Structural and Functional Insights into the Stealth Protein CpsY of Mycobacterium tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Constructs, Expression, and Purification
2.2. Crystallization and Data Collection
2.3. Structure Determination
2.4. Enzymatic Activity Assays
2.5. Molecular Dynamics (MD) Simulations
2.6. Statistical Analysis
2.7. Data Deposition
3. Results
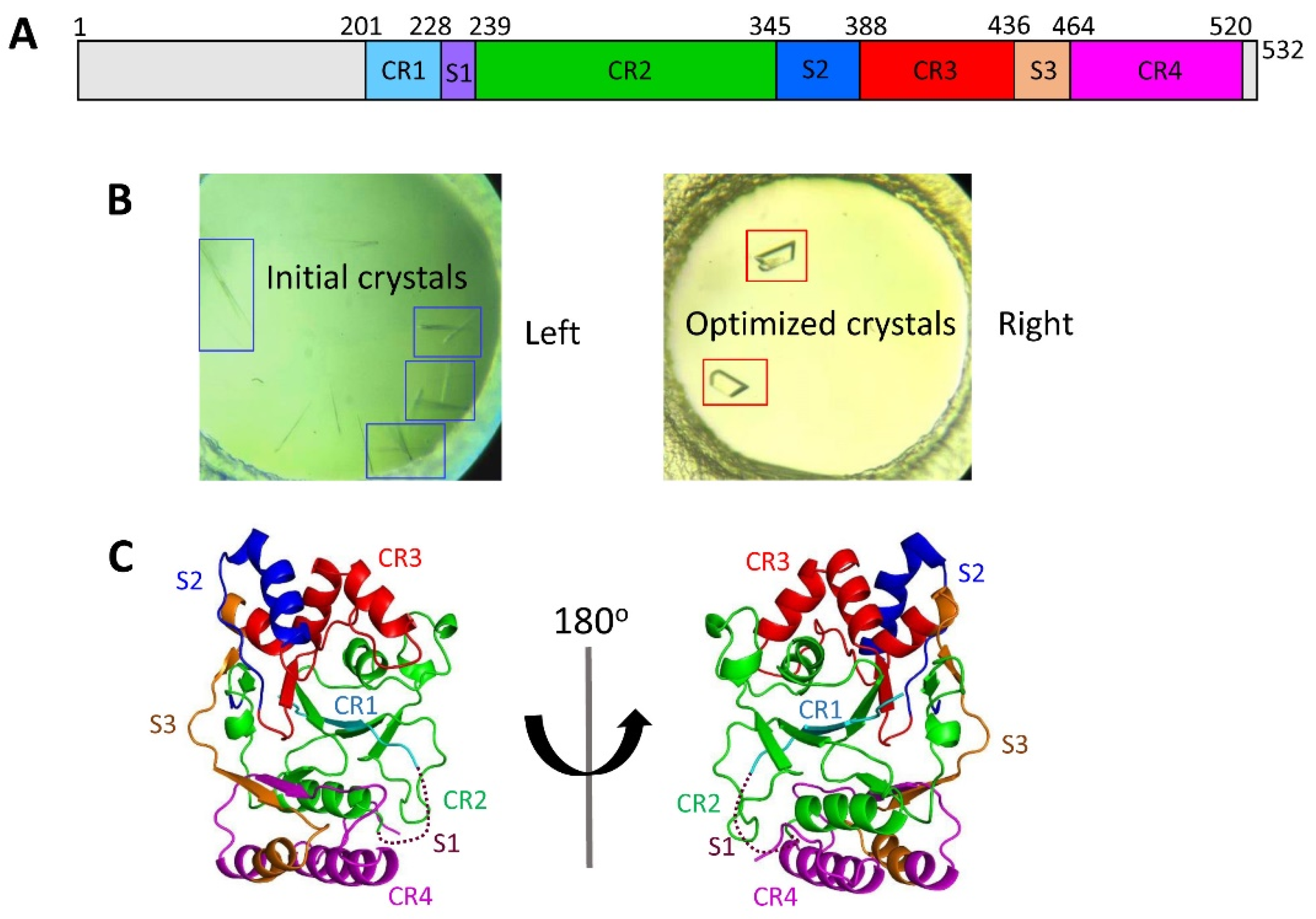
3.1. Preparation and Crystallization of CpsY201−520
3.2. Determination of the Crystal Structure of CpsY201−520
3.3. Structural Comparison between CpsY201−520 and the Zebrafish GNPTAB
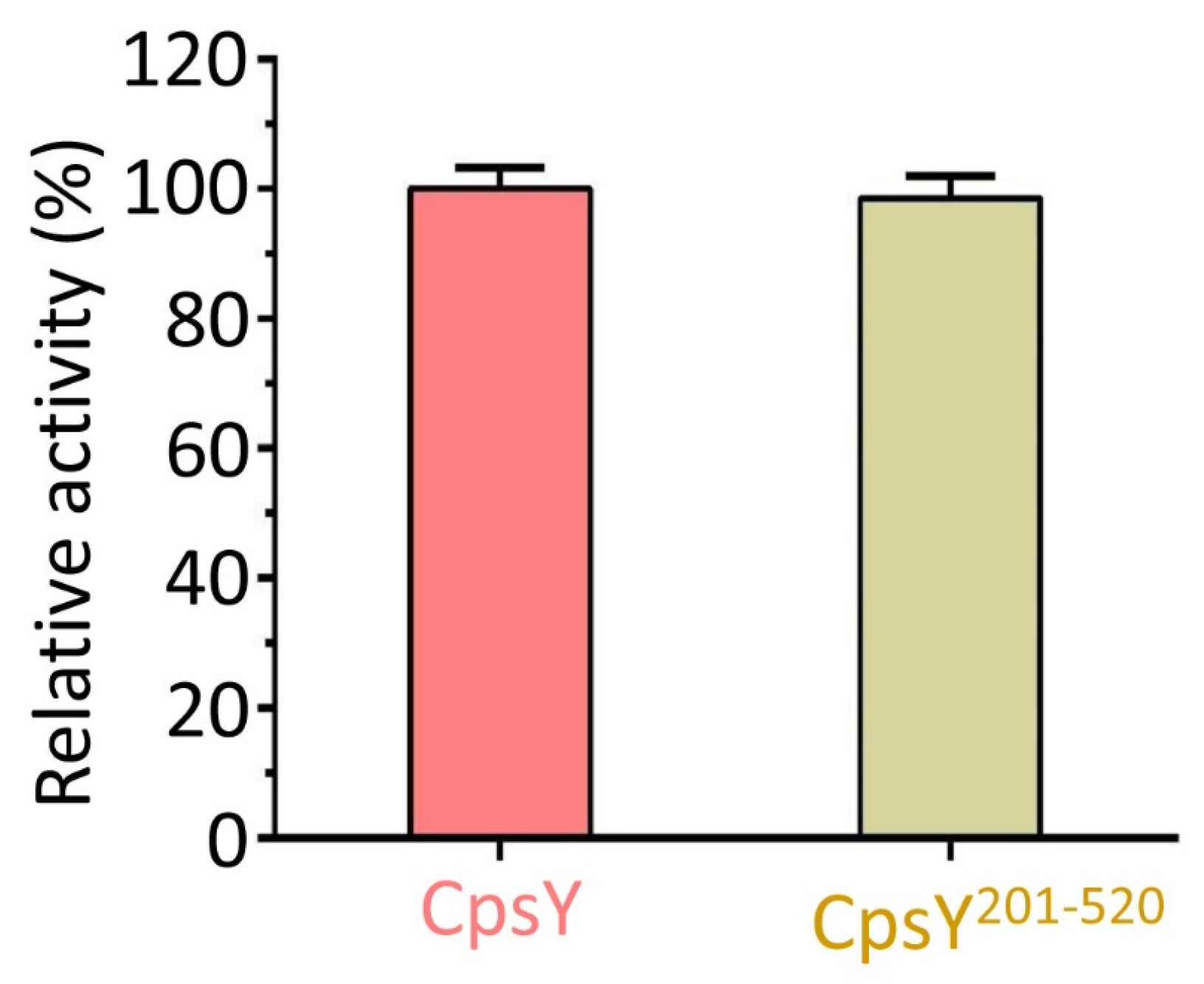
3.4. CpsY201−520 Accounts for the Full Catalytic Activity of Full-Length CpsY
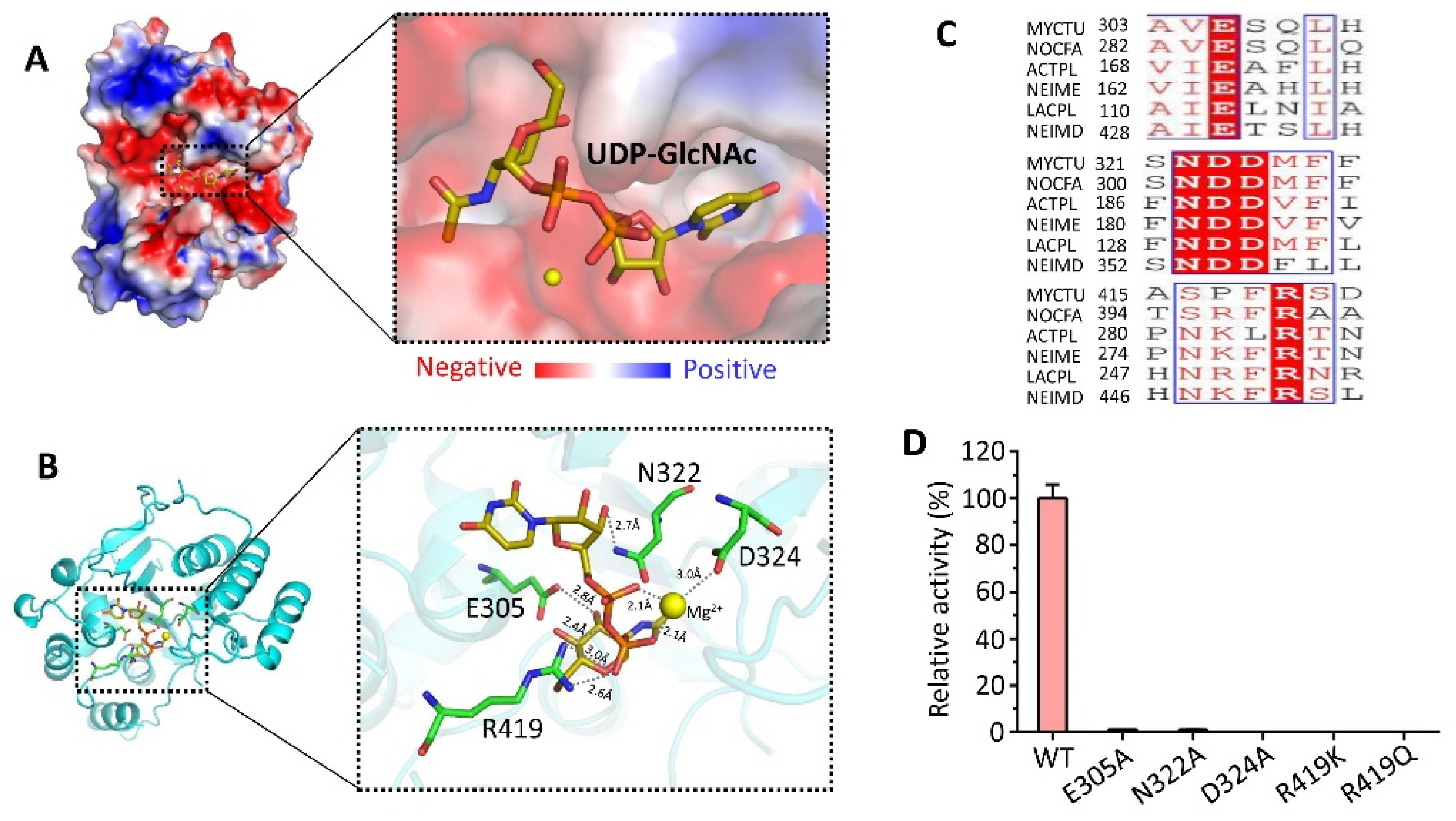
3.5. Substrate UDP-GlcNAc Bound to CpsY201−520
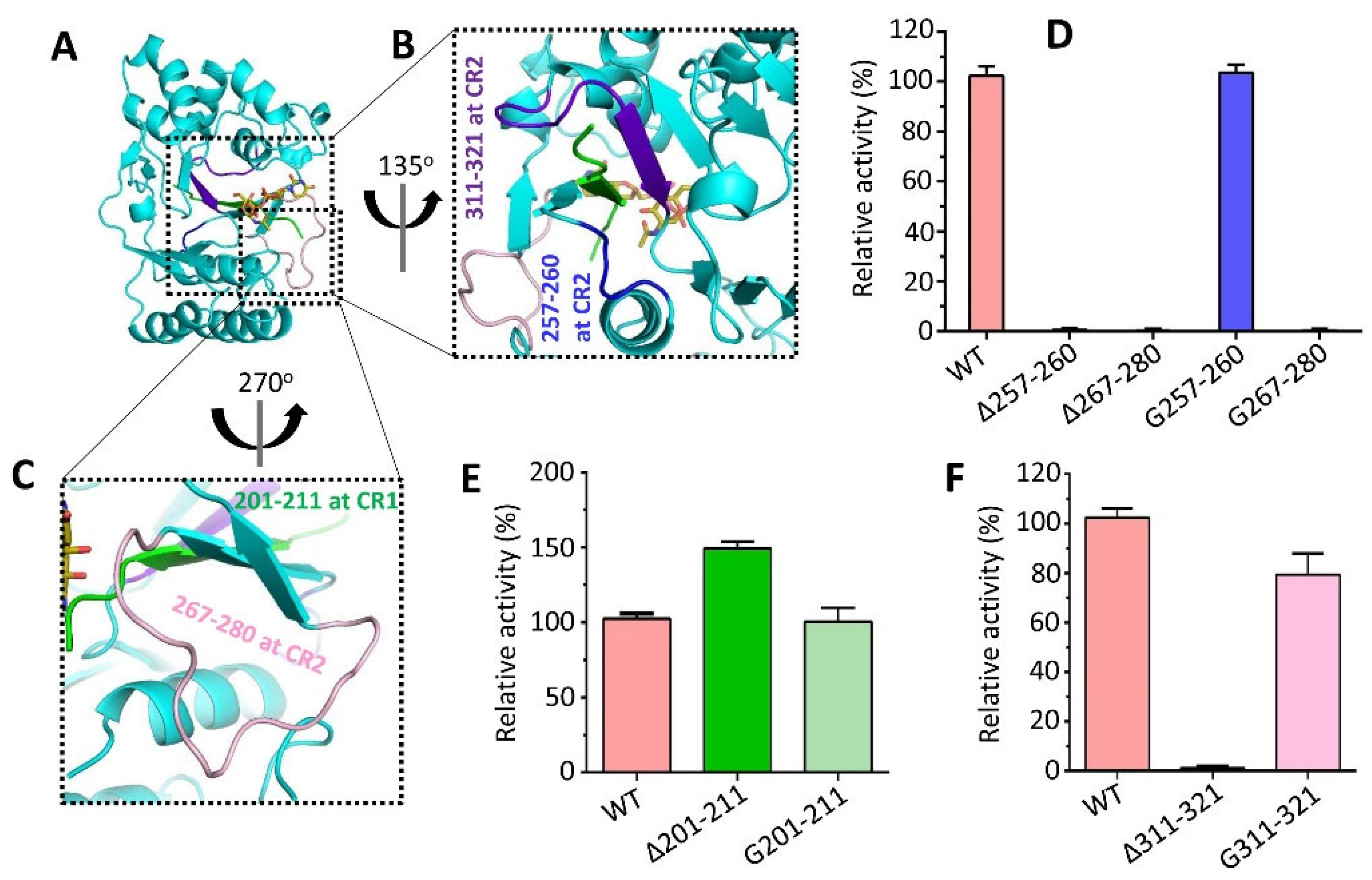
3.6. Conserved Regions Nearby Substrate Binding Site Affect the Activity of CpsY201−520
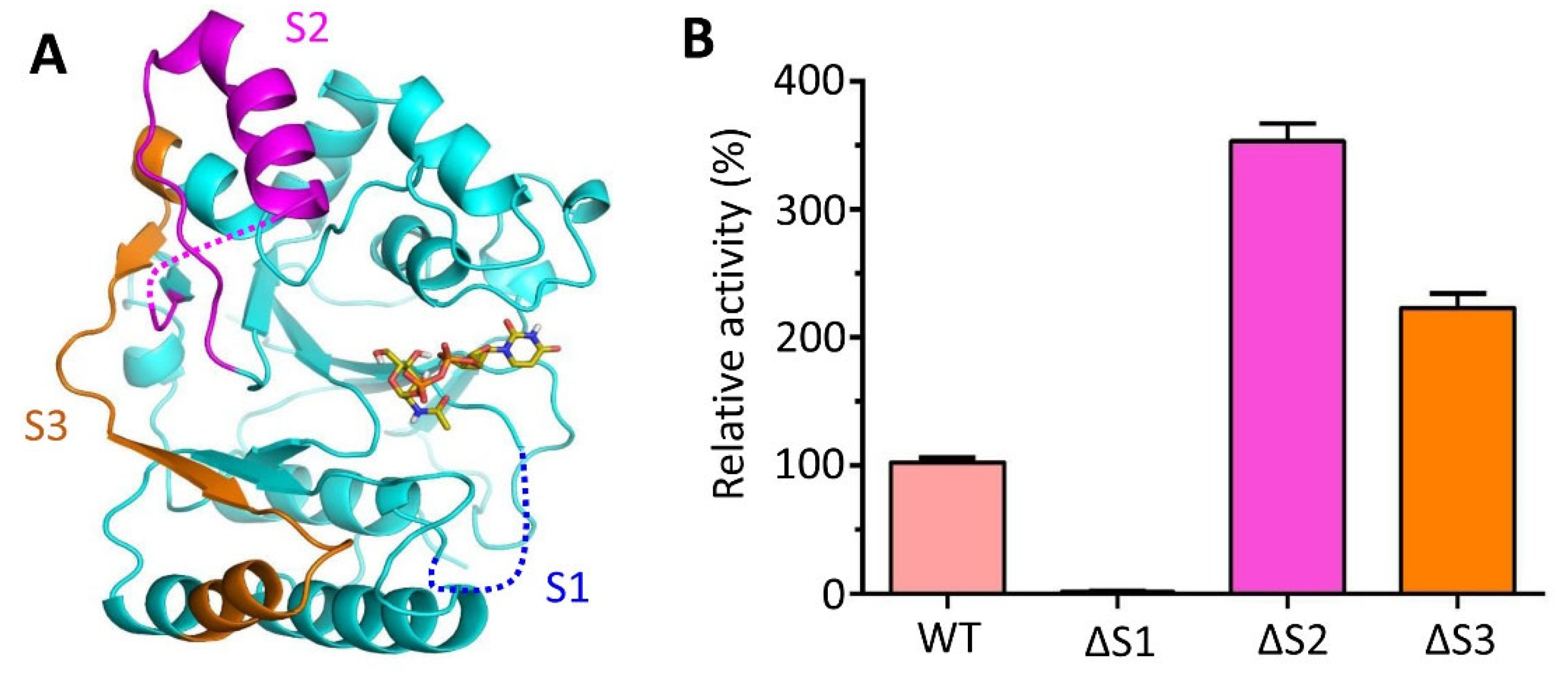
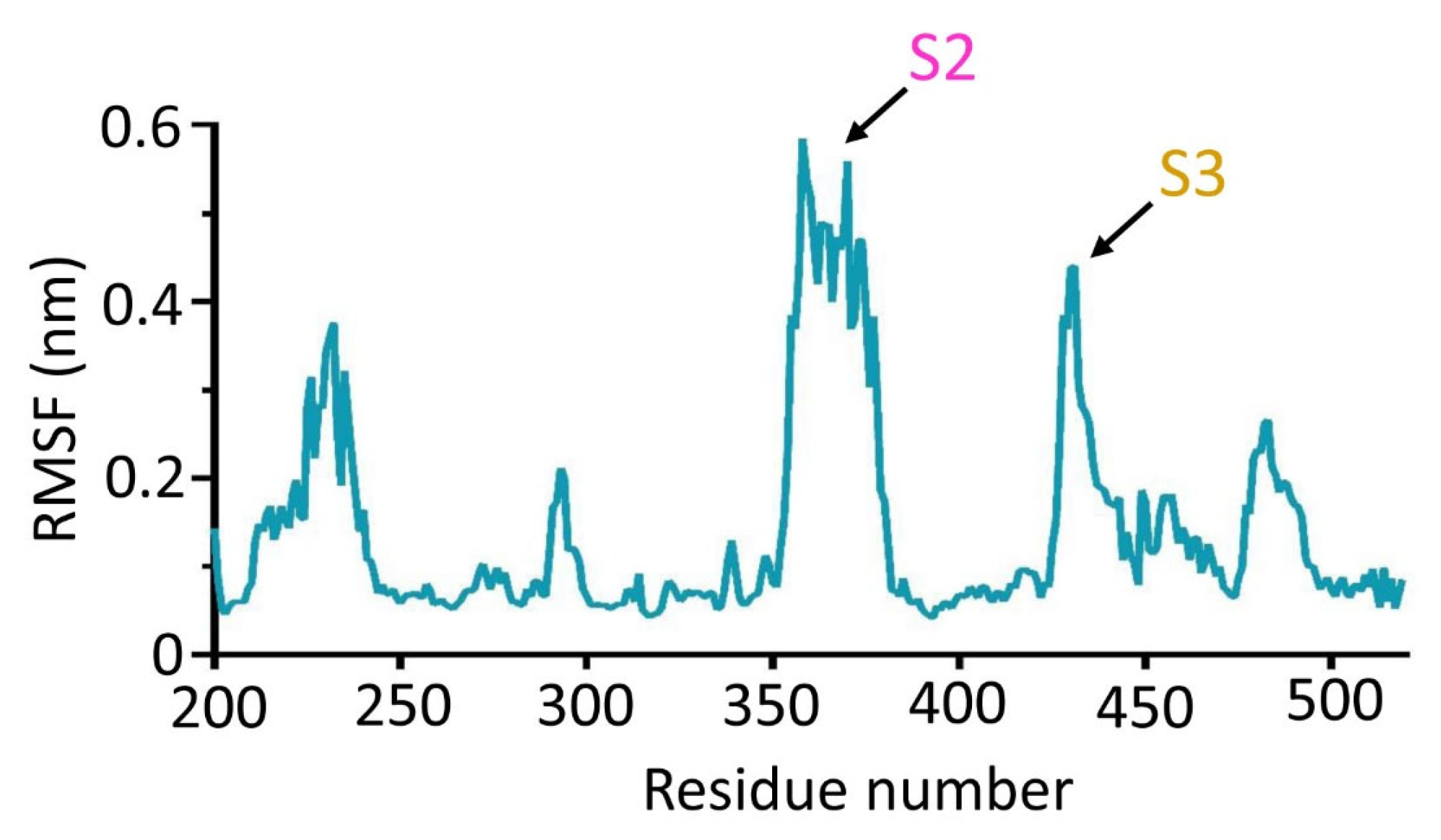
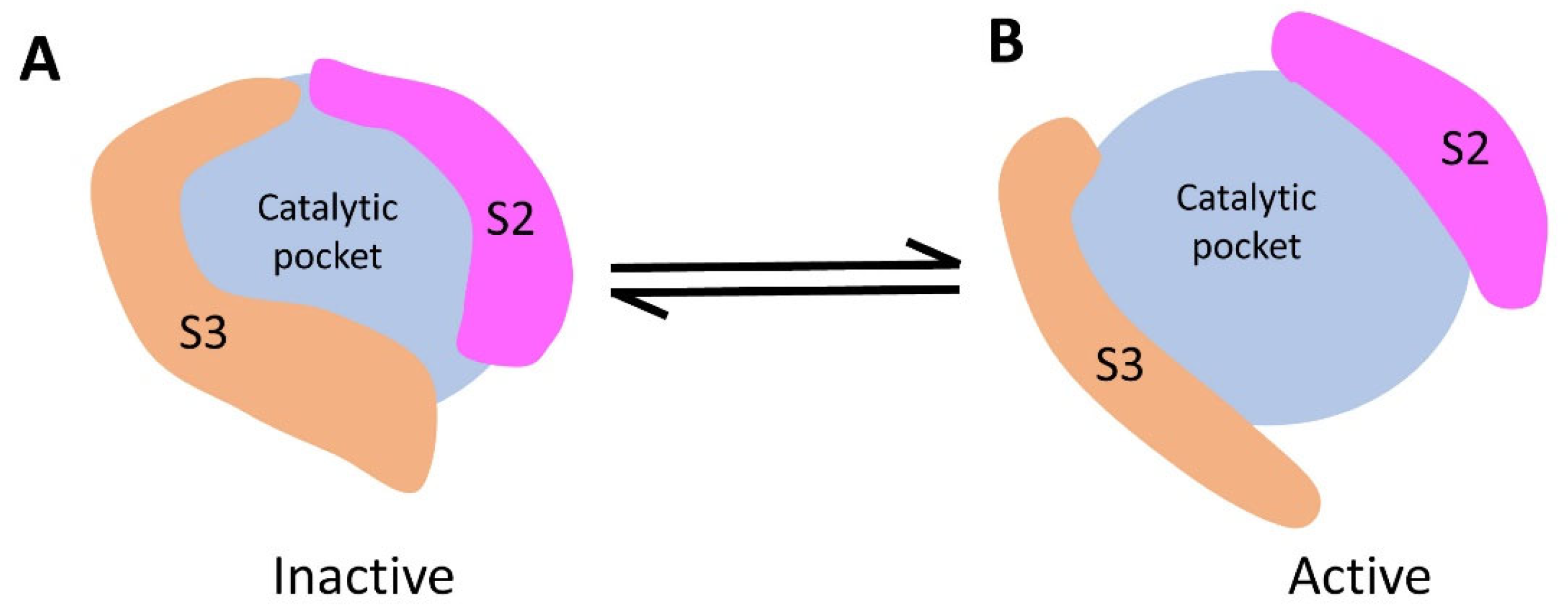
3.7. Deletion of Spacer Segments S2 and S3 Enhances the Catalytic Activity of CpsY201−520
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- Igbokwe, V.; Ruby, L.C.; Sultanli, A.; Belard, S. Post-tuberculosis sequelae in children and adolescents: A systematic review. Lancet Infect. Dis. 2023, 23, e138–e150. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.; Boeree, M.J.; Cambau, E.; Chesov, D.; Conradie, F.; Cox, V.; Dheda, K.; Dudnyk, A.; Farhat, M.R.; Gagneux, S.; et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: A 2023 TBnet/RESIST-TB consensus statement. Lancet Infect Dis 2023, 23, e122–e137. [Google Scholar] [CrossRef]
- Rustage, K.; Lobe, J.; Hayward, S.E.; Kristensen, K.L.; Margineanu, I.; Stienstra, Y.; Goletti, D.; Zenner, D.; Noori, T.; Pareek, M.; et al. Initiation and completion of treatment for latent tuberculosis infection in migrants globally: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Dhana, A.; Hamada, Y.; Kengne, A.P.; Kerkhoff, A.D.; Rangaka, M.X.; Kredo, T.; Baddeley, A.; Miller, C.; Singh, S.; Hanifa, Y.; et al. Tuberculosis screening among ambulatory people living with HIV: A systematic review and individual participant data meta-analysis. Lancet Infect. Dis. 2022, 22, 507–518. [Google Scholar] [CrossRef]
- Rule, R.; Mitton, B.; Govender, N.P.; Hoffmann, D.; Said, M. Spinal epidural abscess caused by Aspergillus spp masquerading as spinal tuberculosis in a person with HIV. Lancet Infect. Dis. 2021, 21, e356–e362. [Google Scholar] [CrossRef]
- Casanova, J.-L.; Abel, L. From rare disorders of immunity to common determinants of infection: Following the mechanistic thread. Cell 2022, 185, 3086–3103. [Google Scholar] [CrossRef]
- Dheda, K.; Perumal, T.; Moultrie, H.; Perumal, R.; Esmail, A.; Scott, A.J.; Udwadia, Z.; Chang, K.C.; Peter, J.; Pooran, A.; et al. The intersecting pandemics of tuberculosis and COVID-19: Population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir. Med. 2022, 10, 603–622. [Google Scholar] [CrossRef]
- Wallis, R.S.; O’Garra, A.; Sher, A.; Wack, A. Host-directed immunotherapy of viral and bacterial infections: Past, present and future. Nat. Rev. Immunol. 2022, 23, 121–133. [Google Scholar] [CrossRef]
- Swanson, R.V.; Gupta, A.; Foreman, T.W.; Lu, L.; Choreno-Parra, J.A.; Mbandi, S.K.; Rosa, B.A.; Akter, S.; Das, S.; Ahmed, M.; et al. Antigen-specific B cells direct T follicular-like helper cells into lymphoid follicles to mediate Mycobacterium tuberculosis control. Nat. Immunol. 2023, 24, 855–868. [Google Scholar] [CrossRef]
- Swanson, R.V. B cells and T follicular helper-like cells within lung granulomas are required for TB control. Nat. Immunol. 2023, 24, 753–754. [Google Scholar]
- Flynn, J.L.; Chan, J. Immune cell interactions in tuberculosis. Cell 2022, 185, 4682–4702. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.R.; Valainis, J.R.; Dubey, M.; Von Boehmer, L.; Sola, E.; Wilhelmy, J.; Guo, J.; Kask, O.; Ohanyan, M.; Sun, M.; et al. NK-like CD8+ γδ T cells are expanded in persistent Mycobacterium tuberculosis infection. Sci. Immunol. 2023, 8, eade3525. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Srikrishna, G.; Klein, S.L.; Bishai, W.R. Genetic and hormonal mechanisms underlying sex-specific immune responses in tuberculosis. Trends Immunol. 2022, 43, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef]
- Chai, Q.; Wang, L.; Liu, C.H.; Ge, B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell. Mol. Immunol. 2020, 17, 901–913. [Google Scholar] [CrossRef]
- Roberts, I.; Saunders, F.; Boulnois, G. Bacterial capsules and interactions with complement and phagocytes. Biochem. Soc. Trans. 1989, 17, 462–464. [Google Scholar] [CrossRef]
- Cross, A.S. The Biologic Significance of Bacterial Encapsulation. Curr. Top. Microbiol. Immunol. 1990, 150, 9. [Google Scholar]
- Moxon, E.R.; Kroll, J.S. The Role of Bacterial Polysaccharide Capsules as Virulence Factors. Curr. Top. Microbiol. Immunol. 1990, 150, 21. [Google Scholar]
- Daffe, M.; Marrakchi, H. Unraveling the Structure of the Mycobacterial Envelope. Microbiol. Spectr. 2019, 7, 4. [Google Scholar] [CrossRef]
- Daffé, M.; Crick, D.C.; Jackson, M.; Hatfull, G.F.; Jacobs, W.R., Jr. Genetics of Capsular Polysaccharides and Cell Envelope (Glyco)lipids. Microbiol. Spectr. 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.P.; Abeygunawardana, C.; Bush, C.A.O.; Cisar, J. The cell wall polysaccharide of Streptococcus gordonii 38: Structure and immunochemical comparison with the receptor polysaccharides of Streptococcus oralis 34 and Streptococcus mitis J22. Glycobiology 1994, 4, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Cisar, J.O.; Sandberg, A.L.; Reddy, G.P.; Abeygunawardana, C.; Bush, C.A. Structural and Antigenic Types of Cell Wall Polysaccharides from Viridans Group Streptococci with Receptors for Oral Actinomyces and Streptococcal Lectins. Infect. Immun. 1997, 65, 5035–5041. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.Q.; Thompson, J.; Cisar, J.O. Genetic loci for coaggregation receptor polysaccharide biosynthesis in Streptococcus gordonii 38. J. Bacteriol. 2003, 185, 5419–5430. [Google Scholar] [CrossRef]
- Krishnan, N.; Malaga, W.; Constant, P.; Caws, M.; Tran, T.H.; Salmons, J.; Nguyen, T.N.; Nguyen, D.B.; Daffe, M.; Young, D.B.; et al. Mycobacterium tuberculosis lineage influences innate immune response and virulence and is associated with distinct cell envelope lipid profiles. PLoS ONE 2011, 6, e23870. [Google Scholar] [CrossRef]
- Geurtsen, J.; Chedammi, S.; Mesters, J.; Cot, M.; Driessen, N.N.; Sambou, T.; Kakutani, R.; Ummels, R.; Maaskant, J.; Takata, H.; et al. Identification of mycobacterial alpha-glucan as a novel ligand for DC-SIGN: Involvement of mycobacterial capsular polysaccharides in host immune modulation. J. Immunol. 2009, 183, 5221–5231. [Google Scholar] [CrossRef]
- Jennings, H.J. Capsular polysaccharides as human vaccines. Adv. Carbohydr. Chem. Biochem. 1983, 41, 155–208. [Google Scholar]
- Dinadayala, P.; Lemassu, A.; Granovski, P.; Cerantola, S.; Winter, N.; Daffe, M. Revisiting the structure of the anti-neoplastic glucans of Mycobacterium bovis Bacille Calmette-Guerin. Structural analysis of the extracellular and boiling water extract-derived glucans of the vaccine substrains. J. Biol. Chem. 2004, 279, 12369–12378. [Google Scholar] [CrossRef]
- Lemassu, A.; Daffe, M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem. J. 1994, 297 Pt 2, 351–357. [Google Scholar] [CrossRef]
- Gill, S.C.; Von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzym. 1997, 276, 307–326. [Google Scholar]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67 Pt 4, 235–242. [Google Scholar] [CrossRef]
- Martinez-Ripoll, M.; Albert, A. Continuous development in macromolecular crystallography with CCP4. Acta Crystallogr. Sect. D Struct. Biol. 2023, 79, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Agirre, J.; Atanasova, M.; Bagdonas, H.; Ballard, C.B.; Baslé, A.; Beilsten-Edmands, J.; Borges, R.J.; Brown, D.G.; Burgos-Mármol, J.J.; Berrisford, J.M.; et al. The CCP4 suite: Integrative software for macromolecular crystallography. Acta Crystallogr. Sect. D Struct. Biol. 2023, 79, 449–461. [Google Scholar] [CrossRef]
- Ballard, C.; Roversi, P.; Walden, H. Molecular replacements. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 2165–2166. [Google Scholar] [CrossRef]
- Millán, C.; Keegan, R.M.; Pereira, J.; Sammito, M.D.; Simpkin, A.J.; McCoy, A.J.; Lupas, A.N.; Hartmann, M.D.; Rigden, D.J.; Read, R.J. Protein-structure prediction revolutionized. Nature 2021, 596, 487–488. [Google Scholar]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66 Pt 4, 486–501. [Google Scholar] [CrossRef]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 2012, 68 Pt 4, 352–367. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef]
- Liebschner, D.; Afonine, P.V.; Baker, M.L.; Bunkóczi, G.; Chen, V.B.; Croll, T.I.; Hintze, B.; Hung, L.-W.; Jain, S.; McCoy, A.J.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. Sect. D Struct. Biol. 2019, 75, 861–877. [Google Scholar] [CrossRef]
- Collaborative Computational Project. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 1994, 50, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, A.; Illes, K.; Bui, K.H.; Nagar, B. Structures of the mannose-6-phosphate pathway enzyme, GlcNAc-1-phosphotransferase. Proc. Natl. Acad. Sci. USA 2022, 119, e2203518119. [Google Scholar] [CrossRef]
- Qian, Y.; Lee, I.; Lee, W.S.; Qian, M.; Kudo, M.; Canfield, W.M.; Lobel, P.; Kornfeld, S. Functions of the alpha, beta, and gamma subunits of UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase. J. Biol. Chem. 2010, 285, 3360–3370. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Canfield, W.M. Structural Requirements for Efficient Processing and Activation of Recombinant Human UDP-N-acetylglucosamine:Lysosomal-enzyme-N-acetylglucosamine-1-phosphotransferase. J. Biol. Chem. 2006, 281, 11761–11768. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Elmendorf, B.J.; Booth, J.L.; Drake, R.R.; Canfield, W.M. Bovine UDP-N-acetylglucosamine:lysosomal-enzyme N-acetylglucosamine-1-phosphotransferase. II. Enzymatic characterization and identification of the catalytic subunit. J. Biol. Chem. 1996, 271, 31446–31451. [Google Scholar] [CrossRef][Green Version]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Deserno, M.; Holm, C. How to mesh up Ewald sums. I. A theoretical and numerical comparison of various particle mesh routines. J. Chem. Phys. 1998, 109, 7678–7693. [Google Scholar] [CrossRef]
- Deserno, M.; Holm, C. How to mesh up Ewald sums. II. An accurate error estimate for the particle–particle–particle-mesh algorithm. J. Chem. Phys. 1998, 109, 7694–7701. [Google Scholar] [CrossRef]
- Hoover, W.G.; Holian, B.L. Kinetic moments method for the canonical ensemble distribution. Phys. Lett. A 1996, 95, 5. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Bork, P.; Sperisen, P.; Schmid, C.D.; Bucher, P.; Zilian, O. Stealth Proteins: In Silico Identification of a Novel Protein Family Rendering Bacterial Pathogens Invisible to Host Immune Defense. PLoS Comput. Biol. 2005, 1, e63. [Google Scholar]
- Mawuenyega, K.G.; Forst, C.V.; Dobos, K.M.; Belisle, J.T.; Chen, J.; Bradbury, E.M.; Bradbury, A.R.M.; Chen, X. Mycobacterium tuberculosis Functional Network Analysis by Global Subcellular Protein Profiling. Mol. Biol. Cell 2005, 16, 396–404. [Google Scholar] [CrossRef]
- Holm, L. DALI and the persistence of protein shape. Protein Sci. 2020, 29, 128–140. [Google Scholar] [CrossRef]
- Waheed, A.; Hasilik, A.; von Figura, K. UDP-N-acetylglucosamine:lysosomal enzyme precursor N-acetylglucosamine-1-phosphotransferase. Partial purification and characterization of the rat liver Golgi enzyme. J. Biol. Chem. 1982, 257, 12322–12331. [Google Scholar] [CrossRef]
- Coutinho, M.F.; Santos Lda, S.; Girisha, K.M.; Satyamoorthy, K.; Lacerda, L.; Prata, M.J.; Alves, S. Mucolipidosis type II alpha/beta with a homozygous missense mutation in the GNPTAB gene. Am. J. Med. Genet. A 2012, 158, 1225–1228. [Google Scholar] [CrossRef]
- Danyukova, T.; Ludwig, N.F.; Velho, R.V.; Harms, F.L.; Gunes, N.; Tidow, H.; Schwartz, I.V.; Tuysuz, B.; Pohl, S. Combined in vitro and in silico analyses of missense mutations in GNPTAB provide new insights into the molecular bases of mucolipidosis II and III alpha/beta. Hum. Mutat. 2020, 41, 133–139. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Z.; Wang, Y.; Yang, F.; Wang, J.; Huang, W.; Qin, J.; Tian, M.; Cai, X.; Liu, X.; et al. Cryo-EM structures of human GMPPA-GMPPB complex reveal how cells maintain GDP-mannose homeostasis. Nat. Struct. Mol. Biol. 2021, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lee, W.-S.; Doray, B.; Kornfeld, S. Engineering of GlcNAc-1-Phosphotransferase for Production of Highly Phosphorylated Lysosomal Enzymes for Enzyme Replacement Therapy. Mol. Ther. Methods Clin. Dev. 2017, 5, 59–65. [Google Scholar] [CrossRef] [PubMed]

| CpsY201−520 (PDB Code: 8J2N) | |
|---|---|
| Data Collection | |
| Wavelength (Å) | 0.987 |
| Resolution range (Å) | 26.10–1.64 (1.68–1.64) |
| Space group | P21 |
| Unit cell (a, b, c) (Å) | 79.88, 41.73, 100.44 |
| Unit cell (α, β, γ) (o) | 90.00, 93.64, 90.00 |
| R-merge | 0.079 (1.133) |
| Multiplicity | 6.7 (6.8) |
| Completeness (%) | 99.9 (99.9) |
| Mean I/sigma(I) a | 13.8 (1.7) |
| CC1/2 | 0.999 (0.720) |
| Total number of observations | 547,798 (40,537) |
| Total number unique | 81,599 (5998) |
| Refinement | |
| Resolution (Å) | 26.10–1.64 |
| Reflections used for R-free | 4075 (4.99%) |
| R-work (%) | 20.28 |
| R-free (%) | 22.62 |
| Wilson B-factor (Å2) | 18.9 |
| Anisotropy | 0.427 |
| RMS lengths (Å) b | 0.0066 |
| RMS angles (o) b | 0.852 |
| Clashscore | 1.5 |
| Ramachandran outliers (%) | 0.00 |
| Ramachandran favored (%) | 97.96 |
| Rotamer outlier (%) | 0.00 |
| No. of atoms | 4255 |
| Construct | UDP-GlcNAc | Glucan | ||
|---|---|---|---|---|
| Km (μM) | Kcat (min−1) | Km (μM) | Kcat (min−1) | |
| CpsY | 25.4 ± 2.1 | 4.3 ± 0.4 | 830.4 ± 29.7 | 23.3 ± 1.2 |
| CpsY201−520 | 26.3 ± 1.9 | 11.2 ± 0.3 | 463.6 ± 11.8 | 67.5 ± 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Yuan, C.; Guo, C.; Huang, M.; Lin, D. Structural and Functional Insights into the Stealth Protein CpsY of Mycobacterium tuberculosis. Biomolecules 2023, 13, 1611. https://doi.org/10.3390/biom13111611
Liu D, Yuan C, Guo C, Huang M, Lin D. Structural and Functional Insights into the Stealth Protein CpsY of Mycobacterium tuberculosis. Biomolecules. 2023; 13(11):1611. https://doi.org/10.3390/biom13111611
Chicago/Turabian StyleLiu, Dafeng, Cai Yuan, Chenyun Guo, Mingdong Huang, and Donghai Lin. 2023. "Structural and Functional Insights into the Stealth Protein CpsY of Mycobacterium tuberculosis" Biomolecules 13, no. 11: 1611. https://doi.org/10.3390/biom13111611
APA StyleLiu, D., Yuan, C., Guo, C., Huang, M., & Lin, D. (2023). Structural and Functional Insights into the Stealth Protein CpsY of Mycobacterium tuberculosis. Biomolecules, 13(11), 1611. https://doi.org/10.3390/biom13111611






