The Effects of Deregulated Ribosomal Biogenesis in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Ribosomes in Cancer Pathogenesis
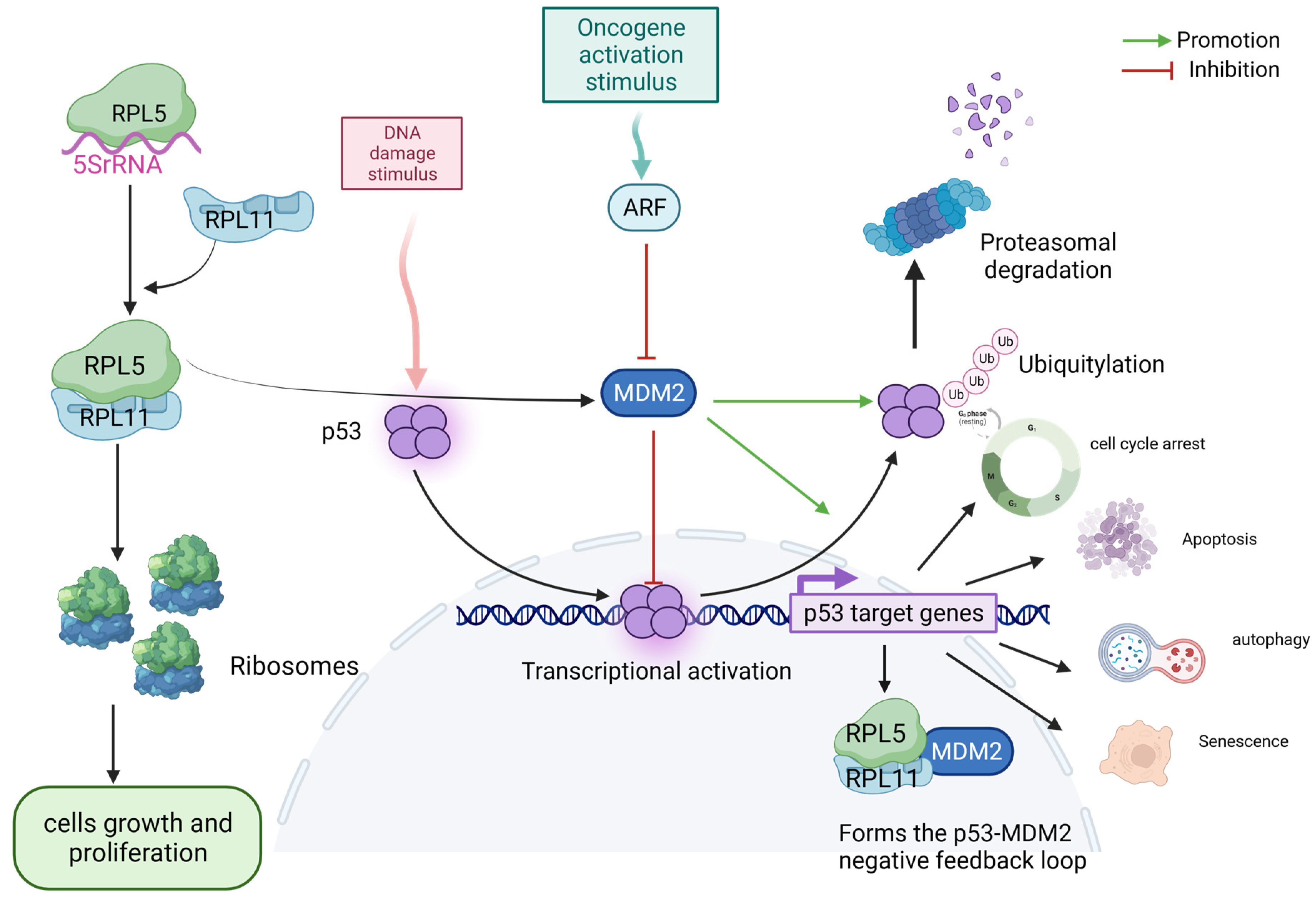
3. RP Gene Deletions Increase Susceptibility to Cancer
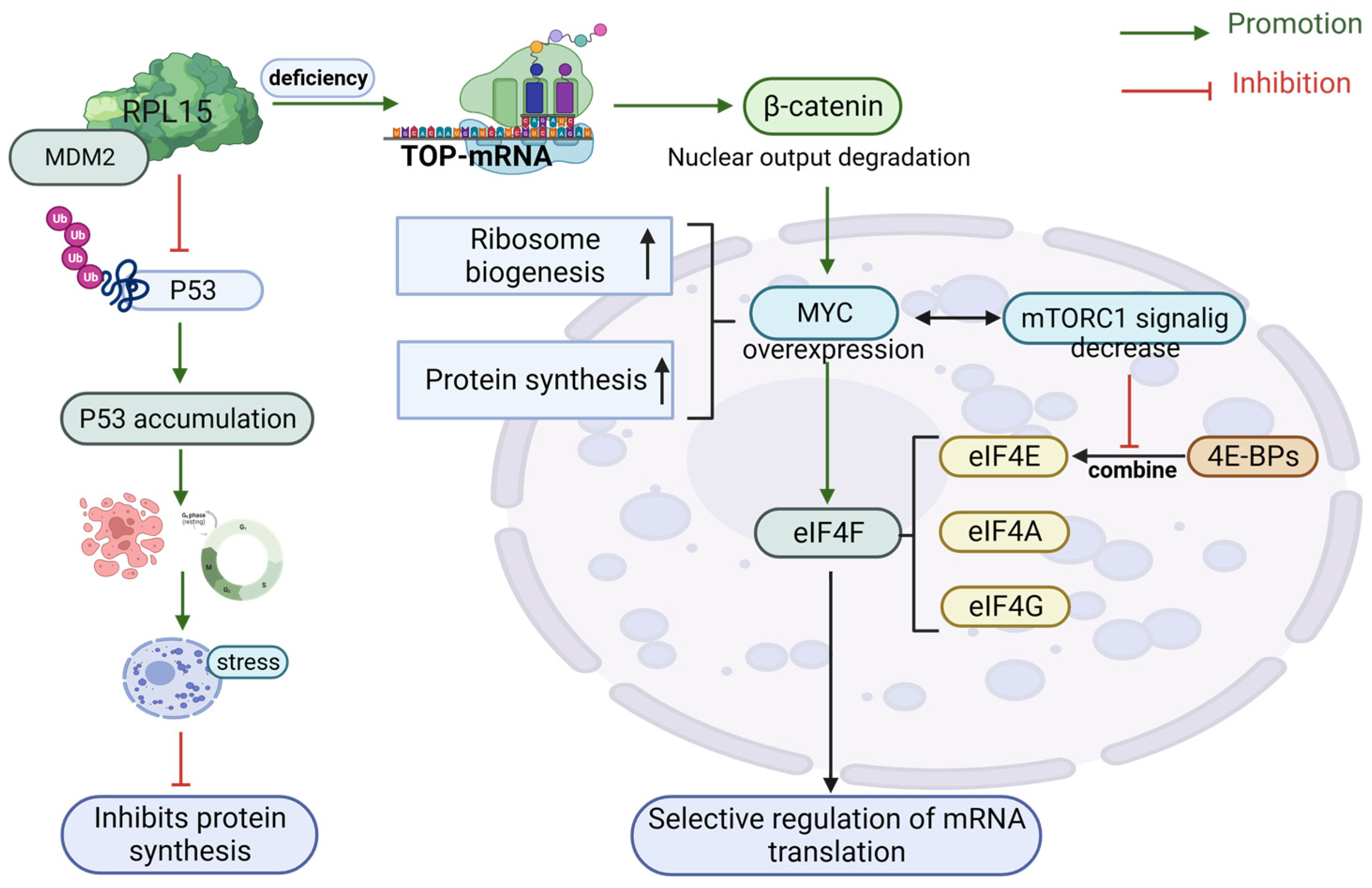
4. Translation and Transcription in RiBi and the Link to p53
5. The 5S RNP–MDM2–p53 IRBC Pathway May Provide a Barrier to the Development of Cancer
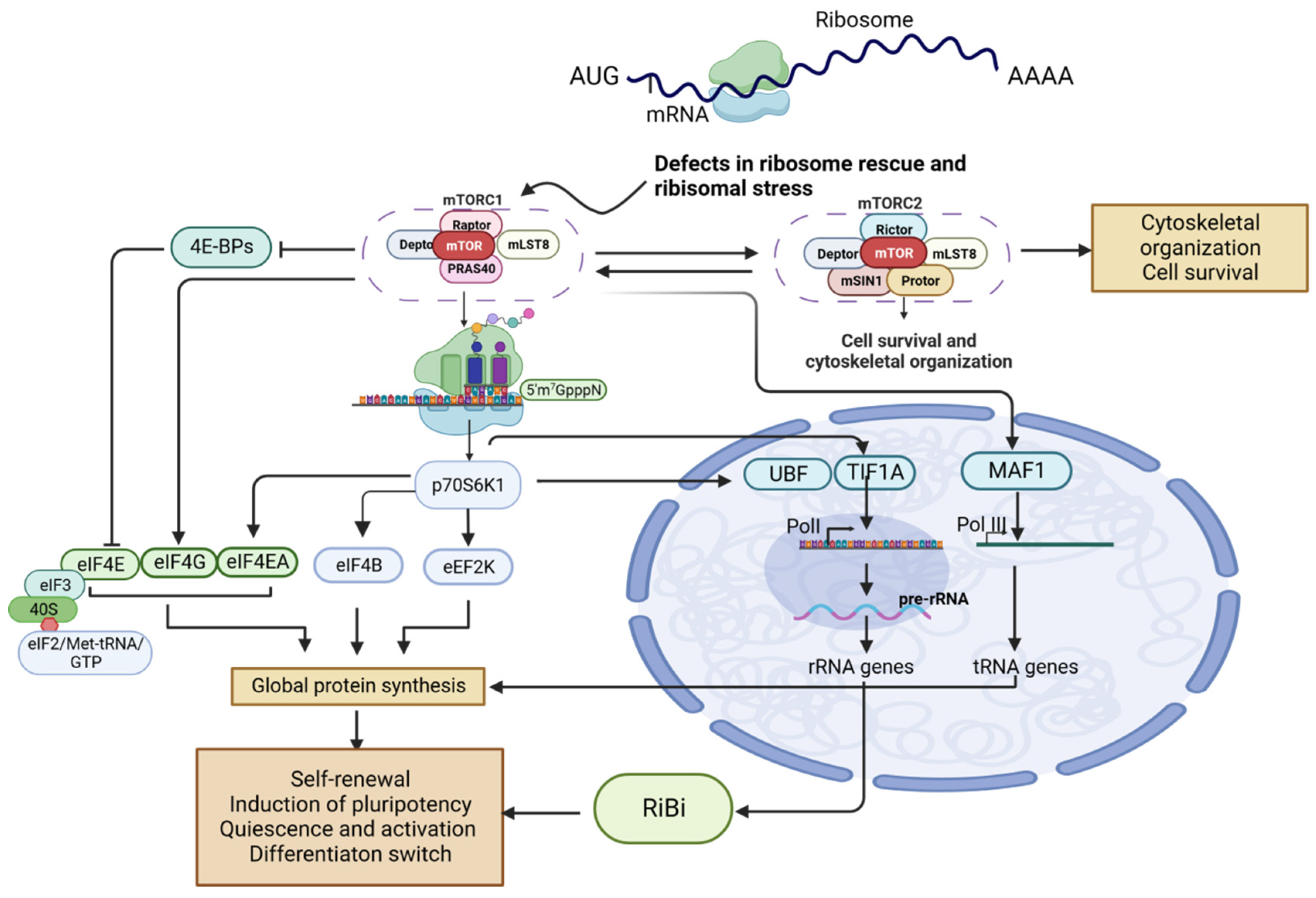
6. mTOR Regulation of RiBi
6.1. mTORC1-Regulated RiBi Is Involved with Tissue Regeneration and Cancer Development
6.2. mTORC1-Directed RiBi Influences Cancer Stem Cell Function
6.3. MYC-Induced Impaired IRBC May Be a Potential Target for Cancer Therapy
6.3.1. Antagonizing RiBi Effects of c-MYC in Cancer
6.3.2. MYC Drives Metabolic Redistribution in Cancer
7. Beta-Catenin and RiBi
8. RiBi Selective Inhibitor
9. Future Perspectives Regarding Activating Nucleolar Stress in Cancer Therapy
Author Contributions
Funding
Conflicts of Interest
References
- Norris, K.; Hopes, T.; Aspden, J.L. Ribosome heterogeneity and specialization in development. Wiley Interdiscip. Rev. RNA 2021, 12, e1644. [Google Scholar] [CrossRef]
- Zolotenkova, E.A.; Gopanenko, A.V.; Tupikin, A.E.; Kabilov, M.R.; Malygin, A.A. Mutation at the Site of Hydroxylation in the Ribosomal Protein uL15 (RPL27a) Causes Specific Changes in the Repertoire of mRNAs Translated in Mammalian Cells. Int. J. Mol. Sci. 2023, 24, 6173. [Google Scholar] [CrossRef] [PubMed]
- Lafita-Navarro, M.; Conacci-Sorrell, M. Nucleolar stress: From development to cancer. Semin. Cell Dev. Biol. 2023, 136, 64–74. [Google Scholar] [CrossRef]
- Elhamamsy, A.R.; Metge, B.J.; Alsheikh, H.A.; Shevde, L.A.; Samant, R.S. Ribosome Biogenesis: A Central Player in Cancer Metastasis and Therapeutic Resistance. Cancer Res. 2022, 82, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Baßler, J.; Hurt, E. Eukaryotic Ribosome Assembly. Annu. Rev. Biochem. 2019, 88, 281–306. [Google Scholar] [CrossRef]
- Gilles, A.; Frechin, L.; Natchiar, K.; Biondani, G.; von Loeffelholz, O.; Holvec, S.; Malaval, J.-L.; Winum, J.-Y.; Klaholz, B.P.; Peyron, J.-F. Targeting the Human 80S Ribosome in Cancer: From Structure to Function and Drug Design for Innovative Adjuvant Therapeutic Strategies. Cells 2020, 9, 629. [Google Scholar] [CrossRef]
- Klinge, S.; Woolford, J.L., Jr. Ribosome assembly coming into focus. Nat. Rev. Mol. Cell Biol. 2019, 20, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.G.; Sibai, D.S.; Valère, M.; Mars, J.-C.; Lessard, F.; Hori, R.T.; Khan, M.M.; Stefanovsky, V.Y.; LeDoux, M.S.; Moss, T. Ribosomal DNA promoter recognition is determined in vivo by cooperation between UBTF1 and SL1 and is compromised in the UBTF-E210K neuroregression syndrome. PLoS Genet. 2022, 18, e1009644. [Google Scholar] [CrossRef]
- Gibbons, J.G.; Branco, A.T.; Godinho, S.A.; Yu, S.; Lemos, B. Concerted copy number variation balances ribosomal DNA dosage in human and mouse genomes. Proc. Natl. Acad. Sci. USA 2015, 112, 2485–2490. [Google Scholar] [CrossRef]
- Dörner, K.; Ruggeri, C.; Zemp, I.; Kutay, U. Ribosome biogenesis factors—From names to functions. EMBO J. 2023, 42, e112699. [Google Scholar] [CrossRef]
- Siemund, A.L.; Hanewald, T.; Kowarz, E.; Marschalek, R. MLL-AF4 and a murinized pSer-variant thereof are turning on the nucleolar stress pathway. Cell Biosci. 2022, 12, 47. [Google Scholar] [CrossRef]
- Friedrich, J.K.; Panov, K.I.; Cabart, P.; Russell, J.; Zomerdijk, J.C.B.M. TBP-TAF Complex SL1 Directs RNA Polymerase I Pre-initiation Complex Formation and Stabilizes Upstream Binding Factor at the rDNA Promoter. J. Biol. Chem. 2005, 280, 29551–29558. [Google Scholar] [CrossRef]
- McCool, M.A.; Bryant, C.J.; Baserga, S.J. MicroRNAs and long non-coding RNAs as novel regulators of ribosome biogenesis. Biochem. Soc. Trans. 2020, 48, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Kopp, K.; Gasiorowski, J.Z.; Chen, D.; Gilmore, R.; Norton, J.T.; Wang, C.; Leary, D.J.; Chan, E.; Dean, D.A.; Huang, S.; et al. Pol I Transcription and Pre-rRNA Processing Are Coordinated in a Transcription-dependent Manner in Mammalian Cells. Mol. Biol. Cell 2007, 18, 394–403. [Google Scholar] [CrossRef]
- Larson, D.E.; Zahradka, P.; Sells, B.H. Control points in eucaryotic ribosome biogenesis. Biochem. Cell Biol. 1991, 69, 5–22. [Google Scholar] [CrossRef]
- Yamada, S.; Kitai, Y.; Tadokoro, T.; Takahashi, R.; Shoji, H.; Maemoto, T.; Ishiura, M.; Muromoto, R.; Kashiwakura, J.-I.; Ishii, K.J.; et al. Identification of RPL15 60S Ribosomal Protein as a Novel Topotecan Target Protein That Correlates with DAMP Secretion and Antitumor Immune Activation. J. Immunol. 2022, 209, 171–179. [Google Scholar] [CrossRef]
- Pelletier, J.; Sonenberg, N. The Organizing Principles of Eukaryotic Ribosome Recruitment. Annu. Rev. Biochem. 2019, 88, 307–335. [Google Scholar] [CrossRef]
- Davuluri, G.; Giusto, M.; Chandel, R.; Welch, N.; Alsabbagh, K.; Kant, S.; Kumar, A.; Kim, A.; Gangadhariah, M.; Ghosh, P.K.; et al. Impaired Ribosomal Biogenesis by Noncanonical Degradation of β-Catenin during Hyperammonemia. Mol. Cell Biol. 2019, 39, e00451-18. [Google Scholar] [CrossRef]
- Orsolic, I.; Jurada, D.; Pullen, N.; Oren, M.; Eliopoulos, A.G.; Volarevic, S. The relationship between the nucleolus and cancer: Current evidence and emerging paradigms. Semin. Cancer Biol. 2016, 37–38, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Liu, Y.; Yu, X.-Y.; Pan, X.; Zhang, Y.; Tu, J.; Song, Y.-H.; Li, Y. Ribosome biogenesis in disease: New players and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 15. [Google Scholar] [CrossRef]
- Parker, M.D.; Karbstein, K. Quality control ensures fidelity in ribosome assembly and cellular health. J. Cell Biol. 2023, 222, e202209115. [Google Scholar] [CrossRef] [PubMed]
- Sulima, S.O.; Kampen, K.R.; De Keersmaecker, K. Cancer Biogenesis in Ribosomopathies. Cells 2019, 8, 229. [Google Scholar] [CrossRef]
- Danilova, N.; Sakamoto, K.M.; Lin, S. Ribosomal protein S19 deficiency in zebrafish leads to developmental abnormalities and defective erythropoiesis through activation of p53 protein family. Blood 2008, 112, 5228–5237. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.D.; Esser, K.A. Wnt/β-catenin signaling activates growth-control genes during overload-induced skeletal muscle hypertrophy. Am. J. Physiol. Cell Physiol. 2005, 289, C853–C859. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Liu, Z. RPL15 promotes hepatocellular carcinoma progression via regulation of RPs-MDM2-p53 signaling pathway. Cancer Cell Int. 2022, 22, 150. [Google Scholar] [CrossRef]
- Farooqee, S.B.U.D.; Christie, J.; Venkatraman, P. PSMD9 ribosomal protein network maintains nucleolar architecture and WT p53 levels. Biochem. Biophys. Res. Commun. 2021, 563, 105–112. [Google Scholar] [CrossRef]
- Ban, N.; Beckmann, R.; Cate, J.H.D.; Dinman, J.D.; Dragon, F.; Ellis, S.R.; Lafontaine, D.L.J.; Lindahl, L.; Liljas, A.; Lipton, J.M.; et al. A new system for naming ribosomal proteins. Curr. Opin. Struct. Biol. 2014, 24, 165–169. [Google Scholar] [CrossRef]
- Fabbri, L.; Chakraborty, A.; Robert, C.; Vagner, S. The plasticity of mRNA translation during cancer progression and therapy resistance. Nat. Rev. Cancer 2021, 21, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, K.; Colis, L.; Liu, H.; Jäämaa, S.; Moore, H.M.; Enbäck, J.; Laakkonen, P.; Vaahtokari, A.; Jones, R.J.; Hällström, T.M.A.; et al. Identification of Novel p53 Pathway Activating Small-Molecule Compounds Reveals Unexpected Similarities with Known Therapeutic Agents. PLoS ONE 2010, 5, e12996. [Google Scholar] [CrossRef]
- Blagotinšek, K.; Rozman, D. Targeting Signalling Pathways in Hepatocellular carcinoma. Curr. Pharm. Des. 2016, 22, 170–175. [Google Scholar] [CrossRef]
- Kang, C.-W.; Blackburn, A.C.; Loh, A.H.P.; Hong, K.C.; Goh, J.Y.; Hein, N.; Drygin, D.; Parish, C.R.; Hannan, R.D.; Hannan, K.M.; et al. Targeting RNA Polymerase I Transcription Activity in Osteosarcoma: Pre-Clinical Molecular and Animal Treatment Studies. Biomedicines 2023, 11, 1133. [Google Scholar] [CrossRef]
- Bustelo, X.R.; Dosil, M. Ribosome biogenesis and cancer: Basic and translational challenges. Curr. Opin. Genet. Dev. 2018, 48, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Hannan, K.M.; Soo, P.; Wong, M.S.; Lee, J.K.; Hein, N.; Poh, P.; Wysoke, K.D.; Williams, T.D.; Montellese, C.; Smith, L.K.; et al. Nuclear stabilization of p53 requires a functional nucleolar surveillance pathway. Cell Rep. 2022, 41, 111571. [Google Scholar] [CrossRef]
- Maracci, C.; Motta, S.; Romagnoli, A.; Costantino, M.; Perego, P.; Di Marino, D. The mTOR/4E-BP1/eIF4E Signalling Pathway as a Source of Cancer Drug Targets. Curr. Med. Chem. 2022, 29, 3501–3529. [Google Scholar] [CrossRef]
- Yusupova, G.; Yusupov, M. High-Resolution Structure of the Eukaryotic 80S Ribosome. Annu. Rev. Biochem. 2014, 83, 467–486. [Google Scholar] [CrossRef]
- Bartish, M.; Abraham, M.J.; Gonçalves, C.; Larsson, O.; Rolny, C.; del Rincón, S.V. The role of eIF4F-driven mRNA translation in regulating the tumour microenvironment. Nat. Rev. Cancer 2023, 23, 408–425. [Google Scholar] [CrossRef]
- Destefanis, F.; Manara, V.; Bellosta, P. Myc as a Regulator of Ribosome Biogenesis and Cell Competition: A Link to Cancer. Int. J. Mol. Sci. 2020, 21, 4037. [Google Scholar] [CrossRef]
- Lin, C.-J.; Cencic, R.; Mills, J.R.; Robert, F.; Pelletier, J. c-Myc and eIF4F Are Components of a Feedforward Loop that Links Transcription and Translation. Cancer Res. 2008, 68, 5326–5334. [Google Scholar] [CrossRef]
- Cheng, Z.; Mugler, C.F.; Keskin, A.; Hodapp, S.; Chan, L.Y.-L.; Weis, K.; Mertins, P.; Regev, A.; Jovanovic, M.; Brar, G.A. Small and Large Ribosomal Subunit Deficiencies Lead to Distinct Gene Expression Signatures that Reflect Cellular Growth Rate. Mol. Cell 2018, 73, 36–47.e10. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, I.; Bursać, S.; Jurada, D.; Hofman, I.D.; Dembić, Z.; Bartek, J.; Mihalek, I.; Volarević, S. Cancer-associated mutations in the ribosomal protein L5 gene dysregulate the HDM2/p53-mediated ribosome biogenesis checkpoint. Oncogene 2020, 39, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Ajore, R.; Raiser, D.; McConkey, M.; Jöud, M.; Boidol, B.; Mar, B.; Saksena, G.; Weinstock, D.M.; Armstrong, S.; Ellis, S.R.; et al. Deletion of ribosomal protein genes is a common vulnerability in human cancer, especially in concert with TP 53 mutations. EMBO Mol. Med. 2017, 9, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Uechi, T.; Kenmochi, N. Guarding the ‘translation apparatus’: Defective ribosome biogenesis and the p53 signaling pathway. Wiley Interdiscip. Rev. RNA 2011, 2, 507–522. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.-Q.; Li, F.-Q.; Chen, J.-N.; Gong, X.; Cao, B.-B.; Wang, W. Triptolide interrupts rRNA synthesis and induces the RPL23-MDM2-p53 pathway to repress lung cancer cells. Oncol. Rep. 2020, 43, 1863–1874. [Google Scholar] [CrossRef]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef]
- Fancello, L.; Kampen, K.R.; Hofman, I.J.; Verbeeck, J.; De Keersmaecker, K. The ribosomal protein gene RPL5 is a haploinsufficient tumor suppressor in multiple cancer types. Oncotarget 2017, 8, 14462–14478. [Google Scholar] [CrossRef]
- Kiparaki, M.; Baker, N.E. Ribosomal protein mutations and cell competition: Autonomous and nonautonomous effects on a stress response. Genetics 2023, 224, iyad080. [Google Scholar] [CrossRef] [PubMed]
- Seithera, P.; Ibena, S.; Grummta, I. Mammalian RNA polymerase I exists as a holoenzyme with associated basal transcription factors. J. Mol. Biol. 1998, 275, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.P.; Galbraith, M.D.; Eduthan, N.P.; Hill, A.A.; Clay, M.R.; Tellez, C.M.; Wilky, B.A.; Elias, A.; Espinosa, J.M.; Sullivan, K.D. Proteasome Inhibition Sensitizes Liposarcoma to MDM2 Inhibition with Nutlin-3 by Activating the ATF4/CHOP Stress Response Pathway. Cancer Res. 2023, 83, 2543–2556. [Google Scholar] [CrossRef]
- James, A.; Wang, Y.; Raje, H.; Rosby, R.; DiMario, P. Nucleolar stress with and without p53. Nucleus 2014, 5, 402–426. [Google Scholar] [CrossRef]
- McNamar, R.; Abu-Adas, Z.; Rothblum, K.; Knutson, B.A.; Rothblum, L.I. Conditional depletion of the RNA polymerase I subunit PAF53 reveals that it is essential for mitosis and enables identification of functional domains. J. Biol. Chem. 2019, 294, 19907–19922. [Google Scholar] [CrossRef]
- Nguyen, T.; Mills, J.C.; Cho, C.J. The coordinated management of ribosome and translation during injury and regeneration. Front. Cell Dev. Biol. 2023, 11, 1186638. [Google Scholar] [CrossRef]
- Jones, N.C.; Lynn, M.L.; Gaudenz, K.; Sakai, D.; Aoto, K.; Rey, J.-P.; Glynn, E.F.; Ellington, L.; Du, C.; Dixon, J.; et al. Prevention of the Neurocristopathy Treacher Collins Syndrome through Inhibition of P53 Function. Nat. Med. 2008, 14, 125–133. [Google Scholar] [CrossRef]
- Abe, Y.; Oda-Sato, E.; Tobiume, K.; Kawauchi, K.; Taya, Y.; Okamoto, K.; Oren, M.; Tanaka, N. Hedgehog signaling overrides p53-mediated tumor suppression by activating Mdm2. Proc. Natl. Acad. Sci. USA 2008, 105, 4838–4843. [Google Scholar] [CrossRef]
- Angrisani, A.; Di Fiore, A.; Di Trani, C.A.; Fonte, S.; Petroni, M.; Severini, L.L.; Bordin, F.; Belloni, L.; Ferretti, E.; Canettieri, G.; et al. Specific Protein 1 and p53 Interplay Modulates the Expression of the KCTD-Containing Cullin3 Adaptor Suppressor of Hedgehog 2. Front. Cell Dev. Biol. 2021, 9, 638508. [Google Scholar] [CrossRef] [PubMed]
- Doheny, D.; Manore, S.G.; Wong, G.L.; Lo, H.-W. Hedgehog Signaling and Truncated GLI1 in Cancer. Cells 2020, 9, 2114. [Google Scholar] [CrossRef]
- Pietrobono, S.; Gagliardi, S.; Stecca, B. Non-canonical Hedgehog Signaling Pathway in Cancer: Activation of GLI Transcription Factors Beyond Smoothened. Front. Genet. 2019, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Lindström, M.S.; Bartek, J.; Maya-Mendoza, A. p53 at the crossroad of DNA replication and ribosome biogenesis stress pathways. Cell Death Differ. 2022, 29, 972–982. [Google Scholar] [CrossRef]
- Cao, P.; Yang, A.; Li, P.; Xia, X.; Han, Y.; Zhou, G.; Wang, R.; Yang, F.; Li, Y.; Zhang, Y.; et al. Genomic gain of RRS1 promotes hepatocellular carcinoma through reducing the RPL11-MDM2-p53 signaling. Sci. Adv. 2021, 7, eabf4304. [Google Scholar] [CrossRef] [PubMed]
- Tcherkezian, J.; Cargnello, M.; Romeo, Y.; Huttlin, E.L.; Lavoie, G.; Gygi, S.P.; Roux, P.P. Proteomic analysis of cap-dependent translation identifies LARP1 as a key regulator of 5′TOP mRNA translation. Genes. Dev. 2014, 28, 357–371. [Google Scholar] [CrossRef]
- Turi, Z.; Senkyrikova, M.; Mistrik, M.; Bartek, J.; Moudry, P. Perturbation of RNA Polymerase I transcription machinery by ablation of HEATR1 triggers the RPL5/RPL11-MDM2-p53 ribosome biogenesis stress checkpoint pathway in human cells. Cell Cycle 2017, 17, 92–101. [Google Scholar] [CrossRef]
- He, S.; Ma, X.; Ye, Y.; Zhang, M.; Zhuang, J.; Song, Y.; Xia, W. HEATR1 modulates cell survival in non-small cell lung cancer via activation of the p53/PUMA signaling pathway. Onco. Targets Ther. 2019, 12, 4001–4011. [Google Scholar] [CrossRef] [PubMed]
- Bursać, S.; Brdovčak, M.C.; Pfannkuchen, M.; Orsolić, I.; Golomb, L.; Zhu, Y.; Katz, C.; Daftuar, L.; Grabušić, K.; Vukelić, I.; et al. Mutual protection of ribosomal proteins L5 and L11 from degradation is essential for p53 activation upon ribosomal biogenesis stress. Proc. Natl. Acad. Sci. USA 2012, 109, 20467–20472. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pagliara, V.; Albano, F.; Esposito, D.; Sagar, V.; Loreni, F.; Irace, C.; Santamaria, R.; Russo, G. Regulatory role of rpL3 in cell response to nucleolar stress induced by Act D in tumor cells lacking functional p53. Cell Cycle. 2016, 15, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Eastham, M.J.; Pelava, A.; Wells, G.R.; Lee, J.K.; Lawrence, I.R.; Stewart, J.; Deichner, M.; Hertle, R.; Watkins, N.J.; Schneider, C. The induction of p53 correlates with defects in the production, but not the levels, of the small ribosomal subunit and stalled large ribosomal subunit biogenesis. Nucleic Acids Res. 2023, 51, 9397–9414. [Google Scholar] [CrossRef]
- Mende, H.; Müller, S. Surveillance of nucleolar homeostasis and ribosome maturation by autophagy and the ubiquitin-proteasome system. Matrix Biol. 2021, 100–101, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gor, K.; Duss, O. Emerging Quantitative Biochemical, Structural, and Biophysical Methods for Studying Ribosome and Protein–RNA Complex Assembly. Biomolecules 2023, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Du, Y.; Chen, D.; Shi, Y.; Hu, M.; Tang, K.; Hong, Z.; Meng, X.; Xu, W.; Wu, G.; et al. Martynoside rescues 5-fluorouracil-impaired ribosome biogenesis by stabilizing RPL27A. Sci. Bull. 2023, 68, 1662–1677. [Google Scholar] [CrossRef]
- Kalita, K.; Makonchuk, D.; Gomes, C.; Zheng, J.-J.; Hetman, M. Inhibition of nucleolar transcription as a trigger for neuronal apoptosis. J. Neurochem. 2008, 105, 2286–2299. [Google Scholar] [CrossRef]
- Pagliara, V.; Saide, A.; Mitidieri, E.; di Villa, B.R.D.E.; Sorrentino, R.; Russo, G.; Russo, A. 5-FU targets rpL3 to induce mitochondrial apoptosis via cystathionine-β-synthase in colon cancer cells lacking p53. Oncotarget 2016, 7, 50333–50348. [Google Scholar] [CrossRef]
- Ferreira, R.; Schneekloth, J.S.; Panov, K.I.; Hannan, K.M.; Hannan, R.D. Targeting the RNA Polymerase I Transcription for Cancer Therapy Comes of Age. Cells 2020, 9, 266. [Google Scholar] [CrossRef]
- Mayer, C.; Grummt, I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 2006, 25, 6384–6391. [Google Scholar] [CrossRef] [PubMed]
- Schofield, A.J.; Duffy, E.E.; Kiefer, L.; Sullivan, M.C.; Simon, M.D. TimeLapse-seq: Adding a temporal dimension to RNA sequencing through nucleoside recoding. Nat. Methods 2018, 15, 221–225. [Google Scholar] [CrossRef]
- Hochstoeger, T.; Chao, J.A. Towards a molecular understanding of the 5′TOP motif in regulating translation of ribosomal mRNAs. Semin. Cell Dev. Biol. 2023, 154 Pt B, 99–104. [Google Scholar] [CrossRef]
- Gandin, V.; Masvidal, L.; Hulea, L.; Gravel, S.-P.; Cargnello, M.; McLaughlan, S.; Cai, Y.; Balanathan, P.; Morita, M.; Rajakumar, A.; et al. nanoCAGE reveals 5′ UTR features that define specific modes of translation of functionally related MTOR-sensitive mRNAs. Genome Res. 2016, 26, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Andreev, D.E.; Dmitriev, S.E.; Loughran, G.; Terenin, I.M.; Baranov, P.V.; Shatsky, I.N. Translation control of mRNAs encoding mammalian translation initiation factors. Gene 2018, 651, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, J.; Roiuk, M.; Teleman, A.A. Phosphorylation of ribosomal protein S6 differentially affects mRNA translation based on ORF length. Nucleic Acids Res. 2021, 49, 13062–13074. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.D.; de Moura, A.P.S.; Ciandrini, L. Gene length as a regulator for ribosome recruitment and protein synthesis: Theoretical insights. Sci. Rep. 2017, 7, 17409. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Chantranupong, L.; Keys, H.R.; Wang, T.; Gray, N.S.; Sabatini, D.M. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012, 485, 109–113. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Shamsuzzaman; Rahman, N.; Gregory, B.; Bommakanti, A.; Zengel, J.M.; Bruno, V.M.; Lindahl, L. Inhibition of Ribosome Assembly and Ribosome Translation Has Distinctly Different Effects on Abundance and Paralogue Composition of Ribosomal Protein mRNAs in Saccharomyces cerevisiae. mSystems 2023, 8, e0109822. [Google Scholar] [CrossRef]
- Li, B.B.; Qian, C.; Gameiro, P.A.; Liu, C.-C.; Jiang, T.; Roberts, T.M.; Struhl, K.; Zhao, J.J. Targeted profiling of RNA translation reveals mTOR-4EBP1/2-independent translation regulation of mRNAs encoding ribosomal proteins. Proc. Natl. Acad. Sci. USA 2018, 115, E9325–E9332. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.C.; Liu, Y.; Edlind, M.P.; Ingolia, N.T.; Janes, M.R.; Sher, A.; Shi, E.Y.; Stumpf, C.R.; Christensen, C.; Bonham, M.J.; et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012, 485, 55–61. [Google Scholar] [CrossRef]
- Fumagalli, S.; Di Cara, A.; Neb-Gulati, A.; Natt, F.; Schwemberger, S.; Hall, J.; Babcock, G.F.; Bernardi, R.; Pandolfi, P.P.; Thomas, G. Absence of nucleolar disruption after impairment of 40S ribosome biogenesis reveals an rpL11-translation-dependent mechanism of p53 induction. Nat. Cell Biol. 2009, 11, 501–508. [Google Scholar] [CrossRef]
- Rahman, N.; Shamsuzzaman; Lindahl, L. Interaction between the assembly of the ribosomal subunits: Disruption of 40S ribosomal assembly causes accumulation of extra-ribosomal 60S ribosomal protein uL18/L5. PLoS ONE 2020, 15, e0222479. [Google Scholar] [CrossRef]
- Jia, J.-J.; Lahr, R.M.; Solgaard, M.T.; Moraes, B.J.; Pointet, R.; Yang, A.-D.; Celucci, G.; Graber, T.E.; Hoang, H.-D.; Niklaus, M.R.; et al. mTORC1 promotes TOP mRNA translation through site-specific phosphorylation of LARP1. Nucleic Acids Res. 2021, 49, 3461–3489. [Google Scholar] [CrossRef]
- Weber, R.; Ghoshdastider, U.; Spies, D.; Duré, C.; Valdivia-Francia, F.; Forny, M.; Ormiston, M.; Renz, P.F.; Taborsky, D.; Yigit, M.; et al. Monitoring the 5′UTR landscape reveals isoform switches to drive translational efficiencies in cancer. Oncogene 2022, 42, 638–650. [Google Scholar] [CrossRef]
- Rao, S.; Hoskins, I.; Tonn, T.; Garcia, P.D.; Ozadam, H.; Cenik, E.S.; Cenik, C. Genes with 5′ terminal oligopyrimidine tracts preferentially escape global suppression of translation by the SARS-CoV-2 Nsp1 protein. RNA 2021, 27, 1025–1045. [Google Scholar] [CrossRef]
- Philippe, L.; Elzen, A.M.G.v.D.; Watson, M.J.; Thoreen, C.C. Global analysis of LARP1 translation targets reveals tunable and dynamic features of 5′ TOP motifs. Proc. Natl. Acad. Sci. USA 2020, 117, 5319–5328. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, L.; Savage, S.R.; Eguez, R.V.; Dou, Y.; Li, Y.; da Veiga Leprevost, F.; Jaehnig, E.J.; Lei, J.T.; Wen, B.; et al. Proteogenomic insights into the biology and treatment of HPV-negative head and neck squamous cell carcinoma. Cancer Cell 2021, 39, 361–379.e16. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.L.; Singh, K.; Zhong, Y.; Drewe, P.; Rajasekhar, V.K.; Sanghvi, V.R.; Mavrakis, K.J.; Jiang, M.; Roderick, J.E.; Van der Meulen, J.; et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature 2014, 513, 65–70. [Google Scholar] [CrossRef]
- Popay, T.M.; Wang, J.; Adams, C.M.; Howard, G.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A.; Thomas, L.R.; Lorey, S.L.; Machida, Y.J.; et al. MYC regulates ribosome biogenesis and mitochondrial gene expression programs through its interaction with host cell factor–1. eLife 2021, 10, e60191. [Google Scholar] [CrossRef]
- Scagliola, A.; Miluzio, A.; Biffo, S. Translational Control of Metabolism and Cell Cycle Progression in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 4885. [Google Scholar] [CrossRef]
- Saez, I.; Gerbracht, J.V.; Koyuncu, S.; Lee, H.J.; Horn, M.; Kroef, V.; Denzel, M.S.; Dieterich, C.; Gehring, N.H.; Vilchez, D. The E3 ubiquitin ligase UBR5 interacts with the H/ACA ribonucleoprotein complex and regulates ribosomal RNA biogenesis in embryonic stem cells. FEBS Lett. 2019, 594, 175–188. [Google Scholar] [CrossRef]
- Katsuno, Y.; Meyer, D.S.; Zhang, Z.; Shokat, K.M.; Akhurst, R.J.; Miyazono, K.; Derynck, R. Chronic TGF-β exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci. Signal. 2019, 12, eaau8544. [Google Scholar] [CrossRef]
- Mayr, C.; Bartel, D.P. Widespread Shortening of 3′UTRs by Alternative Cleavage and Polyadenylation Activates Oncogenes in Cancer Cells. Cell 2009, 138, 673–684. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wu, H.; Xiang, J.; Ruan, X.; Peng, P.; Ruan, Y.; Chen, Y.-G.; Wang, Y.; Yu, Q.; Zhang, H.; et al. Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat. Commun. 2020, 11, 37. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Tang, Y.; Kwiatkowski, D.J.; Henske, E.P. Midkine expression by stem-like tumor cells drives persistence to mTOR inhibition and an immune-suppressive microenvironment. Nat. Commun. 2022, 13, 5018. [Google Scholar] [CrossRef]
- Kim, W.; Kim, H.D.; Jung, Y.; Kim, J.; Chung, J. Drosophila Low Temperature Viability Protein 1 (LTV1) Is Required for Ribosome Biogenesis and Cell Growth Downstream of Drosophila Myc (dMyc). J. Biol. Chem. 2015, 290, 13591–13604. [Google Scholar] [CrossRef] [PubMed]
- Arabi, A.; Wu, S.; Ridderstråle, K.; Bierhoff, H.; Shiue, C.; Fatyol, K.; Fahlén, S.; Hydbring, P.; Söderberg, O.; Grummt, I.; et al. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat. Cell Biol. 2005, 7, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Barna, M.; Pusic, A.; Zollo, O.; Costa, M.; Kondrashov, N.; Rego, E.; Rao, P.H.; Ruggero, D. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature 2008, 456, 971–975. [Google Scholar] [CrossRef]
- Tsoi, H.; You, C.-P.; Leung, M.-H.; Man, E.P.S.; Khoo, U.-S. Targeting Ribosome Biogenesis to Combat Tamoxifen Resistance in ER+ve Breast Cancer. Cancers 2022, 14, 1251. [Google Scholar] [CrossRef]
- Feng, Y.C.; Liu, X.Y.; Teng, L.; Ji, Q.; Wu, Y.; Li, J.M.; Gao, W.; Zhang, Y.Y.; La, T.; Tabatabaee, H.; et al. c-Myc inactivation of p53 through the pan-cancer lncRNA MILIP drives cancer pathogenesis. Nat. Commun. 2020, 11, 4980. [Google Scholar] [CrossRef] [PubMed]
- Morcelle, C.; Menoyo, S.; Morón-Duran, F.D.; Tauler, A.; Kozma, S.C.; Thomas, G.; Gentilella, A. Oncogenic MYC Induces the Impaired Ribosome Biogenesis Checkpoint and Stabilizes p53 Independent of Increased Ribosome Content. Cancer Res. 2019, 79, 4348–4359. [Google Scholar] [CrossRef]
- Miller, S.C.; MacDonald, C.C.; Kellogg, M.K.; Karamysheva, Z.N.; Karamyshev, A.L. Specialized Ribosomes in Health and Disease. Int. J. Mol. Sci. 2023, 24, 6334. [Google Scholar] [CrossRef]
- Comerford, S.A.; Hinnant, E.A.; Chen, Y.; Hammer, R.E. Hepatic ribosomal protein S6 (Rps6) insufficiency results in failed bile duct development and loss of hepatocyte viability; a ribosomopathy-like phenotype that is partially p53-dependent. PLoS Genet. 2023, 19, e1010595. [Google Scholar] [CrossRef]
- Pelletier, J.; Thomas, G.; Volarević, S. Ribosome biogenesis in cancer: New players and therapeutic avenues. Nat. Rev. Cancer 2018, 18, 51–63. [Google Scholar] [CrossRef]
- Stine, Z.E.; Walton, Z.E.; Altman, B.J.; Hsieh, A.L.; Dang, C.V. MYC, Metabolism, and Cancer. Cancer Discov. 2015, 5, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Kang, W.; Yang, M.; Wang, L.; Bao, Q.; Chen, Z.; Dong, Y.; Wang, J.; Jiang, J.; Liu, H.; et al. USP29 coordinates MYC and HIF1α stabilization to promote tumor metabolism and progression. Oncogene 2021, 40, 6417–6429. [Google Scholar] [CrossRef]
- van Riggelen, J.; Yetil, A.; Felsher, D.W. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat. Rev. Cancer 2010, 10, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Clavería-Cabello, A.; Herranz, J.M.; Latasa, M.U.; Arechederra, M.; Uriarte, I.; Pineda-Lucena, A.; Prosper, F.; Berraondo, P.; Alonso, C.; Sangro, B.; et al. Identification and experimental validation of druggable epigenetic targets in hepatoblastoma. J. Hepatol. 2023, 79, 989–1005. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.W.; You, K.S.; Park, J.-S.; Lee, S.-G.; Seong, Y.-S. Ribosomal Protein S6: A Potential Therapeutic Target against Cancer? Int. J. Mol. Sci. 2021, 23, 48. [Google Scholar] [CrossRef]
- Dong, Y.; Tu, R.; Liu, H.; Qing, G. Regulation of cancer cell metabolism: Oncogenic MYC in the driver’s seat. Signal Transduct. Target. Ther. 2020, 5, 124. [Google Scholar] [CrossRef]
- Chalishazar, M.D.; Wait, S.J.; Huang, F.; Ireland, A.S.; Mukhopadhyay, A.; Lee, Y.; Schuman, S.S.; Guthrie, M.R.; Berrett, K.C.; Vahrenkamp, J.M.; et al. MYC-Driven Small-Cell Lung Cancer is Metabolically Distinct and Vulnerable to Arginine Depletion. Clin. Cancer Res. 2019, 25, 5107–5121. [Google Scholar] [CrossRef]
- Huang, F.; Ni, M.; Chalishazar, M.D.; Huffman, K.E.; Kim, J.; Cai, L.; Shi, X.; Cai, F.; Zacharias, L.G.; Ireland, A.S.; et al. Inosine Monophosphate Dehydrogenase Dependence in a Subset of Small Cell Lung Cancers. Cell Metab. 2018, 28, 369–382.e5. [Google Scholar] [CrossRef]
- Huang, F.; Huffman, K.E.; Wang, Z.; Wang, X.; Li, K.; Cai, F.; Yang, C.; Cai, L.; Shih, T.S.; Zacharias, L.G.; et al. Guanosine triphosphate links MYC-dependent metabolic and ribosome programs in small-cell lung cancer. J. Clin. Investig. 2021, 131, e139929. [Google Scholar] [CrossRef]
- Duan, S.; Huang, W.; Liu, X.; Liu, X.; Chen, N.; Xu, Q.; Hu, Y.; Song, W.; Zhou, J. IMPDH2 promotes colorectal cancer progression through activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 signaling pathways. J. Exp. Clin. Cancer Res. 2018, 37, 304. [Google Scholar] [CrossRef] [PubMed]
- Valvezan, A.J.; McNamara, M.C.; Miller, S.K.; Torrence, M.E.; Asara, J.M.; Henske, E.P.; Manning, B.D. IMPDH inhibitors for antitumor therapy in tuberous sclerosis complex. J. Clin. Investig. 2020, 5, e135071. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, I.; Hölzel, M.; Mürnseer, M.; Burtscher, H.; Weidle, U.H.; Eick, D. A role for c-Myc in the regulation of ribosomal RNA processing. Nucleic Acids Res. 2003, 31, 6148–6156. [Google Scholar] [CrossRef]
- Dong, Z.; Li, J.; Dai, W.; Yu, D.; Zhao, Y.; Liu, S.; Li, X.; Zhang, Z.; Zhang, R.; Liang, X.; et al. RRP15 deficiency induces ribosome stress to inhibit colorectal cancer proliferation and metastasis via LZTS2-mediated β-catenin suppression. Cell Death Dis. 2023, 14, 89. [Google Scholar] [CrossRef]
- Huang, W.; Fang, K.; Chen, T.-Q.; Zeng, Z.-C.; Sun, Y.-M.; Han, C.; Sun, L.-Y.; Chen, Z.-H.; Yang, Q.-Q.; Pan, Q.; et al. circRNA circAF4 functions as an oncogene to regulate MLL-AF4 fusion protein expression and inhibit MLL leukemia progression. J. Hematol. Oncol. 2019, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-H.; Ro, E.J.; Yoon, J.-S.; Mizutani, T.; Kang, D.-W.; Park, J.-C.; Kim, T.I.; Clevers, H.; Choi, K.-Y. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/β-catenin pathway activation. Nat. Commun. 2020, 11, 5321. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Niu, W.; Hu, R.; Wang, Y.; Liu, Y.; Liu, L.; Zhong, J.; Zhang, C.; You, H.; Zhang, J.; et al. POLR1D promotes colorectal cancer progression and predicts poor prognosis of patients. Mol. Carcinog. 2019, 58, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Z.; Lin, E.; He, P.; Ru, G. Oxidative damage-induced hyperactive ribosome biogenesis participates in tumorigenesis of offspring by cross-interacting with the Wnt and TGF-β1 pathways in IVF embryos. Exp. Mol. Med. 2021, 53, 1792–1806. [Google Scholar] [CrossRef]
- Chaillou, T.; Kirby, T.J.; McCarthy, J.J. Ribosome Biogenesis: Emerging Evidence for a Central Role in the Regulation of Skeletal Muscle Mass. J. Cell Physiol. 2014, 229, 1584–1594. [Google Scholar] [CrossRef]
- Del Valle, L.; Piña-Oviedo, S. Human Polyomavirus JCPyV and Its Role in Progressive Multifocal Leukoencephalopathy and Oncogenesis. Front. Oncol. 2019, 9, 711. [Google Scholar] [CrossRef]
- Zheng, H.-C.; Xue, H.; Jin, Y.-Z.; Jiang, H.-M.; Cui, Z.-G. The Oncogenic Effects, Pathways, and Target Molecules of JC Polyoma Virus T Antigen in Cancer Cells. Front. Oncol. 2022, 12, 744886. [Google Scholar] [CrossRef]
- Gan, D.-D.; Khalili, K. Interaction between JCV large T-antigen and β-catenin. Oncogene 2004, 23, 483–490. [Google Scholar] [CrossRef]
- Ripple, M.J.; Struckhoff, A.P.; Trillo-Tinoco, J.; Li, L.; Margolin, D.A.; McGoey, R.; Del Valle, L. Activation of c-Myc and Cyclin D1 by JCV T-Antigen and β-Catenin in Colon Cancer. PLoS ONE 2014, 9, e106257. [Google Scholar] [CrossRef]
- Nakanishi, C.; Seimiya, H. G-quadruplex in cancer biology and drug discovery. Biochem. Biophys. Res. Commun. 2020, 531, 45–50. [Google Scholar] [CrossRef]
- Shu, H.; Zhang, R.; Xiao, K.; Yang, J.; Sun, X. G-Quadruplex-Binding Proteins: Promising Targets for Drug Design. Biomolecules 2022, 12, 648. [Google Scholar] [CrossRef] [PubMed]
- Drygin, D.; Siddiqui-Jain, A.; O’Brien, S.; Schwaebe, M.; Lin, A.; Bliesath, J.; Ho, C.B.; Proffitt, C.; Trent, K.; Whitten, J.P.; et al. Anticancer Activity of CX-3543: A Direct Inhibitor of rRNA Biogenesis. Cancer Res. 2009, 69, 7653–7661. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.-Y.; Jiang, Z.-Z.; Guo, M.; Tan, X.-Z.; Chen, F.; Xi, X.-G.; Xu, Y. G-quadruplex DNA: A novel target for drug design. Cell Mol. Life Sci. 2021, 78, 6557–6583. [Google Scholar] [CrossRef]
- Khot, A.; Brajanovski, N.; Cameron, D.P.; Hein, N.; Maclachlan, K.H.; Sanij, E.; Lim, J.; Soong, J.; Link, E.; Blombery, P.; et al. First-in-Human RNA Polymerase I Transcription Inhibitor CX-5461 in Patients with Advanced Hematologic Cancers: Results of a Phase I Dose-Escalation Study. Cancer Discov. 2019, 9, 1036–1049. [Google Scholar] [CrossRef]
- Hein, N.; Cameron, D.P.; Hannan, K.M.; Nguyen, N.-Y.N.; Fong, C.Y.; Sornkom, J.; Wall, M.; Pavy, M.; Cullinane, C.; Diesch, J.; et al. Inhibition of Pol I transcription treats murine and human AML by targeting the leukemia-initiating cell population. Blood 2017, 129, 2882–2895. [Google Scholar] [CrossRef]
- Bywater, M.J.; Poortinga, G.; Sanij, E.; Hein, N.; Peck, A.; Cullinane, C.; Wall, M.; Cluse, L.; Drygin, D.; Anderes, K.; et al. Inhibition of RNA Polymerase I as a Therapeutic Strategy to Promote Cancer-Specific Activation of p53. Cancer Cell 2012, 22, 51–65. [Google Scholar] [CrossRef]
- Xu, H.; Di Antonio, M.; McKinney, S.; Mathew, V.; Ho, B.; O’neil, N.J.; Dos Santos, N.; Silvester, J.; Wei, V.; Garcia, J.; et al. CX-5461 is a DNA G-quadruplex stabilizer with selective lethality in BRCA1/2 deficient tumours. Nat. Commun. 2017, 8, 14432. [Google Scholar] [CrossRef]
- Drygin, D.; Lin, A.; Bliesath, J.; Ho, C.B.; O’Brien, S.E.; Proffitt, C.; Omori, M.; Haddach, M.; Schwaebe, M.K.; Siddiqui-Jain, A.; et al. Targeting RNA Polymerase I with an Oral Small Molecule CX-5461 Inhibits Ribosomal RNA Synthesis and Solid Tumor Growth. Cancer Res. 2011, 71, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Qu, N.; Zhu, R.; Hu, J.; Han, P.; Wu, J.; Tan, L.; Gan, H.; He, C.; Fang, C.; et al. TERT accelerates BRAF mutant–induced thyroid cancer dedifferentiation and progression by regulating ribosome biogenesis. Sci. Adv. 2023, 9, eadg7125. [Google Scholar] [CrossRef]
- Madden, S.K.; de Araujo, A.D.; Gerhardt, M.; Fairlie, D.P.; Mason, J.M. Taking the Myc out of cancer: Toward therapeutic strategies to directly inhibit c-Myc. Mol. Cancer 2021, 20, 3. [Google Scholar] [CrossRef]
- Leung, A.W.; Chen, K.T.; Ryan, G.M.; Anantha, M.; Wretham, N.; Nosrati, Z.; Heroux, D.; Wang, L.; Chow, N.; Dai, Z.; et al. DMPC/Chol liposomal copper CX5461 is therapeutically superior to a DSPC/Chol formulation. J. Control Release 2022, 345, 75–90. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Obinata, D.; Sandhu, S.; Selth, L.A.; Wong, S.Q.; Porter, L.H.; Lister, N.; Pook, D.; Pezaro, C.J.; Goode, D.L.; et al. Patient-derived Models of Abiraterone- and Enzalutamide-resistant Prostate Cancer Reveal Sensitivity to Ribosome-directed Therapy. Eur. Urol. 2018, 74, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Musso, L.; Mazzini, S.; Rossini, A.; Castagnoli, L.; Scaglioni, L.; Artali, R.; Di Nicola, M.; Zunino, F.; Dallavalle, S. c-MYC G-quadruplex binding by the RNA polymerase I inhibitor BMH-21 and analogues revealed by a combined NMR and biochemical Approach. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 615–629. [Google Scholar] [CrossRef]
- Peltonen, K.; Colis, L.; Liu, H.; Trivedi, R.; Moubarek, M.S.; Moore, H.M.; Bai, B.; Rudek, M.A.; Bieberich, C.J.; Laiho, M. A Targeting Modality for Destruction of RNA Polymerase I that Possesses Anticancer Activity. Cancer Cell 2014, 25, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.Q.; Huffines, A.K.; Laiho, M.; Schneider, D.A. The small-molecule BMH-21 directly inhibits transcription elongation and DNA occupancy of RNA polymerase I in vivo and in vitro. J. Biol. Chem. 2021, 298, 101450. [Google Scholar] [CrossRef] [PubMed]
- Ford, B.L.; Wei, T.; Liu, H.; Scull, C.E.; Najmi, S.M.; Pitts, S.; Fan, W.; Schneider, D.A.; Laiho, M. Expression of RNA polymerase I catalytic core is influenced by RPA12. PLoS ONE 2023, 18, e0285660. [Google Scholar] [CrossRef]
- Brogaard, K.; Xi, L.; Wang, J.-P.; Widom, J. A map of nucleosome positions in yeast at base-pair resolution. Nature 2012, 486, 496–501. [Google Scholar] [CrossRef]
- Bruno, P.M.; Lu, M.; Dennis, K.A.; Inam, H.; Moore, C.J.; Sheehe, J.; Elledge, S.J.; Hemann, M.T.; Pritchard, J.R. The primary mechanism of cytotoxicity of the chemotherapeutic agent CX-5461 is topoisomerase II poisoning. Proc. Natl. Acad. Sci. USA 2020, 117, 4053–4060. [Google Scholar] [CrossRef]
- Wittner, M.; Hamperl, S.; Stöckl, U.; Seufert, W.; Tschochner, H.; Milkereit, P.; Griesenbeck, J. Establishment and Maintenance of Alternative Chromatin States at a Multicopy Gene Locus. Cell 2011, 145, 543–554. [Google Scholar] [CrossRef]
- Jacobs, R.Q.; Ingram, Z.M.; Lucius, A.L.; Schneider, D.A. Defining the divergent enzymatic properties of RNA polymerases I and II. J. Biol. Chem. 2021, 296, 100051. [Google Scholar] [CrossRef]
- Schneider, D.A. Quantitative analysis of transcription elongation by RNA polymerase I in vitro. Methods Mol. Biol. 2012, 809, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Sainsbury, S.; Cheung, A.C.; Kostrewa, D.; Cramer, P. RNA polymerase I structure and transcription regulation. Nature 2013, 502, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, A.; Li, Y.; Dobbelstein, M. Fortifying p53—Beyond Mdm2 inhibitors. Aging 2016, 8, 1836–1837. [Google Scholar] [CrossRef][Green Version]
- Fu, X.; Xu, L.; Qi, L.; Tian, H.; Yi, D.; Yu, Y.; Liu, S.; Li, S.; Xu, Y.; Wang, C. BMH-21 inhibits viability and induces apoptosis by p53-dependent nucleolar stress responses in SKOV3 ovarian cancer cells. Oncol. Rep. 2017, 38, 859–865. [Google Scholar] [CrossRef]
- Scull, C.E.; Zhang, Y.; Tower, N.; Rasmussen, L.; Padmalayam, I.; Hunter, R.; Zhai, L.; Bostwick, R.; Schneider, D.A. Discovery of novel inhibitors of ribosome biogenesis by innovative high throughput screening strategies. Biochem. J. 2019, 476, 2209–2219. [Google Scholar] [CrossRef]
- Scull, C.E.; Twa, G.; Zhang, Y.; Yang, N.J.; Hunter, R.N.; Augelli-Szafran, C.E.; Schneider, D.A. Small Molecule RBI2 Disrupts Ribosome Biogenesis through Pre-rRNA Depletion. Cancers 2023, 15, 3303. [Google Scholar] [CrossRef] [PubMed]
- de Estrada, N.M.C.D.; Thoms, M.; Flemming, D.; Hammaren, H.M.; Buschauer, R.; Ameismeier, M.; Baßler, J.; Beck, M.; Beckmann, R.; Hurt, E. Structure of nascent 5S RNPs at the crossroad between ribosome assembly and MDM2–p53 pathways. Nat. Struct. Mol. Biol. 2023, 30, 1119–1131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Wang, S.; Jiao, Y. The Effects of Deregulated Ribosomal Biogenesis in Cancer. Biomolecules 2023, 13, 1593. https://doi.org/10.3390/biom13111593
Lu Y, Wang S, Jiao Y. The Effects of Deregulated Ribosomal Biogenesis in Cancer. Biomolecules. 2023; 13(11):1593. https://doi.org/10.3390/biom13111593
Chicago/Turabian StyleLu, Yiwei, Shizhuo Wang, and Yisheng Jiao. 2023. "The Effects of Deregulated Ribosomal Biogenesis in Cancer" Biomolecules 13, no. 11: 1593. https://doi.org/10.3390/biom13111593
APA StyleLu, Y., Wang, S., & Jiao, Y. (2023). The Effects of Deregulated Ribosomal Biogenesis in Cancer. Biomolecules, 13(11), 1593. https://doi.org/10.3390/biom13111593






