Controlling Trophoblast Cell Fusion in the Human Placenta—Transcriptional Regulation of Suppressyn, an Endogenous Inhibitor of Syncytin-1
Abstract
:1. Introduction
2. Methods
2.1. Cell Cultures
2.2. Vectors and Constructs
2.3. Mutagenesis
2.4. Transient Transfections and Luciferase Assays
2.5. Bisulfite Methylation Analysis
2.6. Statistical Analyses
3. Results
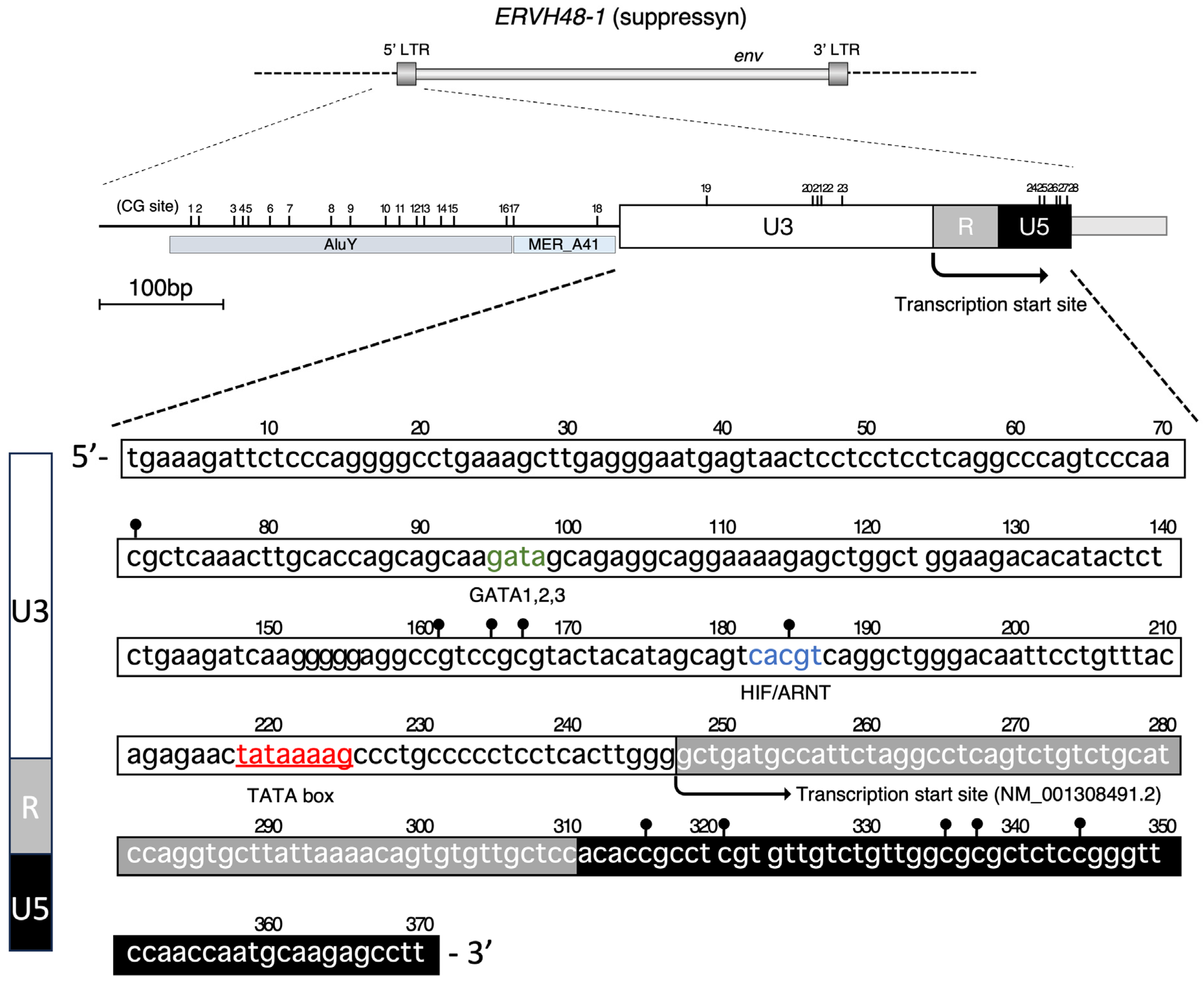
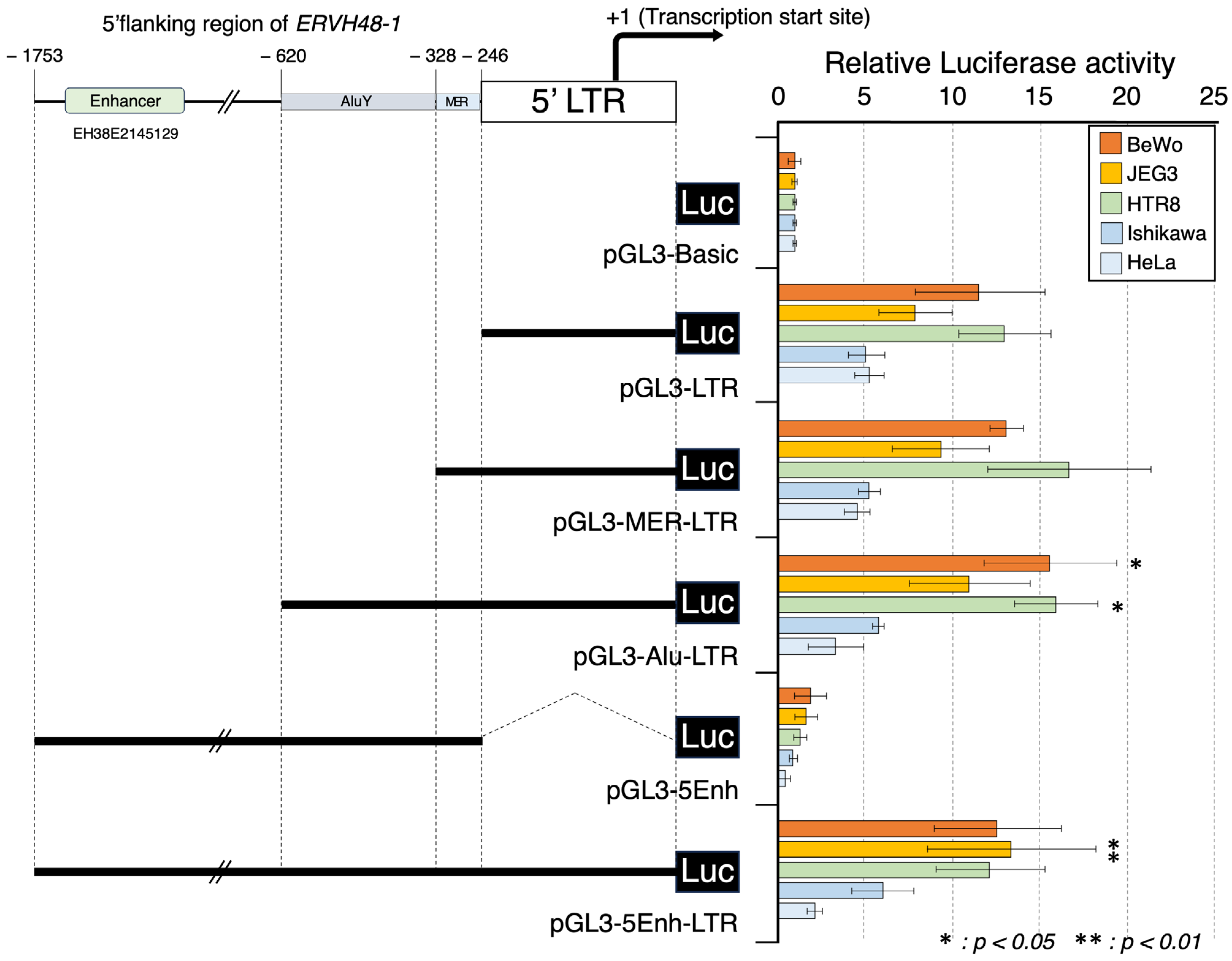
3.1. Promoter Activity of the ERVH48-1 5′ LTR Genome Sequence
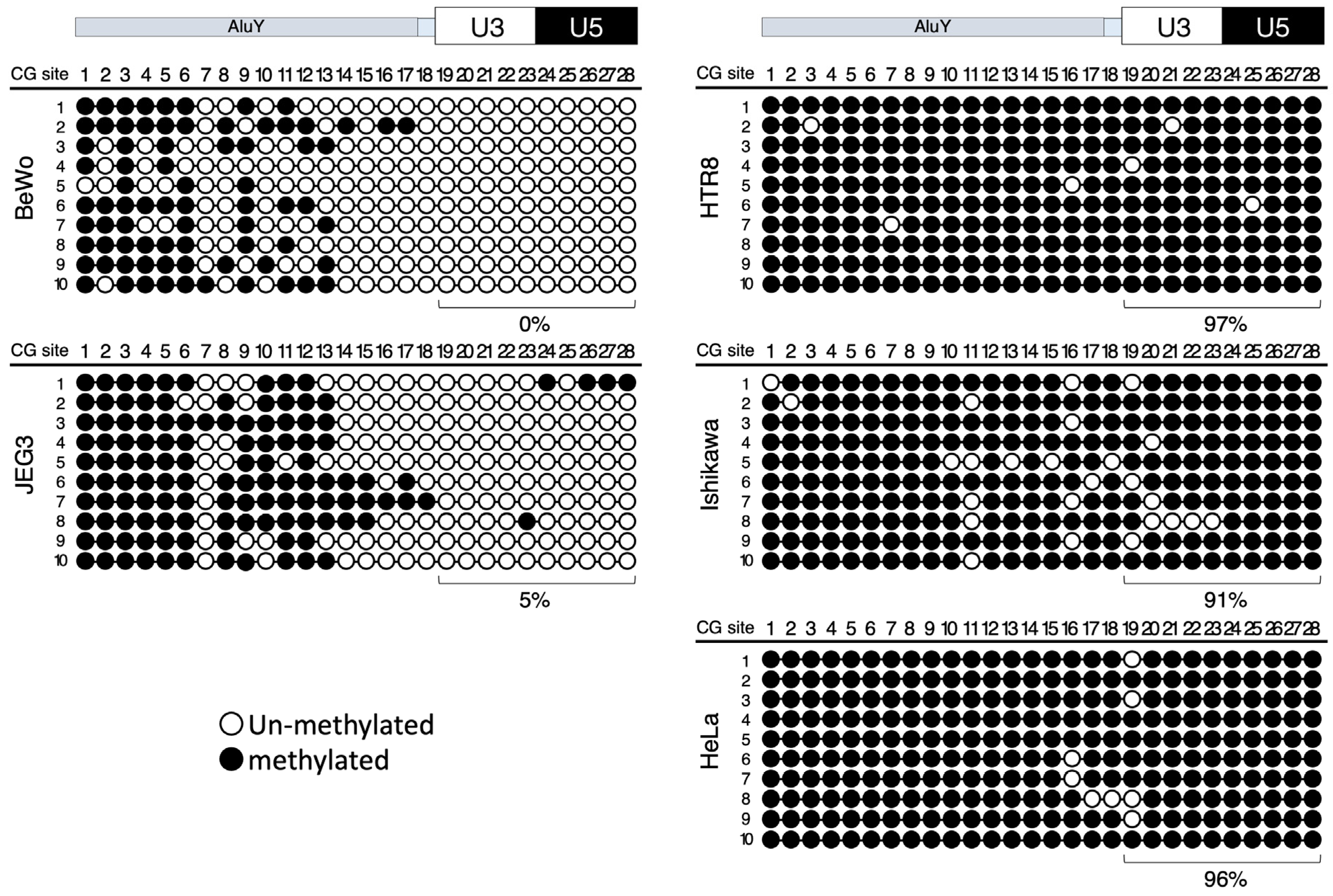
3.2. DNA Methylation Analysis of the 5′ LTR and Upstream Enhancer Sequences
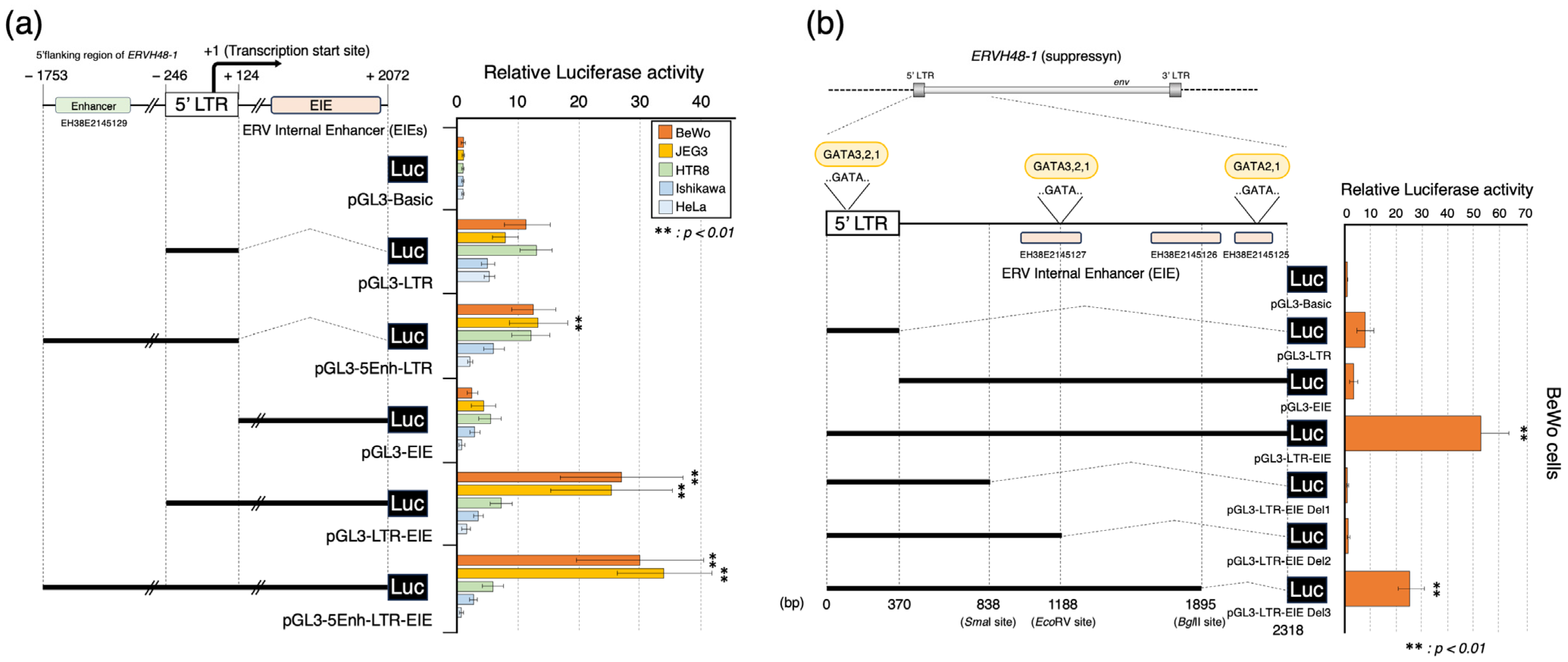
3.3. Placenta-Specific Enhancer Sequences: EIEs
3.4. Oxygen-Related Changes in ERVH48-1 Promoter Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jern, P.; Coffin, J.M. Effects of Retroviruses on Host Genome Function. Annu. Rev. Genet. 2008, 42, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.A. The Discovery of Endogenous Retroviruses. Retrovirology 2006, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R.; Tristem, M. The Evolution, Distribution and Diversity of Endogenous Retroviruses. Virus Genes 2003, 26, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.A.; Singh, M.; Cullen, H.B.; Kirou, R.A.; Benkaddour-Boumzaouad, M.; Cortes, J.L.; Garcia Pérez, J.; Coyne, C.B.; Feschotte, C. Evolution and Antiviral Activity of a Human Protein of Retroviral Origin. Science 2022, 378, 422–428. [Google Scholar] [CrossRef]
- Sugimoto, J.; Sugimoto, M.; Bernstein, H.; Jinno, Y.; Schust, D. A Novel Human Endogenous Retroviral Protein Inhibits Cell-Cell Fusion. Sci. Rep. 2013, 3, 1462. [Google Scholar] [CrossRef] [PubMed]
- Kurth, R.; Bannert, N. Beneficial and Detrimental Effects of Human Endogenous Retroviruses. Int. J. Cancer 2010, 126, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Matousková, M.; Blazková, J.; Pajer, P.; Pavlícek, A.; Hejnar, J. CpG Methylation Suppresses Transcriptional Activity of Human Syncytin-1 in Non-Placental Tissues. Exp. Cell Res. 2006, 312, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, J.; Montgiraud, C.; Oriol, G.; Pichon, J.-P.; Ruel, K.; Tsatsaris, V.; Gerbaud, P.; Frendo, J.-L.; Evain-Brion, D.; Mallet, F. Comparative Methylation of ERVWE1/Syncytin-1 and Other Human Endogenous Retrovirus LTRs in Placenta Tissues. DNA Res. 2009, 16, 195–211. [Google Scholar] [CrossRef]
- Mi, S.; Lee, X.; Li, X.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.Y.; Edouard, P.; Howes, S.; et al. Syncytin Is a Captive Retroviral Envelope Protein Involved in Human Placental Morphogenesis. Nature 2000, 403, 785–789. [Google Scholar] [CrossRef]
- Blond, J.L.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F.L. An Envelope Glycoprotein of the Human Endogenous Retrovirus HERV-W Is Expressed in the Human Placenta and Fuses Cells Expressing the Type D Mammalian Retrovirus Receptor. J. Virol. 2000, 74, 3321–3329. [Google Scholar] [CrossRef]
- Roberts, R.M.; Ezashi, T.; Schulz, L.C.; Sugimoto, J.; Schust, D.J.; Khan, T.; Zhou, J. Syncytins Expressed in Human Placental Trophoblast. Placenta 2021, 113, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ruebner, M.; Strissel, P.L.; Ekici, A.B.; Stiegler, E.; Dammer, U.; Goecke, T.W.; Faschingbauer, F.; Fahlbusch, F.B.; Beckmann, M.W.; Strick, R. Reduced Syncytin-1 Expression Levels in Placental Syndromes Correlates with Epigenetic Hypermethylation of the ERVW-1 Promoter Region. PLoS ONE 2013, 8, e56145. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.-W.; Li, J.; Brost, B.C.; Xia, X.-Y.; Chen, H.B.; Wang, C.-X.; Jiang, S.-W. Decreased Expression and Altered Methylation of Syncytin-1 Gene in Human Placentas Associated with Preeclampsia. Curr. Pharm. Des. 2014, 20, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, A.; Lai Kuen, R.; Malassiné, A.; Tricottet, V.; Blain, P.; Vidaud, M.; Evain-Brion, D. Characterization of Human Villous and Extravillous Trophoblasts Isolated from First Trimester Placenta. Lab. Investig. 2001, 81, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Illsley, N.P.; DaSilva-Arnold, S.C.; Zamudio, S.; Alvarez, M.; Al-Khan, A. Trophoblast Invasion: Lessons from Abnormally Invasive Placenta (Placenta Accreta). Placenta 2020, 102, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Schust, D.J.; Bonney, E.A.; Sugimoto, J.; Ezashi, T.; Roberts, R.M.; Choi, S.; Zhou, J. The Immunology of Syncytialized Trophoblast. Int. J. Mol. Sci. 2021, 22, 1767. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.P.; Aplin, J.D. A Re-Examination of the Origins of Placental Bed Giant Cells. Placenta 2021, 114, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, J.; Choi, S.; Sheridan, M.A.; Koh, I.; Kudo, Y.; Schust, D.J. Could the Human Endogenous Retrovirus-Derived Syncytialization Inhibitor, Suppressyn, Limit Heterotypic Cell Fusion Events in the Decidua? Int. J. Mol. Sci. 2021, 22, 10259. [Google Scholar] [CrossRef]
- James, J.L.; Lissaman, A.; Nursalim, Y.N.S.; Chamley, L.W. Modelling Human Placental Villous Development: Designing Cultures That Reflect Anatomy. Cell. Mol. Life Sci. 2022, 79, 384. [Google Scholar] [CrossRef]
- Jaremek, A.; Shaha, S.; Jeyarajah, M.J.; Jaju Bhattad, G.; Chowdhury, D.; Riddell, M.; Renaud, S.J. Genome-Wide Analysis of Hypoxia-Inducible Factor Binding Reveals Targets Implicated in Impaired Human Placental Syncytiotrophoblast Formation under Low Oxygen. Am. J. Pathol. 2023, 193, 846–865. [Google Scholar] [CrossRef]
- Chang, C.-W.; Wakeland, A.K.; Parast, M.M. Trophoblast Lineage Specification, Differentiation and Their Regulation by Oxygen Tension. J. Endocrinol. 2018, 236, R43–R56. [Google Scholar] [CrossRef]
- Treissman, J.; Yuan, V.; Baltayeva, J.; Le, H.T.; Castellana, B.; Robinson, W.P.; Beristain, A.G. Low Oxygen Enhances Trophoblast Column Growth by Potentiating Differentiation of the Extravillous Lineage and Promoting LOX Activity. Development 2020, 147, dev181263. [Google Scholar] [CrossRef] [PubMed]
- Wakeland, A.K.; Soncin, F.; Moretto-Zita, M.; Chang, C.-W.; Horii, M.; Pizzo, D.; Nelson, K.K.; Laurent, L.C.; Parast, M.M. Hypoxia Directs Human Extravillous Trophoblast Differentiation in a Hypoxia-Inducible Factor-Dependent Manner. Am. J. Pathol. 2017, 187, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Conrad, K.P. Expression, Ontogeny, and Regulation of Hypoxia-Inducible Transcription Factors in the Human Placenta. Biol. Reprod. 2000, 63, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Caniggia, I.; Winter, J.; Lye, S.J.; Post, M. Oxygen and Placental Development during the First Trimester: Implications for the Pathophysiology of Pre-Eclampsia. Placenta 2000, 21 (Suppl. A), S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, J.; Schust, D.J.; Kinjo, T.; Aoki, Y.; Jinno, Y.; Kudo, Y. Suppressyn Localization and Dynamic Expression Patterns in Primary Human Tissues Support a Physiologic Role in Human Placentation. Sci. Rep. 2019, 9, 19502. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, J.; Schust, D.J.; Yamazaki, T.; Kudo, Y. Involvement of the HERV-Derived Cell-Fusion Inhibitor, Suppressyn, in the Fusion Defects Characteristic of the Trisomy 21 Placenta. Sci. Rep. 2022, 12, 10552. [Google Scholar] [CrossRef] [PubMed]
- Okahara, G.; Matsubara, S.; Oda, T.; Sugimoto, J.; Jinno, Y.; Kanaya, F. Expression Analyses of Human Endogenous Retroviruses (HERVs): Tissue-Specific and Developmental Stage-Dependent Expression of HERVs. Genomics 2004, 84, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Toufaily, C.; LeBellego, F.; Rassart, É.; Lafond, J.; Barbeau, B. Reduced Expression of Both Syncytin 1 and Syncytin 2 Correlates with Severity of Preeclampsia. Reprod. Sci. 2011, 18, 1085–1091. [Google Scholar] [CrossRef]
- Priščáková, P.; Svoboda, M.; Feketová, Z.; Hutník, J.; Repiská, V.; Gbelcová, H.; Gergely, L. Syncytin-1, Syncytin-2 and Suppressyn in Human Health and Disease. J. Mol. Med. 2023. [Google Scholar] [CrossRef]
- Graham, C.H.; Hawley, T.S.; Hawley, R.C.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and Characterization of First Trimester Human Trophoblast Cells with Extended Lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Prudhomme, S.; Oriol, G.; Mallet, F. A Retroviral Promoter and a Cellular Enhancer Define a Bipartite Element Which Controls Env ERVWE1 Placental Expression. J. Virol. 2004, 78, 12157–12168. [Google Scholar] [CrossRef] [PubMed]
- Knerr, I.; Schubert, S.W.; Wich, C.; Amann, K.; Aigner, T.; Vogler, T.; Jung, R.; Dötsch, J.; Rascher, W.; Hashemolhosseini, S. Stimulation of GCMa and Syncytin via CAMP Mediated PKA Signaling in Human Trophoblastic Cells under Normoxic and Hypoxic Conditions. FEBS Lett. 2005, 579, 3991–3998. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, M.; Chen, H. Biochemical Characterization of the Human Placental Transcription Factor GCMa/1. Biochem. Cell Biol. 2005, 83, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Ohta, T.; Shioi, G.; Hatanaka, H.; Ogasawara, O.; Okuda, Y.; Kawaji, H.; Nakaki, R.; Sese, J.; Meno, C. ChIP-Atlas: A Data-mining Suite Powered by Full Integration of Public ChIP-seq Data. EMBO Rep. 2018, 19, e46255. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Takagi, T. Estimating Transcription Factor Bindability on DNA. Bioinformatics 1999, 15, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.T.; Roth, M.E.; Groskopf, J.C.; Tsai, F.Y.; Orkin, S.H.; Grosveld, F.; Engel, J.D.; Linzer, D.I. GATA-2 and GATA-3 Regulate Trophoblast-Specific Gene Expression in Vivo. Development 1997, 124, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Sakurai, T.; Kim, M.-S.; Muroi, Y.; Ideta, A.; Aoyagi, Y.; Nakajima, H.; Takahashi, M.; Nagaoka, K.; Imakawa, K. Involvement of GATA Transcription Factors in the Regulation of Endogenous Bovine Interferon-Tau Gene Transcription. Mol. Reprod. Dev. 2009, 76, 1143–1152. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Handwerger, S. A Placenta-Specific Enhancer of the Human Syncytin Gene. Biol. Reprod. 2005, 73, 500–509. [Google Scholar] [CrossRef]
- Bai, H.; Sakurai, T.; Godkin, J.D.; Imakawa, K. Expression and Potential Role of GATA Factors in Trophoblast Development. J. Reprod. Dev. 2013, 59, 1–6. [Google Scholar] [CrossRef]
- Paul, S.; Home, P.; Bhattacharya, B.; Ray, S. GATA Factors: Master Regulators of Gene Expression in Trophoblast Progenitors. Placenta 2017, 60 (Suppl. 1), S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Albadari, N.; Deng, S.; Li, W. The Transcriptional Factors HIF-1 and HIF-2 and Their Novel Inhibitors in Cancer Therapy. Expert Opin. Drug Discov. 2019, 14, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Pringle, K.G.; Kind, K.L.; Sferruzzi-Perri, A.N.; Thompson, J.G.; Roberts, C.T. Beyond Oxygen: Complex Regulation and Activity of Hypoxia Inducible Factors in Pregnancy. Hum. Reprod. Update 2010, 16, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Landers, K.; Mortimer, R.H.; Richard, K. Regulation of Hypoxia Inducible Factors (HIF) in Hypoxia and Normoxia during Placental Development. Placenta 2010, 31, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Colson, A.; Depoix, C.L.; Baldin, P.; Hubinont, C.; Sonveaux, P.; Debiève, F. Hypoxia-Inducible Factor 2 Alpha Impairs Human Cytotrophoblast Syncytialization: New Insights into Placental Dysfunction and Fetal Growth Restriction. FASEB J. 2020, 34, 15222–15235. [Google Scholar] [CrossRef] [PubMed]
- McCracken, S.A.; Seeho, S.K.M.; Carrodus, T.; Park, J.H.; Woodland, N.; Gallery, E.D.M.; Morris, J.M.; Ashton, A.W. Dysregulation of Oxygen Sensing/Response Pathways in Pregnancies Complicated by Idiopathic Intrauterine Growth Restriction and Early-Onset Preeclampsia. Int. J. Mol. Sci. 2022, 23, 2772. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Michael, H.M.; Daftary, A.; Jeyabalan, A.; Gilmour, C.; Conrad, K.P. Proteasomal Activity in Placentas from Women with Preeclampsia and Intrauterine Growth Restriction: Implications for Expression of HIF-Alpha Proteins. Placenta 2008, 29, 290–299. [Google Scholar] [CrossRef]
- Rajakumar, A.; Doty, K.; Daftary, A.; Harger, G.; Conrad, K.P. Impaired Oxygen-Dependent Reduction of HIF-1alpha and -2alpha Proteins in Pre-Eclamptic Placentae. Placenta 2003, 24, 199–208. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugimoto, J.; Schust, D.J.; Sugimoto, M.; Jinno, Y.; Kudo, Y. Controlling Trophoblast Cell Fusion in the Human Placenta—Transcriptional Regulation of Suppressyn, an Endogenous Inhibitor of Syncytin-1. Biomolecules 2023, 13, 1627. https://doi.org/10.3390/biom13111627
Sugimoto J, Schust DJ, Sugimoto M, Jinno Y, Kudo Y. Controlling Trophoblast Cell Fusion in the Human Placenta—Transcriptional Regulation of Suppressyn, an Endogenous Inhibitor of Syncytin-1. Biomolecules. 2023; 13(11):1627. https://doi.org/10.3390/biom13111627
Chicago/Turabian StyleSugimoto, Jun, Danny J. Schust, Makiko Sugimoto, Yoshihiro Jinno, and Yoshiki Kudo. 2023. "Controlling Trophoblast Cell Fusion in the Human Placenta—Transcriptional Regulation of Suppressyn, an Endogenous Inhibitor of Syncytin-1" Biomolecules 13, no. 11: 1627. https://doi.org/10.3390/biom13111627
APA StyleSugimoto, J., Schust, D. J., Sugimoto, M., Jinno, Y., & Kudo, Y. (2023). Controlling Trophoblast Cell Fusion in the Human Placenta—Transcriptional Regulation of Suppressyn, an Endogenous Inhibitor of Syncytin-1. Biomolecules, 13(11), 1627. https://doi.org/10.3390/biom13111627





