Abstract
Ion channels play a crucial role in a wide range of biological processes, including cell cycle regulation and cancer progression. In particular, the transient receptor potential (TRP) family of channels has emerged as a promising therapeutic target due to its involvement in several stages of cancer development and dissemination. TRP channels are expressed in a large variety of cells and tissues, and by increasing cation intracellular concentration, they monitor mechanical, thermal, and chemical stimuli under physiological and pathological conditions. Some members of the TRP superfamily, namely vanilloid (TRPV), canonical (TRPC), melastatin (TRPM), and ankyrin (TRPA), have been investigated in different types of cancer, including breast, prostate, lung, and colorectal cancer. TRP channels are involved in processes such as cell proliferation, migration, invasion, angiogenesis, and drug resistance, all related to cancer progression. Some TRP channels have been mechanistically associated with the signaling of cancer pain. Understanding the cellular and molecular mechanisms by which TRP channels influence cancer provides new opportunities for the development of targeted therapeutic strategies. Selective inhibitors of TRP channels are under initial scrutiny in experimental animals as potential anti-cancer agents. In-depth knowledge of these channels and their regulatory mechanisms may lead to new therapeutic strategies for cancer treatment, providing new perspectives for the development of effective targeted therapies.
1. Introduction
According to the World Health Organization (WHO), cancer comprises a large group of diseases characterized by the rapid growth of abnormal cells that invade neighboring parts of the body, with the capacity to spread to other organs. More than 100 types of cancer have been identified so far [1]. Cancer represents a leading cause of death worldwide, causing nearly 10 million deaths in 2020 [2]. The leading cause in the development of cancer is an abnormal proliferation of cancer cells, which, rather than responding appropriately to the signals that regulate normal cell behavior, grow and divide in an uncontrolled manner. However, because of the large variability in cancers, tissues, organs of origin, predisposing or causal agents, genetic influence, and differential response to pharmacological treatments, the identification of common causes, mechanisms, and treatments is practically impossible. Notwithstanding, an improved knowledge of specific transduction signaling pathways may offer novel possibilities for innovative targeted treatments.
Ion channels are integral membrane proteins containing an aqueous pore that facilitates the mobilization of certain ions between cell compartments playing an essential role in cell functioning [3]. They regulate different cellular pathways, including cell proliferation, migration, apoptosis, and differentiation, to maintain normal tissue homeostasis. During the phenotypic changes that lead from a normal epithelial towards a cancer cell, a series of genetic/epigenetic changes, among other functions, may affect the activity of the ion channels [4]. Ion transport across the cell membrane has a crucial role in fundamental tumor cell functions [5], such as cell migration and cycle progression [6], cell volume regulation, proliferation, and death [7,8], which play critical roles in tumor cell survival and metastasis [9].
Increasing awareness of the ion channel’s role in tumor progression has led to the consideration of cancer as a channelopathy, or as a disease characterized by a profound alteration in ion channel function [10]. A special family of ion channels, called mechano-gated ion channels, includes the prototypical mechanosensitive piezo channels, which respond to mechanical stimuli such as changes in membrane tension or force [11]. Another family of ion channels, the transient receptor potential (TRP) channels, are opened by physical (mechanical and thermal) and chemical stimuli [12]. The great sensitivity of mechanosensitive ion channels to modifications in matrix stiffness is another significant feature [13]. Mechanical signals that operate through mechanosensitive ion channels during tumor growth have an impact on the microenvironment as well as cancer cells [14]. It has been reported that there is an association between gliomas and piezo channels in the regulation of tissue stiffness and tumor mitosis. Piezo1 sustains focal adhesions and supports integrin focal adhesion kinase (FAK) signaling, tissue stiffening, and extracellular matrix control [15].
Calcium (Ca2+), potassium (K+), and sodium (Na+) channels are examples of channels involved in tumor growth and metastasis [16]. Ca2+ is an important second messenger whose intracellular levels control several downstream signaling pathways, such as apoptosis and cell migration, functions that typically affect cancer growth [17]. In some hormone-sensitive cancers, such as breast cancer, the presence of channels in metastatic cells is regulated by positive feedback mechanisms, induced by hormone action [18]. Therefore, those ion channels represent a promising target for tumor treatment [19].
TRP channels are ionic channels permeable to monovalent and divalent cations, with a conserved structure and a higher selectivity for K+, Na+, and Ca2+ [20]. They have a crucial role in several pathologies, including metabolic, cardiovascular, and cancer diseases [21,22]. Recently, in a study evaluating transcriptomic and genomic alterations in TRP genes across more than 10,000 patients, it was found that 27 of 28 TRP genes are correlated with at least one hallmark of cancer in 33 different tumor types [23]. Furthermore, antagonists and agonists of TRPs have been used in association with chemotherapy in many tumor models, although side effects due to a lack of tissue specificity were observed [24,25,26,27,28].
Until now, altered levels in the function of TRP proteins in cancer have been reported, rather than mutations in the TRP genes [29]. Depending on the stage of the cancer, decreased or increased levels of the expression of the normal TRP protein can be detected [30]. Thus, these proteins could represent important markers for predicting tumor progression and, consequently, potential therapeutical targets [31,32,33,34,35]. Changes in TRP channel expression have also been associated with the staging of tumor progression [12,36,37,38]. In this review article, we summarize the latest research on the involvement of different TRP channels in cellular processes, such as proliferation, differentiation, migration, invasion, and angiogenesis, in different cancer subtypes.
2. TRP Channels
The TRP channel family is grouped into seven main subfamilies: ankyrin (with only one representative, TRPA1), canonical (TRPC1–7), melastatin (TRPM1–8), mucolipins (TRPML1–3), non-mechanoreceptor potential C (NOMP-like, TRPN1), polycystins (TRPP1–5), and vanilloid (TRPV1–6) [39] (Figure 1). Except for TRPN1, which has only been detected in fruit flies and zebrafish [3,6], the other TRP channels have also been detected in mammals. The mammalian TRP superfamily comprises 28 channels with a conserved primary structure that consists of six transmembrane domains (S1–S6) containing carboxy and amino terminal regions located on the intracellular side with the pore-forming loop located between S5–S6. The main differences between the seven TRP channel subfamilies are found in the N- and C-terminal cytosolic domains, which contain putative protein interaction and regulatory motifs [40]. TRP channels are activated in several ways: directly by endogenous agents, including diacylglycerols [41], phosphoinositides [42], eicosanoids [43], anandamide [44], and reactive oxygen species (ROS) and their byproducts [45,46], and by an unprecedented series of exogenous compounds, such as capsaicin [47], icilin [48], allicin [49], allyl isothiocyanate (AITC) [50,51], isopetasin [52], umbellulone [53,54], parthenolide [55], and acrolein [56], or indirectly, by intracellular mediators produced by the activation of G protein-coupled receptor (GPCR) or tyrosine kinases receptor [57].
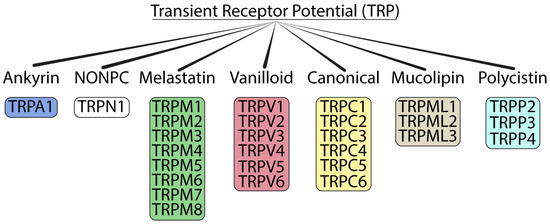
Figure 1.
Tree of the TRP ion channel superfamily.
TRP channels, almost ubiquitously expressed in cells and tissues, play important roles in health and disease [12,58], including neurological, cardiovascular, metabolic, pulmonary, psychiatric, and cancer disorders [28,59].
3. TRPs in Cancer
3.1. TRPA1 in Cancer
TRPA1, the only member of the ankyrin subfamily, is a polymodal channel that can be activated by a wide variety of noxious external stimuli, such as irritants, often associated with pain and inflammation, and intense cold [60,61,62,63]. TRPA1 can also be gated by several endogenously produced reactive chemical species, including oxidative stress by-products, such as ROS, reactive nitrogen (RNS), and carbonylic (RCS) species [45,62]. TRPA1 is predominantly expressed in the primary sensory neurons of the dorsal root (DRG), vagal (VG), and trigeminal (TG) ganglia, where it signals diverse pain stimuli [64,65,66,67,68]. TRPA1 expression has also been reported in non-neuronal cells, including the mouse inner ear and the organ of Corti [69], vascular endothelial cells [70], enterochromaffin cells of the respiratory tract [60,71], keratinocytes and melanocytes, synoviocytes, and dental pulp and gingival fibroblasts [72,73], as well as mast cells, epithelial, and pancreatic β cells [74,75,76,77,78,79,80,81]. More recently, the presence of TRPA1 in glial cells, such as astrocytes [82], oligodendrocytes [83], and Schwann cells [84,85], has been reported. In addition, the expression of TRPA1 has been observed in different cancer cells, including pancreatic adenocarcinoma and melanoma cells [86,87].
In cancer, TRPA1 activation in prostate tumor endothelial cells acts as a modulator of angiogenesis, since its activation promotes neovascularization, endothelial cell migration, and tubulogenesis in vitro in models of human prostate cancer [88]. TRPA1 activation in lung epithelial cancer cells (A549) can induce a decrease in cell invasion by inhibiting the cyclooxygenase-2 (COX-2)/prostaglandin E2 pathway in hypoxic cancer cells in vitro [89]. In breast and lung cancer spheroids, TRPA1 activates Ca2+-dependent antiapoptotic pathways by promoting ROS resistance [90]. Specifically, TRPA1 upregulated by nuclear factor erythroid 2-related factor 2 (NRF2), a transcription factor that, by encoding proteins with antioxidant and anti-inflammatory functions, promotes an adaptive process involving non-canonical oxidative stress defense and canonical ROS defense mechanisms [90]. Furthermore, TRPA1 inhibition attenuates xenograft tumor growth and increases chemosensitivity [90].
The TRPA1 protein has been identified in benign human skin lesions (dermal melanocytic nevi and dysplastic nevi), in cutaneous thin (pT1) and thick (pT4) melanomas, and in two different melanoma cell lines (SK-MEL-28 and WM266-4) [87]. In samples of skin lesion and melanoma, the presence of TRPA1 has been correlated with a progressive increase in oxidative stress and melanocytic transformation, as well as tumor severity. In vitro experiments on melanoma cell lines have shown that TRPA1 activation is associated with the release of H2O2, an observation in line with previous findings that have indicated the channel as an oxidative stress sensor and amplifier, a function that might affect tumor cells and proliferation [87].
3.2. TRPA1 in Cancer in Cancer Pain
Recent studies have underlined the role of TRPA1 in cancer pain. In a mouse model of cancer induced by the subcutaneous inoculation of melanoma B16-F10 cells, neuronal TRPA1 has been proposed to mediate mechanical and cold hypersensitivity and thigmotaxis behavior [91]. However, in mouse models of neuropathic pain induced by sciatic partial nerve ligation or ischemia and reperfusion, a prominent role of TRPA1 expressed in Schwann cells has been proposed [84,92]. Hematogenic macrophages recruited by increases in C-C Motif Chemokine Ligand 2 (CCL2) at sites of nerve injury generated a first burst of oxidative stress that, targeting the peripheral glial cell TRPA1, initiated a feed-forward mechanism that, via a Ca2+-dependent NADPH oxidase-1 (NOX1), amplified the oxidative stress to sustain pain signals [84,92]. Schwann cell TRPA1 has been similarly implicated in cancer-related pain by regulating, via a macrophage colony-stimulating factor (M-CSF), macrophage expansion and oxidative stress amplification, finally targeting neuronal TRPA1 to signal pain. In this mouse cancer model evoked by the inoculation of melanoma cells in the mouse paw, neuroinflammation and mechanical/cold hypersensitivity are maintained by a feed-forward mechanism, which requires continuous interaction between Schwann cell TRPA1 and expanded endoneurial macrophages throughout the entire sciatic nerve trunk [93].
Pain is also a recurrent symptom of cancer that becomes more frequent and debilitating in the presence of bone metastases, which are a common consequence of many primary tumors, including breast cancer [94]. A prominent role for the TRPA1 channel has also been reported in the development of mechanical hypersensitivity in a mouse model of metastatic bone cancer pain induced by the intramammary inoculation of breast carcinoma cells [95,96]. More recently, TRPA1 has been shown to be a regulator of metastatic bone cancer pain via insulin-like growth factor 1 receptor (IGF-1R) signaling in Schwann cells [97]. IGF-1, derived from osteoclast activation in osteolytic lesions caused by metastatic growth, targets its receptor expressed in Schwann cells, thus promoting an endothelial nitric oxide synthase-mediated TRPA1 activation and ROS release that, via M-CSF-mediated endoneurial macrophage expansion, sustains proalgesic responses.
3.3. TRPC
There are seven known members of the TRPC family, from TRPC1 to TRPC7. TRPC channels are widely expressed in many tissues and cells, including neurons, muscle cells, and epithelial cells. TRPC channels are involved in various physiological processes, such as sensory perception, smooth muscle contraction, hormone secretion, and cell migration [28]. TRPC channels can be activated by various signals, including G-protein-coupled receptors (GPCRs), receptor tyrosine kinases, and intracellular second messengers, such as diacylglycerol (DAG) and inositol trisphosphate (IP3) [98,99]. Upon activation, TRPC channels allow for the influx of Ca2+ ions into the cytoplasm, leading to an increase in the intracellular Ca2+ concentration. This Ca2+ influx triggers downstream signaling pathways and modulates the activity of various enzymes and transcription factors, ultimately influencing cell function. Aberrant TRPC channel activity has been associated with several diseases and pathological conditions, including cardiovascular disorders, neurodegenerative diseases, and cancer. Therefore, TRPC channels have emerged as potential therapeutic targets, and efforts are underway to develop drugs that modulate their activity for the treatment of these conditions.
3.3.1. TRPC1
TRPC1 is expressed in various types of cancer, including breast, pancreatic, lung, and glioblastoma multiforme. Its dysregulation has been proposed as a prognostic marker for some types of cancer [100], including breast cancer, as its expression is modulated by tumor development and metastasis [101,102,103]. An increased expression of TRPC1 is has been positively correlated with epithelial–mesenchymal transition (EMT), a complex process that induces tumor cells to spread and fight apoptosis, thus conferring a more aggressive phenotype [104,105]. Recently, it has been reported that TRPC1 overexpression increases markers for an EMT-like phenotype, such as zinc finger proteins, SNAI1 (SNAIL) and SNAI2 (SLUG), and VIMENTIN, by increasing invasiveness in mouse breast cancer cell lines in vitro [106]. TRPC1 is also expressed in human lung carcinoma, and high protein levels have been correlated with cancer differentiation and proliferation [107].
Aberrant Ca2+ signaling has been implicated in glioma pathogenesis and cell biology influencing cell proliferation, migration, invasion, and angiogenesis [108]. TRPC1 channels, acting as Ca2+-permeable channels, have been shown to regulate Ca2+ influx in glioma cells [109]. Increased Ca2+ influx through TRPC1 channels activates downstream signaling pathways, leading to cell proliferation and survival [109]. A loss of function of TRPC1, mediated by pharmacological or genetic inhibition, was found to reduce the proliferation of multinucleated glioma cells, mainly due to the suppression of store-operated Ca2+ entry (SOCE) [109].
TRPC1 channels have been found to promote glioma cell migration through their association with focal adhesion proteins and cytoskeletal rearrangement. Furthermore, TRPC1-mediated Ca2+ signaling can activate proteases and matrix metalloproteinases, facilitating the breakdown of the extracellular matrix and promoting glioma cell invasion [110]. TRPC1 channels have been shown to promote the release of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), from glioma cells, thus inducing endothelial cell proliferation and migration and leading to the formation of new blood vessels within the tumor microenvironment [111].
3.3.2. TRPC3
The TRPC3 protein might contribute to the development of tumor senescent phenotypes. Its downregulation in stromal cells promotes cellular senescence, sustaining inflammation and tumor growth in vivo [112]. TRPC3-mediated Ca2+ influx has been suggested as an endothelial cell attraction factor in prostate cancer, thus promoting angiogenesis [88,113]. TRPC3 overexpression has been detected in triple-negative breast cancer cells, and its activation induces an RAS P21 protein activator 4-mitogen-activated protein kinase (RASA4-MAPK) signaling cascade that plays a crucial functional role in preserving proliferation and resistance to apoptosis. A TRPC3 blocker attenuates proliferation, induces apoptosis, and sensitizes cell death to chemotherapeutic agents [114]. The TRPC3 channel is also highly expressed in gastric cancer specimens, and its expression is correlated with malignant progression by modulating the calcineurin B-like 2/glycogen synthase kinase-3 beta/nuclear factor of the activated T cells 2(CNB2/GSK3β/NFATc2) signaling pathway and controlling cell cycle, apoptosis, and intracellular ROS generation [115].
3.3.3. TRPC5
An aberrant Wnt/β-catenin signaling cascade facilitates cell renewal, proliferation, and differentiation in several cancer types [116]. The activation of this intracellular pathway increases the production of the ATP-biding cassette, subfamily B, member 1 (ABCB1), a multidrug efflux transporter that attenuates the effect of cytotoxic drugs in cancer cells. TRPC5 was found to be overexpressed together with ABCB1 in colorectal cancer cells resistant to 5-fluorouracil (5-Fu). TRPC5 silencing inhibits Wnt/β-catenin signaling, thus reducing ABCB1 and consequently reverting resistance to 5-Fu [117]. In a similar manner, TRPC5 channel expression is increased in breast cancer cell lines together with P-glycoprotein (P-gp), another pump overexpressed by cancer cells to remove cytotoxic drugs. TRPC5 suppression reduces P-gp levels and causes a reversal of drug resistance in cells [118]. The mechanism by which TRPC5 regulates P-gp seems to be specifically controlled through the activation of the nuclear factor of activated T cells isoform c3 (NFATc3) [119]. In addition, in breast cancer cells, TRPC5 activation promotes autophagy and chemoresistance via the Ca2+/calmodulin-dependent protein kinase beta/adenosine monophosphate-activated protein kinase alpha/mechanistic target of rapamycin (CaMKKβ/AMPKα/mTOR) pathway [120]. It has also been shown that TRPC5 is highly expressed in human breast cancer after long-term chemotherapy treatment, and its presence has been correlated with an increase in the transcription of vascular endothelial growth factor, which, in turn, stimulates tumor angiogenesis [121]. The TRPC5 channel seems to promote metastasis in colon cancer. Colon cancer patients with a high expression of TRPC5 display poorer overall and metastasis-free survival [122]. TRPC5 overexpression, by increasing intracellular Ca2+ concentration and mesenchymal biomarker expression, promotes cell migration, invasion, and proliferation [122].
3.3.4. TRPC6
Growing evidence has reported that the pattern of the expression of TRPC6 proteins is upregulated in several pathophysiological conditions, including cancer. TRPC6 has been found to be overexpressed in breast cancer biopsy tissues compared to normal breast tissues [123]. Human breast cancer cells in vitro also display significant levels of TRPC6 expression, and its silencing results in a significant reduction in cell growth [123]. In human hepatocellular carcinoma cells, transforming growth factor beta (TGFβ) is a mediator of motility, invasion, and metastases via the stimulation of Na+/Ca2+ exchanger 1 (NCX1), and TRPC6 activation regulates TGFβ, thus inducing the formation of a TRPC6/NCX1 molecular complex [124]. The expressions of both TRPC6 and NCX1 are markedly increased in human hepatocellular carcinoma tissues, and their expression levels positively correlate with migration, invasion, and intrahepatic metastasis [124]. An increased TRPC6 channel in cervical cancer cell lines induces cell proliferation, suggesting that channel inhibition might reduce the malignant behavior of the cancer. TRPC6 might be a new target for the prevention and treatment of cervical cancer [125].
3.4. TRPM
TRPM channels are a family of ion channels that regulate sensory perception, cellular homeostasis, and signal transduction. TRPM channels respond to a broad array of stimuli, including temperature, touch, pain, osmolarity, and chemical signals. They are expressed in various tissues and cell types throughout the body, highlighting their importance in numerous physiological functions. TRPM channels have gained significant attention in the field of cancer research due to their potential involvement in tumor progression and metastasis. Several members of the TRPM channel family have been implicated in cancer development and are investigated as potential therapeutic targets [126,127].
3.4.1. TRPM1
TRPM1 gene expression has been identified in benign nevi, dysplastic nevi, and cutaneous melanomas, with a negative association between its presence and melanoma aggressiveness [128,129]. In contrast, TRPM1 protein expression is associated with tumor progression and survival in acral melanoma, supposedly because of the activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII), which facilitates the binding of CaMKII with protein kinase B (AKT) and activates AKT, promoting melanoma cell colony formation, mobility, and an increase in tumor growth [130].
3.4.2. TRPM2
TRPM2 is highly expressed in many human cancers (neuroblastoma, breast, gastric, lung, pancreatic, prostate cancer, squamous cell carcinoma, and T-cell leukemia), where its activation increases malignant cell survival [131]. The modulation of TRPM2 via oxidative stress in several pathological conditions has been reported [132]. One additional product derived from oxidative stress, ADP-ribose (ADPR), binds to the C- and N-termini of TRPM2, an action that results in channel activation [133,134,135].
Channel activation also modulates oxidative stress production. TRPM2 opening modulates the hypoxia-inducible transcription factor 1/2α (HIF-1/2α) signaling cascade, including proteins involved in oxidant stress, glycolysis, and mitochondrial function in neuroblastoma xenograft models [136]. TRPM2 inhibition or depletion reduces cell and mitochondria Ca2+ influx and decreases activity, autophagy, antioxidant response, and mitochondrial function, thus impairing tumor cell survival [136,137,138,139,140,141]. TRPM2 also has immunomodulatory functions and can influence the tumor microenvironment. TRPM2 activation in immune cells, such as macrophages and dendritic cells, affects their polarization and cytokine production, leading to a modulation of the anti-tumor immune response [142]. Additionally, TRPM2-mediated Ca2+ influx influences the release of inflammatory mediators that promote tumor growth and angiogenesis.
3.4.3. TRPM3
TRPM3 plays pleiotropic roles in cellular Ca2+ signaling and homeostasis [143]. TRPM3 has been identified in several cancer types in mammals, including kidney cell carcinoma, glioma, melanoma, and melanoma-associated retinopathy (MAR) [144,145,146]. The TRPM3 channel supports the growth of clear cell renal cell carcinoma by promoting autophagy [144]. An increased expression of TRPM3 in renal cell carcinoma leads to Ca2+ influx, which elicits the activation of CaMKII, 5′-AMP-activated protein kinase (AMPK), and Unc-51-like autophagy-activating kinase 1 (ULK1), as well as the formation of phagophore [147].
MiR-204 is an intron micro-RNA (miRNA) located between exons 7 and 8 of the TRPM3 gene. A reduction in miR-204 induced by the higher methylation of host gene TRPM3 in gliomas can promote cell migration and enhance cell stemness [148]. It has been shown that TRPM3 interacts with the signal transducer and activator of transcription 3 (STAT3) via the activation of STAT3-suppressing miR-204 expression. Furthermore, the downregulation of miR-204 via the methylation of the promoter of its host gene TRPM3 leads to the activation of the Src-STAT3-NFAT pathway, promoting glioma stem cell invasion and stem cell-like phenotype [149].
3.4.4. TRPM4
In physiological conditions, TRPM4 controls cell migration [150]. It regulates the activation of T lymphocyte and mast cells, together with the migration of dendritic and mast cells [151,152]. Under inflammatory conditions, TRPM4 is involved in vascular endothelial cell migration and ROS production [153]. TRPM4-mediated effects on cell migration are at least partially due to the activation of Rac family small GTPase 1 (Rac1-GTPase), a key regulator of cytoskeletal dynamics and cell polarity [154].
TRPM4 channel expression has been described in several cancers, including prostate [155,156,157], urinary bladder [158], cervical [159], colorectal [160,161], liver [162], and large B cell lymphoma [163]. In cancer cells, TRPM4 upregulation is associated with cancer cell migration, proliferation, and invasion. A recent study has shown that TRPM4 upregulation and its conductivity control the viability and cell cycle of colorectal cancer cells [164]. Another study revealed that TRPM4 gene defects mechanically engaged intestinal barrier integrity by depressing the generation of ROS and decreasing mucus production, thus promoting chronic bowel inflammation, a risk factor for colorectal cancer [160]. A more recent study indicated that an attenuated expression of TRPM4 is associated with the development of endometrial carcinoma and breast cancer through the hyperactivation of the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway, which regulates cell transcription, translation, migration, metabolism, proliferation, and survival [165]. In addition, within normal lymphoid tissues, including the tonsils, lymph nodes, and appendix, human normal B cells express low levels of TRPM4, while in diffuse large B cell lymphoma, a higher TRPM4 protein level has been detected, which confers significantly poorer patient outcomes [163]. Similarly, a higher level of TRPM4 protein correlates with a higher risk of recurrence following radical prostatectomy [157].
3.4.5. TRPM6
TRPM6 is mostly expressed in the kidneys, distal small intestine, and colon [166]. The TRPM6 channel is permeable to magnesium (Mg2+), thus assuming a relevant role in epithelial Mg2+ transport and active Mg2+ absorption, especially in the gut and kidneys [167]. Hypomagnesemia is evidenced in cancer patients after cisplatin-based chemotherapies [168], and it has emerged as the most notable adverse effect of the anti-epidermal growth factor receptor (EGFR) monoclonal antibody, cetuximab, which is used widely for the treatment of advanced colorectal cancer cells [169]. The downregulation of the TRPM6 channel is present in 80% of primary tumors in colorectal cancer cells, whereas its high expression increases patient survival [170].
3.4.6. TRPM8
TRPM8, also defined as a “cold receptor”, as it is activated by chemical cooling agents (such as menthol) [171], exhibits an increased expression in several cancer subtypes, including colon, breast, and prostate tumors, and it is considered to be a useful prognostic marker [172,173,174]. TRPM8 channels in cancer prognosis are associated with the modulation of cell viability, proliferation, migration, and apoptosis. For example, TRPM8 activation may regulate AMPK activity by modifying cellular autophagy to control the proliferation and migration of breast cancer cells. TRPM8 knockdown decreases basal autophagy, while TRPM8 overexpression increases basal autophagy in several mammalian cancer cell types. The activation of autophagy-associated signaling pathways for AMPK and unc-51-like kinase 1 (ULK1), as well as the production of phagophores, are part of the TRPM8 strategy for controlling autophagy [175].
TRPM8 involvement in the death and apoptosis of bladder cancer cells is due to the modulation of mitochondrial activity [176,177]. It has also been reported that, in glioblastoma, TRPM8 channels modulate the expression of apoptosis-related factors through the p38/MAPK pathway [178,179]. In colon, oral, esophageal, bladder, and breast cancers, TRPM8 seems directly involved in invasiveness and metastasis via the regulation of the epithelial–mesenchymal transition process (EMT) [173,180,181,182].
3.5. TRPML
The endolysosomal TRPML subfamily consists of the TRPML1, TRPML2, and TRPML3 proteins. TRPMLs share roughly 40% of amino acid sequence similarity and have important roles in ion homeostasis, membrane trafficking, exocytosis, and autophagy [183,184]. Two pore channels (TPCs) and TRPMLs are endolysosomal channels regulating the autophagy/lysosome system, which is intensely associated with both cancer progression and cancer escape from immunosurveillance [185]. Several recent reports have clearly proved an emerging role for the TRPML channel in cancer development and progression, and a clinical prognostic role has been suggested [25,185,186].
3.5.1. TRPML1
TRPML1, mainly located in lysosomes, promotes cation efflux into the cytosol [187], thus controlling lysosomal storage, transportation, and pH homeostasis. TRPML1 mutations alter lysosomal storage, and lysosomal impairment is responsible for autophagy distortions. TRPML1 can also be negatively regulated by the target of rapamycin (TOR) with a consequent autophagy reduction, thus supporting a central role of TRPML1 in this process [188]. TRPML1 also regulates the exocytosis of intracellular contents via the endosomal lysosomal pathway [189,190]. Recent studies have revealed that TRPML1 is significantly increased in HRAS-positive tumors and oppositely correlated with patient prognosis. The knockdown, or selective inhibition, of TRPML1 abolishes the proliferation of cancer cells that express oncogenic HRAS [191]. An increased expression of the TRPML1 gene is also observed in melanoma cells compared to normal melanocytes, and TRPML1-deficient melanoma cells exhibit decreased survival, proliferation, and tumor growth [192].
Recently, an increased TRPML1 expression level has been correlated with poor clinical outcomes of pancreatic ductal adenocarcinoma patients [193], significantly lowering overall survival. A role of TRPML1 in pancreatic ductal adenocarcinoma progression has been further investigated by using an in vitro cell model, and the results show that the proliferation of pancreatic ductal adenocarcinoma cells is blocked by TRPML1 depletion. Parallel to this result, in a pancreatic ductal adenocarcinoma mouse model, TRPML1 was crucial for the formation and growth of tumors [193]. Altogether, these studies suggest that TRPML1 is upregulated in cancer cells to promote tumorigenesis. In line with this conclusion, another study suggests that TRPML1-mediated lysosomal exocytosis, which releases high levels of ATP to the extracellular space, promotes triple-negative breast cancer cell invasion and metastasis [194]. Conversely, another recent study proposes that TRPML1 activation in glioblastoma cell lines reduces cell viability by inducing caspase-3-dependent apoptosis [195]. Thus, the loss of TRPML1 expression is strongly correlated with a short survival in glioblastoma patients, suggesting that a reduction in TRPML1 expression represents a negative prognostic factor in glioblastoma patients [195].
3.5.2. TRPML2
While TRPML1 is mainly localized in late endosomes and lysosomes in all tissues, TRPML2 is primarily expressed in myeloid and lymphoid cell lineages as recycling endosomes [196]. Due to its higher expression in immune cells compared to other endolysosomal ion channels, a role for the channel in innate immune responses has been postulated [197,198]. TRPML2-knockout mice display an impaired recruitment of peripheral macrophages in response to inflammation, suggesting a potential defect in the immune response.
A link between TRPML2 expression and cancer has been investigated in different tumor types. Overall, a pro-tumorigenic role of TRPML2 has recently been proposed [199]. TRPML2 silencing decreases proliferation and cell viability by abolishing AKT/ERK1/2 phosphorylation and provokes apoptosis in glioma cell lines. Moreover, a role for TRPML2 in prostate cancer has recently been reported [200]. Via the regulation of the interleukin-1 beta/factor nuclear kappa B (IL-1β/NF-κB) pathway, TRPML2 activation promotes cancer cell proliferation, migration, and invasion.
3.5.3. TRPML3
Differing from TRPML1, which is ubiquitously expressed in all tissues, TRPML3 is mainly expressed in specific organs, such as the kidneys, lungs, back skin, and thymus [201,202]. TRPML3 was discovered in plasma membrane and multiple intracellular compartments, including autophagosomes, early and late endosomes, and lysosomes, where channel activation is involved in autophagy regulation [203]. Although its role in cancer has been poorly explored, a detailed analysis in ‘The Cancer Genome Atlas’ (TCGA) revealed that the downregulation of TRPML3/MCOLN3 is associated with a relatively better survival in several types of cancers, including adrenocortical, breast invasive, uterine corpus endometrial, kidney renal clear cell, and kidney papillary cell carcinomas, colon, lung, lung squamous cell, rectal, and stomach adenocarcinomas, pheochromocytoma, and paraganglioma, thymoma, and uterine carcinosarcoma [204].
3.6. TRPP
The TRPP family consists of three members: TRPP2, TRPP3, and TRPP5. TRPP2 has been demonstrated to be an active coordinator in TRP channel heteromerization. The physical interaction of TRPP2, also known as polycystin-2 (PKD2), with polycystin-1 (PKD1) was identified 26 years ago [205]. A study reported that PKD1 is present in stem cells of variable origins. The overexpression of PKD1 enhances the cell mobility and differentiation in umbilical-cord-blood-derived stem cells [206]. Functionally, TRPP2 is involved in the regulation of smooth muscle contraction, cell proliferation, and mechanical sensation [207]. It has been reported that TRPP2 activation enhances the metastasis of laryngeal squamous cell carcinoma, regulating the EMT [208]. The silencing of TRPP2 significantly suppresses ATP-induced Ca2+ release, wound healing, and cell invasion, which collaboratively diminishes the SMAD family member 4 (Smad4), STAT3, SNAIL, SLUG, and TWIST expression.
3.7. TRPV
TRPV channels are a group of ion channels that play crucial roles in sensory signaling and are involved in a wide range of physiological processes. These channels are named after their founding member, vanilloid 1 (TRPV1), which deorphanized the so-called receptor for capsaicin, the spicy ingredient of chili peppers [209,210]. TRPV channels are widespread in various tissues throughout the body, including neurons, epithelial cells, and immune cells. They are non-selective cation channels, allowing for the influx of Ca2+, Na+, and K+ across the cell membrane. TRPV channels are characterized by their sensitivity to multiple physical and chemical stimuli, including temperature, pH, mechanical stress, and various endogenous and exogenous compounds.
TRPV channels have been increasingly involved in cancer development and progression. Emerging evidence suggests that these channels play significant roles in various aspects of cancer biology, including tumor cell proliferation, migration, invasion, angiogenesis, and resistance to therapy. The dysregulation of TRPV channels in cancer suggests their potential as therapeutic targets. Modulating the activity of TRPV channels could help to inhibit tumor growth and metastasis and enhance the efficacy of existing therapies.
3.7.1. TRPV1
The TRPV1 channel is the selective molecular target of the vanilloid capsaicin, but it can also be gated by acid (pH < 6.5), ethanol, and heat [209,211,212]. Although TRPV1 is mainly localized to the plasma membrane, it can also be detected in the endoplasmic and sarcoplasmic reticulum [213,214], providing a pathway for the release of Ca2+ from these intracellular stores. The capsaicin-induced stimulation of TRPV1 triggers the apoptosis of human urothelial cancer cells via the activation of the ataxia telangiectasia mutated/CHK2/p53DNA damage response and Fas/CD95-mediated apoptotic pathways [215]. In tumor cells, proliferation, invasion, and metastasis are controlled by Ca2+ signaling. TRPV1 is functionally expressed in human esophageal squamous cells, and thermo-TRPVs might play an important role in the development of esophageal squamous cells [216]. It has been reported that TRPV1 overactivation supports the proliferation and/or migration of esophageal squamous cells.
An elevated TRPV1 expression has been proven in squamous cell carcinoma of the human tongue, lung cancer, and breast cancer [217,218,219]. TRPV1 exceptionally suppresses the development of gastric cancer through a novel Ca2+/CaMKKβ/AMPK pathway, and its downregulation has been associated with poor survival in human gastric cancer patients. Thus, TRPV1 upregulation and its downstream signaling may represent promising targets for gastric cancer prevention and therapy [220]. Little is known about TRPV1 and cancer-induced chemosensitivity.
3.7.2. TRPV2
While TRPV1 expression is primarily localized to the plasma membrane [221], TRPV2 is found in intracellular membranes [222]. TRPV2 is implicated in the signaling pathways that mediate cell survival, proliferation, and metastasis. In leukemia and bladder cancer, the oncogenic activity of TRPV2 relates to a different expression profile of the receptor. It can be overexpressed in cancerous cells, increasing tumor aggressivity, and its silencing or blocking can trigger apoptosis and cell cycle arrest [223]. Oxidative stress generated by TRPV2 in human hepatoma cell lines induces cell death, involving the inhibition of pro-survival signaling proteins and the activation of pro-death signaling proteins [224]. The higher expression of TRPV2 in gastric cancer patients has been proposed as a prognostic biomarker and potential therapeutic target [225]. TRPV2 expression has been evaluated in epidermal melanocytes, two human malignant melanoma, and two metastatic melanoma cell lines [226]. TRPV2-mediated melanoma cell death via channel activation favors the antitumor process.
In multiple myeloma patients, TRPV2 overexpression is associated with bone tissue damage and a poor prognosis. A loss or inactivation of TRPV2 also increases glioblastoma cell proliferation and provokes resistance to CD95-induced apoptotic cell death [145]. TRPV2 mediates cell adhesion, migration, and invasion by stimulating adrenomedullin in prostate and urothelial cancer [227]. Adrenomedullin induces prostate and urothelial cancer cell migration and invasion through TRPV2 translocation to the plasma membrane and an ensuing increase in the resting Ca2+ level. In prostate cancer, TRPV2 overexpression is also correlated with castration-resistant phenotype and metastasis [228]. Recent analyses have demonstrated that high expressions of TRPV2 and TRPM4 are negatively correlated with the prognosis of uveal melanoma patients [165]. TRPV2 overexpression is also linked to high relapse-free survival in triple-negative breast cancer, where the reverse is found in patients with esophageal squamous cell carcinoma or gastric cancer. Overall, these findings validate TRPV2 as a potential candidate for a cancer biomarker and future therapeutic target [223].
3.7.3. TRPV3
TRPV3 is a Ca2+-permeable nonselective cation channel broadly expressed in skin keratinocytes, along with oral and nasal epithelia [229]. Although the role of TRPV3 in cancer has not been extensively studied, emerging evidence suggests its potential implications in cancer development and progression. Several studies have reported altered expression patterns of TRPV3 in various cancer types, indicating its possible involvement in oncogenic processes. In some cancers, such as non-small lung cancer, melanoma, squamous cell carcinoma, and breast cancer, TRPV3 expression has been found to be upregulated. An increased TRPV3 expression has been associated with cancer cell proliferation, survival, and invasion [230,231]. The activation of TRPV3 has been shown to promote cancer cell growth by triggering the intracellular signaling pathways involved in cell proliferation, such as the ERK1/2 pathway. Additionally, TRPV3 activation can induce the expressions of genes associated with cancer progression and metastasis, including matrix metalloproteinases (MMPs) and VEGF.
3.7.4. TRPV4
The TRPV4 channel is a non-selective cation channel that is extensively expressed in several tissues, where it acts as a molecular sensor and a transducer that regulates a variety of functional activities [232,233,234,235,236]. Like other channels, the modulation of TRPV4 channel expression is observed to be closely related to tumor formation progression and metastasis. TRPV4 is overexpressed in colorectal, lung, and gastric cancer cells, but in other tumors, including prostate, skin, and esophageal cancer cells, TRPV4 channel expression appears to be normal [237,238]. The latest findings indicate that TRPV4 induces apoptosis via p38 MAPK in human lung cancer cells. TRPV4 overexpression in human lung cancer cell lines induces cell death and inhibits cell proliferation and migration. The inhibition of p38 MAPK reduces TRPV4’s effects on the cell proliferation, apoptosis, and migration of those cells. Collectively, this can imply that TRPV4 is a candidate target for human lung cancer therapy [239].
An abnormal TRPV4 expression is linked to gastric, liver, pancreatic, colorectal, lung, and breast cancers [237,240]. The upregulation of TRPV4 has been identified in breast cancer cell lines with the potential to metastasize, and its expression appears to increase with tumor grade and size, and to correlate with poor survival [241,242]. Furthermore, TRPV4 has been linked to cell proliferation through the CaMKII pathway and the regulation of apoptosis in distinct cancer models [243,244]. According to recent studies, tumor growth and metastasis are significantly increased in a syngeneic Lewis lung carcinoma tumor model of endothelial-specific TRPV4 knockout (TRPV4-ECKO) mice compared to wild-type mice [245]. This tumor growth is accompanied by increased tumor angiogenesis and metastasis compatible with the abnormal leaky vessels observed. Mechanistically, in TRPV4-ECKO mouse tumors, proteins that are related to endothelial structure and cell proliferation, such as vascular endothelial growth factor receptor 2 (VEGFR2), p-ERK, and MMP-9 expression, are increased. Thus, endothelial TRPV4 emerges as a subtle modulator of vascular integrity and an inhibitor of tumor angiogenesis, given that the deletion of TRPV4 promotes tumor angiogenesis, growth, and metastasis [245]. TRPV4 expression is higher in breast metastatic lesions compared to normal breast tissue and invasive ductal carcinomas, and its expression increases with tumor grade and size [246]. Conversely, TRPV4 is markedly downregulated in keratinocytes in the premalignant lesions of non-melanoma skin cancer, such as solar keratosis and Bowen’s disease, and in basal and squamous cell carcinoma [247]. The suppression of TRPV4 expression in keratinocytes has been correlated with the increase in cytokines and prostaglandins within the tumor milieu [247].
3.7.5. TRPV5
TRPV5 is a Ca2+-selective ion channel widely expressed in many tissues, including urinary bladder and kidney, where it acts as a gatekeeper of active Ca2+ reabsorption [248,249]. Altered TRPV5 expression has been identified among the different renal cell carcinoma histopathological subtypes. An altered vitamin D receptor expression may be associated with renal cell carcinoma carcinogenesis via TRPV5/6 [250].
Ca2+ deficiency triggers abnormal colonic growth and increases colon cancer risk with well-known mechanisms [251]. The parathyroid glands play an overall regulatory role in systemic Ca2+ homeostasis. There was a presence of TRPV5 and TRPV6 in sporadic parathyroid adenomas and normal parathyroid glands co-localized with Ca2+-sensing receptors on the membrane surface, although immunoreactivity was present in the cytosol and around the nuclei. An increased expression of both channels in adenoma compared to normal glands implies a relationship between cell Ca2+ signaling and pathological processes [252]. A recent study showed that low Ca2+-induced IGF signaling is mediated by TRPV5-associated membrane depolarization. These results disclose a novel signaling mechanism that results in abnormal epithelial proliferation associated with Ca2+ deficiency [253]. A reduced TRPV5 expression in tumor tissues is detected in non-small-cell lung cancer patients and associated with a shorter median survival time after surgical resection. The combined expression of TRPV5 and TRPV6 in tumor tissues exhibited promising prognostic value in non-small-cell lung cancer patients [254].
3.7.6. TRPV6
TRPV6 is a membrane Ca2+ channel widely expressed by the epithelial tissues of many tissues, such as the intestines, kidneys, placenta, epididymis, and exocrine glands [255]. The expression of the TRPV6 gene is remarkably upregulated in several human malignancies, including the most common cancers: prostate and breast cancer [34,256]. TRPV6 appears to be expressed in various cancer cell lines, but a direct identification of the TRPV6 protein using mass spectrometry has only been shown in the human breast cancer cell line, T47D [257], and in the human lymph node prostate cancer cell line, LNCaP [258].
Mechanistically, TRPV6-mediated Ca2+ influx maintains the inactive state by targeting IGF-mediated AKT-TOR and ERK signaling. A recent study showed that, in zebrafish epithelia and human colon carcinoma cells, TRPV6 diminishes intracellular Ca2+ levels and activates protein phosphatase 2A (PP2A), which downregulates IGF signaling and endorses the inactive state. This suggests that TRPV6 mediates a constitutive Ca2+ influx into epithelial cells to continuously suppress growth factor signaling and maintain the quiescent state [259]. In addition, TRPV6 activates NFATc2 by increasing the nuclear factor of activated T cells 2 interacting protein (NFATc2IP) phosphorylation, and CDK5 may be the candidate for performing this phosphorylation. Consequently, activated NFATc2 escalates breast cancer metastasis by upregulating ADAM metallopeptidase with thrombospondin type 1 motif 6 (ADAMTS6) expression. These findings indicate that TRPV6 increases NFATc2 transcriptional activity by targeting NFATc2IP phosphorylation, which finally upregulates ADAMTS6 expression to stimulate breast cancer metastasis [260]. One study has revealed a co-expression pattern between TRPV5 and TRPV6 channels in the human myeloid leukemia cell line K562, which proposes that the channel interaction contributes to intracellular Ca2+ signaling [261] (Table 1).

Table 1.
TRP channels and cancer types.
4. Translational Approaches
First, promising clinical studies were directed to test novel TRP channel modulators for treating inflammation and pain. However, the improved understanding of the role of these channels in different pathophysiological functions has indicated their major involvement in specific phases of cancer progression, thus suggesting that they are promising as potential targets.
To date, there are only two clinical trials for TRPA1 related to cancer. An ongoing interventional study (NCT05024383) aims to investigate the role of the TRPA1 channel in oral cancer pain. In particular, the study evaluates the mechanical (von Frey testing) and chemical (capsaicin and AITC) response in oral cancer patients and compares their sensitivities to healthy subjects. Another interventional study (NCT04923412) aims to investigate TRPA1 and TRPV1 expression in non-small cell lung cancer (NSCLC) patients before and after surgery to quantitatively measure injuries of the vagus nerve during mediastinal lymph node dissection. A lobectomy with lymph node dissection represents the standard surgical method. However, about 60% of patients experience postoperative chronic cough during the first year after surgery. In this trial, the investigators provide a new basis for safe and effective new surgical techniques, as well as possible new biomarkers (TRPA1 and TRPV1) related to postoperative cough and pulmonary complications.
Regarding TRPV1, two cancer clinical trials have been reported so far. A phase III study (NCT04572776) is currently investigating the role of TRPV1 in intractable cancer pain through a single epidural administration of resiniferatoxin vs. the standard of care. A small prospective study (NCT02666976) evaluates the modulation of inflammatory biomarkers, including TRPV1, in patients affected by gastrointestinal subepithelial tumors. One observational study (NCT05507879) evaluates the role of TRPC6 as a predictive biomarker of chemotherapy-related cardiac toxicity in patients with breast cancer. A small interventional phase I clinical trial (NCT01578564) tests the safety and tolerability of a TRPV6 inhibitor in subjects with advanced ovarian cancer or other cancers known to overexpress the TRPV6 channel.
5. Conclusions
Over the years, researchers have revealed that TRP channels play crucial roles in cellular homeostasis and represent an efficient and timely interface between the environment and the body. The dysregulation of TRP channels has been associated with numerous cancer types, highlighting their potential as therapeutic targets [22,23].. The role of TRPA1 in sustaining chronic cancer pain in rodent models is supported by robust data [92,94]. Emerging evidence suggests that TRP channels are implicated in cancer progression and metastatic processes. TRP channels have been also proposed as biomarkers for cancer diagnosis and prognosis, providing valuable insights into patient stratification and personalized medicine approaches [23,24,25,26,27,28]. However, due to the broad tissue distribution and multiple functions of TRPs in the same types of cancer and healthy tissues, their inhibition may result in systemic toxicity. Thus, the identification of the specific TRP channel and its selective cellular localization implicated in a given pro-oncogenic function is of paramount importance for the development of safer and better anticancer drugs. New insights into tumor biology driven by TRP channels clearly require further effort and dedicated study.
Author Contributions
M.M., M.T. and D.S.M.d.A. created the outline and wrote the manuscript, and P.G., R.N. and F.D.L. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) under IG 2020—ID 24503 (R.N.), European Union—Next Generation EU. National Recovery and Resilience Plan, Mission 4 Component 2—Investment 1.3—Mnesys A multiscale integrated approach to the study of the nervous system in health and disease—CUP-B83C22004910002 (F.D.L. and P.G.). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
F.D.L., P.G. and R.N. are the founding scientists of FloNext Srl. The other authors declare no conflict of interest.
References
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef]
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 7 June 2023).
- Kessler, D.; Gruen, G.C.; Heider, D.; Morgner, J.; Reis, H.; Schmid, K.W.; Jendrossek, V. The action of small GTPases Rab11 and Rab25 in vesicle trafficking during cell migration. Cell. Physiol. Biochem. 2012, 29, 647–656. [Google Scholar] [CrossRef]
- Kunzelmann, K. Ion channels and cancer. J. Membr. Biol. 2005, 205, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.M. Oncochannels. Cell Calcium 2013, 53, 241–255. [Google Scholar] [CrossRef]
- Becchetti, A. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am. J. Physiol. Cell Physiol. 2011, 301, C255–C265. [Google Scholar] [CrossRef]
- Lang, F.; Hoffmann, E.K. Role of ion transport in control of apoptotic cell death. Compr. Physiol. 2012, 2, 2037–2061. [Google Scholar] [CrossRef]
- Turner, K.L.; Sontheimer, H. Cl− and K+ channels and their role in primary brain tumour biology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130095. [Google Scholar] [CrossRef]
- Djamgoz, M.B.; Onkal, R. Persistent current blockers of voltage-gated sodium channels: A clinical opportunity for controlling metastatic disease. Recent. Pat. Anticancer. Drug Discov. 2013, 8, 66–84. [Google Scholar] [CrossRef]
- Litan, A.; Langhans, S.A. Cancer as a channelopathy: Ion channels and pumps in tumor development and progression. Front. Cell. Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef]
- Delmas, P.; Parpaite, T.; Coste, B. PIEZO channels and newcomers in the mammalian mechanosensitive ion channel family. Neuron 2022, 110, 2713–2727. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.; Lynch, C.D.; Sheetz, M.P. Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism. PLoS ONE 2009, 4, e6361. [Google Scholar] [CrossRef] [PubMed]
- Karska, J.; Kowalski, S.; Saczko, J.; Moisescu, M.G.; Kulbacka, J. Mechanosensitive Ion Channels and Their Role in Cancer Cells. Membranes 2023, 13, 167. [Google Scholar] [CrossRef]
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.K.; Hu, M.; Draghici, D.; et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron 2018, 100, 799–815.e797. [Google Scholar] [CrossRef]
- Lang, F.; Stournaras, C. Ion channels in cancer: Future perspectives and clinical potential. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130108. [Google Scholar] [CrossRef]
- Canales, J.; Morales, D.; Blanco, C.; Rivas, J.; Díaz, N.; Angelopoulos, I.; Cerda, O. A TR(i)P to Cell Migration: New Roles of TRP Channels in Mechanotransduction and Cancer. Front. Physiol. 2019, 10, 757. [Google Scholar] [CrossRef]
- Fraser, S.P.; Ozerlat-Gunduz, I.; Brackenbury, W.J.; Fitzgerald, E.M.; Campbell, T.M.; Coombes, R.C.; Djamgoz, M.B. Regulation of voltage-gated sodium channel expression in cancer: Hormones, growth factors and auto-regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130105. [Google Scholar] [CrossRef]
- Oosterwijk, E.; Gillies, R.J. Targeting ion transport in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130107. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef]
- Saldías, M.P.; Maureira, D.; Orellana-Serradell, O.; Silva, I.; Lavanderos, B.; Cruz, P.; Torres, C.; Cáceres, M.; Cerda, O. TRP Channels Interactome as a Novel Therapeutic Target in Breast Cancer. Front. Oncol. 2021, 11, 621614. [Google Scholar] [CrossRef]
- Smani, T.; Shapovalov, G.; Skryma, R.; Prevarskaya, N.; Rosado, J.A. Functional and physiopathological implications of TRP channels. Biochim. Biophys. Acta 2015, 1853, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Gao, Y.; Xu, G.; Zhou, P.; Li, S.; Guo, J.; Zou, H.; Xu, Q.; Huang, X.; Xu, J.; et al. Pan-cancer analyses reveal the genetic and pharmacogenomic landscape of transient receptor potential channels. NPJ Genom. Med. 2022, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Maggi, F.; Morelli, M.B.; Santoni, M.; Marinelli, O. Transient Receptor Potential Cation Channels in Cancer Therapy. Med. Sci. 2019, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Farfariello, V. TRP channels and cancer: New targets for diagnosis and chemotherapy. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 54–67. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef]
- Steinritz, D.; Stenger, B.; Dietrich, A.; Gudermann, T.; Popp, T. TRPs in Tox: Involvement of Transient Receptor Potential-Channels in Chemical-Induced Organ Toxicity-A Structured Review. Cells 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Stokłosa, P.; Borgström, A.; Kappel, S.; Peinelt, C. TRP Channels in Digestive Tract Cancers. Int. J. Mol. Sci. 2020, 21, 1877. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochim. Biophys. Acta 2007, 1772, 937–946. [Google Scholar] [CrossRef]
- Duncan, L.M.; Deeds, J.; Hunter, J.; Shao, J.; Holmgren, L.M.; Woolf, E.A.; Tepper, R.I.; Shyjan, A.W. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998, 58, 1515–1520. [Google Scholar]
- Tsavaler, L.; Shapero, M.H.; Morkowski, S.; Laus, R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001, 61, 3760–3769. [Google Scholar] [PubMed]
- Fuessel, S.; Sickert, D.; Meye, A.; Klenk, U.; Schmidt, U.; Schmitz, M.; Rost, A.K.; Weigle, B.; Kiessling, A.; Wirth, M.P. Multiple tumor marker analyses (PSA, hK2, PSCA, trp-p8) in primary prostate cancers using quantitative RT-PCR. Int. J. Oncol. 2003, 23, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Fixemer, T.; Wissenbach, U.; Flockerzi, V.; Bonkhoff, H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: A novel prognostic marker for tumor progression. Oncogene 2003, 22, 7858–7861. [Google Scholar] [CrossRef]
- Bödding, M. TRP proteins and cancer. Cell. Signal 2007, 19, 617–624. [Google Scholar] [CrossRef]
- Bai, S.; Wei, Y.; Liu, R.; Chen, Y.; Ma, W.; Wang, M.; Chen, L.; Luo, Y.; Du, J. The role of transient receptor potential channels in metastasis. Biomed. Pharmacother. 2023, 158, 114074. [Google Scholar] [CrossRef]
- Gkika, D.; Prevarskaya, N. TRP channels in prostate cancer: The good, the bad and the ugly? Asian J. Androl. 2011, 13, 673–676. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in tumour metastasis: New roles for known actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef]
- Minke, B. The history of the Drosophila TRP channel: The birth of a new channel superfamily. J. Neurogenet. 2010, 24, 216–233. [Google Scholar] [CrossRef]
- Fiorio Pla, A.; Munaron, L. Functional properties of ion channels and transporters in tumour vascularization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130103. [Google Scholar] [CrossRef]
- Sokabe, T.; Bradshaw, H.B.; Tominaga, M.; Leishman, E.; Chandel, A.; Montell, C. Endocannabinoids produced in photoreceptor cells in response to light activate Drosophila TRP channels. Sci. Signal 2022, 15, eabl6179. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Morales-Lázaro, S.L. Regulation of ThermoTRP Channels by PIP2 and Cholesterol. Adv. Exp. Med. Biol. 2023, 1422, 245–277. [Google Scholar] [CrossRef] [PubMed]
- Minic, Z.; O’Leary, D.S.; Reynolds, C.A. Spinal Reflex Control of Arterial Blood Pressure: The Role of TRP Channels and Their Endogenous Eicosanoid Modulators. Front. Physiol. 2022, 13, 838175. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.A.; Sehgal, A. Anandamide Metabolites Protect against Seizures through the TRP Channel Water Witch in Drosophila melanogaster. Cell Rep. 2020, 31, 107710. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andrè, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Mori, Y. TRP Channels as Sensors and Signal Integrators of Redox Status Changes. Front. Pharmacol. 2011, 2, 58. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Fajardo, O.; Meseguer, V.; Belmonte, C.; Viana, F. TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: Pharmacological and genetic evidence. J. Neurosci. 2008, 28, 7863–7875. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef]
- Takahashi, N.; Mizuno, Y.; Kozai, D.; Yamamoto, S.; Kiyonaka, S.; Shibata, T.; Uchida, K.; Mori, Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels 2008, 2, 287–298. [Google Scholar] [CrossRef]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Kleeberg-Hartmann, J.; Vogler, B.; Messlinger, K. Petasin and isopetasin reduce CGRP release from trigeminal afferents indicating an inhibitory effect on TRPA1 and TRPV1 receptor channels. J. Headache Pain. 2021, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Materazzi, S.; Vriens, J.; Prenen, J.; Benemei, S.; De Siena, G.; la Marca, G.; Andrè, E.; Preti, D.; Avonto, C.; et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012, 135, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Minassi, A.; Prenen, J.; Taglialatela-Scafati, O.; Appendino, G.; Nilius, B. Umbellulone modulates TRP channels. Pflug. Arch. 2011, 462, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Andersson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X.; et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain 2013, 154, 2750–2758. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef]
- Dietrich, A. Transient Receptor Potential (TRP) Channels in Health and Disease. Cells 2019, 8, 413. [Google Scholar] [CrossRef]
- Lee, K.; Jo, Y.Y.; Chung, G.; Jung, J.H.; Kim, Y.H.; Park, C.K. Functional Importance of Transient Receptor Potential (TRP) Channels in Neurological Disorders. Front. Cell Dev. Biol. 2021, 9, 611773. [Google Scholar] [CrossRef]
- Mukhopadhyay, I.; Gomes, P.; Aranake, S.; Shetty, M.; Karnik, P.; Damle, M.; Kuruganti, S.; Thorat, S.; Khairatkar-Joshi, N. Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J. Recept. Signal Transduct. Res. 2011, 31, 350–358. [Google Scholar] [CrossRef]
- Cvetkov, T.L.; Huynh, K.W.; Cohen, M.R.; Moiseenkova-Bell, V.Y. Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J. Biol. Chem. 2011, 286, 38168–38176. [Google Scholar] [CrossRef]
- Landini, L.; Souza Monteiro de Araujo, D.; Titiz, M.; Geppetti, P.; Nassini, R.; De Logu, F. TRPA1 Role in Inflammatory Disorders: What Is Known So Far? Int. J. Mol. Sci. 2022, 23, 4529. [Google Scholar] [CrossRef]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a therapeutic target for nociceptive pain. Expert. Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Appendino, G.; Owsianik, G. The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflug. Arch. 2012, 464, 425–458. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Fusi, C.; Materazzi, S.; Coppi, E.; Tuccinardi, T.; Marone, I.M.; De Logu, F.; Preti, D.; Tonello, R.; Chiarugi, A.; et al. The TRPA1 channel mediates the analgesic action of dipyrone and pyrazolone derivatives. Br. J. Pharmacol. 2015, 172, 3397–3411. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; De Logu, F.; Geppetti, P.; De Cesaris, F. The role of TRP ion channels in migraine and headache. Neurosci. Lett. 2022, 768, 136380. [Google Scholar] [CrossRef]
- De Logu, F.; Li Puma, S.; Landini, L.; Tuccinardi, T.; Poli, G.; Preti, D.; De Siena, G.; Patacchini, R.; Tsagareli, M.G.; Geppetti, P.; et al. The acyl-glucuronide metabolite of ibuprofen has analgesic and anti-inflammatory effects via the TRPA1 channel. Pharmacol. Res. 2019, 142, 127–139. [Google Scholar] [CrossRef]
- De Logu, F.; Trevisan, G.; Marone, I.M.; Coppi, E.; Padilha Dalenogare, D.; Titiz, M.; Marini, M.; Landini, L.; Souza Monteiro de Araujo, D.; Li Puma, S.; et al. Oxidative stress mediates thalidomide-induced pain by targeting peripheral TRPA1 and central TRPV4. BMC Biol. 2020, 18, 197. [Google Scholar] [CrossRef]
- García-Añoveros, J.; Duggan, A. Frontiers in Neuroscience. TRPA1 in Auditory and Nociceptive Organs. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; Copyright© 2023; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Oxford, UK, 2007. [Google Scholar]
- Earley, S.; Gonzales, A.L.; Crnich, R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ. Res. 2009, 104, 987–994. [Google Scholar] [CrossRef]
- Nassini, R.; Pedretti, P.; Moretto, N.; Fusi, C.; Carnini, C.; Facchinetti, F.; Viscomi, A.R.; Pisano, A.R.; Stokesberry, S.; Brunmark, C.; et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS ONE 2012, 7, e42454. [Google Scholar] [CrossRef]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef]
- Maglie, R.; Souza Monteiro de Araujo, D.; Antiga, E.; Geppetti, P.; Nassini, R.; De Logu, F. The Role of TRPA1 in Skin Physiology and Pathology. Int. J. Mol. Sci. 2021, 22, 3065. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.L.; Meotti, F.C.; Calixto, J.B. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2012, 133, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Preti, D.; Materazzi, S.; Geppetti, P. Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J. Med. Chem. 2010, 53, 5085–5107. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Kammel, L.G.; Zimmerman, A.L.; Oancea, E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 2383–2388. [Google Scholar] [CrossRef]
- Bellono, N.W.; Oancea, E. UV light phototransduction depolarizes human melanocytes. Channels 2013, 7, 243–248. [Google Scholar] [CrossRef]
- Büch, T.R.; Schäfer, E.A.; Demmel, M.T.; Boekhoff, I.; Thiermann, H.; Gudermann, T.; Steinritz, D.; Schmidt, A. Functional expression of the transient receptor potential channel TRPA1, a sensor for toxic lung inhalants, in pulmonary epithelial cells. Chem. Biol. Interact. 2013, 206, 462–471. [Google Scholar] [CrossRef]
- Earley, S. TRPA1 channels in the vasculature. Br. J. Pharmacol. 2012, 167, 13–22. [Google Scholar] [CrossRef]
- Oh, M.H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J. Immunol. 2013, 191, 5371–5382. [Google Scholar] [CrossRef]
- Prasad, P.; Yanagihara, A.A.; Small-Howard, A.L.; Turner, H.; Stokes, A.J. Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J. Immunol. 2008, 181, 5024–5034. [Google Scholar] [CrossRef]
- Takizawa, M.; Harada, K.; Nakamura, K.; Tsuboi, T. Transient receptor potential ankyrin 1 channels are involved in spontaneous peptide hormone release from astrocytes. Biochem. Biophys. Res. Commun. 2018, 501, 988–995. [Google Scholar] [CrossRef]
- Hamilton, N.B.; Kolodziejczyk, K.; Kougioumtzidou, E.; Attwell, D. Proton-gated Ca2+-permeable TRP channels damage myelin in conditions mimicking ischaemia. Nature 2016, 529, 523–527. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Nassini, R.; Materazzi, S.; Carvalho Gonçalves, M.; Nosi, D.; Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017, 8, 1887. [Google Scholar] [CrossRef]
- De Logu, F.; Li Puma, S.; Landini, L.; Portelli, F.; Innocenti, A.; de Araujo, D.S.M.; Janal, M.N.; Patacchini, R.; Bunnett, N.W.; Geppetti, P.; et al. Schwann cells expressing nociceptive channel TRPA1 orchestrate ethanol-evoked neuropathic pain in mice. J. Clin. Investig. 2019, 129, 5424–5441. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, F.; Şelescu, T.; Domocoş, D.; Măruţescu, L.; Chiritoiu, G.; Chelaru, N.R.; Dima, S.; Mihăilescu, D.; Babes, A.; Cucu, D. Functional expression of the transient receptor potential ankyrin type 1 channel in pancreatic adenocarcinoma cells. Sci. Rep. 2021, 11, 2018. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Souza Monteiro de Araujo, D.; Ugolini, F.; Iannone, L.F.; Vannucchi, M.; Portelli, F.; Landini, L.; Titiz, M.; De Giorgi, V.; Geppetti, P.; et al. The TRPA1 Channel Amplifies the Oxidative Stress Signal in Melanoma. Cells 2021, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, M.; Brossa, A.; Chinigo, G.; Grolez, G.P.; Trimaglio, G.; Allart, L.; Hulot, A.; Marot, G.; Genova, T.; Joshi, A.; et al. Transient Receptor Potential Channel Expression Signatures in Tumor-Derived Endothelial Cells: Functional Roles in Prostate Cancer Angiogenesis. Cancers 2019, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shim, M.K.; Jin, M.; Rhyu, M.R.; Lee, Y. Methyl syringate, a TRPA1 agonist represses hypoxia-induced cyclooxygenase-2 in lung cancer cells. Phytomedicine 2016, 23, 324–329. [Google Scholar] [CrossRef]
- Takahashi, N.; Chen, H.Y.; Harris, I.S.; Stover, D.G.; Selfors, L.M.; Bronson, R.T.; Deraedt, T.; Cichowski, K.; Welm, A.L.; Mori, Y.; et al. Cancer Cells Co-opt the Neuronal Redox-Sensing Channel TRPA1 to Promote Oxidative-Stress Tolerance. Cancer Cell 2018, 33, 985–1003. [Google Scholar] [CrossRef]
- Antoniazzi, C.T.D.; Nassini, R.; Rigo, F.K.; Milioli, A.M.; Bellinaso, F.; Camponogara, C.; Silva, C.R.; de Almeida, A.S.; Rossato, M.F.; De Logu, F.; et al. Transient receptor potential ankyrin 1 (TRPA1) plays a critical role in a mouse model of cancer pain. Int. J. Cancer 2019, 144, 355–365. [Google Scholar] [CrossRef]
- De Logu, F.; De Prá, S.D.; de David Antoniazzi, C.T.; Kudsi, S.Q.; Ferro, P.R.; Landini, L.; Rigo, F.K.; de Bem Silveira, G.; Silveira, P.C.L.; Oliveira, S.M.; et al. Macrophages and Schwann cell TRPA1 mediate chronic allodynia in a mouse model of complex regional pain syndrome type I. Brain Behav. Immun. 2020, 88, 535–546. [Google Scholar] [CrossRef]
- De Logu, F.; Marini, M.; Landini, L.; Souza Monteiro de Araujo, D.; Bartalucci, N.; Trevisan, G.; Bruno, G.; Marangoni, M.; Schmidt, B.L.; Bunnett, N.W.; et al. Peripheral Nerve Resident Macrophages and Schwann Cells Mediate Cancer-Induced Pain. Cancer Res. 2021, 81, 3387–3401. [Google Scholar] [CrossRef]
- Rades, D.; Schild, S.E.; Abrahm, J.L. Treatment of painful bone metastases. Nat. Rev. Clin. Oncol. 2010, 7, 220–229. [Google Scholar] [CrossRef]
- de Almeida, A.S.; Rigo, F.K.; De Prá, S.D.; Milioli, A.M.; Pereira, G.C.; Lückemeyer, D.D.; Antoniazzi, C.T.; Kudsi, S.Q.; Araújo, D.; Oliveira, S.M.; et al. Role of transient receptor potential ankyrin 1 (TRPA1) on nociception caused by a murine model of breast carcinoma. Pharmacol. Res. 2020, 152, 104576. [Google Scholar] [CrossRef]
- de Almeida, A.S.; Pereira, G.C.; Brum, E.D.S.; Silva, C.R.; Antoniazzi, C.T.D.; Ardisson-Araújo, D.; Oliveira, S.M.; Trevisan, G. Role of TRPA1 expressed in bone tissue and the antinociceptive effect of the TRPA1 antagonist repeated administration in a breast cancer pain model. Life Sci. 2021, 276, 119469. [Google Scholar] [CrossRef]
- Landini, L.; Marini, M.; Souza Monteiro de Araujo, D.; Romitelli, A.; Montini, M.; Albanese, V.; Titiz, M.; Innocenti, A.; Bianchini, F.; Geppetti, P.; et al. Schwann cell insulin-like growth factor receptor type-1 mediates metastatic bone cancer pain in mice. Brain Behav. Immun. 2023, 110, 348–364. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Jeon, J.P.; Myeong, J.; Wie, J.; Hong, C.; Kim, H.J.; Jeon, J.H.; So, I. The roles of G proteins in the activation of TRPC4 and TRPC5 transient receptor potential channels. Channels 2012, 6, 333–343. [Google Scholar] [CrossRef]
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells 2020, 9, 1983. [Google Scholar] [CrossRef]
- Elzamzamy, O.M.; Penner, R.; Hazlehurst, L.A. The Role of TRPC1 in Modulating Cancer Progression. Cells 2020, 9, 388. [Google Scholar] [CrossRef]
- Dhennin-Duthille, I.; Gautier, M.; Faouzi, M.; Guilbert, A.; Brevet, M.; Vaudry, D.; Ahidouch, A.; Sevestre, H.; Ouadid-Ahidouch, H. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: Correlation with pathological parameters. Cell. Physiol. Biochem. 2011, 28, 813–822. [Google Scholar] [CrossRef]
- Azimi, I.; Milevskiy, M.J.G.; Kaemmerer, E.; Turner, D.; Yapa, K.; Brown, M.A.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. TRPC1 is a differential regulator of hypoxia-mediated events and Akt signalling in PTEN-deficient breast cancer cells. J. Cell Sci. 2017, 130, 2292–2305. [Google Scholar] [CrossRef]
- Zhang, Y.; Lun, X.; Guo, W. Expression of TRPC1 and SBEM protein in breast cancer tissue and its relationship with clinicopathological features and prognosis of patients. Oncol. Lett. 2020, 20, 392. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, C.; De Clercq, K.; Vriens, J. Transient Receptor Potential Channels in the Epithelial-to-Mesenchymal Transition. Int. J. Mol. Sci. 2021, 22, 8188. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, M.; Mimori, K.; Yokobori, T.; Ishi, H.; Beppu, T.; Nakamori, S.; Baba, H.; Mori, M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010, 101, 293–299. [Google Scholar] [CrossRef]
- Tai, Y.K.; Chan, K.K.W.; Fong, C.H.H.; Ramanan, S.; Yap, J.L.Y.; Yin, J.N.; Yip, Y.S.; Tan, W.R.; Koh, A.P.F.; Tan, N.S.; et al. Modulated TRPC1 Expression Predicts Sensitivity of Breast Cancer to Doxorubicin and Magnetic Field Therapy: Segue Towards a Precision Medicine Approach. Front. Oncol. 2021, 11, 783803. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.N.; Zeng, B.; Zhang, Y.; Daskoulidou, N.; Fan, H.; Qu, J.M.; Xu, S.Z. Involvement of TRPC channels in lung cancer cell differentiation and the correlation analysis in human non-small cell lung cancer. PLoS ONE 2013, 8, e67637. [Google Scholar] [CrossRef]
- Maklad, A.; Sharma, A.; Azimi, I. Calcium Signaling in Brain Cancers: Roles and Therapeutic Targeting. Cancers 2019, 11, 145. [Google Scholar] [CrossRef]
- Bomben, V.C.; Sontheimer, H. Disruption of transient receptor potential canonical channel 1 causes incomplete cytokinesis and slows the growth of human malignant gliomas. Glia 2010, 58, 1145–1156. [Google Scholar] [CrossRef]
- Bomben, V.C.; Turner, K.L.; Barclay, T.T.; Sontheimer, H. Transient receptor potential canonical channels are essential for chemotactic migration of human malignant gliomas. J. Cell Physiol. 2011, 226, 1879–1888. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Meng, X.; Zou, F. Hypoxia up-regulates vascular endothelial growth factor in U-87 MG cells: Involvement of TRPC1. Neurosci. Lett. 2009, 459, 132–136. [Google Scholar] [CrossRef]
- Farfariello, V.; Gordienko, D.V.; Mesilmany, L.; Touil, Y.; Germain, E.; Fliniaux, I.; Desruelles, E.; Gkika, D.; Roudbaraki, M.; Shapovalov, G.; et al. TRPC3 shapes the ER-mitochondria Ca2+ transfer characterizing tumour-promoting senescence. Nat. Commun. 2022, 13, 956. [Google Scholar] [CrossRef]
- Sakellakis, M.; Chalkias, A. The Role οf Ion Channels in the Development and Progression of Prostate Cancer. Mol. Diagn. Ther. 2023, 27, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, Y.X.; Qi, Z.; Tsang, S.Y. TRPC3 Regulates the Proliferation and Apoptosis Resistance of Triple Negative Breast Cancer Cells through the TRPC3/RASA4/MAPK Pathway. Cancers 2019, 11, 558. [Google Scholar] [CrossRef]
- Lin, D.C.; Zheng, S.Y.; Zhang, Z.G.; Luo, J.H.; Zhu, Z.L.; Li, L.; Chen, L.S.; Lin, X.; Sham, J.S.K.; Lin, M.J.; et al. TRPC3 promotes tumorigenesis of gastric cancer via the CNB2/GSK3β/NFATc2 signaling pathway. Cancer Lett. 2021, 519, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.; Zhu, Y.; Pan, Q.; Liu, Y.; Qi, X.; Jin, L.; Jin, J.; Ma, X.; Hua, D. Inhibition of transient receptor potential channel 5 reverses 5-Fluorouracil resistance in human colorectal cancer cells. J. Biol. Chem. 2015, 290, 448–456. [Google Scholar] [CrossRef]
- Ma, X.; Cai, Y.; He, D.; Zou, C.; Zhang, P.; Lo, C.Y.; Xu, Z.; Chan, F.L.; Yu, S.; Chen, Y.; et al. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc. Natl. Acad. Sci. USA 2012, 109, 16282–16287. [Google Scholar] [CrossRef]
- Dong, Y.; Pan, Q.; Jiang, L.; Chen, Z.; Zhang, F.; Liu, Y.; Xing, H.; Shi, M.; Li, J.; Li, X.; et al. Tumor endothelial expression of P-glycoprotein upon microvesicular transfer of TrpC5 derived from adriamycin-resistant breast cancer cells. Biochem. Biophys. Res. Commun. 2014, 446, 85–90. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Li, H.; Chen, Z.; Yao, X.; Jin, J.; Ma, X. TRPC5-induced autophagy promotes drug resistance in breast carcinoma via CaMKKβ/AMPKα/mTOR pathway. Sci. Rep. 2017, 7, 3158. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Q.; Meng, H.; Jiang, Y.; Mao, A.; Wang, T.; Hua, D.; Yao, X.; Jin, J.; Ma, X. Enhancement of vascular endothelial growth factor release in long-term drug-treated breast cancer via transient receptor potential channel 5-Ca2+-hypoxia-inducible factor 1α pathway. Pharmacol. Res. 2015, 93, 36–42. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Dong, Y.; Zhang, P.; Han, X.; Jin, J.; Ma, X. Overexpression of TrpC5 promotes tumor metastasis via the HIF-1α-Twist signaling pathway in colon cancer. Clin. Sci. 2017, 131, 2439–2450. [Google Scholar] [CrossRef]
- Aydar, E.; Yeo, S.; Djamgoz, M.; Palmer, C. Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: A potential target for breast cancer diagnosis and therapy. Cancer Cell Int. 2009, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, Y.; Xie, R.; Liu, J.; Nie, X.; An, J.; Wen, G.; Liu, X.; Jin, H.; Tuo, B. The NCX1/TRPC6 Complex Mediates TGFβ-Driven Migration and Invasion of Human Hepatocellular Carcinoma Cells. Cancer Res. 2018, 78, 2564–2576. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.P.; Chen, Y.L.; Zheng, A. Pharmacological targeting transient receptor potential canonical channel 6 modulates biological behaviors for cervical cancer HeLa and SiHA cell. Cancer Cell Int. 2022, 22, 145. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liao, P. TRPM4 channel and cancer. Cancer Lett. 2019, 454, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef]
- Deeds, J.; Cronin, F.; Duncan, L.M. Patterns of melastatin mRNA expression in melanocytic tumors. Hum. Pathol. 2000, 31, 1346–1356. [Google Scholar] [CrossRef]
- Erickson, L.A.; Letts, G.A.; Shah, S.M.; Shackelton, J.B.; Duncan, L.M. TRPM1 (Melastatin-1/MLSN1) mRNA expression in Spitz nevi and nodular melanomas. Mod. Pathol. 2009, 22, 969–976. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Su, Y.C.; Jiang, K.Y.; Ito, T.; Li, T.W.; Kaku-Ito, Y.; Cheng, S.T.; Chen, L.T.; Hwang, D.Y.; Shen, C.H. TRPM1 promotes tumor progression in acral melanoma by activating the Ca2+/CaMKIIδ/AKT pathway. J. Adv. Res. 2023, 43, 45–57. [Google Scholar] [CrossRef]
- Miller, B.A. TRPM2 in Cancer. Cell Calcium 2019, 80, 8–17. [Google Scholar] [CrossRef]
- Simon, F.; Varela, D.; Cabello-Verrugio, C. Oxidative stress-modulated TRPM ion channels in cell dysfunction and pathological conditions in humans. Cell. Signal. 2013, 25, 1614–1624. [Google Scholar] [CrossRef]
- Hara, Y.; Wakamori, M.; Ishii, M.; Maeno, E.; Nishida, M.; Yoshida, T.; Yamada, H.; Shimizu, S.; Mori, E.; Kudoh, J.; et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 2002, 9, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Sumoza-Toledo, A.; Penner, R. TRPM2: A multifunctional ion channel for calcium signalling. J. Physiol. 2011, 589, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Lü, W.; Du, J. The N-terminal domain in TRPM2 channel is a conserved nucleotide binding site. J. Gen. Physiol. 2020, 152, e201912555. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Chen, S.J.; Conrad, K.; Keefer, K.; Abraham, T.; Lee, J.P.; Wang, J.; Zhang, X.Q.; Hirschler-Laszkiewicz, I.; Wang, H.G.; et al. Depletion of the Human Ion Channel TRPM2 in Neuroblastoma Demonstrates Its Key Role in Cell Survival through Modulation of Mitochondrial Reactive Oxygen Species and Bioenergetics. J. Biol. Chem. 2016, 291, 24449–24464. [Google Scholar] [CrossRef]
- Miller, B.A.; Zhang, W. TRP channels as mediators of oxidative stress. Adv. Exp. Med. Biol. 2011, 704, 531–544. [Google Scholar] [CrossRef]
- Zhang, W.; Chu, X.; Tong, Q.; Cheung, J.Y.; Conrad, K.; Masker, K.; Miller, B.A. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J. Biol. Chem. 2003, 278, 16222–16229. [Google Scholar] [CrossRef]
- Chen, S.J.; Zhang, W.; Tong, Q.; Conrad, K.; Hirschler-Laszkiewicz, I.; Bayerl, M.; Kim, J.K.; Cheung, J.Y.; Miller, B.A. Role of TRPM2 in cell proliferation and susceptibility to oxidative stress. Am. J. Physiol. Cell Physiol. 2013, 304, C548–C560. [Google Scholar] [CrossRef]
- Chen, S.J.; Hoffman, N.E.; Shanmughapriya, S.; Bao, L.; Keefer, K.; Conrad, K.; Merali, S.; Takahashi, Y.; Abraham, T.; Hirschler-Laszkiewicz, I.; et al. A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2α. J. Biol. Chem. 2014, 289, 36284–36302. [Google Scholar] [CrossRef]
- Hirschler-Laszkiewicz, I.; Chen, S.J.; Bao, L.; Wang, J.; Zhang, X.Q.; Shanmughapriya, S.; Keefer, K.; Madesh, M.; Cheung, J.Y.; Miller, B.A. The human ion channel TRPM2 modulates neuroblastoma cell survival and mitochondrial function through Pyk2, CREB, and MCU activation. Am. J. Physiol. Cell Physiol. 2018, 315, C571–C586. [Google Scholar] [CrossRef]
- Gershkovitz, M.; Caspi, Y.; Fainsod-Levi, T.; Katz, B.; Michaeli, J.; Khawaled, S.; Lev, S.; Polyansky, L.; Shaul, M.E.; Sionov, R.V.; et al. TRPM2 Mediates Neutrophil Killing of Disseminated Tumor Cells. Cancer Res. 2018, 78, 2680–2690. [Google Scholar] [CrossRef]
- Grimm, C.; Kraft, R.; Sauerbruch, S.; Schultz, G.; Harteneck, C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J. Biol. Chem. 2003, 278, 21493–21501. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.P.; Cost, N.G.; Hegde, S.; Kellner, E.; Mikhaylova, O.; Stratton, Y.; Ehmer, B.; Abplanalp, W.A.; Pandey, R.; Biesiada, J.; et al. TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell 2014, 26, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Chinigò, G.; Castel, H.; Chever, O.; Gkika, D. TRP Channels in Brain Tumors. Front. Cell Dev. Biol. 2021, 9, 617801. [Google Scholar] [CrossRef] [PubMed]
- Duvoisin, R.M.; Haley, T.L.; Ren, G.; Strycharska-Orczyk, I.; Bonaparte, J.P.; Morgans, C.W. Autoantibodies in Melanoma-Associated Retinopathy Recognize an Epitope Conserved Between TRPM1 and TRPM3. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2732–2738. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Blessing, A.M.; Pulliam, T.L.; Shi, Y.; Wilkenfeld, S.R.; Han, J.J.; Murray, M.M.; Pham, A.H.; Duong, K.; Brun, S.N.; et al. Inhibition of CAMKK2 impairs autophagy and castration-resistant prostate cancer via suppression of AMPK-ULK1 signaling. Oncogene 2021, 40, 1690–1705. [Google Scholar] [CrossRef]
- Ying, Z.; Li, Y.; Wu, J.; Zhu, X.; Yang, Y.; Tian, H.; Li, W.; Hu, B.; Cheng, S.Y.; Li, M. Loss of miR-204 expression enhances glioma migration and stem cell-like phenotype. Cancer Res. 2013, 73, 990–999. [Google Scholar] [CrossRef]
- Bian, Z.; Ji, W.; Xu, B.; Huo, Z.; Huang, H.; Huang, J.; Jiao, J.; Shao, J.; Zhang, X. Noncoding RNAs involved in the STAT3 pathway in glioma. Cancer Cell Int. 2021, 21, 445. [Google Scholar] [CrossRef]
- Cáceres, M.; Ortiz, L.; Recabarren, T.; Romero, A.; Colombo, A.; Leiva-Salcedo, E.; Varela, D.; Rivas, J.; Silva, I.; Morales, D.; et al. TRPM4 Is a Novel Component of the Adhesome Required for Focal Adhesion Disassembly, Migration and Contractility. PLoS ONE 2015, 10, e0130540. [Google Scholar] [CrossRef]
- Barbet, G.; Demion, M.; Moura, I.C.; Serafini, N.; Léger, T.; Vrtovsnik, F.; Monteiro, R.C.; Guinamard, R.; Kinet, J.P.; Launay, P. The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat. Immunol. 2008, 9, 1148–1156. [Google Scholar] [CrossRef]
- Vennekens, R.; Olausson, J.; Meissner, M.; Bloch, W.; Mathar, I.; Philipp, S.E.; Schmitz, F.; Weissgerber, P.; Nilius, B.; Flockerzi, V.; et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat. Immunol. 2007, 8, 312–320. [Google Scholar] [CrossRef]
- Sarmiento, D.; Montorfano, I.; Cerda, O.; Cáceres, M.; Becerra, A.; Cabello-Verrugio, C.; Elorza, A.A.; Riedel, C.; Tapia, P.; Velásquez, L.A.; et al. Increases in reactive oxygen species enhance vascular endothelial cell migration through a mechanism dependent on the transient receptor potential melastatin 4 ion channel. Microvasc. Res. 2015, 98, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Chinigò, G.; Fiorio Pla, A.; Gkika, D. TRP Channels and Small GTPases Interplay in the Main Hallmarks of Metastatic Cancer. Front. Pharmacol. 2020, 11, 581455. [Google Scholar] [CrossRef]
- Holzmann, C.; Kappel, S.; Kilch, T.; Jochum, M.M.; Urban, S.K.; Jung, V.; Stöckle, M.; Rother, K.; Greiner, M.; Peinelt, C. Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget 2015, 6, 41783–41793. [Google Scholar] [CrossRef] [PubMed]
- Sagredo, A.I.; Sagredo, E.A.; Pola, V.; Echeverría, C.; Andaur, R.; Michea, L.; Stutzin, A.; Simon, F.; Marcelain, K.; Armisén, R. TRPM4 channel is involved in regulating epithelial to mesenchymal transition, migration, and invasion of prostate cancer cell lines. J. Cell Physiol. 2019, 234, 2037–2050. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.D.; Soldini, D.; Jung, M.; Dietrich, D.; Stephan, C.; Jung, K.; Dietel, M.; Vainer, B.; Kristiansen, G. TRPM4 protein expression in prostate cancer: A novel tissue biomarker associated with risk of biochemical recurrence following radical prostatectomy. Virchows Arch. 2016, 468, 345–355. [Google Scholar] [CrossRef]
- Ceylan, G.G.; Önalan, E.E.; Kuloğlu, T.; Aydoğ, G.; Keleş, İ.; Tonyali, Ş.; Ceylan, C. Potential role of melastatin-related transient receptor potential cation channel subfamily M gene expression in the pathogenesis of urinary bladder cancer. Oncol. Lett. 2016, 12, 5235–5239. [Google Scholar] [CrossRef]
- Narayan, G.; Bourdon, V.; Chaganti, S.; Arias-Pulido, H.; Nandula, S.V.; Rao, P.H.; Gissmann, L.; Dürst, M.; Schneider, A.; Pothuri, B.; et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: Identification of candidate amplified and overexpressed genes. Genes. Chromosomes Cancer 2007, 46, 373–384. [Google Scholar] [CrossRef]
- Zhu, L.; Miao, B.; Dymerska, D.; Kuswik, M.; Bueno-Martínez, E.; Sanoguera-Miralles, L.; Velasco, E.A.; Paramasivam, N.; Schlesner, M.; Kumar, A.; et al. Germline Variants of CYBA and TRPM4 Predispose to Familial Colorectal Cancer. Cancers 2022, 14, 670. [Google Scholar] [CrossRef]
- Pérez-Riesgo, E.; Gutiérrez, L.G.; Ubierna, D.; Acedo, A.; Moyer, M.P.; Núñez, L.; Villalobos, C. Transcriptomic Analysis of Calcium Remodeling in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 922. [Google Scholar] [CrossRef]
- Chen, J.; Luan, Y.; Yu, R.; Zhang, Z.; Zhang, J.; Wang, W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Biosci. Trends 2014, 8, 1–10. [Google Scholar] [CrossRef]
- Loo, S.K.; Ch’ng, E.S.; Md Salleh, M.S.; Banham, A.H.; Pedersen, L.M.; Møller, M.B.; Green, T.M.; Wong, K.K. TRPM4 expression is associated with activated B cell subtype and poor survival in diffuse large B cell lymphoma. Histopathology 2017, 71, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Stokłosa, P.; Borgström, A.; Hauert, B.; Baur, R.; Peinelt, C. Investigation of Novel Small Molecular TRPM4 Inhibitors in Colorectal Cancer Cells. Cancers 2021, 13, 5400. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, S.; Liang, S.; Qian, C.; Dong, Y.; Pei, M.; Wang, H.; Wan, G. TRPM4 and TRPV2 are two novel prognostic biomarkers and promising targeted therapy in UVM. Front. Mol. Biosci. 2022, 9, 985434. [Google Scholar] [CrossRef] [PubMed]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lee, J.M. Transcriptional Control of Trpm6 by the Nuclear Receptor FXR. Int. J. Mol. Sci. 2022, 23, 1980. [Google Scholar] [CrossRef]
- Finkel, M.; Goldstein, A.; Steinberg, Y.; Granowetter, L.; Trachtman, H. Cisplatinum nephrotoxicity in oncology therapeutics: Retrospective review of patients treated between 2005 and 2012. Pediatr. Nephrol. 2014, 29, 2421–2424. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Segaert, S.; Safont, M.J.; Demonty, G.; Prenen, H. Management of adverse events during treatment of gastrointestinal cancers with epidermal growth factor inhibitors. Crit. Rev. Oncol. Hematol. 2017, 114, 102–113. [Google Scholar] [CrossRef]
- Xie, B.; Zhao, R.; Bai, B.; Wu, Y.; Xu, Y.; Lu, S.; Fang, Y.; Wang, Z.; Maswikiti, E.P.; Zhou, X.; et al. Identification of key tumorigenesis-related genes and their microRNAs in colon cancer. Oncol. Rep. 2018, 40, 3551–3560. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Chodon, D.; Guilbert, A.; Dhennin-Duthille, I.; Gautier, M.; Telliez, M.S.; Sevestre, H.; Ouadid-Ahidouch, H. Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer 2010, 10, 212. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Shuai, S.; Ding, D.; Li, R.; Luo, R. TRPM8 promotes aggressiveness of breast cancer cells by regulating EMT via activating AKT/GSK-3β pathway. Tumour Biol. 2014, 35, 8969–8977. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xu, Z.; Zou, C.; Wu, D.; Wang, Y.; Yao, X.; Ng, C.F.; Chan, F.L. Ion channel TRPM8 promotes hypoxic growth of prostate cancer cells via an O2 -independent and RACK1-mediated mechanism of HIF-1α stabilization. J. Pathol. 2014, 234, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.; Jia, Z.; Zhao, W.; Zhou, C.; Zhang, R.; Ali, D.W.; Michalak, M.; Chen, X.Z.; Tang, J. Transient Receptor Potential Melastatin 8 (TRPM8) Channel Regulates Proliferation and Migration of Breast Cancer Cells by Activating the AMPK-ULK1 Pathway to Enhance Basal Autophagy. Front. Oncol. 2020, 10, 573127. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Yang, Z.; Wang, B.; Li, S. Menthol induces cell death via the TRPM8 channel in the human bladder cancer cell line T24. Oncology 2009, 77, 335–341. [Google Scholar] [CrossRef]
- Xiao, N.; Jiang, L.M.; Ge, B.; Zhang, T.Y.; Zhao, X.K.; Zhou, X. Over-expression of TRPM8 is associated with poor prognosis in urothelial carcinoma of bladder. Tumour Biol. 2014, 35, 11499–11504. [Google Scholar] [CrossRef]
- Alptekin, M.; Eroglu, S.; Tutar, E.; Sencan, S.; Geyik, M.A.; Ulasli, M.; Demiryurek, A.T.; Camci, C. Gene expressions of TRP channels in glioblastoma multiforme and relation with survival. Tumour Biol. 2015, 36, 9209–9213. [Google Scholar] [CrossRef]
- Zeng, J.; Wu, Y.; Zhuang, S.; Qin, L.; Hua, S.; Mungur, R.; Pan, J.; Zhu, Y.; Zhan, R. Identification of the role of TRPM8 in glioblastoma and its effect on proliferation, apoptosis and invasion of the U251 human glioblastoma cell line. Oncol. Rep. 2019, 42, 1517–1526. [Google Scholar] [CrossRef]
- Liu, J.J.; Li, L.Z.; Xu, P. Upregulation of TRPM8 can promote the colon cancer liver metastasis through mediating Akt/GSK-3 signal pathway. Biotechnol. Appl. Biochem. 2022, 69, 230–239. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Y.; Li, J.; Zheng, X.; Zhou, Q.; Turner, A.; Wu, C.; Lu, B.; Jiang, J. PD-L1 Expression Promotes Epithelial to Mesenchymal Transition in Human Esophageal Cancer. Cell. Physiol. Biochem. 2017, 42, 2267–2280. [Google Scholar] [CrossRef]
- Wang, G.; Cao, R.; Qian, K.; Peng, T.; Yuan, L.; Chen, L.; Cheng, S.; Xiong, Y.; Ju, L.; Wang, X.; et al. TRPM8 Inhibition Regulates the Proliferation, Migration and ROS Metabolism of Bladder Cancer Cells. Onco Targets Ther. 2020, 13, 8825–8835. [Google Scholar] [CrossRef]
- Zhou, X.; Li, M.; Su, D.; Jia, Q.; Li, H.; Li, X.; Yang, J. Cryo-EM structures of the human endolysosomal TRPML3 channel in three distinct states. Nat. Struct. Mol. Biol. 2017, 24, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Wong, C.O.; Zhu, M.X. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium 2015, 58, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Santoni, M.; Maggi, F.; Marinelli, O.; Morelli, M.B. Emerging Role of Mucolipins TRPML Channels in Cancer. Front. Oncol. 2020, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Bartel, K.; Vollmar, A.M.; Biel, M. Endolysosomal Cation Channels and Cancer-A Link with Great Potential. Pharmaceuticals 2018, 11, 4. [Google Scholar] [CrossRef]
- Morgan, A.J.; Platt, F.M.; Lloyd-Evans, E.; Galione, A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem. J. 2011, 439, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Onyenwoke, R.U.; Sexton, J.Z.; Yan, F.; Díaz, M.C.; Forsberg, L.J.; Major, M.B.; Brenman, J.E. The mucolipidosis IV Ca2+ channel TRPML1 (MCOLN1) is regulated by the TOR kinase. Biochem. J. 2015, 470, 331–342. [Google Scholar] [CrossRef]
- Samie, M.; Wang, X.; Zhang, X.; Goschka, A.; Li, X.; Cheng, X.; Gregg, E.; Azar, M.; Zhuo, Y.; Garrity, A.G.; et al. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev. Cell 2013, 26, 511–524. [Google Scholar] [CrossRef]
- LaPlante, J.M.; Sun, M.; Falardeau, J.; Dai, D.; Brown, E.M.; Slaugenhaupt, S.A.; Vassilev, P.M. Lysosomal exocytosis is impaired in mucolipidosis type IV. Mol. Genet. Metab. 2006, 89, 339–348. [Google Scholar] [CrossRef]
- Jung, J.; Cho, K.J.; Naji, A.K.; Clemons, K.N.; Wong, C.O.; Villanueva, M.; Gregory, S.; Karagas, N.E.; Tan, L.; Liang, H.; et al. HRAS-driven cancer cells are vulnerable to TRPML1 inhibition. EMBO Rep. 2019, 20, e46685. [Google Scholar] [CrossRef]
- Kasitinon, S.Y.; Eskiocak, U.; Martin, M.; Bezwada, D.; Khivansara, V.; Tasdogan, A.; Zhao, Z.; Mathews, T.; Aurora, A.B.; Morrison, S.J. TRPML1 Promotes Protein Homeostasis in Melanoma Cells by Negatively Regulating MAPK and mTORC1 Signaling. Cell Rep. 2019, 28, 2293–2305.e1–e9. [Google Scholar] [CrossRef]
- Hu, Z.D.; Yan, J.; Cao, K.Y.; Yin, Z.Q.; Xin, W.W.; Zhang, M.F. MCOLN1 Promotes Proliferation and Predicts Poor Survival of Patients with Pancreatic Ductal Adenocarcinoma. Dis. Markers 2019, 2019, 9436047. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Almasi, S.; Yang, Y.; Yan, C.; Sterea, A.M.; Rizvi Syeda, A.K.; Shen, B.; Richard Derek, C.; Huang, P.; Gujar, S.; et al. The lysosomal TRPML1 channel regulates triple negative breast cancer development by promoting mTORC1 and purinergic signaling pathways. Cell Calcium 2019, 79, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.B.; Amantini, C.; Tomassoni, D.; Nabissi, M.; Arcella, A.; Santoni, G. Transient Receptor Potential Mucolipin-1 Channels in Glioblastoma: Role in Patient’s Survival. Cancers 2019, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Cuajungco, M.P.; Silva, J.; Habibi, A.; Valadez, J.A. The mucolipin-2 (TRPML2) ion channel: A tissue-specific protein crucial to normal cell function. Pflug. Arch. 2016, 468, 177–192. [Google Scholar] [CrossRef]
- Sun, L.; Hua, Y.; Vergarajauregui, S.; Diab, H.I.; Puertollano, R. Novel Role of TRPML2 in the Regulation of the Innate Immune Response. J. Immunol. 2015, 195, 4922–4932. [Google Scholar] [CrossRef]
- García-Añoveros, J.; Wiwatpanit, T. TRPML2 and mucolipin evolution. Handb. Exp. Pharmacol. 2014, 222, 647–658. [Google Scholar] [CrossRef]
- Morelli, M.B.; Nabissi, M.; Amantini, C.; Tomassoni, D.; Rossi, F.; Cardinali, C.; Santoni, M.; Arcella, A.; Oliva, M.A.; Santoni, A.; et al. Overexpression of transient receptor potential mucolipin-2 ion channels in gliomas: Role in tumor growth and progression. Oncotarget 2016, 7, 43654–43668. [Google Scholar] [CrossRef]
- Yu, H.; Xie, M.; Meng, Z.; Lo, C.Y.; Chan, F.L.; Jiang, L.; Meng, X.; Yao, X. Endolysosomal ion channel MCOLN2 (Mucolipin-2) promotes prostate cancer progression via IL-1β/NF-κB pathway. Br. J. Cancer 2021, 125, 1420–1431. [Google Scholar] [CrossRef]
- Cheng, X.; Shen, D.; Samie, M.; Xu, H. Mucolipins: Intracellular TRPML1-3 channels. FEBS Lett. 2010, 584, 2013–2021. [Google Scholar] [CrossRef]
- Spix, B.; Castiglioni, A.J.; Remis, N.N.; Flores, E.N.; Wartenberg, P.; Wyatt, A.; Boehm, U.; Gudermann, T.; Biel, M.; García-Añoveros, J.; et al. Whole-body analysis of TRPML3 (MCOLN3) expression using a GFP-reporter mouse model reveals widespread expression in secretory cells and endocrine glands. PLoS ONE 2022, 17, e0278848. [Google Scholar] [CrossRef]
- Kim, H.J.; Soyombo, A.A.; Tjon-Kon-Sang, S.; So, I.; Muallem, S. The Ca2+ channel TRPML3 regulates membrane trafficking and autophagy. Traffic 2009, 10, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, X.; Zhang, T.; Liu, Z.; Zhao, Y. Identification of a Nine-Gene Signature and Establishment of a Prognostic Nomogram Predicting Overall Survival of Pancreatic Cancer. Front. Oncol. 2019, 9, 996. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Germino, F.J.; Cai, Y.; Zhang, X.; Somlo, S.; Germino, G.G. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 1997, 16, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; You, J.E.; Choi, S.W.; Kang, K.S.; Cho, J.Y.; Lyu, J.; Kim, P.H. Polycystin-1 Enhances Stemmness Potential of Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 4868. [Google Scholar] [CrossRef]
- Streets, A.J.; Needham, A.J.; Gill, S.K.; Ong, A.C. Protein kinase D-mediated phosphorylation of polycystin-2 (TRPP2) is essential for its effects on cell growth and calcium channel activity. Mol. Biol. Cell 2010, 21, 3853–3865. [Google Scholar] [CrossRef]
- Wu, K.; Shen, B.; Jiang, F.; Xia, L.; Fan, T.; Qin, M.; Yang, L.; Guo, J.; Li, Y.; Zhu, M.; et al. TRPP2 Enhances Metastasis by Regulating Epithelial-Mesenchymal Transition in Laryngeal Squamous Cell Carcinoma. Cell. Physiol. Biochem. 2016, 39, 2203–2215. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef]
- Cesare, P.; Moriondo, A.; Vellani, V.; McNaughton, P.A. Ion channels gated by heat. Proc. Natl. Acad. Sci. USA 1999, 96, 7658–7663. [Google Scholar] [CrossRef]
- Trevisani, M.; Smart, D.; Gunthorpe, M.J.; Tognetto, M.; Barbieri, M.; Campi, B.; Amadesi, S.; Gray, J.; Jerman, J.C.; Brough, S.J.; et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat. Neurosci. 2002, 5, 546–551. [Google Scholar] [CrossRef]
- Xin, H.; Tanaka, H.; Yamaguchi, M.; Takemori, S.; Nakamura, A.; Kohama, K. Vanilloid receptor expressed in the sarcoplasmic reticulum of rat skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 332, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Olah, Z.; Szabo, T.; Karai, L.; Hough, C.; Fields, R.D.; Caudle, R.M.; Blumberg, P.M.; Iadarola, M.J. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J. Biol. Chem. 2001, 276, 11021–11030. [Google Scholar] [CrossRef]
- Amantini, C.; Ballarini, P.; Caprodossi, S.; Nabissi, M.; Morelli, M.B.; Lucciarini, R.; Cardarelli, M.A.; Mammana, G.; Santoni, G. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis 2009, 30, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, F.; Yang, Y.; Ma, W.; Lin, Z.; Cheng, N.; Long, Y.; Deng, S.; Li, Z. Recurrent activations of transient receptor potential vanilloid-1 and vanilloid-4 promote cellular proliferation and migration in esophageal squamous cell carcinoma cells. FEBS Open Bio 2019, 9, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Marincsák, R.; Tóth, B.I.; Czifra, G.; Márton, I.; Rédl, P.; Tar, I.; Tóth, L.; Kovács, L.; Bíró, T. Increased expression of TRPV1 in squamous cell carcinoma of the human tongue. Oral. Dis. 2009, 15, 328–335. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Xiang, Q.; Fan, S.; Xiao, T.; Chen, Y.; Zheng, D. Transient Receptor Potential Cation Channel Subfamily V Member 1 Expression Promotes Chemoresistance in Non-Small-Cell Lung Cancer. Front. Oncol. 2022, 12, 773654. [Google Scholar] [CrossRef]
- Weber, L.V.; Al-Refae, K.; Wölk, G.; Bonatz, G.; Altmüller, J.; Becker, C.; Gisselmann, G.; Hatt, H. Expression and functionality of TRPV1 in breast cancer cells. Breast Cancer Targets Ther. 2016, 8, 243–252. [Google Scholar] [CrossRef]
- Gao, N.; Yang, F.; Chen, S.; Wan, H.; Zhao, X.; Dong, H. The role of TRPV1 ion channels in the suppression of gastric cancer development. J. Exp. Clin. Cancer Res. 2020, 39, 206. [Google Scholar] [CrossRef]
- Perálvarez-Marín, A.; Doñate-Macian, P.; Gaudet, R. What do we know about the transient receptor potential vanilloid 2 (TRPV2) ion channel? FEBS J. 2013, 280, 5471–5487. [Google Scholar] [CrossRef]
- Cohen, M.R.; Huynh, K.W.; Cawley, D.; Moiseenkova-Bell, V.Y. Understanding the cellular function of TRPV2 channel through generation of specific monoclonal antibodies. PLoS ONE 2013, 8, e85392. [Google Scholar] [CrossRef]
- Siveen, K.S.; Nizamuddin, P.B.; Uddin, S.; Al-Thani, M.; Frenneaux, M.P.; Janahi, I.A.; Steinhoff, M.; Azizi, F. TRPV2: A Cancer Biomarker and Potential Therapeutic Target. Dis. Markers 2020, 2020, 8892312. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, C.; Yin, S.; Liu, J.; Gao, C.; Lin, Z.; Huang, R.; Huang, J.; Li, Z. Novel role of TRPV2 in promoting the cytotoxicity of H2O2-mediated oxidative stress in human hepatoma cells. Free Radic. Biol. Med. 2015, 89, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Zoppoli, P.; Calice, G.; Laurino, S.; Ruggieri, V.; La Rocca, F.; La Torre, G.; Ciuffi, M.; Amendola, E.; De Vita, F.; Petrillo, A.; et al. TRPV2 Calcium Channel Gene Expression and Outcomes in Gastric Cancer Patients: A Clinically Relevant Association. J. Clin. Med. 2019, 8, 662. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, F.; Du, S.; Li, M.; Wu, T.; Tan, X.; Cheng, W. Mechanism for Regulation of Melanoma Cell Death via Activation of Thermo-TRPV4 and TRPV2. J. Oncol. 2019, 2019, 7362875. [Google Scholar] [CrossRef]
- Oulidi, A.; Bokhobza, A.; Gkika, D.; Vanden Abeele, F.; Lehen’kyi, V.; Ouafik, L.; Mauroy, B.; Prevarskaya, N. TRPV2 mediates adrenomedullin stimulation of prostate and urothelial cancer cell adhesion, migration and invasion. PLoS ONE 2013, 8, e64885. [Google Scholar] [CrossRef]
- Feng, E.; Rydzewski, N.R.; Zhang, M.; Lundberg, A.; Bootsma, M.; Helzer, K.T.; Lang, J.M.; Aggarwal, R.; Small, E.J.; Quigley, D.A.; et al. Intrinsic Molecular Subtypes of Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2022, 28, 5396–5404. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, M.X. TRPV3. Handb. Exp. Pharmacol. 2014, 222, 273–291. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Fan, K.; Li, B.; Li, H.; Qi, H.; Guo, J.; Cao, Y.; Sun, H. Overexpression of TRPV3 Correlates with Tumor Progression in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2016, 17, 437. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Li, Z.; Wang, T.; Yu, D.; Jin, H.; Cao, Y. TRPV3 promotes the angiogenesis through HIF-1α-VEGF signaling pathway in A549 cells. Acta Histochem. 2022, 124, 151955. [Google Scholar] [CrossRef]
- Boudaka, A.; Al-Suleimani, M.; Al-Lawati, I.; Baomar, H.; Al-Siyabi, S.; Zadjali, F. Downregulation of endothelial transient receptor potential vanilloid type 4 channel underlines impaired endothelial nitric oxide-mediated relaxation in the mesenteric arteries of hypertensive rats. Physiol. Res. 2019, 68, 219–231. [Google Scholar] [CrossRef]
- Shibasaki, K.; Tominaga, M.; Ishizaki, Y. Hippocampal neuronal maturation triggers post-synaptic clustering of brain temperature-sensor TRPV4. Biochem. Biophys. Res. Commun. 2015, 458, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Lawhorn, B.G.; Brnardic, E.J.; Behm, D.J. Recent advances in TRPV4 agonists and antagonists. Bioorg Med. Chem. Lett. 2020, 30, 127022. [Google Scholar] [CrossRef] [PubMed]
- Boudaka, A.; Tominaga, M. Physiological and Pathological Significance of Esophageal TRP Channels: Special Focus on TRPV4 in Esophageal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 4550. [Google Scholar] [CrossRef]
- De Logu, F.; Maglie, R.; Titiz, M.; Poli, G.; Landini, L.; Marini, M.; Souza Monteiro de Araujo, D.; De Siena, G.; Montini, M.; Cabrini, D.A.; et al. miRNA-203b-3p Induces Acute and Chronic Pruritus through 5-HTR2B and TRPV4. J. Investig. Dermatol. 2023, 143, 142–153.e110. [Google Scholar] [CrossRef]
- Yu, S.; Huang, S.; Ding, Y.; Wang, W.; Wang, A.; Lu, Y. Transient receptor potential ion-channel subfamily V member 4: A potential target for cancer treatment. Cell Death Dis. 2019, 10, 497. [Google Scholar] [CrossRef]
- Shikano, M.; Ueda, T.; Kamiya, T.; Ishida, Y.; Yamada, T.; Mizushima, T.; Shimura, T.; Mizoshita, T.; Tanida, S.; Kataoka, H.; et al. Acid inhibits TRPV4-mediated Ca²⁺ influx in mouse esophageal epithelial cells. Neurogastroenterol. Motil. 2011, 23, 1020–1028.e1497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Liu, X. TRPV4 induces apoptosis via p38 MAPK in human lung cancer cells. Braz. J. Med. Biol. Res. 2021, 54, e10867. [Google Scholar] [CrossRef]
- Yang, D.; Kim, J. Emerging role of transient receptor potential (TRP) channels in cancer progression. BMB Rep. 2020, 53, 125–132. [Google Scholar] [CrossRef]
- Lee, W.H.; Choong, L.Y.; Mon, N.N.; Lu, S.; Lin, Q.; Pang, B.; Yan, B.; Krishna, V.S.; Singh, H.; Tan, T.Z.; et al. TRPV4 Regulates Breast Cancer Cell Extravasation, Stiffness and Actin Cortex. Sci. Rep. 2016, 6, 27903. [Google Scholar] [CrossRef]
- So, C.L.; Milevskiy, M.J.G.; Monteith, G.R. Transient receptor potential cation channel subfamily V and breast cancer. Lab. Investig. 2020, 100, 199–206. [Google Scholar] [CrossRef]
- Olivan-Viguera, A.; Garcia-Otin, A.L.; Lozano-Gerona, J.; Abarca-Lachen, E.; Garcia-Malinis, A.J.; Hamilton, K.L.; Gilaberte, Y.; Pueyo, E.; Köhler, R. Pharmacological activation of TRPV4 produces immediate cell damage and induction of apoptosis in human melanoma cells and HaCaT keratinocytes. PLoS ONE 2018, 13, e0190307. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Tajiri, Y.; Hasegawa, K.; Matsumoto, S.; Yoshimoto, R.U.; Wada, H.; Kishida, S.; Kido, M.A.; Yoshikawa, H.; Ozeki, S.; et al. The TRPV4-AKT axis promotes oral squamous cell carcinoma cell proliferation via CaMKII activation. Lab. Investig. 2020, 100, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Kanugula, A.K.; Adapala, R.K.; Jamaiyar, A.; Lenkey, N.; Guarino, B.D.; Liedtke, W.; Yin, L.; Paruchuri, S.; Thodeti, C.K. Endothelial TRPV4 channels prevent tumor growth and metastasis via modulation of tumor angiogenesis and vascular integrity. Angiogenesis 2021, 24, 647–656. [Google Scholar] [CrossRef]
- Lee, W.H.; Choong, L.Y.; Jin, T.H.; Mon, N.N.; Chong, S.; Liew, C.S.; Putti, T.; Lu, S.Y.; Harteneck, C.; Lim, Y.P. TRPV4 plays a role in breast cancer cell migration via Ca2+-dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncogenesis 2017, 6, e338. [Google Scholar] [CrossRef]
- Fusi, C.; Materazzi, S.; Minocci, D.; Maio, V.; Oranges, T.; Massi, D.; Nassini, R. Transient receptor potential vanilloid 4 (TRPV4) is downregulated in keratinocytes in human non-melanoma skin cancer. J. Investig. Dermatol. 2014, 134, 2408–2417. [Google Scholar] [CrossRef]
- Dang, S.; van Goor, M.K.; Asarnow, D.; Wang, Y.; Julius, D.; Cheng, Y.; van der Wijst, J. Structural insight into TRPV5 channel function and modulation. Proc. Natl. Acad. Sci. USA 2019, 116, 8869–8878. [Google Scholar] [CrossRef]
- van der Wijst, J.; van Goor, M.K.; Schreuder, M.F.; Hoenderop, J.G. TRPV5 in renal tubular calcium handling and its potential relevance for nephrolithiasis. Kidney Int. 2019, 96, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Miyamoto, T.; Li, K.; Nakagomi, H.; Sawada, N.; Kira, S.; Kobayashi, H.; Zakohji, H.; Tsuchida, T.; Fukazawa, M.; et al. Decreased expression of the epithelial Ca2+ channel TRPV5 and TRPV6 in human renal cell carcinoma associated with vitamin D receptor. J. Urol. 2011, 186, 2419–2425. [Google Scholar] [CrossRef]
- Wang, W.; Yu, S.; Huang, S.; Deng, R.; Ding, Y.; Wu, Y.; Li, X.; Wang, A.; Wang, S.; Chen, W.; et al. A Complex Role for Calcium Signaling in Colorectal Cancer Development and Progression. Mol. Cancer Res. 2019, 17, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Giusti, L.; Cetani, F.; Da Valle, Y.; Pardi, E.; Ciregia, F.; Donadio, E.; Gargini, C.; Piano, I.; Borsari, S.; Jaber, A.; et al. First evidence of TRPV5 and TRPV6 channels in human parathyroid glands: Possible involvement in neoplastic transformation. J. Cell. Mol. Med. 2014, 18, 1944–1952. [Google Scholar] [CrossRef]
- Dai, W.; Bai, Y.; Hebda, L.; Zhong, X.; Liu, J.; Kao, J.; Duan, C. Calcium deficiency-induced and TRP channel-regulated IGF1R-PI3K-Akt signaling regulates abnormal epithelial cell proliferation. Cell Death Differ. 2014, 21, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Shen, Y.X.; Yuan, Y.F. Expression and prognostic roles of TRPV5 and TRPV6 in non-small cell lung cancer after curative resection. Asian Pac. J. Cancer Prev. 2014, 15, 2559–2563. [Google Scholar] [CrossRef] [PubMed]
- Lehen’kyi, V.; Raphaël, M.; Prevarskaya, N. The role of the TRPV6 channel in cancer. J. Physiol. 2012, 590, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Bolanz, K.A.; Hediger, M.A.; Landowski, C.P. The role of TRPV6 in breast carcinogenesis. Mol. Cancer Ther. 2008, 7, 271–279. [Google Scholar] [CrossRef]
- Fecher-Trost, C.; Wissenbach, U.; Beck, A.; Schalkowsky, P.; Stoerger, C.; Doerr, J.; Dembek, A.; Simon-Thomas, M.; Weber, A.; Wollenberg, P.; et al. The in vivo TRPV6 protein starts at a non-AUG triplet, decoded as methionine, upstream of canonical initiation at AUG. J. Biol. Chem. 2013, 288, 16629–16644. [Google Scholar] [CrossRef]
- Wissenbach, U.; Niemeyer, B.A.; Fixemer, T.; Schneidewind, A.; Trost, C.; Cavalie, A.; Reus, K.; Meese, E.; Bonkhoff, H.; Flockerzi, V. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J. Biol. Chem. 2001, 276, 19461–19468. [Google Scholar] [CrossRef]
- Xin, Y.; Malick, A.; Hu, M.; Liu, C.; Batah, H.; Xu, H.; Duan, C. Cell-autonomous regulation of epithelial cell quiescence by calcium channel Trpv6. Elife 2019, 8, e48003. [Google Scholar] [CrossRef]
- Xu, X.; Li, N.; Wang, Y.; Yu, J.; Mi, J. Calcium channel TRPV6 promotes breast cancer metastasis by NFATC2IP. Cancer Lett. 2021, 519, 150–160. [Google Scholar] [CrossRef]
- Semenova, S.B.; Vassilieva, I.O.; Fomina, A.F.; Runov, A.L.; Negulyaev, Y.A. Endogenous expression of TRPV5 and TRPV6 calcium channels in human leukemia K562 cells. Am. J. Physiol. Cell Physiol. 2009, 296, C1098–C1104. [Google Scholar] [CrossRef]
- Ochoa, S.V.; Casas, Z.; Albarracín, S.L.; Sutachan, J.J.; Torres, Y.P. Therapeutic potential of TRPM8 channels in cancer treatment. Front. Pharmacol. 2023, 14, 1098448. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).