Neutrophil to Lymphocyte Ratio as a Prognostic Marker in Amyotrophic Lateral Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Cohort’s Characteristics
3.2. Correlation of Blood Cell Count with Parameters of Disease Progression at Diagnosis
3.3. NLR as a Relevant Parameter to Understanding Pathophysiology
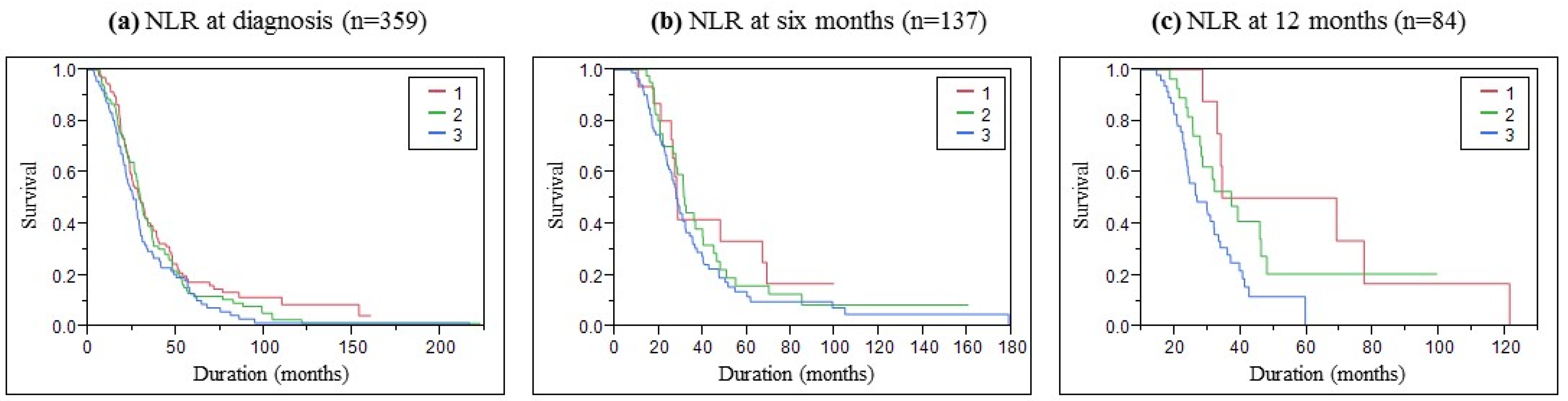
3.4. NLR as a Performant Parameter to Help in ALS Prognostic
4. Discussion
4.1. Correlation between NLR and Clinical Parameters
4.2. NLR as a Risk Factor for Poor Survival
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bjornevik, K.; O’Reilly, E.J.; Molsberry, S.; Kolonel, L.N.; Le Marchand, L.; Paganoni, S.; Schwarzschild, M.A.; Benkert, P.; Kuhle, J.; Ascherio, A. Prediagnostic Neurofilament Light Chain Levels in Amyotrophic Lateral Sclerosis. Neurology 2021, 97, e1466–e1474. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Steinacker, P.; Weishaupt, J.H.; Kassubek, J.; Oeckl, P.; Halbgebauer, S.; Tumani, H.; Von Arnim, C.A.F.; Dorst, J.; Feneberg, E.; et al. Neurofilament Light Chain in Serum for the Diagnosis of Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; Van den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gascon, R.; Miller, R.G.; Gelinas, D.F.; Mass, J.; Hadlock, K.; Jin, X.; Reis, J.; Narvaez, A.; McGrath, M.S. Evidence for Systemic Immune System Alterations in Sporadic Amyotrophic Lateral Sclerosis (sALS). J. Neuroimmunol. 2005, 159, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Appel, S.H. Immune Dysregulation in Amyotrophic Lateral Sclerosis: Mechanisms and Emerging Therapies. Lancet Neurol. 2019, 18, 211–220. [Google Scholar] [CrossRef] [PubMed]
- McGill, R.B.; Steyn, F.J.; Ngo, S.T.; Thorpe, K.A.; Heggie, S.; Ruitenberg, M.J.; Henderson, R.D.; McCombe, P.A.; Woodruff, T.M. Monocytes and Neutrophils Are Associated with Clinical Features in Amyotrophic Lateral Sclerosis. Brain Commun. 2020, 2, fcaa013. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.-Q.; Hou, Y.-B.; Zhang, L.-Y.; Ou, R.-W.; Cao, B.; Chen, Y.-P.; Shang, H.-F. Neutrophil-to-Lymphocyte Ratio in Sporadic Amyotrophic Lateral Sclerosis. Neural. Regen. Res. 2022, 17, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.A.; Mandrioli, J.; Russo, S.; Cucovici, A.; Gianferrari, G.; Lisnic, V.; Muresanu, D.F.; Giuliani, F.; Copetti, M. The Pooled Resource Open-Access Als Clinical Trials Consortium, null; et al. Neutrophils-to-Lymphocyte Ratio Is Associated with Progression and Overall Survival in Amyotrophic Lateral Sclerosis. Biomedicines 2022, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Grassano, M.; Manera, U.; De Marchi, F.; Cugnasco, P.; Matteoni, E.; Daviddi, M.; Solero, L.; Bombaci, A.; Palumbo, F.; Vasta, R.; et al. The Role of Peripheral Immunity in ALS: A Population-based Study. Ann. Clin. Transl. Neurol. 2023, 10, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial Revisited: Revised Criteria for the Diagnosis of Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.J.; Goutman, S.A.; Boss, J.; Kim, S.; Feldman, E.L. Amyotrophic Lateral Sclerosis Survival Associates With Neutrophils in a Sex-Specific Manner. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e953. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Hong, Y.-H.; Kim, S.-M.; Shin, J.-Y.; Suh, Y.J.; Sung, J.-J. High Neutrophil-to-Lymphocyte Ratio Predicts Short Survival Duration in Amyotrophic Lateral Sclerosis. Sci. Rep. 2020, 10, 428. [Google Scholar] [CrossRef]
- Arbel, Y.; Finkelstein, A.; Halkin, A.; Birati, E.Y.; Revivo, M.; Zuzut, M.; Shevach, A.; Berliner, S.; Herz, I.; Keren, G.; et al. Neutrophil/Lymphocyte Ratio Is Related to the Severity of Coronary Artery Disease and Clinical Outcome in Patients Undergoing Angiography. Atherosclerosis 2012, 225, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Pirozzolo, G.; Gisbertz, S.S.; Castoro, C.; Van Berge Henegouwen, M.I.; Scarpa, M. Neutrophil-to-Lymphocyte Ratio as Prognostic Marker in Esophageal Cancer: A Systematic Review and Meta-Analysis. J. Thorac. Dis. 2019, 11, 3136–3145. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Neutrophil-to-Lymphocyte Ratio, Past, Present and Future Perspectives. BLL 2021, 122, 474–488. [Google Scholar] [CrossRef] [PubMed]
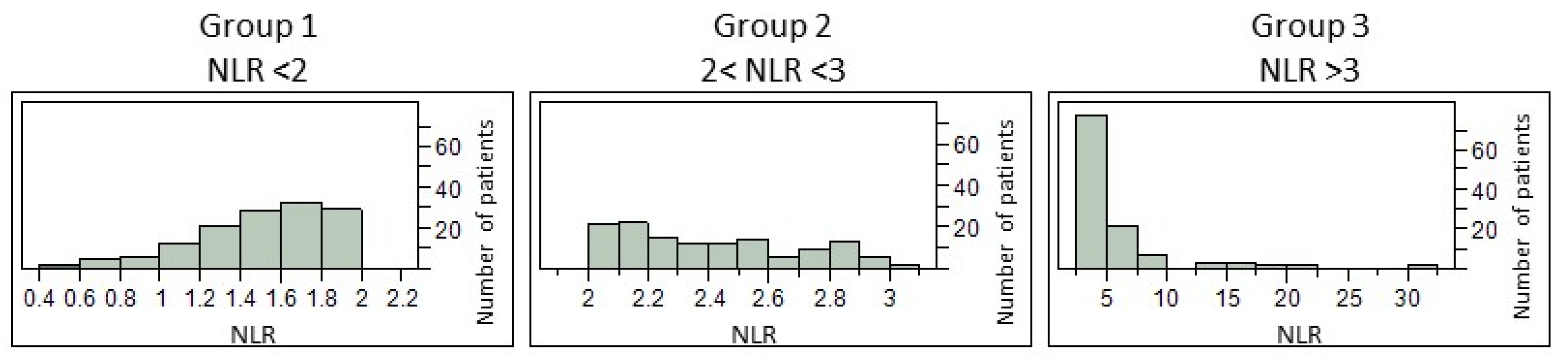
| Patients with NLR = 359 | Group 1 n = 127 | Group 2 n = 122 | Group 3 n = 110 | p-Value |
|---|---|---|---|---|
| Age diagnostic (years) | 65.9 ± 11.9 | 67.3 ± 10.6 | 68.9 ± 11.6 | 0.1288 |
| Weight (kg) | 66.7 ± 15.1 | 69.3 ± 14.8 | 64.5 ± 14.2 | 0.0474 |
| Sex (%) | Female: 59.1 | Female: 48.4 | Female: 44.5 | 0.0649 |
| Male: 40.1 | Male: 51.6 | Male: 55.5 | ||
| Onset site (%) | Bulbar: 34.6 | Bulbar: 38.0 | Bulbar: 30.9 | 0.5258 |
| Spinal: 65.4 | Spinal: 62.0 | Spinal: 69.1 | ||
| Duration (month) | 34.5 ± 27.8 | 34.0 ± 28.1 | 28.5 ± 26 | 0.0281 |
| FVC (%) | 93.8 ± 27.1 | 91.1 ± 26.7 | 80.8 ± 30.3 | 0.0098 |
| ALSFRS | 38.2 ± 5.4 | 38.1 ± 6.5 | 35.1 ± 7.6 | 0.0010 * |
| BMI kg/m2 | 24.6 ± 4.9 | 24.9 ± 4.4 | 23.5 ± 4.5 | 0.0485 |
| PNN (G/L) | 3.2 ± 0.9 | 4.11 ± 1.1 | 6.0 ± 2.4 | <0.0001 * |
| Hemoglobin (g/L) | 139.2 ± 12.5 | 141.1 ± 12.0 | 140.9 ± 14.1 | 0.3510 |
| Red blood cells (T/L) | 4.5 ± 0.4 | 4.6 ± 0.4 | 4.6 ± 0.5 | 0.1313 |
| Hematocrit (%) | 41.5 ± 3.7 | 42.0 ± 3.5 | 42.2 ± 4.3 | 0.2548 |
| MCV (fL) | 92.1 ± 4.2 | 91.3 ± 3.9 | 92.1 ± 4.1 | 0.1275 |
| MCHC (g/dL) | 33.6 ± 0.8 | 33.5 ± 0.7 | 33.4 ± 0.8 | 0.1536 |
| MCH (pg) | 30.9 ± 1.6 | 30.7 ± 1.5 | 30.8 ± 1.6 | 0.2311 |
| PMV (fL) | 8.9 ± 1.0 | 9.0 ± 0.9 | 8.9 ± 1.1 | 0.5314 |
| Leucocytes (G/L) | 6.0 ± 1.5 | 6.5 ± 1.6 | 8.1 ± 2.6 | <0.0001 * |
| PNE (G/L) | 0.18 ± 0.12 | 0.15 ± 0.11 | 0.12 ± 0.09 | 0.0004 * |
| PNB (G/L) | 0.041 ± 0.022 | 0.037 ± 0.019 | 0.036 ± 0.029 | 0.0120 |
| Lymphocytes (G/L) | 2.1 ± 0.6 | 1.7 ± 0.4 | 1.3 ± 0.4 | <0.0001 * |
| Monocytes (G/L) | 0.49 ± 0.15 | 0.53 ± 0.18 | 0.63 ± 0.21 | <0.0001 * |
| Platelets (G/L) | 234.8 ± 58.7 | 235.7 ± 66.3 | 254.4 ± 70.9 | 0.0193 |
| NMR | 6.9 ± 2.4 | 8.2 ± 2.3 | 10.1 ± 3.4 | <0.0001 * |
| Survival | Median in Months (Confidence Interval at 95%) | ||
|---|---|---|---|
| At Inclusion | At 6 Months | At 12 Months | |
| Group 1 (NLR < 2) | 29 (24.3–34.3) | 28.2 (21–67) | 51.75 (28.6–121.3) |
| Group 2 (NLR 2–3) | 30 (26.9–33.8) | 31.9 (27.7–40.2) | 37.3 (27.8–46.2) |
| Group 3 (NLR < 3) | 25.7 (21.5–29.1) | 28 (24.6–32.1) | 26.9 (23.6–33.4) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| NLR_M0 | 1.066 (1.013–1.111) | 0.0158 * | 1.057 (0.954–1.159) | 0.2764 |
| NLR_M6 | 1.131 (1.038–1.215) | 0.0062 * | 1.258 (1.036–1.510) | 0.0213 * |
| NLR_M12 | 1.172 (1.075–1.269) | 0.0006 * | 1.368 (1.022–1.829) | 0.0358 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotet, C.; Alarcan, H.; Hérault, O.; Corcia, P.; Vourc’h, P.; Andres, C.R.; Blasco, H.; Veyrat-Durebex, C. Neutrophil to Lymphocyte Ratio as a Prognostic Marker in Amyotrophic Lateral Sclerosis. Biomolecules 2023, 13, 1689. https://doi.org/10.3390/biom13121689
Cotet C, Alarcan H, Hérault O, Corcia P, Vourc’h P, Andres CR, Blasco H, Veyrat-Durebex C. Neutrophil to Lymphocyte Ratio as a Prognostic Marker in Amyotrophic Lateral Sclerosis. Biomolecules. 2023; 13(12):1689. https://doi.org/10.3390/biom13121689
Chicago/Turabian StyleCotet, Camille, Hugo Alarcan, Olivier Hérault, Philippe Corcia, Patrick Vourc’h, Christian R. Andres, Hélène Blasco, and Charlotte Veyrat-Durebex. 2023. "Neutrophil to Lymphocyte Ratio as a Prognostic Marker in Amyotrophic Lateral Sclerosis" Biomolecules 13, no. 12: 1689. https://doi.org/10.3390/biom13121689
APA StyleCotet, C., Alarcan, H., Hérault, O., Corcia, P., Vourc’h, P., Andres, C. R., Blasco, H., & Veyrat-Durebex, C. (2023). Neutrophil to Lymphocyte Ratio as a Prognostic Marker in Amyotrophic Lateral Sclerosis. Biomolecules, 13(12), 1689. https://doi.org/10.3390/biom13121689








