A Nexus between Genetic and Non-Genetic Mechanisms Guides KRAS Inhibitor Resistance in Lung Cancer
Abstract
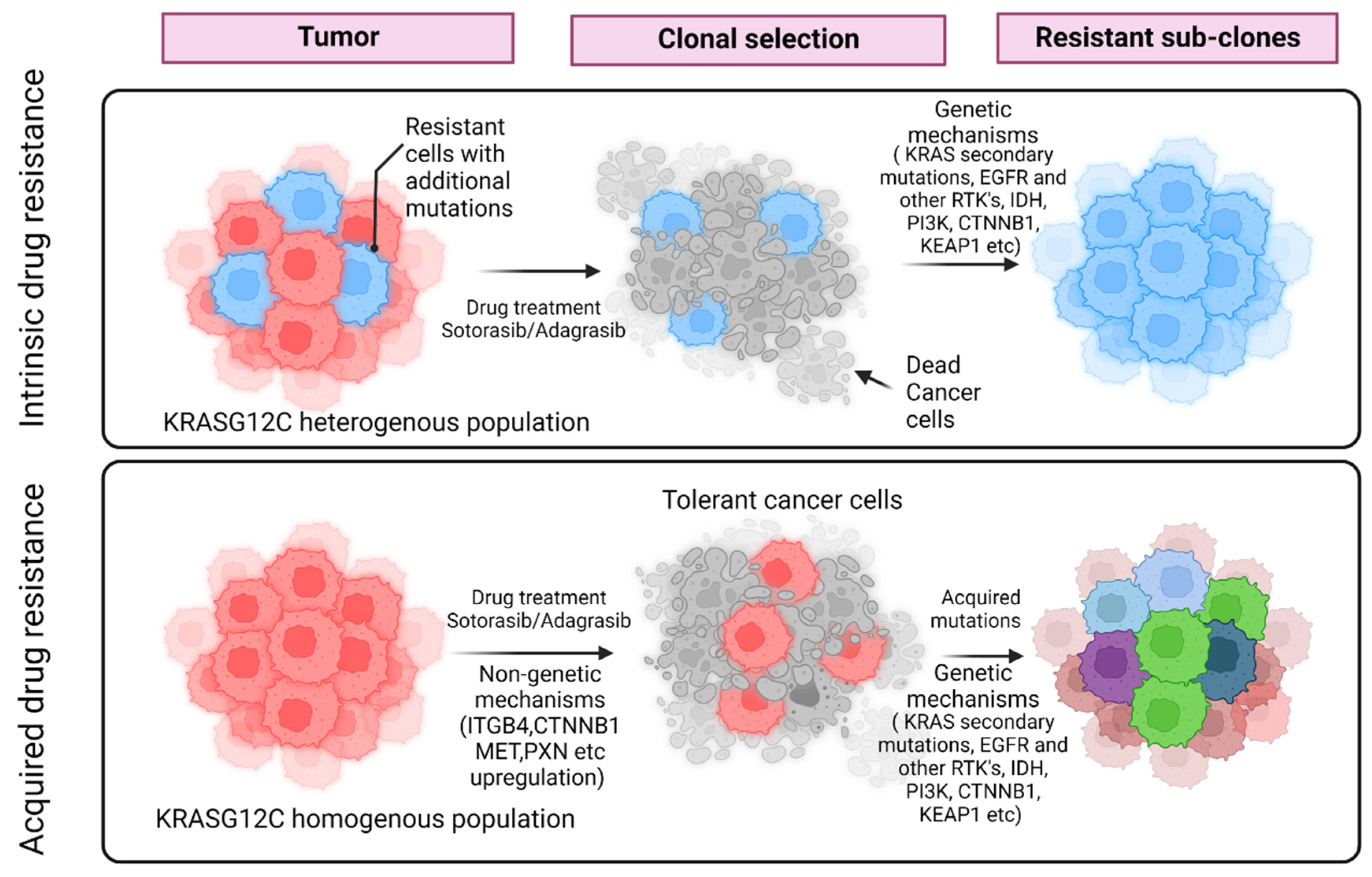
:1. Introduction
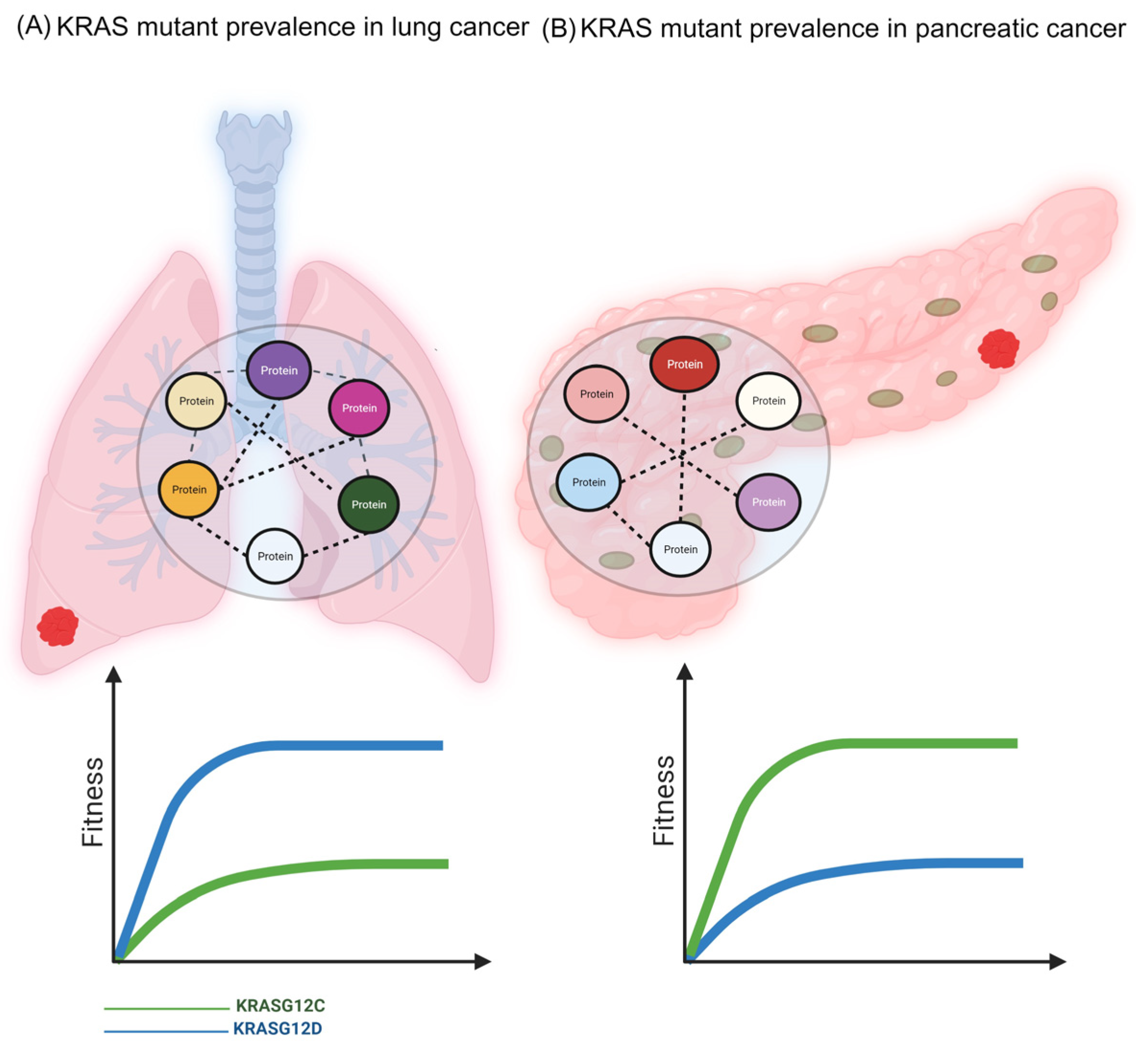
The KRAS Inhibitor Resistance Paradigm
2. Phenotypic Plasticity and Drug Sensitivity
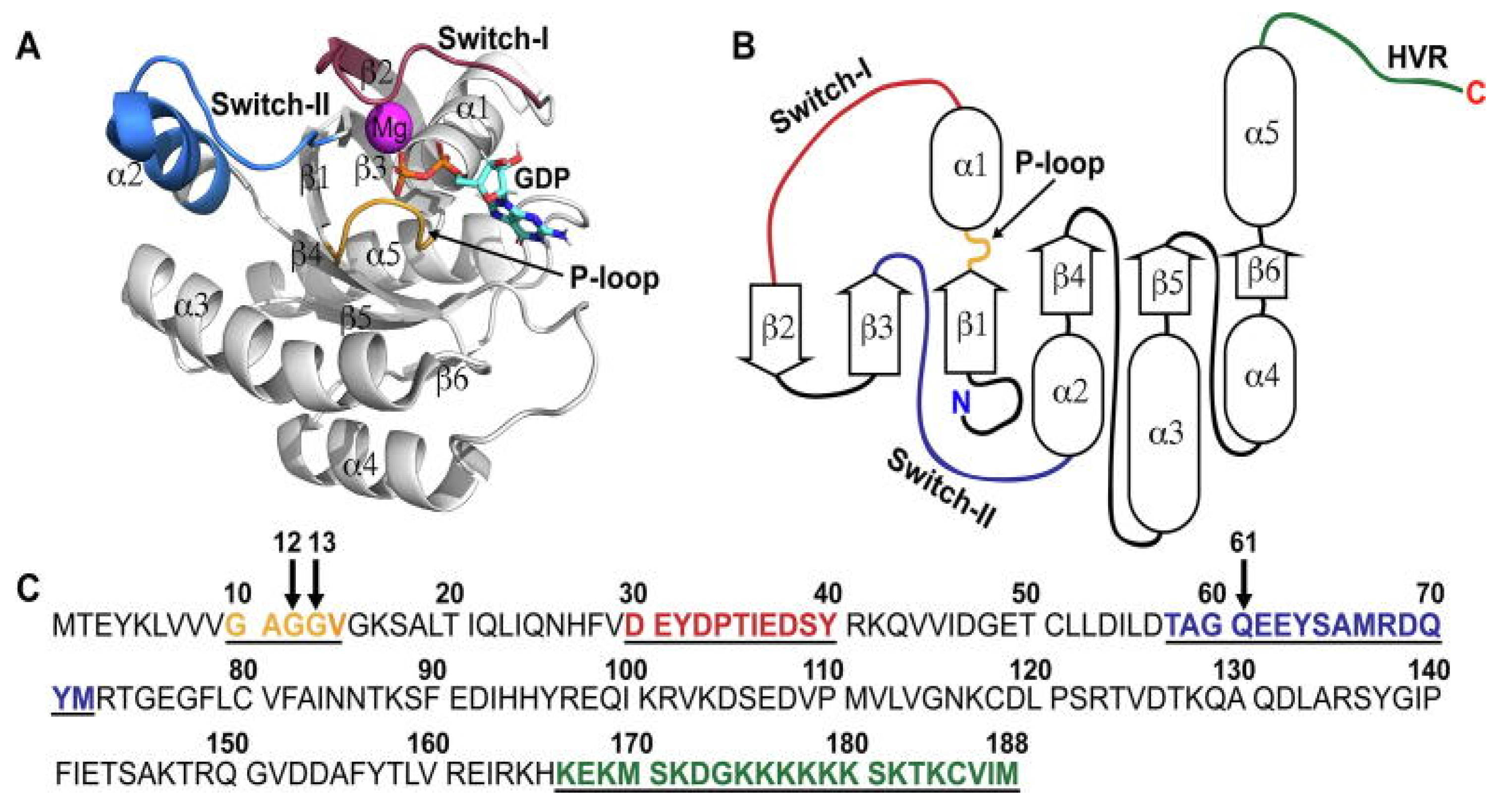
3. KRAS Conformational Dynamics and Its Sensitivity to Inhibitors
4. The Focal Adhesion Complex and Drug Resistance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boveri, T. Zur Frage der Entstehung Maligner Tumoren; Verlag von Gustav Fischer: Jena, Germany, 1914. [Google Scholar]
- Boveri, T. The Origin of Malignant Tumors; Boveri, M., Translator; Williams and Wilkins: Baltimore, MD, USA, 1929. [Google Scholar]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nat. Med. 2004, 10, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. The Path to Cancer—Three Strikes and You’re Out. N. Engl. J. Med. 2015, 373, 1895–1898. [Google Scholar] [CrossRef]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the definition of cancer. Mol. Cancer Res. 2023, 6, MCR-23-0411. [Google Scholar] [CrossRef] [PubMed]
- Salgia, R.; Pharaon, R.; Mambetsariev, I.; Nam, A.; Sattler, M. The improbable targeted therapy: KRAS as an emerging target in non-small cell lung cancer (NSCLC). Cell Rep. Med. 2021, 2, 100186. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.L.; Gong, Y.; Chitale, D.; Golas, B.; McLellan, M.D.; Kasai, Y.; Ding, L.; Mardis, E.R.; Wilson, R.K.; Solit, D.; et al. Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res. 2008, 68, 5524–5528. [Google Scholar] [CrossRef] [PubMed]
- Shachaf, C.M.; Kopelman, A.M.; Arvanitis, C.; Karlsson, A.; Beer, S.; Mandl, S.; Bachmann, M.H.; Borowsky, A.D.; Ruebner, B.; Cardiff, R.D.; et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature 2004, 431, 1112–1117. [Google Scholar] [CrossRef]
- Mahmoudabadi, G.; Rajagopalan, K.; Getzenberg, R.H.; Hannenhalli, S.; Rangarajan, G.; Kulkarni, P. Intrinsically disordered proteins and conformational noise: Implications in cancer. Cell Cycle 2013, 12, 26–31. [Google Scholar] [CrossRef]
- Kulkarni, P.; Salgia, R.; Rangarajan, G. Intrinsically disordered proteins and conformational noise: The hypothesis a decade later. iScience 2023, 26, 107109. [Google Scholar] [CrossRef]
- Jolly, M.K.; Kulkarni, P.; Weninger, K.; Orban, J.; Levine, H. Phenotypic Plasticity, Bet-Hedging, and Androgen Independence in Prostate Cancer: Role of Non-Genetic Heterogeneity. Front. Oncol. 2018, 8, 50. [Google Scholar] [CrossRef]
- Gunnarsson, E.B.; De, S.; Leder, K.; Foo, J. Understanding the role of phenotypic switching in cancer drug resistance. J. Theor. Biol. 2020, 490, 110162. [Google Scholar] [CrossRef]
- Cassidy, T.; Nichol, D.; Robertson-Tessi, M.; Craig, M.; Anderson, A.R.A. The role of memory in non-genetic inheritance and its impact on cancer treatment resistance. PLoS Comput. Biol. 2021, 17, e1009348. [Google Scholar] [CrossRef] [PubMed]
- Oren, Y.; Tsabar, M.; Cuoco, M.S.; Amir-Zilberstein, L.; Cabanos, H.F.; Hütter, J.C.; Hu, B.; Thakore, P.I.; Tabaka, M.; Fulco, C.P.; et al. Cycling cancer persister cells arise from lineages with distinct programs. Nature 2021, 596, 576–582. [Google Scholar] [CrossRef]
- Gomez, K.; Rabadan, R. A persistent look at how tumours evade therapy. Nature 2021, 596, 491–493. [Google Scholar] [CrossRef]
- Ebi, H. Drug-Tolerant Persister Cells After EGFR Tyrosine Kinase Inhibitor Treatment: Their Origin and the Influences from the Tumor Microenvironment. J. Thorac. Oncol. 2023, 8, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Vagner, S.; Robert, C. Persistent Cancer Cells: The Deadly Survivors. Cell 2020, 183, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Nagasaka, M.; Li, Y.; Sukari, A.; Ou, S.I.; Al-Hallak, M.N.; Azmi, A.S. KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat. Rev. 2020, 84, 101974. [Google Scholar] [CrossRef]
- Anonymous. Another KRAS Inhibitor Holds Its Own. Cancer Discov. 2020, 10, OF2. [Google Scholar] [CrossRef]
- Zhang, Z.; Guiley, K.Z.; Shokat, K.M. Chemical acylation of an acquired serine suppresses oncogenic signaling of K-Ras(G12S). Nat. Chem. Biol. 2022, 18, 1177–1183. [Google Scholar] [CrossRef]
- Patricelli, M.P.; Janes, M.R.; Li, L.S.; Hansen, R.; Peters, U.; Kessler, L.V.; Chen, Y.; Kucharski, J.M.; Feng, J.; Ely, T.; et al. Selective Inhibition of Oncogenic KRAS Output with Small Molecules Targeting the Inactive State. Cancer Discov. 2016, 6, 316–329. [Google Scholar] [CrossRef]
- Hallin, J.; Engstrom, L.D.; Hargis, L.; Calinisan, A.; Aranda, R.; Briere, D.M.; Sudhakar, N.; Bowcut, V.; Baer, B.R.; Ballard, J.A.; et al. The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020, 10, 54–71. [Google Scholar] [CrossRef]
- Kingwell, K. A non-covalent inhibitor with pan-KRAS potential. Nat. Rev. Drug Discov. 2023, 22, 622. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef]
- Jänne, P.A.; Smit, E.F.; de Marinis, F.; Laskin, J.; Domine Gomez, M.; Gadgeel, S.; Garassino, M.C.; Lu, S.; Spira, A.S.; Kang, V.; et al. LBA4—Preliminary safety and efficacy of adagrasib with pembrolizumab in treatment-naïve patients with advanced non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. Ann. Oncol. 2022, 16 (Suppl. S1), 100104. [Google Scholar] [CrossRef]
- Ramalingam, S.; Fakih, M.; Strickler, J.; Govindan, R.; Li, B.T.; Goldberg, S.; Gandara, D.; Burns, T.; Barve, M.; Shu, C.; et al. A phase 1b study evaluating the safety and efficacy of sotorasib, a KRAS G12C inhibitor, in combination with trametinib, a MEK inhibitor, in KRAS p.G12C-mutated solid tumors. In Proceedings of the American Association for Cancer Research—National Cancer Institute-European Organization for Research and Treatment of Cancer (AACR-NCI-EORTC) Virtual International Conference on Molecular Targets and Cancer Therapeutics, Virtual Meeting, 7–10 October 2021. [Google Scholar]
- Mohanty, A.; Nam, A.; Srivastava, S.; Jones, J.; Lomenick, B.; Singhal, S.S.; Guo, L.; Cho, H.; Li, A.; Behal, A.; et al. Acquired resistance to KRAS G12C small molecule inhibitors via genetic/non-genetic mechanisms in lung cancer. Sci. Adv. 2023, 9, eade3816. [Google Scholar] [CrossRef]
- Adachi, Y.; Ito, K.; Hayashi, Y.; Kimura, R.; Tan, T.Z.; Yamaguchi, R.; Ebi, H. Epithelial-to-Mesenchymal Transition is a Cause of Both Intrinsic and Acquired Resistance to KRAS G12C Inhibitor in KRAS G12C-Mutant Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 5962–5973. [Google Scholar] [CrossRef]
- Debaugnies, M.; Rodríguez-Acebes, S.; Blondeau, J.; Parent, M.A.; Zocco, M.; Song, Y.; de Maertelaer, V.; Moers, V.; Latil, M.; Dubois, C.; et al. RHOJ controls EMT-associated resistance to chemotherapy. Nature 2023, 616, 168–175. [Google Scholar] [CrossRef]
- Foo, J.; Basanta, D.; Rockne, R.C.; Strelez, C.; Shah, C.; Ghaffarian, K.; Mumenthaler, S.M.; Mitchell, K.; Lathia, J.D.; Frankhouser, D.; et al. Roadmap on plasticity and epigenetics in cancer. Phys. Biol. 2022, 19, 031501. [Google Scholar] [CrossRef]
- Toyokawa, G.; Bersani, F.; Bironzo, P.; Picca, F.; Tabbò, F.; Haratake, N.; Takenaka, T.; Seto, T.; Yoshizumi, T.; Novello, S.; et al. Tumor plasticity and therapeutic resistance in oncogene-addicted non-small cell lung cancer: From preclinical observations to clinical implications. Crit. Rev. Oncol. Hematol. 2023, 184, 103966. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Marti, T.M.; Dorn, P.; Peng, R.W. Non-genetic adaptive resistance to KRASG12C inhibition: EMT is not the only culprit. Front. Oncol. 2022, 12, 1004669. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Mishra, A.; Kaur, H.; Hari, K.; Muralidharan, S.; Mandal, S.; Jolly, M.K. A mechanistic model captures the emergence and implications of non-genetic heterogeneity and reversible drug resistance in ER+ breast cancer cells. NAR Cancer 2021, 3, zcab027. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Liu, S.; Rybkin, I.I.; Arbour, K.C.; Dilly, J.; Zhu, V.W.; Johnson, M.L.; Heist, R.S.; Patil, T.; Riely, G.J.; et al. Acquired Resistance to KRASG12C Inhibition in Cancer. N. Engl. J. Med. 2021, 384, 2382–2393. [Google Scholar] [CrossRef]
- Salgia, R.; Kulkarni, P. The Genetic/Non-genetic Duality of Drug ‘Resistance’ in Cancer. Trends Cancer 2018, 4, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Salgia, R.; Kulkarni, P.; Levine, H.; Loughlin, D.T. Quantifying Cancer: More Than Just a Numbers Game. Trends Cancer 2021, 7, 267–269. [Google Scholar] [CrossRef]
- Mohanty, A.; Nam, A.; Pozhitkov, A.; Yang, L.; Srivastava, S.; Nathan, A.; Wu, X.; Mambetsariev, I.; Nelson, M.; Subbalakshmi, A.R.; et al. A Non-genetic Mechanism Involving the Integrin β4/Paxillin Axis Contributes to Chemoresistance in Lung Cancer. iScience 2020, 23, 101496. [Google Scholar] [CrossRef]
- Kulkarni, P.; Mohanty, A.; Bhattacharya, S.; Singhal, S.; Guo, L.; Ramisetty, S.; Mirzapoiazova, T.; Mambetsariev, B.; Mittan, S.; Malhotra, J.; et al. Addressing Drug Resistance in Cancer: A Team Medicine Approach. J. Clin. Med. 2022, 11, 5701. [Google Scholar] [CrossRef]
- Nam, A.; Mohanty, A.; Bhattacharya, S.; Kotnala, S.; Achuthan, S.; Hari, K.; Srivastava, S.; Guo, L.; Nathan, A.; Chatterjee, R.; et al. Dynamic Phenotypic Switching and Group Behavior Help Non-Small Cell Lung Cancer Cells Evade Chemotherapy. Biomolecules 2021, 12, 8. [Google Scholar] [CrossRef]
- Kulkarni, P.; Wiley, H.S.; Levine, H.; Sauro, H.; Anderson, A.; Wong, S.T.C.; Meyer, A.S.; Iyengar, P.; Corlette, K.; Swanson, K.; et al. Addressing the genetic/nongenetic duality in cancer with systems biology. Trends Cancer 2023, 9, 185–187. [Google Scholar] [CrossRef]
- Sharma, S.V.; Lee, D.Y.; Li, B.; Quinlan, M.P.; Takahashi, F.; Maheswaran, S.; McDermott, U.; Azizian, N.; Zou, L.; Fischbach, M.A.; et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 2010, 141, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Marine, J.C.; Dawson, S.J.; Dawson, M.A. Non-genetic mechanisms of therapeutic resistance in cancer. Nat. Rev. Cancer 2020, 20, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Marin-Bejar, O.; Rogiers, A.; Dewaele, M.; Femel, J.; Karras, P.; Pozniak, J.; Bervoets, G.; Van Raemdonck, N.; Pedri, D.; Swings, T.; et al. Evolutionary predictability of genetic versus nongenetic resistance to anticancer drugs in melanoma. Cancer Cell 2021, 39, 1135–1149.e8. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Rajal, A.; Marzec, K.A.; McCloy, R.A.; Nobis, M.; Chin, V.; Hastings, J.F.; Lai, K.; Kennerson, M.; Hughes, W.E.; Vaghjiani, V.; et al. A non-genetic, cell cycle-dependent mechanism of platinum resistance in lung adenocarcinoma. eLife 2021, 10, e65234. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mohanty, A.; Achuthan, S.; Kotnala, S.; Jolly, M.K.; Kulkarni, P.; Salgia, R. Group Behavior and Emergence of Cancer Drug Resistance. Trends Cancer 2021, 7, 323–334. [Google Scholar] [CrossRef]
- Dunnett-Kane, V.; Nicola, P.; Blackhall, F.; Lindsay, C. Mechanisms of Resistance to KRASG12C Inhibitors. Cancers 2021, 13, 151. [Google Scholar] [CrossRef]
- Tanaka, N.; Lin, J.J.; Li, C.; Ryan, M.B.; Zhang, J.; Kiedrowski, L.A.; Michel, A.G.; Syed, M.U.; Fella, K.A.; Sakhi, M.; et al. Clinical Acquired Resistance to KRASG12C Inhibition through a Novel KRAS Switch-II Pocket Mutation and Polyclonal Alterations Converging on RAS-MAPK Reactivation. Cancer Discov. 2021, 11, 1913–1922. [Google Scholar] [CrossRef]
- Koga, T.; Suda, K.; Fujino, T.; Ohara, S.; Hamada, A.; Nishino, M.; Chiba, M.; Shimoji, M.; Takemoto, T.; Arita, T.; et al. KRAS Secondary Mutations That Confer Acquired Resistance to KRAS G12C Inhibitors, Sotorasib and Adagrasib, and Overcoming Strategies: Insights from the In-Vitro Experiments. J. Thorac. Oncol. 2021, 16, 1321–1332. [Google Scholar] [CrossRef]
- Addeo, A.; Banna, G.L.; Friedlaender, A. KRAS G12C Mutations in NSCLC: From Target to Resistance. Cancers 2021, 13, 2541. [Google Scholar] [CrossRef]
- Reck, M.; Carbone, D.P.; Garassino, M.; Barlesi, F. Targeting KRAS in non-small-cell lung cancer: Recent progress and new approaches. Ann. Oncol. 2021, 32, 1101–1110. [Google Scholar] [CrossRef]
- Xue, J.Y.; Zhao, Y.; Aronowitz, J.; Mai, T.T.; Vides, A.; Qeriqi, B.; Kim, D.; Li, C.; de Stanchina, E.; Mazutis, L.; et al. Rapid non-uniform adaptation to conformation. Nature 2020, 577, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.; Mohanty, A.; Kulkarni, P.; Salgia, R. Precision oncology provides opportunities for targeting KRAS-inhibitor resistance. Trends Cancer 2023, 9, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Pantsar, T. The current understanding of KRAS protein structure and dynamics. Comput. Struct. Biotechnol. J. 2019, 18, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pantsar, T.; Rissanen, S.; Dauch, D.; Laitinen, T.; Vattulainen, I.; Poso, A. Assessment of mutation probabilities of KRAS G12 missense mutants and their long-timescale dynamics by atomistic molecular simulations and Markov state modeling. PLoS Comput. Biol. 2018, 14, e1006458. [Google Scholar] [CrossRef]
- Heyne, M.; Shirian, J.; Cohen, I.; Peleg, Y.; Radisky, E.S.; Papo, N.; Shifman, J.M. Climbing Up and Down Binding Landscapes through Deep Mutational Scanning of Three Homologous Protein-Protein Complexes. J. Am. Chem. Soc. 2021, 143, 17261–17275. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Deng, J.; Akbay, E.A.; Hai, J.; Ambrogio, C.; Zhang, L.; Zhou, F.; Jenkins, R.W.; Adeegbe, D.O.; et al. Assessing Therapeutic Efficacy of MEK Inhibition in a KRASG12C-Driven Mouse Model of Lung Cancer. Clin. Cancer Res. 2018, 24, 4854–4864. [Google Scholar] [CrossRef]
- Tu, G.; Liu, Q.; Qiu, Y.; Leung, E.L.; Yao, X. In Silico Study of the Acquired Resistance Caused by the Secondary Mutations of KRAS G12C Protein Using Long Time Molecular Dynamics Simulation and Markov State Model Analysis. Int. J. Mol. Sci. 2022, 23, 13845. [Google Scholar] [CrossRef]
- Kulkarni, P.; Jolly, M.K.; Jia, D.; Mooney, S.M.; Bhargava, A.; Kagohara, L.T.; Chen, Y.; Hao, P.; He, Y.; Veltri, R.W.; et al. Phosphorylation-induced conformational dynamics in an intrinsically disordered protein and potential role in phenotypic heterogeneity. Proc. Natl. Acad. Sci. USA 2017, 114, E2644–E2653. [Google Scholar] [CrossRef]
- Kulkarni, P.; Achuthan, S.; Bhattacharya, S.; Jolly, M.K.; Kotnala, S.; Leite, V.B.P.; Mohanty, A.; Orban, J.; Roy, S.; Rangarajan, G.; et al. Protein conformational dynamics and phenotypic switching. Biophys. Rev. 2021, 13, 1127–1138. [Google Scholar] [CrossRef]
- Mohanty, A.; Nam, A.; Pozhitkov, A.; Bhattacharya, S.; Yang, L.; Nathan, A.; Wu, X.; Srivastava, S.; Mambetsariev, I.; Nelson, M.; et al. A Non-Genetic Mechanism for Chemoresistance in Lung Cancer: The Role of Integrin β4/Paxillin Axis. Available online: https://www.biorxiv.org/content/10.1101/781807v1 (accessed on 1 September 2023).
- Waddington, C.H. The Strategy of the Genes: A Discussion of Some Aspects of Theoretical Discussion; Allen & Unwin: Crows Nest, Australia, 1957. [Google Scholar]
- Saigusa, T.; Tero, A.; Nakagaki, T.; Kuramoto, Y. Amoebae anticipate periodic events. Phys. Rev. Lett. 2008, 100, 018101. [Google Scholar] [CrossRef]
- Nakagaki, T.; Yamada, H.; Tóth, A. Maze-solving by an amoeboid organism. Nature 2000, 407, 470. [Google Scholar] [CrossRef]
- Tero, A.; Takagi, S.; Saigusa, T.; Ito, K.; Bebber, D.P.; Fricker, M.D.; Yumiki, K.; Kobayashi, R.; Nakagaki, T. Rules for biologically inspired adaptive network design. Science 2010, 327, 439–442. [Google Scholar] [CrossRef]
- Gershman, S.J.; Balbi, P.E.; Gallistel, C.R.; Gunawardena, J. Reconsidering the evidence for learning in single cells. eLife 2021, 10, e61907. [Google Scholar] [CrossRef]
- Klumpe, H.E.; Langley, M.A.; Linton, J.M.; Su, C.J.; Antebi, Y.E.; Elowitz, M.B. The context-dependent, combinatorial logic of BMP signaling. Cell Syst. 2022, 13, 388–407.e10. [Google Scholar] [CrossRef]
- Noble, D. Central Dogma or Central Debate? Physiology 2018, 33, 246–249. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Brown, J.S. The Evolution and Ecology of Resistance in Cancer Therapy. Cold Spring Harb. Perspect. Med. 2020, 10, a040972. [Google Scholar] [CrossRef]
- Stanková, K.; Brown, J.S.; Dalton, W.S.; Gatenby, R.A. Optimizing Cancer Treatment Using Game Theory: A Review. JAMA Oncol. 2019, 5, 96–103. [Google Scholar] [CrossRef]
| Number | Combination | Clinical Study | Reference |
|---|---|---|---|
| 1 | Adagrasib + immune checkpoint inhibitor pembrolizumab | Yes | [28] |
| 2 | Sotorasib + MEK inhibitor trametinib | Yes | [29] |
| 3 | Sotorasib + proteasome inhibitor carfilzomib | No | [30] |
| 4 | Adagrasib + proteasome inhibitor carfilzomib | No | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, P.; Mohanty, A.; Ramisetty, S.; Duvivier, H.; Khan, A.; Shrestha, S.; Tan, T.; Merla, A.; El-Hajjaoui, M.; Malhotra, J.; et al. A Nexus between Genetic and Non-Genetic Mechanisms Guides KRAS Inhibitor Resistance in Lung Cancer. Biomolecules 2023, 13, 1587. https://doi.org/10.3390/biom13111587
Kulkarni P, Mohanty A, Ramisetty S, Duvivier H, Khan A, Shrestha S, Tan T, Merla A, El-Hajjaoui M, Malhotra J, et al. A Nexus between Genetic and Non-Genetic Mechanisms Guides KRAS Inhibitor Resistance in Lung Cancer. Biomolecules. 2023; 13(11):1587. https://doi.org/10.3390/biom13111587
Chicago/Turabian StyleKulkarni, Prakash, Atish Mohanty, Sravani Ramisetty, Herbert Duvivier, Ajaz Khan, Sagun Shrestha, Tingting Tan, Amartej Merla, Michelle El-Hajjaoui, Jyoti Malhotra, and et al. 2023. "A Nexus between Genetic and Non-Genetic Mechanisms Guides KRAS Inhibitor Resistance in Lung Cancer" Biomolecules 13, no. 11: 1587. https://doi.org/10.3390/biom13111587
APA StyleKulkarni, P., Mohanty, A., Ramisetty, S., Duvivier, H., Khan, A., Shrestha, S., Tan, T., Merla, A., El-Hajjaoui, M., Malhotra, J., Singhal, S., & Salgia, R. (2023). A Nexus between Genetic and Non-Genetic Mechanisms Guides KRAS Inhibitor Resistance in Lung Cancer. Biomolecules, 13(11), 1587. https://doi.org/10.3390/biom13111587







