18. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; He, X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a007880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bu, G. LRP5/6 in Wnt signaling and tumorigenesis. Future Oncol. 2005, 1, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef]
- Veltri, A.; Lang, C.; Lien, W.H. Concise Review: Wnt Signaling Pathways in Skin Development and Epidermal Stem Cells. Stem Cells 2018, 36, 22–35. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. Comparative genomics on Wnt8a and Wnt8b genes. Int. J. Oncol. 2005, 26, 1129–1133. [Google Scholar] [CrossRef]
- Lin, B.J.; Lin, G.Y.; Zhu, J.Y.; Yin, G.Q.; Huang, D.; Yan, Y.Y. LncRNA-PCAT1 maintains characteristics of dermal papilla cells and promotes hair follicle regeneration by regulating miR-329/Wnt10b axis. Exp. Cell Res. 2020, 394, 112031. [Google Scholar] [CrossRef]
- Ledwon, J.K.; Vaca, E.E.; Huang, C.C.; Kelsey, L.J.; McGrath, J.L.; Topczewski, J.; Gosain, A.K.; Topczewska, J.M. Langerhans cells and SFRP2/Wnt/beta-catenin signalling control adaptation of skin epidermis to mechanical stretching. J. Cell Mol. Med. 2022, 26, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Le, S.; Yu, M.; Yan, J. Phosphorylation Reduces the Mechanical Stability of the alpha-Catenin/beta-Catenin Complex. Angew. Chem. Int. Ed. Engl. 2019, 58, 18663–18669. [Google Scholar] [CrossRef] [PubMed]
- Archbold, H.C.; Yang, Y.X.; Chen, L.; Cadigan, K.M. How do they do Wnt they do?: Regulation of transcription by the Wnt/beta-catenin pathway. Acta Physiol. 2012, 204, 74–109. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Maan, Z.N.; Whittam, A.J.; Siemers, F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. Organogenesis 2015, 11, 95–104. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Grigoryan, T.; Wend, P.; Klaus, A.; Birchmeier, W. Deciphering the function of canonical Wnt signals in development and disease: Conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes. Dev. 2008, 22, 2308–2341. [Google Scholar] [CrossRef] [PubMed]
- Salahshor, S.; Woodgett, J.R. The links between axin and carcinogenesis. J. Clin. Pathol. 2005, 58, 225–236. [Google Scholar] [CrossRef]
- Mi, K.; Dolan, P.J.; Johnson, G.V. The low density lipoprotein receptor-related protein 6 interacts with glycogen synthase kinase 3 and attenuates activity. J. Biol. Chem. 2006, 281, 4787–4794. [Google Scholar] [CrossRef]
- Ku, A.T.; Miao, Q.; Nguyen, H. Monitoring Wnt/beta-Catenin Signaling in Skin. Methods Mol. Biol. 2016, 1481, 127–140. [Google Scholar] [CrossRef]
- Nusse, R. Developmental biology. Making head or tail of Dickkopf. Nature 2001, 411, 255–256. [Google Scholar] [CrossRef]
- Du, G.; Kataoka, K.; Sakaguchi, M.; Abarzua, F.; Than, S.S.; Sonegawa, H.; Makino, T.; Shimizu, T.; Huh, N.H. Expression of REIC/Dkk-3 in normal and hyperproliferative epidermis. Exp. Dermatol. 2011, 20, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Bazzi, H.; Fantauzzo, K.A.; Richardson, G.D.; Jahoda, C.A.; Christiano, A.M. The Wnt inhibitor, Dickkopf 4, is induced by canonical Wnt signaling during ectodermal appendage morphogenesis. Dev. Biol. 2007, 305, 498–507. [Google Scholar] [CrossRef]
- Reddy, S.; Andl, T.; Bagasra, A.; Lu, M.M.; Epstein, D.J.; Morrisey, E.E.; Millar, S.E. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 2001, 107, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.J.; Conrad, W.H.; Moon, R.T. A Wnt survival guide: From flies to human disease. J. Investig. Dermatol. 2009, 129, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Krupnik, V.E.; Sharp, J.D.; Jiang, C.; Robison, K.; Chickering, T.W.; Amaravadi, L.; Brown, D.E.; Guyot, D.; Mays, G.; Leiby, K.; et al. Functional and structural diversity of the human Dickkopf gene family. Gene 1999, 238, 301–313. [Google Scholar] [CrossRef]
- Jiang, P.; Wei, K.; Chang, C.; Zhao, J.; Zhang, R.; Xu, L.; Jin, Y.; Xu, L.; Shi, Y.; Guo, S.; et al. SFRP1 Negatively Modulates Pyroptosis of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis: A Review. Front. Immunol. 2022, 13, 903475. [Google Scholar] [CrossRef]
- Esteve, P.; Sandonis, A.; Ibanez, C.; Shimono, A.; Guerrero, I.; Bovolenta, P. Secreted frizzled-related proteins are required for Wnt/beta-catenin signalling activation in the vertebrate optic cup. Development 2011, 138, 4179–4184. [Google Scholar] [CrossRef]
- Lin, H.; Angeli, M.; Chung, K.J.; Ejimadu, C.; Rosa, A.R.; Lee, T. sFRP2 activates Wnt/beta-catenin signaling in cardiac fibroblasts: Differential roles in cell growth, energy metabolism, and extracellular matrix remodeling. Am. J. Physiol. Cell Physiol. 2016, 311, C710–C719. [Google Scholar] [CrossRef]
- Bhanot, P.; Brink, M.; Samos, C.H.; Hsieh, J.C.; Wang, Y.; Macke, J.P.; Andrew, D.; Nathans, J.; Nusse, R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996, 382, 225–230. [Google Scholar] [CrossRef]
- Wu, Q.; Yin, X.; Zhao, W.; Xu, W.; Chen, L. Downregulation of SFRP2 facilitates cancer stemness and radioresistance of glioma cells via activating Wnt/beta-catenin signaling. PLoS ONE 2021, 16, e0260864. [Google Scholar] [CrossRef] [PubMed]
- van Loon, K.; Huijbers, E.J.M.; Griffioen, A.W. Secreted frizzled-related protein 2: A key player in noncanonical Wnt signaling and tumor angiogenesis. Cancer Metastasis Rev. 2021, 40, 191–203. [Google Scholar] [CrossRef]
- Bayle, J.; Fitch, J.; Jacobsen, K.; Kumar, R.; Lafyatis, R.; Lemaire, R. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: Role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J. Investig. Dermatol. 2008, 128, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Gay, D.; Ghinatti, G.; Guerrero-Juarez, C.F.; Ferrer, R.A.; Ferri, F.; Lim, C.H.; Murakami, S.; Gault, N.; Barroca, V.; Rombeau, I.; et al. Phagocytosis of Wnt inhibitor SFRP4 by late wound macrophages drives chronic Wnt activity for fibrotic skin healing. Sci. Adv. 2020, 6, eaay3704. [Google Scholar] [CrossRef] [PubMed]
- Dees, C.; Schlottmann, I.; Funke, R.; Distler, A.; Palumbo-Zerr, K.; Zerr, P.; Lin, N.Y.; Beyer, C.; Distler, O.; Schett, G.; et al. The Wnt antagonists DKK1 and SFRP1 are downregulated by promoter hypermethylation in systemic sclerosis. Ann. Rheum. Dis. 2014, 73, 1232–1239. [Google Scholar] [CrossRef]
- Fang, L.; Gao, C.; Bai, R.X.; Wang, H.F.; Du, S.Y. Overexpressed sFRP3 exerts an inhibitory effect on hepatocellular carcinoma via inactivation of the Wnt/beta-catenin signaling pathway. Cancer Gene Ther. 2021, 28, 875–891. [Google Scholar] [CrossRef]
- Zou, D.P.; Chen, Y.M.; Zhang, L.Z.; Yuan, X.H.; Zhang, Y.J.; Inggawati, A.; Kieu Nguyet, P.T.; Gao, T.W.; Chen, J. SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the Wnt/beta-catenin signaling. Genes. Dis. 2021, 8, 677–688. [Google Scholar] [CrossRef]
- Bafico, A.; Liu, G.; Yaniv, A.; Gazit, A.; Aaronson, S.A. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 2001, 3, 683–686. [Google Scholar] [CrossRef]
- Mao, B.; Niehrs, C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene 2003, 302, 179–183. [Google Scholar] [CrossRef]
- Kim, M.; Han, J.H.; Kim, J.H.; Park, T.J.; Kang, H.Y. Secreted Frizzled-Related Protein 2 (sFRP2) Functions as a Melanogenic Stimulator; the Role of sFRP2 in UV-Induced Hyperpigmentary Disorders. J. Investig. Dermatol. 2016, 136, 236–244. [Google Scholar] [CrossRef]
- Vonica, A.; Bhat, N.; Phan, K.; Guo, J.; Iancu, L.; Weber, J.A.; Karger, A.; Cain, J.W.; Wang, E.C.E.; DeStefano, G.M.; et al. Apcdd1 is a dual BMP/Wnt inhibitor in the developing nervous system and skin. Dev. Biol. 2020, 464, 71–87. [Google Scholar] [CrossRef]
- Shimomura, Y.; Agalliu, D.; Vonica, A.; Luria, V.; Wajid, M.; Baumer, A.; Belli, S.; Petukhova, L.; Schinzel, A.; Brivanlou, A.H.; et al. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature 2010, 464, 1043–1047. [Google Scholar] [CrossRef]
- Bielefeld, K.A.; Amini-Nik, S.; Alman, B.A. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell Mol. Life Sci. 2013, 70, 2059–2081. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.A.; Hemmati-Brivanlou, A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 1995, 376, 331–333. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- M’Boneko, V.; Merker, H.J. Development and morphology of the periderm of mouse embryos (days 9–12 of gestation). Acta Anat. 1988, 133, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef]
- Rendl, M.; Polak, L.; Fuchs, E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes. Dev. 2008, 22, 543–557. [Google Scholar] [CrossRef]
- Lim, X.; Tan, S.H.; Koh, W.L.; Chau, R.M.; Yan, K.S.; Kuo, C.J.; van Amerongen, R.; Klein, A.M.; Nusse, R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 2013, 342, 1226–1230. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, I.S.; Kim, H.; Lee, J.S.; Kim, K.; Yim, H.Y.; Jeong, J.; Kim, J.H.; Kim, J.Y.; Lee, H.; et al. RORalpha attenuates Wnt/beta-catenin signaling by PKCalpha-dependent phosphorylation in colon cancer. Mol. Cell 2010, 37, 183–195. [Google Scholar] [CrossRef]
- Witte, F.; Bernatik, O.; Kirchner, K.; Masek, J.; Mahl, A.; Krejci, P.; Mundlos, S.; Schambony, A.; Bryja, V.; Stricker, S. Negative regulation of Wnt signaling mediated by CK1-phosphorylated Dishevelled via Ror2. FASEB J. 2010, 24, 2417–2426. [Google Scholar] [CrossRef]
- Lim, X.; Nusse, R. Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008029. [Google Scholar] [CrossRef] [PubMed]
- Ohtola, J.; Myers, J.; Akhtar-Zaidi, B.; Zuzindlak, D.; Sandesara, P.; Yeh, K.; Mackem, S.; Atit, R. beta-Catenin has sequential roles in the survival and specification of ventral dermis. Development 2008, 135, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jarrell, A.; Guo, C.; Lang, R.; Atit, R. Dermal beta-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 2012, 139, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Hsu, W. Epidermal Wnt controls hair follicle induction by orchestrating dynamic signaling crosstalk between the epidermis and dermis. J. Investig. Dermatol. 2013, 133, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Thulabandu, V.; Chen, D.; Atit, R.P. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e307. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef]
- Plikus, M.V.; Mayer, J.A.; de la Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef]
- Driskell, R.R.; Watt, F.M. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015, 25, 92–99. [Google Scholar] [CrossRef]
- Gupta, K.; Levinsohn, J.; Linderman, G.; Chen, D.; Sun, T.Y.; Dong, D.; Taketo, M.M.; Bosenberg, M.; Kluger, Y.; Choate, K.; et al. Single-Cell Analysis Reveals a Hair Follicle Dermal Niche Molecular Differentiation Trajectory that Begins Prior to Morphogenesis. Dev. Cell 2019, 48, 17–31.e16. [Google Scholar] [CrossRef]
- Mok, K.W.; Saxena, N.; Heitman, N.; Grisanti, L.; Srivastava, D.; Muraro, M.J.; Jacob, T.; Sennett, R.; Wang, Z.; Su, Y.; et al. Dermal Condensate Niche Fate Specification Occurs Prior to Formation and Is Placode Progenitor Dependent. Dev. Cell 2019, 48, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.W.; Devarajan, M.; Atit, R.P. Stage-specific roles of Ezh2 and Retinoic acid signaling ensure calvarial bone lineage commitment. Dev. Biol. 2018, 443, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Mirzamohammadi, F.; Papaioannou, G.; Inloes, J.B.; Rankin, E.B.; Xie, H.; Schipani, E.; Orkin, S.H.; Kobayashi, T. Polycomb repressive complex 2 regulates skeletal growth by suppressing Wnt and TGF-beta signalling. Nat. Commun. 2016, 7, 12047. [Google Scholar] [CrossRef] [PubMed]
- Phan, Q.M.; Fine, G.M.; Salz, L.; Herrera, G.G.; Wildman, B.; Driskell, I.M.; Driskell, R.R. Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. eLife 2020, 9, e60066. [Google Scholar] [CrossRef] [PubMed]
- Augustin, I. Wnt signaling in skin homeostasis and pathology. J. Dtsch. Dermatol. Ges. 2015, 13, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. Bioessays 2005, 27, 247–261. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef]
- Rompolas, P.; Mesa, K.R.; Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 2013, 502, 513–518. [Google Scholar] [CrossRef]
- Van Amerongen, R.; Fuerer, C.; Mizutani, M.; Nusse, R. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev. Biol. 2012, 369, 101–114. [Google Scholar] [CrossRef]
- Lei, M.; Guo, H.; Qiu, W.; Lai, X.; Yang, T.; Widelitz, R.B.; Chuong, C.M.; Lian, X.; Yang, L. Modulating hair follicle size with Wnt10b/DKK1 during hair regeneration. Exp. Dermatol. 2014, 23, 407–413. [Google Scholar] [CrossRef]
- Frances, D.; Niemann, C. Stem cell dynamics in sebaceous gland morphogenesis in mouse skin. Dev. Biol. 2012, 363, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.Y.; Yin, M.; Sima, J.; Childress, V.; Michel, M.; Piao, Y.; Schlessinger, D. Involvement of Wnt, Eda and Shh at defined stages of sweat gland development. Development 2014, 141, 3752–3760. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Davidson, J.M.; Kirsner, R.S.; Bornstein, P.; Herman, I.M. Dynamic reciprocity in the wound microenvironment. Wound Repair. Regen. 2011, 19, 134–148. [Google Scholar] [CrossRef]
- Mahdavian Delavary, B.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Bielefeld, K.A.; Amini-Nik, S.; Whetstone, H.; Poon, R.; Youn, A.; Wang, J.; Alman, B.A. Fibronectin and beta-catenin act in a regulatory loop in dermal fibroblasts to modulate cutaneous healing. J. Biol. Chem. 2011, 286, 27687–27697. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef]
- Green, K.J.; Gaudry, C.A. Are desmosomes more than tethers for intermediate filaments? Nat. Rev. Mol. Cell Biol. 2000, 1, 208–216. [Google Scholar] [CrossRef]
- Kemler, R. From cadherins to catenins: Cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993, 9, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M. The stem cell compartment in human interfollicular epidermis. J. Dermatol. Sci. 2002, 28, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Brem, H.; Vouthounis, C.; Lee, B.; Fallon, J.; Stallcup, M.; Merchant, A.; Galiano, R.D.; Tomic-Canic, M. Molecular pathogenesis of chronic wounds: The role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am. J. Pathol. 2005, 167, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Brantjes, H.; Barker, N.; van Es, J.; Clevers, H. TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol. Chem. 2002, 383, 255–261. [Google Scholar] [CrossRef]
- Blanpain, C.; Horsley, V.; Fuchs, E. Epithelial stem cells: Turning over new leaves. Cell 2007, 128, 445–458. [Google Scholar] [CrossRef]
- Rahmani, W.; Abbasi, S.; Hagner, A.; Raharjo, E.; Kumar, R.; Hotta, A.; Magness, S.; Metzger, D.; Biernaskie, J. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Dev. Cell 2014, 31, 543–558. [Google Scholar] [CrossRef]
- Abbasi, S.; Sinha, S.; Labit, E.; Rosin, N.L.; Yoon, G.; Rahmani, W.; Jaffer, A.; Sharma, N.; Hagner, A.; Shah, P.; et al. Distinct Regulatory Programs Control the Latent Regenerative Potential of Dermal Fibroblasts during Wound Healing. Cell Stem Cell 2020, 27, 396–412.e396. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Choo, H.; He, J.; Wang, X.; Wu, Y.; Chen, X. beta-Catenin Signaling Evokes Hair Follicle Senescence by Accelerating the Differentiation of Hair Follicle Mesenchymal Progenitors. Front. Cell Dev. Biol. 2022, 10, 839519. [Google Scholar] [CrossRef]
- Veniaminova, N.A.; Jia, Y.Y.; Hartigan, A.M.; Huyge, T.J.; Tsai, S.Y.; Grachtchouk, M.; Nakagawa, S.; Dlugosz, A.A.; Atwood, S.X.; Wong, S.Y. Distinct mechanisms for sebaceous gland self-renewal and regeneration provide durability in response to injury. Cell Rep. 2023, 42, 113121. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Choo, H.; Chen, Y.; Zhang, X.; Xu, R.H.; Wang, X.; Wu, Y. Distinct bulge stem cell populations maintain the pilosebaceous unit in a beta-catenin-dependent manner. iScience 2023, 26, 105805. [Google Scholar] [CrossRef]
- Chua, A.W.; Ma, D.; Gan, S.U.; Fu, Z.; Han, H.C.; Song, C.; Sabapathy, K.; Phan, T.T. The role of R-spondin2 in keratinocyte proliferation and epidermal thickening in keloid scarring. J. Investig. Dermatol. 2011, 131, 644–654. [Google Scholar] [CrossRef]
- Whyte, J.L.; Smith, A.A.; Liu, B.; Manzano, W.R.; Evans, N.D.; Dhamdhere, G.R.; Fang, M.Y.; Chang, H.Y.; Oro, A.E.; Helms, J.A. Augmenting endogenous Wnt signaling improves skin wound healing. PLoS ONE 2013, 8, e76883. [Google Scholar] [CrossRef]
- Kobayashi, T.; Naik, S.; Nagao, K. Choreographing Immunity in the Skin Epithelial Barrier. Immunity 2019, 50, 552–565. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Tian, R.; Zhang, Y.; Drutskaya, M.S.; Wang, C.; Ge, J.; Fan, Z.; Kong, D.; Wang, X.; et al. Macrophages induce AKT/beta-catenin-dependent Lgr5(+) stem cell activation and hair follicle regeneration through TNF. Nat. Commun. 2017, 8, 14091. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, W.; Liu, Y.; Rosin, N.L.; Kline, A.; Raharjo, E.; Yoon, J.; Stratton, J.A.; Sinha, S.; Biernaskie, J. Macrophages Promote Wound-Induced Hair Follicle Regeneration in a CX(3)CR1- and TGF-beta1-Dependent Manner. J. Investig. Dermatol. 2018, 138, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, X.; Han, J.; Fan, Z.; Sadia, S.; Zhang, R.; Guo, Y.; Jiang, Y.; Wu, Y. AKT and its related molecular feature in aged mice skin. PLoS ONE 2017, 12, e0178969. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, X.; Chen, Y.; Han, J.; Kong, D.; Zhu, M.; Fu, X.; Wu, Y. Pten loss in Lgr5(+) hair follicle stem cells promotes SCC development. Theranostics 2019, 9, 8321–8331. [Google Scholar] [CrossRef]
- Jere, S.W.; Houreld, N.N. Regulatory Processes of the Canonical Wnt/beta-Catenin Pathway and Photobiomodulation in Diabetic Wound Repair. Int. J. Mol. Sci. 2022, 23, 4210. [Google Scholar] [CrossRef]
- Collins, C.A.; Kretzschmar, K.; Watt, F.M. Reprogramming adult dermis to a neonatal state through epidermal activation of beta-catenin. Development 2011, 138, 5189–5199. [Google Scholar] [CrossRef]
- Shi, Y.; Shu, B.; Yang, R.; Xu, Y.; Xing, B.; Liu, J.; Chen, L.; Qi, S.; Liu, X.; Wang, P.; et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res. Ther. 2015, 6, 120. [Google Scholar] [CrossRef]
- Ponnusamy, Y.; Chear, N.J.; Ramanathan, S.; Lai, C.S. Polyphenols rich fraction of Dicranopteris linearis promotes fibroblast cell migration and proliferation in vitro. J. Ethnopharmacol. 2015, 168, 305–314. [Google Scholar] [CrossRef]
- Caraci, F.; Gili, E.; Calafiore, M.; Failla, M.; La Rosa, C.; Crimi, N.; Sortino, M.A.; Nicoletti, F.; Copani, A.; Vancheri, C. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol. Res. 2008, 57, 274–282. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Sun, C.; Wang, T.; Shen, Y.; Cai, W.; Sun, J.; Chi, L.; Wang, H.; Song, N.; et al. Feedback Activation of Basic Fibroblast Growth Factor Signaling via the Wnt/beta-Catenin Pathway in Skin Fibroblasts. Front. Pharmacol. 2017, 8, 32. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, C.; Shi, C.; Sun, F.; Xu, X.; Qian, W.; Nie, S.; Han, X. Activated Wnt signaling induces myofibroblast differentiation of mesenchymal stem cells, contributing to pulmonary fibrosis. Int. J. Mol. Med. 2014, 33, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.; Poon, R.; Yu, C.; Khoury, M.; Shenker, R.; Fish, J.; Alman, B.A. Prolonged beta-catenin stabilization and tcf-dependent transcriptional activation in hyperplastic cutaneous wounds. Lab. Investig. 2005, 85, 416–425. [Google Scholar] [CrossRef]
- Sato, M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm. Venereol. 2006, 86, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, T.; Hu, M.S.; Marshall, C.D.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Scarless wound healing: Finding the right cells and signals. Cell Tissue Res. 2016, 365, 483–493. [Google Scholar] [CrossRef]
- Wei, Q.; Yokota, C.; Semenov, M.V.; Doble, B.; Woodgett, J.; He, X. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J. Biol. Chem. 2007, 282, 15903–15911. [Google Scholar] [CrossRef]
- Ding, J.; Lei, L.; Liu, S.; Zhang, Y.; Yu, Z.; Su, Y.; Ma, X. Macrophages are necessary for skin regeneration during tissue expansion. J. Transl. Med. 2019, 17, 36. [Google Scholar] [CrossRef]
- Azadgoli, B.; Fahradyan, A.; Wolfswinkel, E.M.; Tsuha, M.; Magee, W., 3rd; Hammoudeh, J.A.; Urata, M.M.; Howell, L.K. External Port Tissue Expansion in the Pediatric Population: Confirming Its Safety and Efficacy. Plast. Reconstr. Surg. 2018, 141, 883e–890e. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.A.; Elhadi, H.M.; Kitzmiller, W.J.; Billmire, D.A.; Yakuboff, K.P. Tissue expander complications in the pediatric burn patient: A 10-year follow-up. Ann. Plast. Surg. 2014, 72, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, Y.; Xiong, S.; Wang, T.; Liu, W.; Yu, Z.; Ma, X. Mechanical Stretch Induced Skin Regeneration: Molecular and Cellular Mechanism in Skin Soft Tissue Expansion. Int. J. Mol. Sci. 2022, 23, 9622. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.H.; Kim, S.Y.; Lee, H.; Lim, H.K.; Kim, J.W.; Lee, U.L.; Lee, J.B.; Park, S.H.; Kim, S.J.; Song, J.D.; et al. Soft tissue expander for vertically atrophied alveolar ridges: Prospective, multicenter, randomized controlled trial. Clin. Oral. Implants Res. 2020, 31, 585–594. [Google Scholar] [CrossRef]
- Gonzalez Ruiz, Y.; Lopez Gutierrez, J.C. Multiple Tissue Expansion for Giant Congenital Melanocytic Nevus. Ann. Plast. Surg. 2017, 79, e37–e40. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, Z.; Song, Y.; Zhang, Y.; Du, J.; Su, Y.; Ma, X. Hair follicle bulge-derived stem cells promote tissue regeneration during skin expansion. Biomed. Pharmacother. 2020, 132, 110805. [Google Scholar] [CrossRef]
- Razzak, M.A.; Hossain, M.S.; Radzi, Z.B.; Yahya, N.A.; Czernuszka, J.; Rahman, M.T. Cellular and Molecular Responses to Mechanical Expansion of Tissue. Front. Physiol. 2016, 7, 540. [Google Scholar] [CrossRef]
- Pamplona, D.C.; Weber, H.I.; Leta, F.R. Optimization of the use of skin expanders. Skin. Res. Technol. 2014, 20, 463–472. [Google Scholar] [CrossRef]
- Aragona, M.; Sifrim, A.; Malfait, M.; Song, Y.; Van Herck, J.; Dekoninck, S.; Gargouri, S.; Lapouge, G.; Swedlund, B.; Dubois, C.; et al. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 2020, 584, 268–273. [Google Scholar] [CrossRef]
- Ledwon, J.K.; Kelsey, L.J.; Vaca, E.E.; Gosain, A.K. Transcriptomic analysis reveals dynamic molecular changes in skin induced by mechanical forces secondary to tissue expansion. Sci. Rep. 2020, 10, 15991. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Hu, J.; Sheng, R.; Lin, Q.; He, X.; Guo, M. Volumetric Compression Induces Intracellular Crowding to Control Intestinal Organoid Growth via Wnt/beta-Catenin Signaling. Cell Stem Cell 2021, 28, 63–78.e67. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, J.; Papasaikas, P.; Ferralli, J.; Nakamura, Y.; Smallwood, S.; Tsiairis, C.D. Mechanical oscillations orchestrate axial patterning through Wnt activation in Hydra. Sci. Adv. 2021, 7, eabj6897. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liang, X.; Zhou, Y.; Li, H.; Du, H.; Suo, Y.; Liu, W.; Jin, R.; Chai, B.; Duan, R.; et al. CDH1 is Identified as A Therapeutic Target for Skin Regeneration after Mechanical Loading. Int. J. Biol. Sci. 2021, 17, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, S.; Cui, J.; Song, Y.; Wang, T.; Song, B.; Peng, P.; Ma, X. Early histological and ultrastructural changes in expanded murine scalp. Ultrastruct. Pathol. 2020, 44, 141–152. [Google Scholar] [CrossRef]
- Takei, T.; Rivas-Gotz, C.; Delling, C.A.; Koo, J.T.; Mills, I.; McCarthy, T.L.; Centrella, M.; Sumpio, B.E. Effect of strain on human keratinocytes in vitro. J. Cell Physiol. 1997, 173, 64–72. [Google Scholar] [CrossRef]
- Liu, S.; Ding, J.; Zhang, Y.; Cheng, X.; Dong, C.; Song, Y.; Yu, Z.; Ma, X. Establishment of a Novel Mouse Model for Soft Tissue Expansion. J. Surg. Res. 2020, 253, 238–244. [Google Scholar] [CrossRef]
- Huo, R.; Yang, W.; Shangbin, L.; Tingting, L.; Yang, Z.; Feng, G.; Qingping, Y.; Wenhao, Z. A microscopic and biomechanical study of skin and soft tissue after repeated expansion. Dermatol. Surg. 2009, 35, 72–79. [Google Scholar] [CrossRef]
- Huang, C.; Miyazaki, K.; Akaishi, S.; Watanabe, A.; Hyakusoku, H.; Ogawa, R. Biological effects of cellular stretch on human dermal fibroblasts. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, e351–e361. [Google Scholar] [CrossRef]
- Liu, W.; Xiong, S.; Zhang, Y.; Du, J.; Dong, C.; Yu, Z.; Ma, X. Transcriptome Profiling Reveals Important Transcription Factors and Biological Processes in Skin Regeneration Mediated by Mechanical Stretch. Front. Genet. 2021, 12, 757350. [Google Scholar] [CrossRef]
- Tiede, S.; Kloepper, J.E.; Bodo, E.; Tiwari, S.; Kruse, C.; Paus, R. Hair follicle stem cells: Walking the maze. Eur. J. Cell Biol. 2007, 86, 355–376. [Google Scholar] [CrossRef]
- Chu, S.Y.; Chou, C.H.; Huang, H.D.; Yen, M.H.; Hong, H.C.; Chao, P.H.; Wang, Y.H.; Chen, P.Y.; Nian, S.X.; Chen, Y.R.; et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat. Commun. 2019, 10, 1524. [Google Scholar] [CrossRef]
- Simon, P.J.; Anderson, L.S.; Manstein, M.E. Increased hair growth and density following controlled expansion of guinea pig skin and soft tissue. Ann. Plast. Surg. 1987, 19, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Huang, X.; Zhou, Y.; Jin, R.; Li, Q. Mechanical Stretching Promotes Skin Tissue Regeneration via Enhancing Mesenchymal Stem Cell Homing and Transdifferentiation. Stem Cells Transl. Med. 2016, 5, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.H. Ontogeny and function of murine epidermal Langerhans cells. Nat. Immunol. 2017, 18, 1068–1075. [Google Scholar] [CrossRef]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert. Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Castellana, D.; Paus, R.; Perez-Moreno, M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 2014, 12, e1002002. [Google Scholar] [CrossRef]
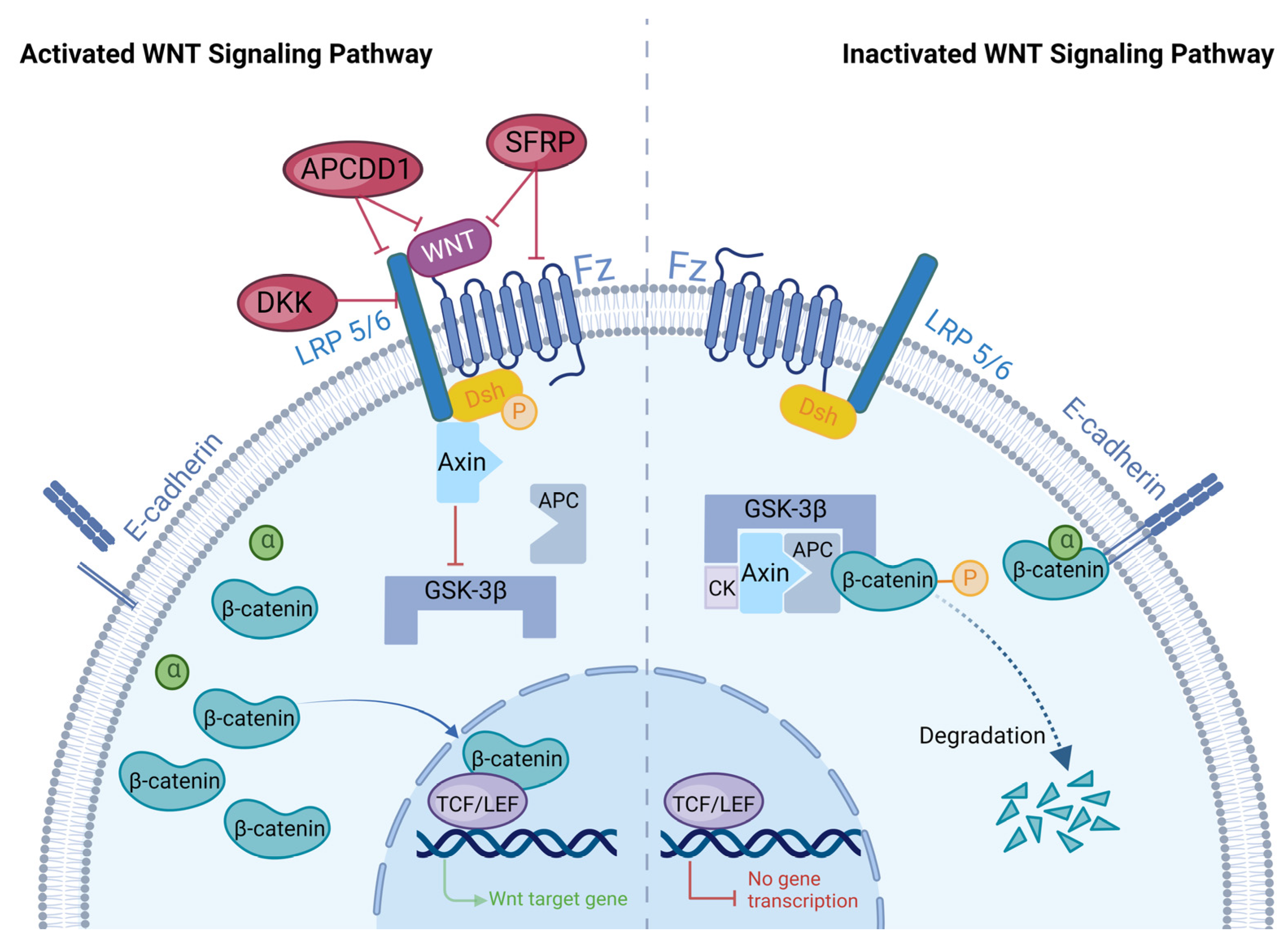
| Regulators | Function | References | |
|---|---|---|---|
| DKK | DKK1 | DKK1 inhibits WNT/β-catenin signaling by binding to LRP5/6. | [38] |
| DKK2 | DKK2 is an environment-dependent WNT inhibitor. The expression levels of different DKK receptors determine the ability of DKK2 to act as an activator or inhibitor of WNT/β-catenin signaling. | [25,39] | |
| DKK3 | DKK3 does not participate in WNT/β-catenin signaling. However, DKK3 is considered to be a marker of hair follicle stem cells (HFSCs). | [21,25] | |
| DKK4 | DKK4 transforms classic WNT signaling into non-canonical WNT signaling. | [23] | |
| SFRP | SFRP1 | SFRP1 is one of the WNT signaling pathway antagonists. The structure of SFRP1 is highly homologous to the FZ receptor and can bind WNT proteins and the FZ receptor. | [27] |
| SFRP2 | Overexpression of SFRP2 chelates WNT ligands and prevents the binding of WNT ligands to FZ receptors, thereby reducing β-catenin levels and preventing excessive activation of the WNT/β-catenin pathway. SFRP2 can also inhibit WNT signaling by directly binding to FZ receptors. | [31] | |
| SFRP3 | Overexpression of SFRP3 can inactivate the WNT/β-catenin signaling pathway. | [36] | |
| SFRP4 | SFRP4 can inhibit the WNT/β-catenin signaling pathway. | [40] | |
| SFRP5 | SFRP5 is an inhibitor of WNT signaling. SFRP5 is structurally very similar to the FZ receptor and can inhibit WNT signaling activity by competitively inhibiting the FZ receptor. | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.; Guo, Y.; Liu, W.; Song, Y.; Yu, Z.; Ma, X. The Roles of WNT Signaling Pathways in Skin Development and Mechanical-Stretch-Induced Skin Regeneration. Biomolecules 2023, 13, 1702. https://doi.org/10.3390/biom13121702
Bai R, Guo Y, Liu W, Song Y, Yu Z, Ma X. The Roles of WNT Signaling Pathways in Skin Development and Mechanical-Stretch-Induced Skin Regeneration. Biomolecules. 2023; 13(12):1702. https://doi.org/10.3390/biom13121702
Chicago/Turabian StyleBai, Ruoxue, Yaotao Guo, Wei Liu, Yajuan Song, Zhou Yu, and Xianjie Ma. 2023. "The Roles of WNT Signaling Pathways in Skin Development and Mechanical-Stretch-Induced Skin Regeneration" Biomolecules 13, no. 12: 1702. https://doi.org/10.3390/biom13121702
APA StyleBai, R., Guo, Y., Liu, W., Song, Y., Yu, Z., & Ma, X. (2023). The Roles of WNT Signaling Pathways in Skin Development and Mechanical-Stretch-Induced Skin Regeneration. Biomolecules, 13(12), 1702. https://doi.org/10.3390/biom13121702






