The Role of Sphingosine-1-Phosphate Receptor 2 in Mouse Retina Light Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Electroretinography (ERG)
2.3. SD-OCT Analysis of Retinal Layers in Mice
2.4. Immunohistochemistry (IHC)
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
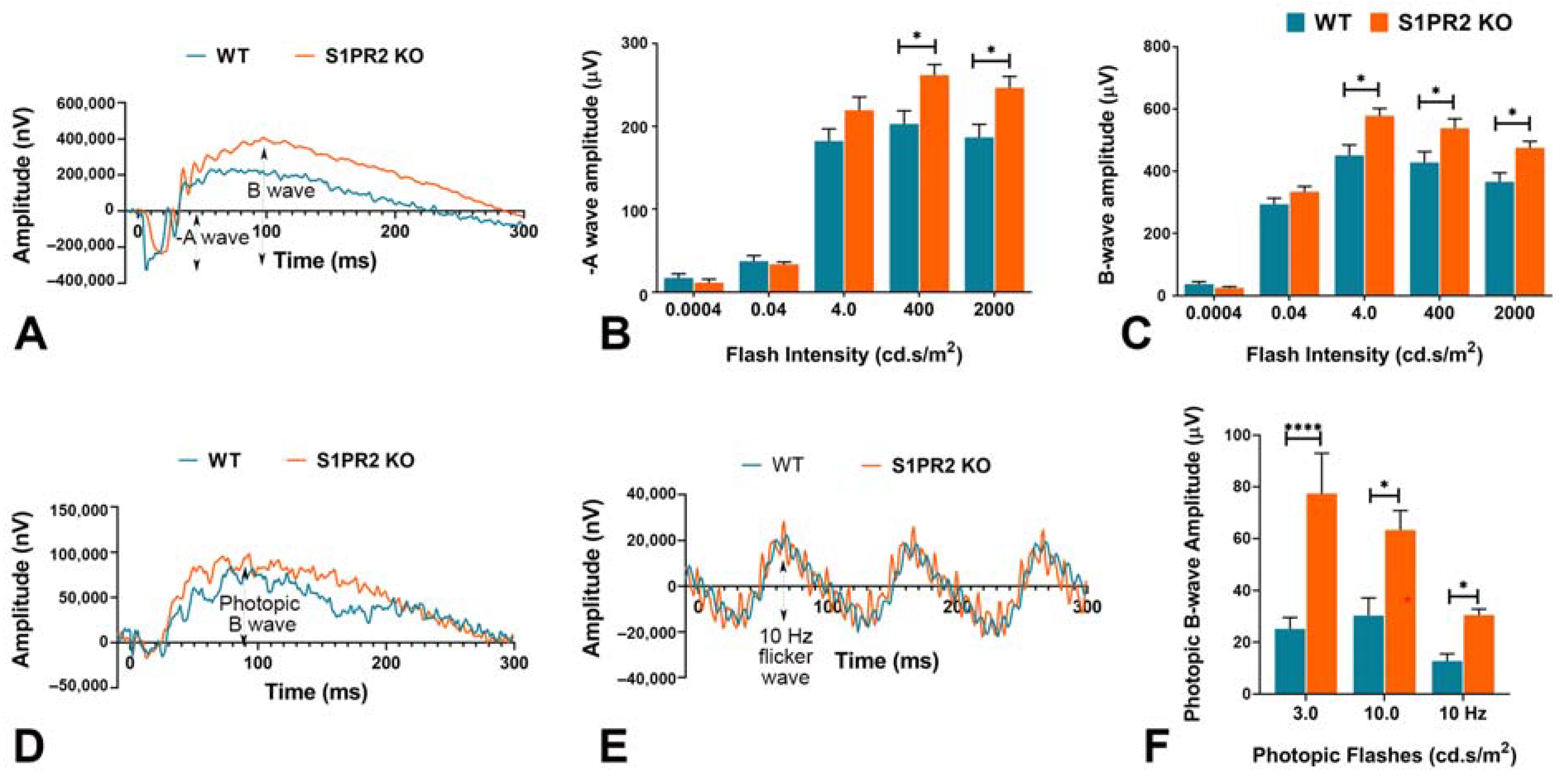
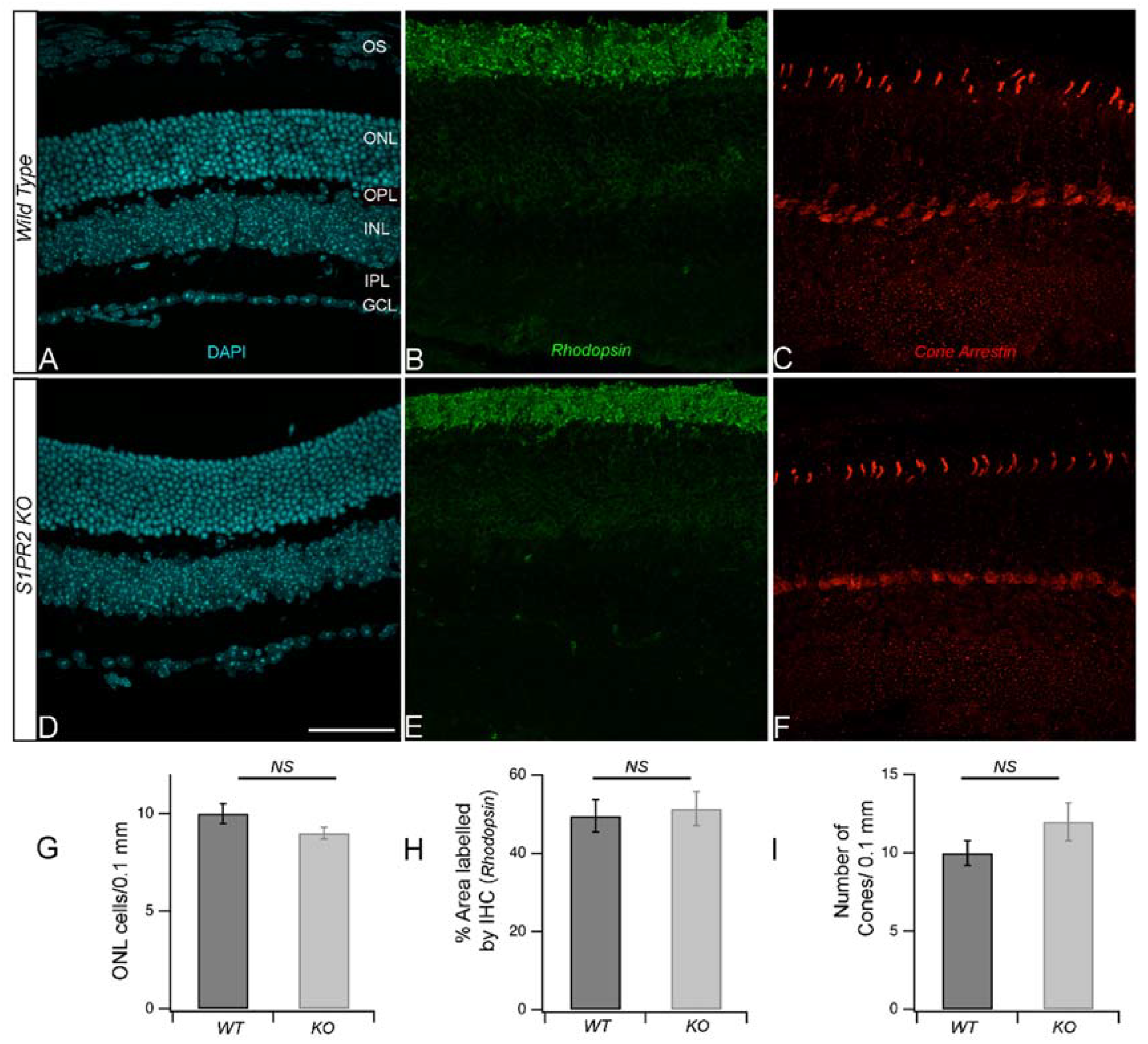
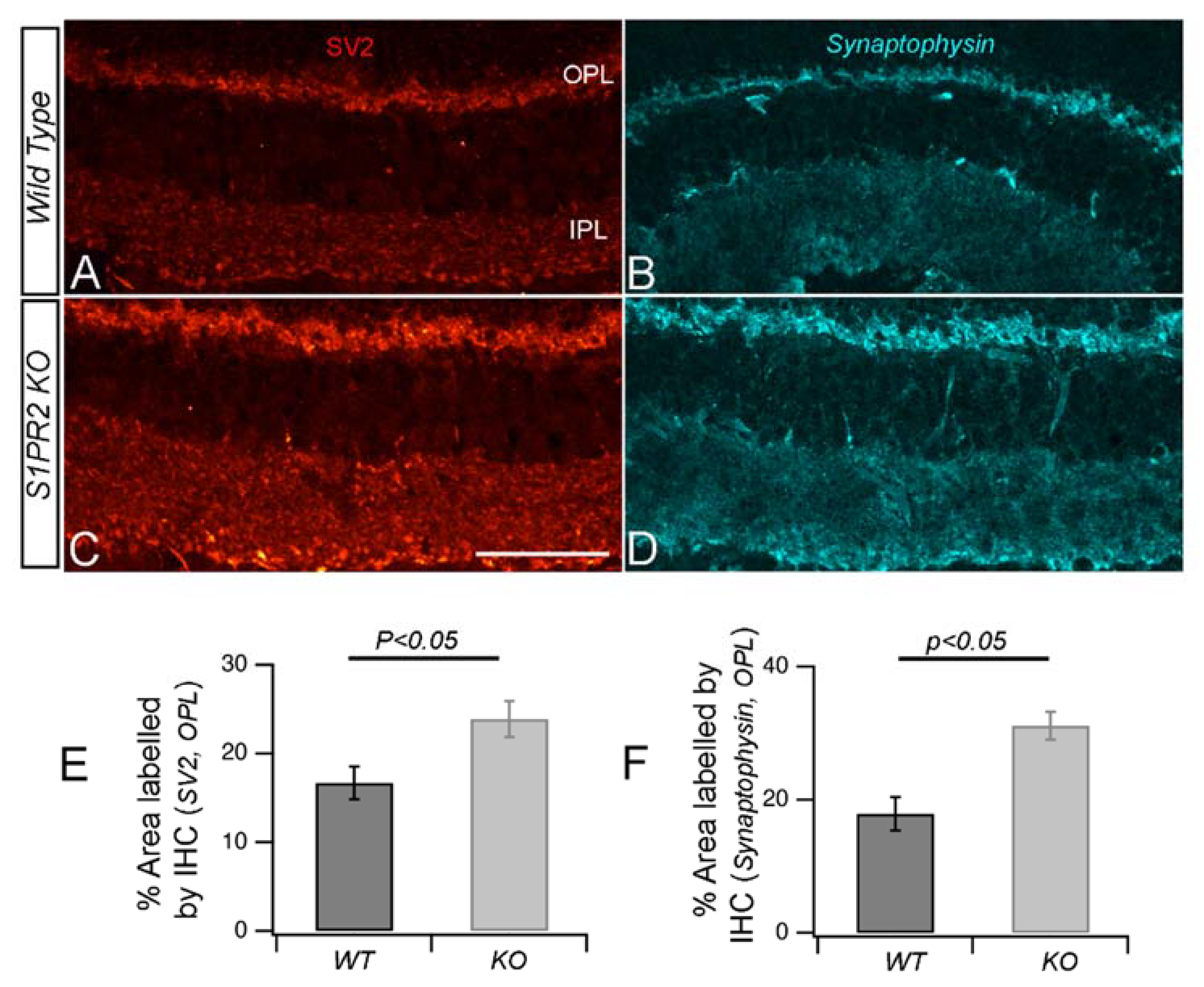
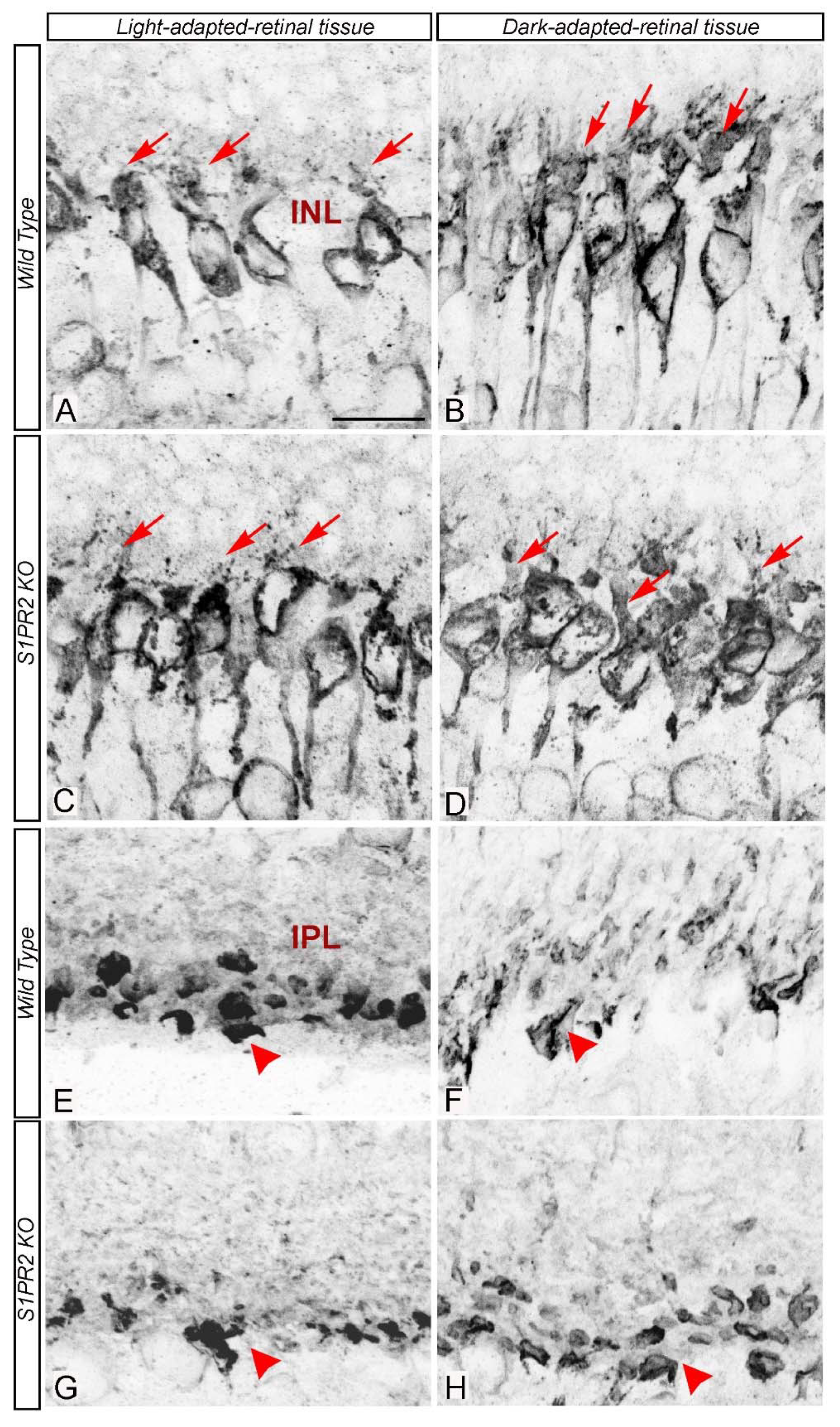
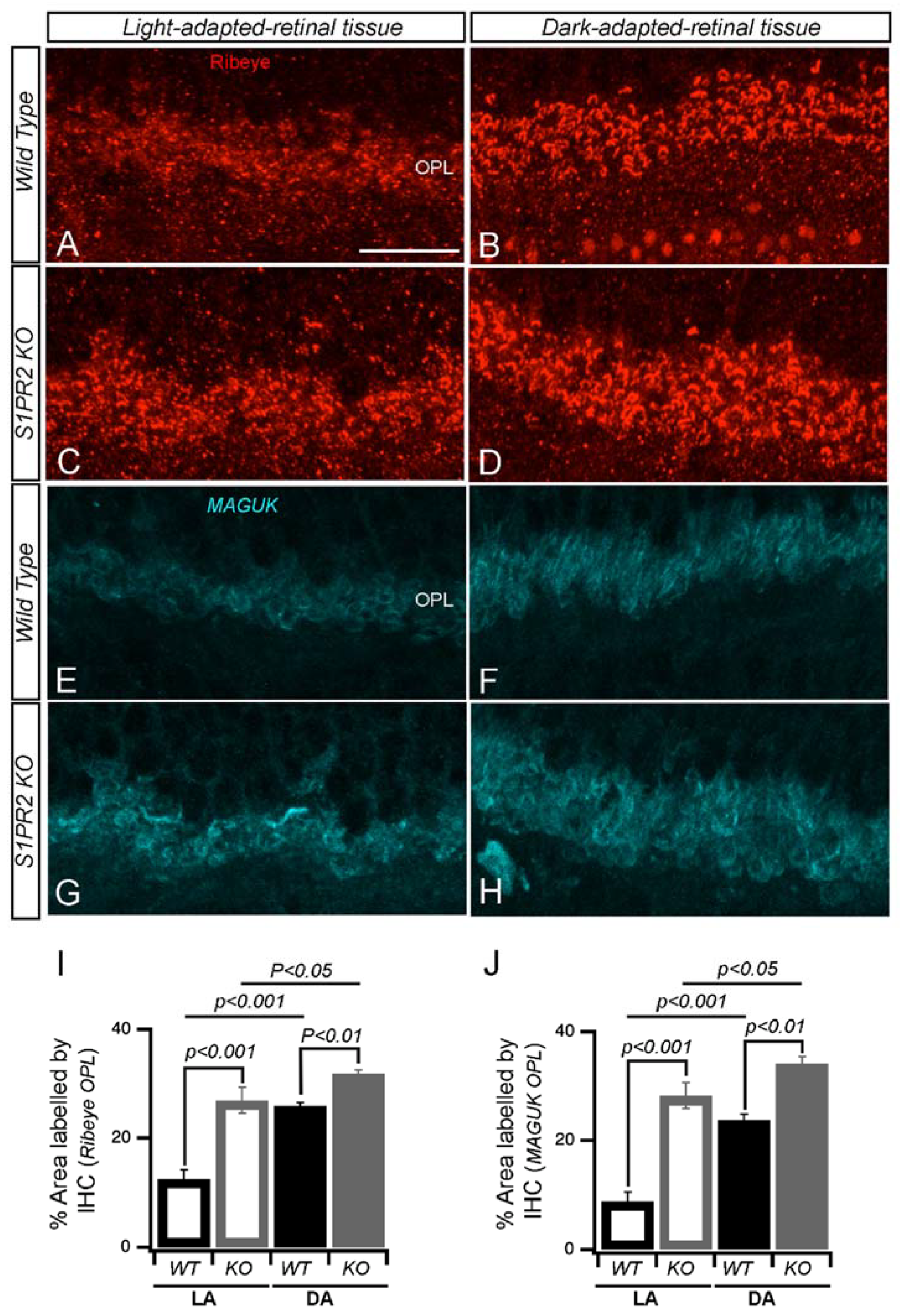
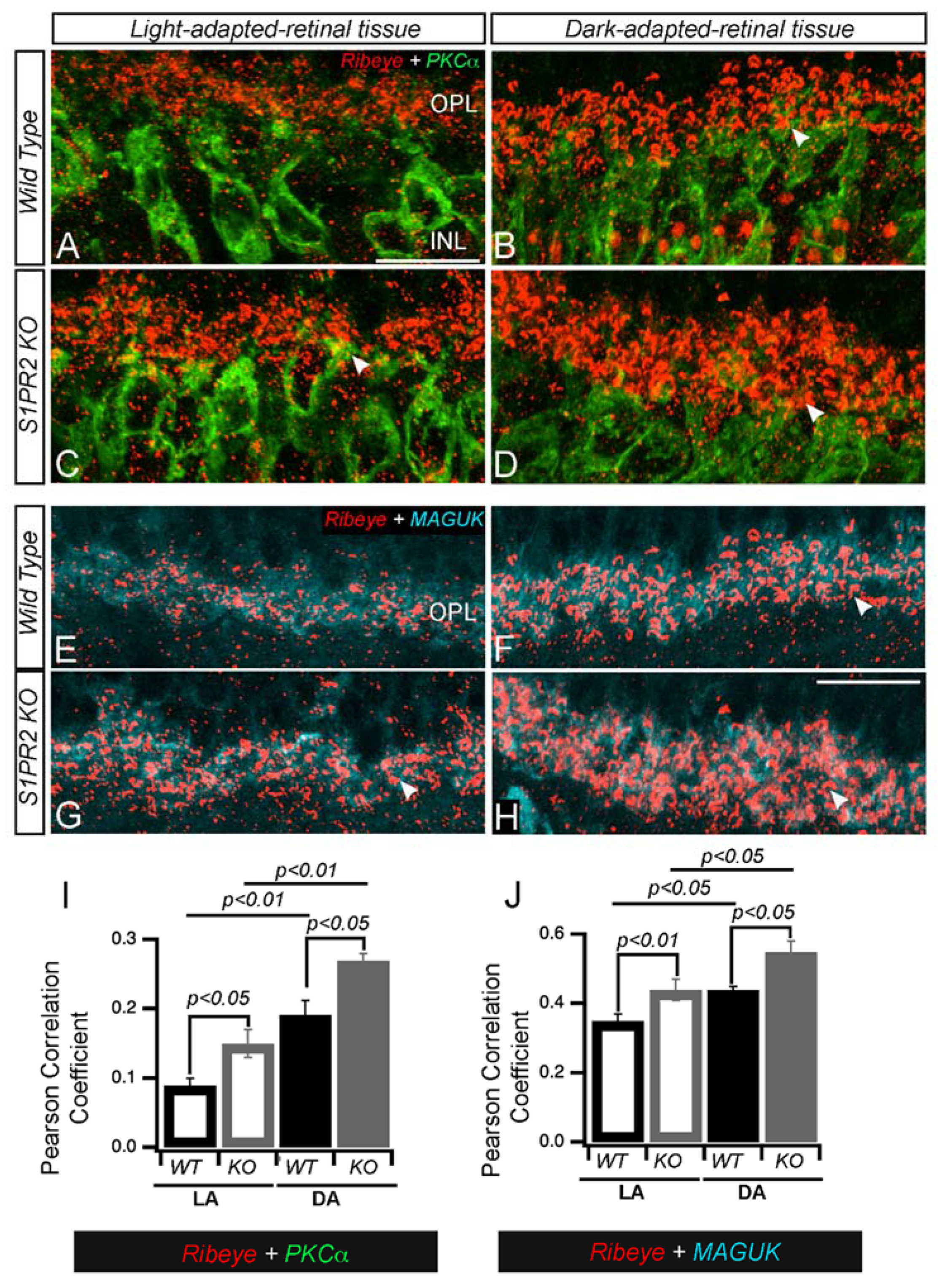
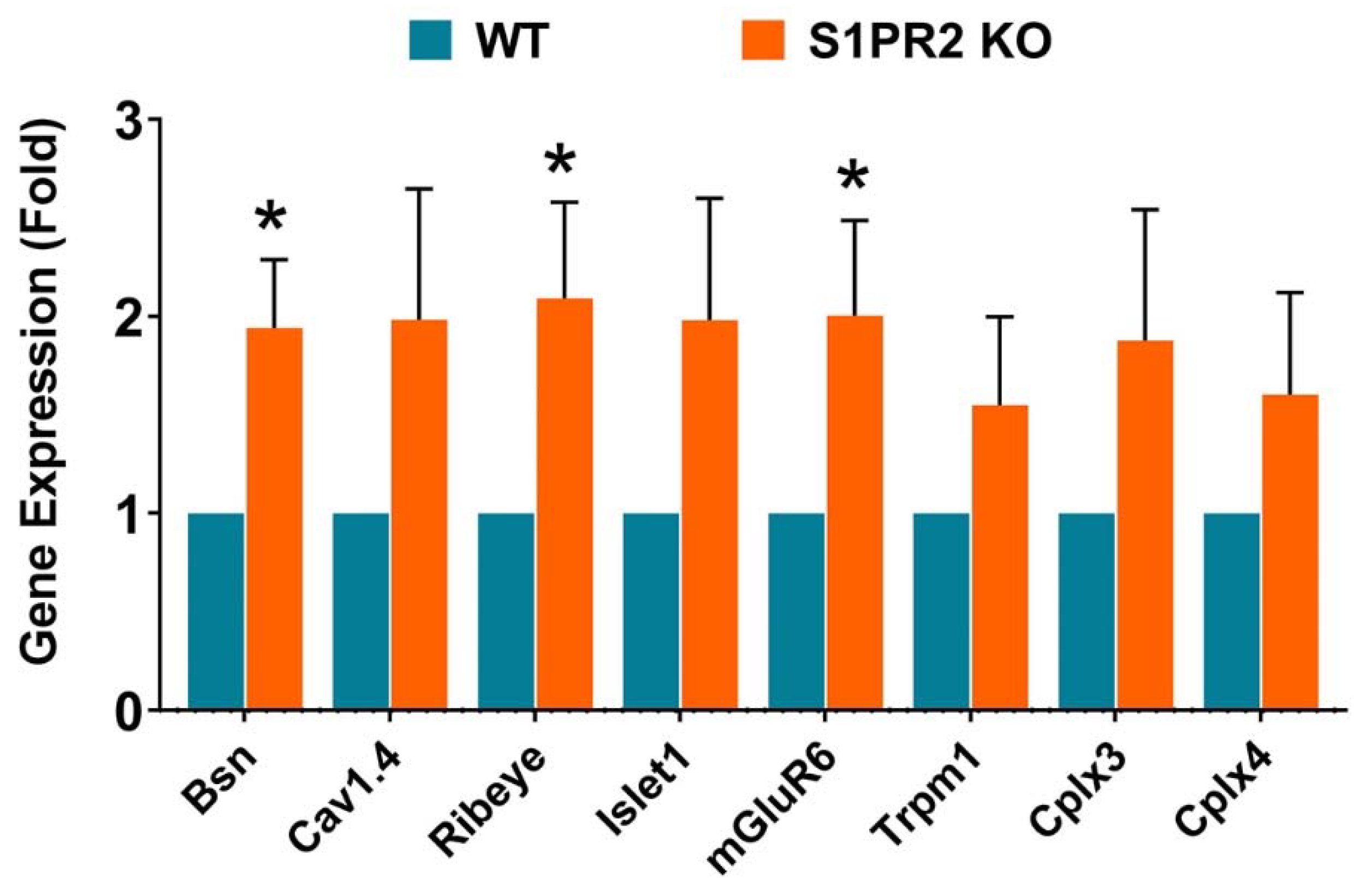
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, H.; Qi, H.; Prabhu, N.; Grambergs, R.; McRae, J.; Hopiavuori, B.; Mandal, N. Characterizing Sphingosine Kinases and Sphingosine 1-Phosphate Receptors in the Mammalian Eye and Retina. Int. J. Mol. Sci. 2018, 19, 3885. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Sankala, H.; Hait, N.C.; Le Stunff, H.; Liu, H.; Toman, R.; Collier, C.; Zhang, M.; Satin, L.S.; Merrill, A.H.; et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005, 280, 37118–37129. [Google Scholar] [CrossRef] [PubMed]
- Ensari Delioğlu, E.N.; Uğurlu, N.; Erdal, E.; Malekghasemi, S.; Çağıl, N. Evaluation of sphingolipid metabolism on diabetic retinopathy. Indian J. Ophthalmol. 2021, 69, 3376–3380. [Google Scholar] [CrossRef]
- Pujol-Lereis, L.M.; Liebisch, G.; Schick, T.; Lin, Y.; Grassmann, F.; Uchida, K.; Zipfel, P.F.; Fauser, S.; Skerka, C.; Weber, B.H.F. Evaluation of serum sphingolipids and the influence of genetic risk factors in age-related macular degeneration. PLoS ONE 2018, 13, e0200739. [Google Scholar] [CrossRef]
- Simón, M.V.; Prado Spalm, F.H.; Vera, M.S.; Rotstein, N.P. Sphingolipids as Emerging Mediators in Retina Degeneration. Front. Cell. Neurosci. 2019, 13, 246. [Google Scholar] [CrossRef]
- Mendelson, K.; Evans, T.; Hla, T. Sphingosine 1-phosphate signalling. Development 2014, 141, 5–9. [Google Scholar] [CrossRef]
- Bryan, A.M.; Del Poeta, M. Sphingosine-1-phosphate receptors and innate immunity. Cell. Microbiol. 2018, 20, e12836. [Google Scholar] [CrossRef]
- Yanagida, K.; Engelbrecht, E.; Niaudet, C.; Jung, B.; Gaengel, K.; Holton, K.; Swendeman, S.; Liu, C.H.; Levesque, M.V.; Kuo, A.; et al. Sphingosine 1-Phosphate Receptor Signaling Establishes AP-1 Gradients to Allow for Retinal Endothelial Cell Specialization. Dev. Cell 2020, 52, 779–793.e7. [Google Scholar] [CrossRef]
- Kono, M.; Mi, Y.; Liu, Y.; Sasaki, T.; Allende, M.L.; Wu, Y.P.; Yamashita, T.; Proia, R.L. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 2004, 279, 29367–29373. [Google Scholar] [CrossRef]
- Qi, H.; Cole, J., 2nd; Grambergs, R.C.; Gillenwater, J.R.; Mondal, K.; Khanam, S.; Dutta, S.; Stiles, M.; Proia, R.L.; Allegood, J.; et al. Sphingosine Kinase 2 Phosphorylation of FTY720 is Unnecessary for Prevention of Light-Induced Retinal Damage. Sci. Rep. 2019, 9, 7771. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.M.; Mahroo, O.A.; Lamb, T.D. Dark adaptation of human rod bipolar cells measured from the b-wave of the scotopic electroretinogram. J. Physiol. 2006, 575, 507–526. [Google Scholar] [CrossRef]
- Miller, R.F.; Dowling, J.E. Intracellular responses of the Müller (glial) cells of mudpuppy retina: Their relation to b-wave of the electroretinogram. J. Neurophysiol. 1970, 33, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tran, J.T.; Eckerd, A.; Huynh, T.P.; Elliott, M.H.; Brush, R.S.; Mandal, N.A. Inhibition of de novo ceramide biosynthesis by FTY720 protects rat retina from light-induced degeneration. J. Lipid Res. 2013, 54, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Garanto, A.; Mandal, N.A.; Egido-Gabas, M.; Marfany, G.; Fabrias, G.; Anderson, R.E.; Casas, J.; Gonzalez-Duarte, R. Specific sphingolipid content decrease in Cerkl knockdown mouse retinas. Exp. Eye Res. 2013, 110, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Mandal, N.A.; Tran, J.T.; Saadi, A.; Rahman, A.K.; Huynh, T.P.; Klein, W.H.; Cho, J.H. Expression and localization of CERKL in the mammalian retina, its response to light-stress, and relationship with NeuroD1 gene. Exp. Eye Res. 2013, 106, 24–33. [Google Scholar] [CrossRef]
- Lenahan, C.; Sanghavi, R.; Huang, L.; Zhang, J.H. Rhodopsin: A Potential Biomarker for Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 326. [Google Scholar] [CrossRef]
- Zhu, X.; Li, A.; Brown, B.; Weiss, E.R.; Osawa, S.; Craft, C.M. Mouse cone arrestin expression pattern: Light induced translocation in cone photoreceptors. Mol. Vis. 2002, 8, 462–471. [Google Scholar]
- Kolb, H.; Famiglietti, E.V. Rod and cone pathways in the inner plexiform layer of cat retina. Science 1974, 186, 47–49. [Google Scholar] [CrossRef]
- Wässle, H.; Peichl, L.; Airaksinen, M.S.; Meyer, M. Calcium-binding proteins in the retina of a calbindin-null mutant mouse. Cell Tissue Res. 1998, 292, 211–218. [Google Scholar] [CrossRef]
- Christakos, S.; Liu, Y. Biological actions and mechanism of action of calbindin in the process of apoptosis. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 401–404. [Google Scholar] [CrossRef]
- Piatigorsky, J. Dual use of the transcriptional repressor (CtBP2)/ribbon synapse (RIBEYE) gene: How prevalent are multifunctional genes? Trends Neurosci. 2001, 24, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.; Königstorfer, A.; Südhof, T.C. RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron 2000, 28, 857–872. [Google Scholar] [CrossRef]
- Kerov, V.; Laird, J.G.; Joiner, M.L.; Knecht, S.; Soh, D.; Hagen, J.; Gardner, S.H.; Gutierrez, W.; Yoshimatsu, T.; Bhattarai, S.; et al. α(2)δ-4 Is Required for the Molecular and Structural Organization of Rod and Cone Photoreceptor Synapses. J. Neurosci. 2018, 38, 6145–6160. [Google Scholar] [CrossRef] [PubMed]
- Boeckers, T.M. The postsynaptic density. Cell Tissue Res. 2006, 326, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.P. The role of the MAGUK protein CASK in neural development and synaptic function. Curr. Med. Chem. 2006, 13, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.P.; Trimmer, J.S.; Gilthorpe, J.D.; Smith, S.J. Characterization of zebrafish PSD-95 gene family members. J. Neurobiol. 2005, 63, 91–105. [Google Scholar] [CrossRef]
- Ciruelas, K.; Marcotulli, D.; Bajjalieh, S.M. Synaptic vesicle protein 2: A multi-faceted regulator of secretion. Semin. Cell Dev. Biol. 2019, 95, 130–141. [Google Scholar] [CrossRef]
- Chen, D.; Tong, J.; Wang, H.; Zeng, L.; Zhou, J.; Huang, J.; Luo, X. Synaptophysin expression in rat retina following acute high intraocular pressure. Acta Histochem. Cytochem. 2008, 41, 173–178. [Google Scholar] [CrossRef][Green Version]
- Xiong, W.H.; Pang, J.J.; Pennesi, M.E.; Duvoisin, R.M.; Wu, S.M.; Morgans, C.W. The Effect of PKCα on the Light Response of Rod Bipolar Cells in the Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4961–4974. [Google Scholar] [CrossRef]
- Behrens, U.D.; Kasten, P.; Wagner, H.J. Adaptation-dependent plasticity of rod bipolar cell axon terminal morphology in the rat retina. Cell Tissue Res. 1998, 294, 243–251. [Google Scholar] [CrossRef]
- Yazulla, S.; Studholme, K.M. Light-dependent plasticity of the synaptic terminals of Mb bipolar cells in goldfish retina. J. Comp. Neurol. 1992, 320, 521–530. [Google Scholar] [CrossRef]
- Behrens, U.D.; Wagner, H.J. Adaptation-dependent changes of bipolar cell terminals in fish retina: Effects on overall morphology and spinule formation in Ma and Mb cells. Vis. Res. 1996, 36, 3901–3911. [Google Scholar] [CrossRef] [PubMed]
- Dembla, E.; Dembla, M.; Maxeiner, S.; Schmitz, F. Synaptic ribbons foster active zone stability and illumination-dependent active zone enrichment of RIM2 and Cav1.4 in photoreceptor synapses. Sci. Rep. 2020, 10, 5957. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.; Studholme, K.; Yazulla, S.; von Gersdorff, H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J. Neurophysiol. 2006, 96, 2025–2033. [Google Scholar] [CrossRef]
- Vollrath, L.; Spiwoks-Becker, I. Plasticity of retinal ribbon synapses. Microsc. Res. Tech. 1996, 35, 472–487. [Google Scholar] [CrossRef]
- Emran, F.; Dowling, J.E. Larval zebrafish turn off their photoreceptors at night. Commun. Integr. Biol. 2010, 3, 430–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abe, H.; Yamamoto, T.Y. Diurnal changes in synaptic ribbons of rod cells of the turtle. J. Ultrastruct. Res. 1984, 86, 246–251. [Google Scholar] [CrossRef]
- Mukherjee, K.; Yang, X.; Gerber, S.H.; Kwon, H.-B.; Ho, A.; Castillo, P.E.; Liu, X.; Südhof, T.C. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proc. Natl. Acad. Sci. USA 2010, 107, 6504–6509. [Google Scholar] [CrossRef]
- Tian, L.; Kammermeier, P.J. G protein coupling profile of mGluR6 and expression of Gα proteins in retinal ON bipolar cells. Vis. Neurosci. 2006, 23, 909–916. [Google Scholar] [CrossRef]
- Brandli, A.; Stone, J. Using the Electroretinogram to Assess Function in the Rodent Retina and the Protective Effects of Remote Limb Ischemic Preconditioning. J. Vis. Exp. 2015, 10, e52658. [Google Scholar] [CrossRef]
- Breton, M.E.; Schueller, A.W.; Lamb, T.D.; Pugh, E.N., Jr. Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Investig. Ophthalmol. Vis. Sci. 1994, 35, 295–309. [Google Scholar]
- Velten, I.M.; Horn, F.K.; Korth, M.; Velten, K. The b-wave of the dark adapted flash electroretinogram in patients with advanced asymmetrical glaucoma and normal subjects. Br. J. Ophthalmol. 2001, 85, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Fricke, S.; Metzdorf, K.; Ohm, M.; Haak, S.; Heine, M.; Korte, M.; Zagrebelsky, M. Fast Regulation of GABA(A)R Diffusion Dynamics by Nogo-A Signaling. Cell Rep. 2019, 29, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Rutherford, M.A.; Strenzke, N.; Neef, A.; Pangršič, T.; Khimich, D.; Fejtova, A.; Gundelfinger, E.D.; Liberman, M.C.; Harke, B.; et al. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron 2010, 68, 724–738. [Google Scholar] [CrossRef]
- Pernet, V. Nogo-A in the visual system development and in ocular diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1300–1311. [Google Scholar] [CrossRef]
- Park, S.; Park, J.H.; Kang, U.B.; Choi, S.K.; Elfadl, A.; Ullah, H.M.A.; Chung, M.J.; Son, J.Y.; Yun, H.H.; Park, J.M.; et al. Nogo-A regulates myogenesis via interacting with Filamin-C. Cell Death Discov. 2021, 7, 1. [Google Scholar] [CrossRef]
- Gordon, W.C.; Casey, D.M.; Lukiw, W.J.; Bazan, N.G. DNA damage and repair in light-induced photoreceptor degeneration. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3511–3521. [Google Scholar]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef]
- McCall, M.A.; Lukasiewicz, P.D.; Gregg, R.G.; Peachey, N.S. Elimination of the rho1 subunit abolishes GABA(C) receptor expression and alters visual processing in the mouse retina. J. Neurosci. 2002, 22, 4163–4174. [Google Scholar] [CrossRef]
- Vigh, J.; von Gersdorff, H. Prolonged reciprocal signaling via NMDA and GABA receptors at a retinal ribbon synapse. J. Neurosci. 2005, 25, 11412–11423. [Google Scholar] [CrossRef]



| Antigen | Antiserum | Host | Dilution | Source (Number) | Marker for |
|---|---|---|---|---|---|
| Rhodopsin | 4D2, monoclonal | Mouse | 1:500 | Sigma (MABN15) | Rod photoreceptors |
| Cone Arrestin | Polyclonal anti- Cone Arrestin n | Rabbit | 1:1000 | Sigma (AB15282MI) | Cone photoreceptors |
| Secretagogin | Polyclonal anti-secretagogin | Sheep | 1:100 | Fisher (50-165-6430) | Cone-bipolar cells |
| PKC | Monoclonal anti-PKC | Mouse | 1:250 | Santa Cruz (sc-17769) | Rod-type bipolar cells |
| Ribeye | Polyclonal anti-ribeye | Guinea pig | 1:500 | Synaptic Systems, (192 104) | Synaptic ribbons (IPL) |
| Calbindin | Monoclonal anti-calbindin | Mouse | 1:500 | ThermoFisher (MA5-32993) | Horizontal cells |
| Pan- MAGUK | Monoclonal pan-MAGUK, clone K28/86 | Mouse | 1:250 | Millipore Sigma (MABN72MI) | Post-synaptic excitatory glutamatergic synapses |
| Synaptophysin | Polyclonal anti-synaptophysin | Rabbit | 1:250 | Alomone lab (ANR-013) | Synaptic vesicles |
| SV2 | Monoclonal anti-SV2 | Mouse | 1:250 | DSHB, (SV2) | Synaptic vesicles |
| Antibody | Conjugation | Source (Number) |
|---|---|---|
| Donkey anti-guinea pig | Cy3 | Jackson Immunoresearch, #706-165-148 |
| Donkey anti-mouse | Alexa Fluor 647 | Southern Biotech, #6440-31 |
| Donkey anti-rabbit | Alexa Fluor 488 | Southern Biotech, #6440-30 |
| Donkey Anti-Sheep | Alexa Fluor 488 | ThermoFisher, A11015 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Bsn (Bassoon pre-synaptic cytomatrix protein) | GGGCTACATGTTGGATGATT | CTTCTGGGAGGAGGAGAC |
| Cav1.4 (Calcium channel, voltage-dependent, alpha 1F subunit) | CCTCCCTTTACAGTGATGAG | CTTCACCCACTGTAGATTCC |
| Ribeye (Ctbp2) | CTCCAAATCTCATCTGCACA | GAGCTGAAGTCACGAAGAAT |
| Islet1 (Insulin Gene Enhancer Protein ISL-1) | TCATGAAGGAGCAACTAGTG | TTAGCCTGTAAACCACCATC |
| mGluR6 (Glutamate receptor, metabotropic 6) | CACCTGTATTATCTGGCTGG | GTAGGTTTTGGGCACATAGA |
| Trpm1 (Transient receptor potential cation channel) | AAGCCAGATCTTCGTCTTTG | TCCTCTTTCACTTTGCCTTT |
| Cplx3 (Complexin 3) | CGAGAAGAGTACGAGGAGTA | GGCTTTCATCTGTTTCGTTC |
| Cplx4 (Complexin 4) | GGTGGGTCTGAAGAAAAGAA | CTCTTTGGAAGCCTGTACTT |
| CD68 (Macrosialin) | CAAGCAGCACAGTGGACATT | GGCAGCAAGAGAGATTGGTC |
| Opn1mw (Opsin 1, medium-wave-sensitive) | TGAGATTTGATGCTAAGCTGG | TGCCGGTTCATAAAGACATAG |
| Opn1sw (Opsin 1, short-wave-sensitive) | CATCATTCCTCTTTCCCTCAT | TGTTTTCTGAGAGCCAGACAC |
| PKCα (Protein kinase C-alpha) | TCAATGTCCCTAGCCTCTGC | GCTCTCATTCTTGGGGTCAG |
| Syntaxin 1A | GCCCTCAGTGAGATCGAGAC | GTACTTGACGGCCTTCTTGG |
| Thy1 (Thymus cell antigen 1) | CCAACTTCACCACCAAGGAT | AAGGAGAGGGAAAGCAGGAG |
| Rod arrestin | CCAACTTCACCACCAAGGAT | AAGGAGAGGGAAAGCAGGAG |
| Rhodopsin | CTTCCTGATCTGCTGGCTTC | ACAGTCTCTGGCCAGGCTTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, A.P.; Stiles, M.; Grambergs, R.C.; Boff, J.M.; Madireddy, S.; Mondal, K.; Rajmanna, R.; Porter, H.; Sherry, D.M.; Proia, R.L.; et al. The Role of Sphingosine-1-Phosphate Receptor 2 in Mouse Retina Light Responses. Biomolecules 2023, 13, 1691. https://doi.org/10.3390/biom13121691
Shrestha AP, Stiles M, Grambergs RC, Boff JM, Madireddy S, Mondal K, Rajmanna R, Porter H, Sherry DM, Proia RL, et al. The Role of Sphingosine-1-Phosphate Receptor 2 in Mouse Retina Light Responses. Biomolecules. 2023; 13(12):1691. https://doi.org/10.3390/biom13121691
Chicago/Turabian StyleShrestha, Abhishek P., Megan Stiles, Richard C. Grambergs, Johane M. Boff, Saivikram Madireddy, Koushik Mondal, Rhea Rajmanna, Hunter Porter, David M. Sherry, Richard L. Proia, and et al. 2023. "The Role of Sphingosine-1-Phosphate Receptor 2 in Mouse Retina Light Responses" Biomolecules 13, no. 12: 1691. https://doi.org/10.3390/biom13121691
APA StyleShrestha, A. P., Stiles, M., Grambergs, R. C., Boff, J. M., Madireddy, S., Mondal, K., Rajmanna, R., Porter, H., Sherry, D. M., Proia, R. L., Vaithianathan, T., & Mandal, N. (2023). The Role of Sphingosine-1-Phosphate Receptor 2 in Mouse Retina Light Responses. Biomolecules, 13(12), 1691. https://doi.org/10.3390/biom13121691







