Abstract
Ischemic heart disease (IHD), especially myocardial infarction (MI), is a leading cause of death worldwide. Although coronary reperfusion is the most straightforward treatment for limiting the MI size, it has nevertheless been shown to exacerbate ischemic myocardial injury. Therefore, identifying and developing therapeutic strategies to treat IHD is a major medical challenge. Snake venoms contain biologically active proteins and peptides that are of major interest for pharmacological applications in the cardiovascular system (CVS). This has led to their use for the development and design of new drugs, such as the first-in-class angiotensin-converting enzyme inhibitor captopril, developed from a peptide present in Bothrops jararaca snake venom. This review discusses the potential usefulness of snake venom toxins for developing effective treatments against IHD and related diseases such as hypertension and atherosclerosis. It describes their biological effects at the molecular scale, their mechanisms of action according to their different pharmacological properties, as well as their subsequent molecular pathways and therapeutic targets. The molecules reported here have either been approved for human medical use and are currently available on the drug market or are still in the clinical or preclinical developmental stages. The information summarized here may be useful in providing insights into the development of future snake venom-derived drugs.
1. Introduction
Ischemic heart disease (IHD) is the most common cause of death worldwide, accounting for 16% of total diseases [1,2]. IHD includes acute coronary syndrome (ACS), which is composed of unstable angina, myocardial infarction (MI) and sudden cardiac death [3]. MI, which is the major IHD manifestation and cause of death, induces myocardial necrosis and apoptosis in a short time, leading to organ dysfunction and HF with a poor prognosis [3]. IHD may arise for various causes, including hypertension, a major contributor to the high IHD prevalence, and atherosclerosis with coronary blood flow reduction to the heart muscle due to plaque formation and rupture [4,5]. Although myocardial reperfusion is essential to safeguard viable myocardium in MI patients, the process of restoring coronary blood flow to the ischemic myocardium can paradoxically induce myocardial injury in itself—a manifestation that has been termed “myocardial reperfusion injury” [6]. However, the progression of coronary revascularization techniques and angioplasty as well as the significant advances in the MI diagnosis and treatment, such as the development of novel antiplatelet and antithrombotic agents, remain insufficient [7]. This emphasizes the need to identify and develop new therapeutic strategies to reduce the prevalence of IHD as well as hypertension and atherosclerosis.
Some drug development strategies have shown promising results in the use of natural products, such as snake venom, to treat many diseases, including IHD [7,8,9,10]. The concept of using snake venom as a treatment for cardiovascular diseases (CVDs) emerged from the observation that snake bite envenoming is associated with a number of cardiovascular effects, including hypotension/hypertension, cardiotoxicity, MI, cardiac arrest, arrhythmias, coagulopathy and circulatory shock [11]. These potentially lethal manifestations caused by snake venom components have led to their use for thousands of years in traditional medicine as the basis of preparations and decoctions meant to cure cardiovascular disorders [12]. Since 1837, cobra venom has been used to treat vascular problems and ACS [12]. In the 20th century, the first animal toxin-derived drug, captopril, was designed based on bradykinin-potentiating peptides (BPPs) from the venom of the Bothrops jararaca snake and approved in 1981 for the treatment of hypertension [13]. Since then, other venom drugs have been developed based on or inspired by snake venom toxins, such as eptifibatide and tirofiban, and used to prevent heart attacks and thrombotic diseases [14]. Today, there is a renewed interest in snake-venom-based therapies, particularly due to the improvements in high-throughput approaches to the discovery of new toxin-based drugs. Some of these venom-based compounds are currently in clinical trials and many others may appear in the future as studies continue to investigate snake venom [15,16,17,18,19,20]. Snake venom is composed of highly selective and affine compounds [17,21] and mainly include proteins and peptides (90% to 95% of the venom dry weight) that target receptors (acetylcholine receptors, membrane transporters, enzymes) and ion channels (Na+, K+ and Ca2+ channels) [22]. In the CVS, snake toxins may have multiple targets such as cardiac muscle and vascular smooth muscle or the capillary vascular bed, and they include (i) proteins and peptides without enzymatic activity, such as BPPs, natriuretic peptides (NPs), disintegrins, C-type lectin-like proteins (CTLs), three-finger toxins (3FTx), vascular endothelial growth factors (svVEGFs), sarafotoxins (SFTXs), alternagin-C and cysteine-rich secretory proteins (CRISPs); and (ii) proteins with enzymatic activity, such as fibrinolytic enzymes (metalloproteinases and serine proteinases) and phospholipases A2 (PLA2s) [23,24] (Figure 1 and Table 1).
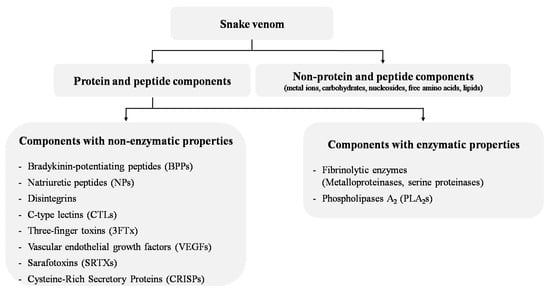
Figure 1.
Main snake venom components with cardiovascular activities.
Overall, cardiovascular research on snake toxins has focused on three main areas related to the management of (i) MI and ACS, (ii) hypertension and (iii) atherosclerosis with the prevention of platelet aggregation and antithrombotic therapy. Therefore, this review describes the snake venom components that interact with the CVS and their use for the potential treatment of IHD and associated diseases (hypertension and atherosclerosis) by emphasizing their targets and mechanisms of action (Table 1). Venom toxins that do not directly interact with the CVS, such as proteins and peptides affecting blood cells and enzyme systems, are not discussed in this review.

Table 1.
Cardiovascular properties of snake venom components.
Table 1.
Cardiovascular properties of snake venom components.
| Proteins and Peptides | Molecular Mass (kDa) | Main Biological Target | Effects on Cardiovascular System | ||
|---|---|---|---|---|---|
| Effects in Ischemic Heart Disease | Effects on Blood Pressure | Effects on Atherosclerosis | |||
| Non-enzymatic toxins | |||||
| Bradykinin-potentiating peptides (BPPs) | 1.5–3.0 | Angiotensin-converting enzyme (ACE). |
|
|
|
| Natriuretic peptides (NPs) | 2.5–5.5 | Natriuretic peptide receptor A, B and C. |
|
| |
| Disintegrins | 4–15 | Platelet receptors αIIbβ3 integrin (glycoprotein IIb/IIIa receptors). |
|
| |
| C-type lectins (CTLs) | 30 | Platelet receptors GPIb and GPIa/IIa (α2β1 integrin). |
| ||
| Three-finger toxins (3FTx) | 5.2–8.0 | Platelet receptors αIIbβ3 integrin, cholinergic receptors. |
|
|
|
| Vascular endothelial growth factors (svVEGFs) | 24–25 | Receptor tyrosine kinases VEGFR-1, VEGFR-2 and VEGFR-3. |
|
| |
| Sarafotoxins (SFTXs) | 2.3–2.7 | Endothelin type-A (ETA) and -B (ETB) receptors. |
|
| |
| Alternagin-C | 21.7 | Integrin α2ß1 and VEGFR-2. |
| ||
| Cysteine-rich secretory proteins (CRISPs) | 23–25 | Voltage-gated ion channels. |
| ||
| Enzymatic toxins | |||||
| Fibrinolytic enzymes (metalloproteinases and serine proteinases) | 8–104 | Fibrinogen (α- and β chains), fibrin. |
|
| |
| Phospholipases A2 (PLA2s) | 13–14 | Cell membrane, secretory PLA2 receptors. |
|
|
|
The upper part of the table presents protein compounds without enzymatic activity: bradykinin-potentiating peptides (BPPs), natriuretic peptides (NPs), disintegrins, C-type lectin-like proteins (CTLs), three-finger toxins (3FTx), vascular endothelial growth factors (svVEGFs), sarafotoxins (SFTXs), alternagin-C and cysteine-rich secretory proteins (CRISPs). The lower part of the table presents protein compounds with enzymatic activity: fibrinolytic enzymes (metalloproteinases and serine proteinases) and phospholipases A2 (PLA2s). ACE, angiotensin-converting enzyme; ACS, acute coronary syndrome; Ang II, angiotensin II; BK, bradykinin; BP, blood pressure; MI, myocardial infarction; HF, heart failure; IHD, ischemic heart disease.
The review focuses on major toxins that directly affect the CVS, including four categories that are classified based on their cardiovascular therapeutic usefulness in humans: toxin-based approved drugs, toxin-based orphan drugs (i.e., synthetic pharmaceutical products that remain commercially undeveloped), toxins of interest in experimental and pre-clinical studies and toxins with limited experimental data (Table 2).
It should be noted that several molecules that were successful in animal assays were not approved in subsequent clinical trials. Several orphan drugs derived from snake venom also reached advanced clinical phases, but were stopped before market approval [82,83]. This is mainly due to the extrapolation of biological data across species, which is a key aspect of biomedical research and drug development. Caution should also be considered in interpreting animal experiments, as the use of in vitro assays in artificial conditions lacking a normal physiological environment and neurohumoral connections may influence study results and bias the subsequent in vivo data. This explains why some studies discussed in this review report conflicting results regarding venom compounds.

Table 2.
Classification of main snake venom toxins according to their usefulness in cardiovascular therapy or research.
Table 2.
Classification of main snake venom toxins according to their usefulness in cardiovascular therapy or research.
| Toxin-Based Approved Drugs | ||||
|---|---|---|---|---|
| Peptide/Protein | Origin | Drug | Mode of Action | Cardiovascular Indications |
| BPP-5a, BPP-9a (Bradykinin-potentiating peptides, BPPs) | Bothrops jararaca | Captopril/Enalapril | ACE inhibitor | Hypertension, MI, HF [84]. |
| Barbourin (Disintegrin) | Sistrurus m. barbouri | Integrilin/Eptifibatide | GPIIb/IIIa antagonist | ACS and antithrombotic therapy [14,41]. |
| Echistatin (Disintegrin) | Echis carinatus | Aggrastat/Tirofiban | GPIIb/IIIa antagonist | ACS and antithrombotic therapy [14,41]. |
| Batroxobin (Fibrinolytic enzyme) | Bothrops moojeni Bothrops atrox | Defibrase | Cleavage of fibrinogen Aα subunit | Anticoagulant therapy in ACS [85,86]. |
| Toxin-based orphan drugs | ||||
| Peptide/Protein | Origin | Drug | Mode of action | Cardiovascular indications |
| DNP (Natriuretic peptide) | Dendroaspis angusticeps | Cenderitide (CD-NP) | NPR-A and -B agonist | HF [37,38]. |
| Fibrolase (Fibrinolytic enzyme) | Agkistrodon contortrix contortrix | Alfimeprase | Cleavage of fibrinogen α- and β-chains | Acute ischemic stroke, acute peripheral arterial occlusion, catheter occlusion [87,88,89]. |
| Ancrod (Fibrinolytic enzyme) | Calloselasma rhodostoma | Viprinex | Cleavage of fibrinogen α-chain | Anticoagulant therapy in thrombosis [88,90]. |
| Toxins of interest in experimental and pre-clinical studies | ||||
| Peptide/Protein family | Origin | Molecule | Mode of action | Cardiovascular indications |
| Natriuretic peptides | Macrovipera lebetina | Lebetin 2 (L2) | NPR-A and -B agonist | Increased BP, MI [15,17,18]. |
| Disintegrins | Rhinocerophis alternates | Alternagin-C | GPIa/IIa (α2β1 integrin) antagonist VEGFR-2 inhibition | MI [62,91]. |
| C-type Lectins (CTLs) | Vipera palaestinae | Vipegitide | GPIa/IIa (α2β1 integrin) antagonist | Antithrombotic therapy [14,92]. |
| Three-finger toxins (3FTx) | Dendroaspis jamesoni kaimosae | Dendroaspin (mambin) | GPIIb/IIIa (Integrin αIIbβ3) antagonist | Antithrombotic therapy [54]. |
| Vascular endothelial growth factors (svVEGFs) | Macrovipera lebetina | ICPP | VEGF-A agonist | Increased BP, acute MI [16]. |
| Sarafotoxins (SRTXs) | Atractaspis engaddensis | SRTX-6c | Endothelin receptors ETB agonist | MI [58,59,60,61]. |
| Toxins with limited experimental data | ||||
| Peptide/Protein family | Origin | Molecule | Mode of action | Cardiovascular indications |
| Cysteine-rich secretory proteins (CRISPs) | Gloydius blomhoffii | Ablomin | L-type voltage-gated Ca2+ channel blockade | Hypertension [63,64]. |
| Phospholipases A2 (PLA2s) | Oxyuranus scutellatus Vipera palaestinae | OSC3 PLA2 isoforms | Hypertension [93]. Atherosclerosis [81]. | |
ACE, angiotensin-converting enzyme; ACS, acute coronary syndrome; DNP, Dendroaspis natriuretic peptide; HF, heart failure; ICPP, increasing capillary permeability protein; MI, myocardial infarction; NPR-A and -B, natriuretic peptide receptors A and B; VEGR-2, vascular endothelial growth factor receptor 2.
In the following part of the review, the order of toxins is presented as indicated in Table 1: proteins and peptides without enzymatic activity are described first (Section 2, Section 3, Section 4, Section 5, Section 6, Section 7, Section 8 and Section 9), followed by compounds with enzymatic activity (Section 10 and Section 11).
2. Bradykinin-Potentiating Peptides
Bradykinin-potentiating peptides (BPPs) are proline-rich oligopeptides of 5 to 14 amino acid residues, predominantly generated by the enzymatic action of kallikreins on endogenous kininogen [94]. They are found in some snake venoms, mainly in Bothrops species, where they represent the first natural bradykinin (BK) agonists and ACE inhibitors [84,95]. In the organism, BPPs increase BK-induced hypotension and decrease vasopressor effects linked to angiotensin I (Ang I) by inhibiting ACE [96,97] (Figure 2). However, snake BPPs enhance BK-induced effects by interacting directly with BK receptors rather than inhibiting BK degradation through ACE inhibition [98]. Accordingly, BK receptor B2 (B2-R) stimulation induces vasodilation as well as anti-fibrosis, anti-inflammatory and anti-reactive oxygen species effects through various intracellular signaling pathways, while the inhibition of angiotensin II (Ang II) production prevents vasoconstriction, inflammation and cardiovascular damage [99,100]. The first BPP was isolated from the venom of the Brazilian viper Bothrops jararaca and then developed into a drug, captopril, against hypertension based on the structure of the peptides BPP-5a and BPP-9a [101]. Captopril was a significant milestone in several aspects: it was (i) the first drug developed from animal venom by converting the toxic effect into therapeutic action; (ii) one of the first examples of ligand-based drug discovery; (iii) the first ACE-targeting drug, becoming a blockbuster and inspiring many other ACE-inhibitor drugs based on the BPP-5a binding motif, like enalapril, lisinopril, quinapril, ramipril, trandolapril and moexipril [102,103]; and (iv) the drug that initiated the development of the next class of antihypertensive drugs, the Ang II-receptor antagonists (ARBs). Considering the therapeutic success of captopril, other snake BPPs have been investigated, particularly due to their specificity for the ACE C-domain. ACE has two homologous catalytic domains (N and C) with different substrate specificities: BK is hydrolyzed approximately equally by both catalytic domains, while Ang I is hydrolyzed approximately three times more efficiently by the C-domain than by the N-domain [104]. Thus, a C-domain-selective inhibitor would be more relevant as it mainly decreases the conversion of Ang I to Ang II by the C-domain and decreases the degradation of BK while preventing its accumulation by preserving ACE N-domain activity. As conventional ACE inhibitors, such as captopril, are not domain-selective and thus induce a higher risk of developing BK-mediated angioedema, this property of C-domain selectivity of BPPs makes them more beneficial than ACE inhibitors such as captopril [105]. Nevertheless, studies have shown that the complete inhibition of Ang I cleavage requires the blockade of both active sites of ACE [106,107,108]. Selectivity of action of the C-domain has been reported for certain snake BPPs, such as R-BPP and Y-BPP derived from Azemiops feae venom [109] and the decapeptide BPP-10c from Bothrops jararaca venom, which was found to be 400-fold more selective for the C-domain (Ki = 0.5 nM) than for the N-domain (Ki = 200 nM) [110]. In contrast, an opposite pattern was found for BPP-12b from the same snake, which was shown to be more selective for the N-domain (Ki = 5 nM) than for C-domain (Ki = 150 nM) [111]. Additionally, other BPPs with associated neuroendocrine functions have been discovered from the venom of Bothrops, Crotalus and Lachesis [112,113,114,115,116,117] and are suggested to belong to a novel class of endogenous neuropeptides [84,118].
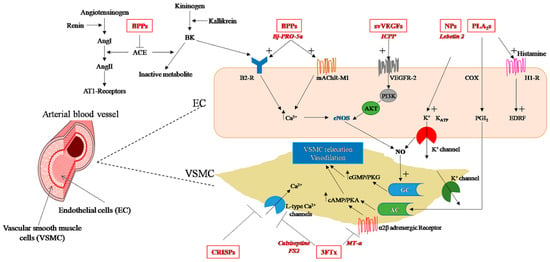
Figure 2.
Snake venom toxins with hypotensive properties (highlighted in red): mechanisms of action in endothelial cells (EC) and vascular smooth muscle cells (VSMC) (details are included in the text). AC, adenylyl cyclase; ACE, angiotensin-converting enzyme; AKT, protein kinase B; AMP, adenosine monophosphate; Ang, angiotensin I; Ang II, angiotensin II; AT1-receptors, Angiotensin II receptor type 1; B2-R, bradykinin receptor B2; BK, bradykinin; BPPs, bradykinin-potentiating peptides; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; COX, cyclooxygenase; CRISPs, cysteine-rich secretory proteins; EDRF, endothelium-derived relaxing factor; eNOS, endothelial nitric oxide synthase; GC, guanylate cyclase; H1-R, histamine H1 receptor; mAChR-M1, M1 muscarinic acetylcholine receptor; MT-α, muscarinic toxin; NO, nitric oxide; PGI2 prostaglandin I2 (prostacyclin); PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; PKG, protein kinase G; PLA2s, phospholipases A2; NPs, snake venom natriuretic peptides; PI3K, phosphatidylinositol-3-kinase; svVEGFs, snake venom vascular endothelial growth factors; 3FTx, three finger toxins; VEGFR-2, vascular endothelial growth factor receptor type 2.
2.1. BPPs and Cardioprotection in Ischemia–Reperfusion Injury
Initially indicated for the treatment of arterial hypertension, the use of BPP-based drugs such as captopril has been extended to prevent, treat or improve symptoms in conditions such as coronary artery disease and HF [29,30]. These drugs showed beneficial effects in limiting myocardial injury and necrosis in various models of coronary artery occlusion [9,25,26,27,28] and in clinical trials [29,30], where they decreased by more than 20% the risk of cardiac arrest and increased survival rates after MI [31]. Captopril has also been shown to slow the progression of arteriosclerotic lesions in clinical and preclinical studies by improving endothelial vasomotor dysfunction, preventing myointimal proliferation after vascular injury, reducing aortic cholesterol content and the progression of carotid and coronary lesions in high-cholesterol diet models [32,33,36,119]. Several lines of evidence indicate that the actions of ACE inhibitors in cardiac ischemia are due to their effect on the inhibition of endogenous BK degradation rather than their effect on Ang II inhibition [8,9,120,121]. BK has been suggested to be a key mediator in ischemic and pharmacological cardioprotective maneuvers via the inhibition of platelet aggregation and plasminogen activation, which could contribute to ACE-inhibitor-induced cardiovascular protection in IHD [9]. The BP effects of ACE inhibitors may also play a role; however, captopril has been found to be cardioprotective in hypertensive as well as in normotensive rats, suggesting that the cardiac effect of captopril may also involve the suppression of Ang II [119]. Recently, other BPPs, such as BPP-10c, which kinetically modulate argininosuccinate synthase activity with nitric oxide (NO) production in endothelial cells, have been shown to reduce hydrogen peroxide-induced oxidative stress [122,123]. Since post-MI reperfusion induces oxidative stress, NO deficiency and endothelial dysfunction leading to severe cardiac injury, BPP-10c could be further investigated as a promising therapeutic strategy for IHD.
2.2. BPPs with Potential in Hypertension Therapy
A persistent increase in BP is a sign of hypertension that can lead, if untreated, to IHD [4,5]. To date, numerous antihypertensive drugs with different mechanisms of action have been developed. Snake venom BPPs decrease BP by enhancing the action of the endogenous vasodilator BK and inhibiting the vasoconstrictor Ang II [96,97] (Figure 2). However, it is not yet well established whether the cardiovascular effects of BPPs are only due to ACE inhibition [84]. Some studies have revealed that there are BPPs, such as BPP-10c, that possess non-ACE-inhibition mechanisms, such as the stimulation of argininosuccinate synthase, which leads to the production of NO in endothelial cells and a decrease in BP [84]. The BPP-5a peptide has also been shown to exert a long-lasting antihypertensive effect in spontaneously hypertensive rats (SHRs) via a unique target involving an NO-dependent mechanism [124,125,126]. In another study, the BPP-5a peptide was found to promote vasodilation by interacting with M1 muscarinic cholinergic receptors and the BK receptor B2-R and triggering NO synthesis by the endothelium [125]. Other members of the BPPs with a common hypotensive effect have been identified in different snake species, including Bothrops jararaca (BPP-7a) [127], Bothrops insularis [128], Lachesis muta (Lm-BPP 1–5) [115], Agkistrodon bilineatus [129], Lachesis muta rhombeata (LmrBPP-9) [130], and Crotalus durissus cascavella (BPP-Cdc) [131]. Different physiological mechanisms underlie the hypotensive effect of BPPs. Therefore, these studies contribute to the understanding of BP regulation and the identification of new therapeutic targets useful in the treatment of CVDs.
3. Natriuretic Peptides
Venom natriuretic peptides (NPs) are part of the NP family, which is composed of three mammalian NP isoforms, namely atrial NP (ANP), B-type NP (BNP), C-type NP (CNP) and an isoform originating from the Dendroaspis snake venom (DNP) [132]. These peptides are composed of approximately 20 to 50 amino acid residues and contain a conserved 17-residue disulfide ring [132]. NPs target membrane NP receptors (NPRs) of the reno-cardiovascular system to induce natriuresis, diuresis and vasorelaxation, thereby reducing blood pressure and volume [130,131] (Figure 2). NPs isolated from snake venom, although of a wide variety, nevertheless exhibit the same cardio-renal properties, allowing them to exert a broad spectrum of physiological effects that can be potentially used to treat CVDs [133]. Various snake venom NPs have been identified, such as Lebetin 2 (L2) from the blunt-nosed viper (Macrovipera lebetina) [134]; NP2_Casca from the cascavella rattlesnake (Crotalus durissus cascavella) [135]; PtNP-a from the eastern brown snake (Pseudonaja textilis) [136]; PaNP-c from the king brown snake (Pseudechis australis) [136]; PNP from the Persian horned viper (Pseudocerastes persicus) [137]; three natriuretic-like peptides, TNP-a, TNP-b, and TNP-c, from the venom of the inland taipan (Oxyuranus microlepidotus) [138]; KNP from Bungarus flaviceps [139] and Lm-CNP from Lachesis muta [115]. These venom peptides have been widely used as a promising basis for the design of cardiovascular drugs with improved activity, affinity and selectivity as well as longer stability and half-life [140,141].
3.1. Natriuretic Peptides and Cardioprotection in Ischemia–Reperfusion Injury
Recently, a drug developed by Novartis on the basis of its ability to potentiate endogenous NPs was commercialized to reduce the risk of cardiovascular death and hospitalization after HF [142]. Nesiritide is another drug used as a recombinant human BNP to improve the hemodynamic status of HF patients. However it induces severe adverse effects, such as hypotension, which currently limits its clinical use, highlighting the need for developing NP analogs without these side effects [143]. In this context, venom NPs have been used to develop analogues with the prospect of clinical application; among these, cenderitide is the most advanced [141]. It should be noted that NPs have the common property of inducing an NO increase and protein kinase G activation, which mediates their vasorelaxant effect [138,144]. Cenderitide (formerly CD-NP) was designed to overcome the hypotensive properties of NPs while improving cardiovascular function through the activation of the particulate guanylyl cyclases NPR-A and -B [141]. The chimeric peptide, composed of the 15-residue C-terminal tail of DNP and CNP, showed less adverse hypotensive effects than current NP drugs in preclinical studies as well as in the late phases of clinical trials [37,38], but the licensing agreement to further develop this peptide was recently terminated [82]. More recently, L2 from Macrovipera lebetina [134,145] has been proved to improve cardiac function and reduce necrosis, fibrosis and inflammation in a reperfused MI model [15,18]. Cardiac effects were shown to be mediated through an NPR-A–cyclic guanosine monophosphate (cGMP)-dependent pathway, downstream activation of mitochondrial KATP channels, and inhibition of the mitochondrial permeability transition pore (mPTP) at the time of reperfusion [15,17,18]. These results also showed that L2 can use a previously undiscovered mechanism involving M2 macrophage polarization and activation of the NPR-A–cGMP–Interleukin 10 axis to exert its post-MI anti-inflammatory effects [20]. Other ongoing experiments suggest that L2 could exert at least part of its preclinical effects via the involvement of the natriuretic receptor NPR-C, classically associated with the clearance of NPs, thus confirming the mechanistic role of NPR-C in NP cardiovascular effects [17,18]. Thus, these features demonstrate that venom compounds such as L2 may be of great therapeutic significance, especially in MIs with intense and prolonged inflammatory responses. These studies also provide novel insights into the mechanisms of myocardial repair triggered by NPs and could have good prospects for discovering new therapeutic targets and options in the treatment of IHD.
3.2. Natriuretic Peptides with Potential in Hypertension Therapy
Snake venom NPs exhibit the same hemodynamic properties as their mammalian counterparts. They decrease BP by inducing diuresis, natriuresis and vasodilation via endothelium-dependent vasorelaxation and by inhibiting the renin–angiotensin–aldosterone system [146]. Although NP hemodynamic properties have been proven, NPs are nevertheless not used clinically for the treatment of hypertension. In fact, potential NP-based drugs have been synthesized to overcome these hypotensive properties, as was the case with DNP [141]. However, several preclinical studies have demonstrated the vasorelaxant and vasodilator effect of natriuretic toxins. Fon instance, venom-derived L2 induces a dose-dependent decrease in the arterial BP in mice [18] (Figure 2). NP2_Casca was also shown to induce an endothelium-dependent relaxant effect on the thoracic aortic rings, likely involving K+ channels, along with a decrease in heart rate (HR) and arterial BP and an improvement in renal function [135]. Coa_NP, identified in Crotalus oreganus abyssus venom, also produces endothelium-dependent vasorelaxation in the thoracic aortic rings by increased NO production but without binding to the natriuretic receptor NPR-A [39]. For some other snake venom NPs, such as PaNP-c and PtNP-a, a dual mechanism was observed with ACE inhibition together with an increase in intracellular cGMP concentration [136].
4. Disintegrins
Snake venom disintegrins are small cysteine-rich proteins (4–15 kDa) that have been demonstrated to be potent inhibitors of various integrins. They can act on platelet integrin αIIbβ3, also known as glycoprotein IIb/IIIa receptors, to inhibit platelet aggregation in vitro and in vivo by blocking fibrinogen and von Willebrand factor binding to the GPIIb/GPIIIa complex on the surface of platelets [147] (Figure 3). Thus, antiplatelet drugs targeting this receptor could be indicated in the case of ACS and in percutaneous coronary interventions [14]. Snake venom disintegrins have RGD or KGD motifs that allow them to bind integrin and prevent fibrinogen binding to platelets [148,149]. An RGD tripeptide sequence is the main recognition site for the αIIbβ3 integrin receptor. As such, RGD disintegrins have been the most studied, and research on these have resulted in the design and synthesis of novel antiplatelet pharmaceutical compounds [148]. Typical antiplatelet drugs found in clinics to treat ACS include Integrilin (eptifibatide), a heptapeptide derived from barbourin, a protein found in the venom of the American Southeastern pygmy rattlesnake (Sistrurus miliarius barbourin) and Aggrastat (tirofiban), a small molecule based on the structure of echistatin that is found in the venom of the saw-scaled viper (Echis carinatus) [14]. Several other disintegrins with common antiplatelet aggregation potential have been purified from venoms of snakes such as the common bamboo viper (Trimeresurus gramineus) [147], the Malayan pit viper (Calloselasma rhodostoma) [150], the Halys pit viper (Gloydius halys) [151] and the fer-de-lance (Bothrops asper) [152]. These disintegrins have been described as highly potent and selective GPIIb/IIIa antagonists in vitro and in vivo [147,153]. In addition to their antiplatelet effect, some disintegrins have angiogenic properties. For instance, CC5 and CC8, two highly homologous disintegrins isolated from the North African viper Cerastes cerastes venom, inhibit angiogenesis by disrupting αvβ3 and α5β1 binding in vitro in human microvascular endothelial cells (HMEC-1) and human brain microvascular endothelial cells (HBMEC) [154], ex vivo in a rat aortic ring assay and in vivo in a chick embryo chorioallantoic membrane model (CAM). These effects, which require an RGD-loop disintegrin, have been shown to be mediated by pro-apoptotic pathways involving the downregulation of the FAK/AKT/PI3K axis and caspase activation. Similarly, alternagin-C, a disintegrin-like peptide isolated from Bothrops alternatus snake venom, impairs angiogenesis following binding to α2β1 integrin and inhibition of VEGF/VEGFR-2 signaling [91].
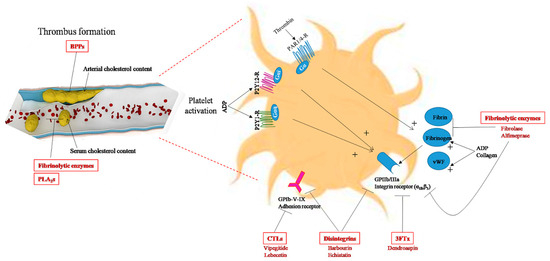
Figure 3.
Snake-venom toxins with anti-atherogenic properties (highlighted in red). Snake-venom-derived molecules may exert anti-atherogenic effects by lowering cholesterol levels in the blood or in the arterial wall (BPPs, fibrinolytic enzymes, PLA2s), or by inhibiting arterial thrombosis by blocking platelet aggregation (disintegrins, CTLs, 3FTx, fibrinolytic enzymes,) or by activating fibrinolysis in blood clots (fibrinolytic enzymes: metalloproteases and serine proteases). Members of the disintegrin and fibrinolytic enzyme families are currently the main toxins used as antithrombotic drugs. Disintegrins act on the platelet surface via various receptors and glycoproteins, and fibrinolytic enzymes act by digesting fibrin to inhibit thrombus formation. ADP, adenosine diphosphate; BPPs, bradykinin-potentiating peptides; CTLs, C-type lectins, GPIb-V-IX, platelet glycoprotein adhesion receptor; GPIIb/IIIa, platelet-specific integrin receptor (αIIbβ3); P2Y12-R, P2Y1-R, platelet G-protein-coupled receptors for ADP; PAR1/4-R, protease-activated receptors (G-protein-coupled receptors for thrombin); PLA2s, phospholipases A2; 3FTx, three-finger toxins; vWF: von Willebrand factor.
4.1. Disintegrins and Cardioprotection in Ischemia–Reperfusion Injury
The snake venom disintegrins-based drugs Integrilin (or eptifibatide) and Aggrastat (or tirofiban) designed from barbourin and echistatin, respectively, are the most frequently studied GPIIb/IIIa inhibitors [41]. They were approved by the FDA in 1988 for the treatment of ACS [14]. Both drugs are specific αIIbβ3 antagonists, designed based on the KGD pharmacophore for eptifibatide and the RGD pharmacophore for tirofiban. They exert their actions by preventing the binding of fibrinogen to αIIbβ3 of human platelets and thereby inhibiting platelet aggregation and thrombus formation (Figure 3). In preclinical studies, eptifibatide and tirofiban have been shown to improve microvascular flow, reduce the infarct size and decrease the number of infiltrating platelets and leucocyte recruitment and accumulation in the ischemic myocardium at the onset of reperfusion [40]. Clinically, these drugs reduce the risk of IHD and prevent thrombotic complications and major adverse cardiac events that can occur during and after percutaneous coronary intervention [41,42]. However, despite the beneficial effects of GPIIb/IIIa inhibitors, they are not included in the current therapeutic protocol for MI patients. Due to the risk of bleeding complications associated with GPIIb/IIIa inhibitors, only patients with a high risk of thrombosis are currently treated with GPIIb/IIIa inhibitors [40]. Consequently, numerous preclinical studies have focused on the therapeutic potential of other snake-venom disintegrins in IR injury. Some of them are under experimental study and have good prospects for the treatment of IHD and related diseases, such as alternagin-C from the Bothrops alternatus venom, which has been found to protect cardiomyocytes against hypoxia–reoxygenation-induced negative inotropism [62].
4.2. Disintegrins in Atherosclerosis Therapy
Most cases of acute MI are caused by the rupture of an atherosclerotic plaque associated with subsequent thrombus formation [155]. Atherosclerosis is a fibroproliferative and inflammatory process occurring in arterial walls in response to several factors, such as hypertension and hypercholesterolemia. Low-density-lipoprotein (LDL) cholesterol is an important causal risk factor for atherosclerotic CVDs. In reperfused MI, elevated LDL cholesterol promotes thromboinflammation through excess microvascular endothelial von Willebrand factor and platelet adhesion, resulting in less microvascular reflow and a larger infarct size [156]. The rupture of an atherosclerotic plaque leads to platelet aggregation, thrombosis and an increased risk of ACS [155,157]. Thus, in the presence of elevated LDL cholesterol, therapies that suppress endothelial-associated von Willebrand factor and platelet aggregation may promote recovery of the left ventricular function and protect against IR and subsequent remodeling. In this context, chronic treatment with tirofiban and other antiplatelet drugs has been shown to decrease carotid atherosclerotic plaque inflammation and reduce serum levels of inflammatory markers such as Hs-CRP, IL-6 and sICAM-1 in ACS patients [43,44]. In addition, the combination of tirofiban and other drugs has been demonstrated to have a synergistic effect and significantly attenuate the inflammation response and improve the prognosis of ACS patients [45], which may lead to stabilization of the plaque, highlighting an anti-atherogenic role of disintegrin-based antiplatelet drugs.
5. C-Type Lectin-like Proteins
C-type lectins (CTLs) are Ca2+-dependent carbohydrate-binding proteins that share structural homology in their carbohydrate-recognition domains (CRDs). CTLs are widely represented in nature and are classified into several types according to their structure, cellular target and mode of action [47]. However, those found in snake venom mainly present a heterodimeric structure, with two subunits α and β that are responsible for the inhibitory effect on platelet function [47]. Heterodimeric CTLs have been shown to inhibit platelet aggregation by inhibiting receptor GPIb binding to von Willebrand factor platelet [47] (Figure 2). Among the toxins interacting with the GPIb receptor, several molecules have been characterized, such as lebecetin from Macrovipera lebetina [158], CHH-A and B from Crotalus horridus horridus [159], echicetin from Echis carinatus [160], TSV-GPIB-BP from Trimeresurus stejnegeri [161], tokaracetin from Trimeresurus tokarensis [162] and agkistin from Agkistrodon acutus [163]. Other snake CTLs inhibit platelets by binding to GPIa/IIa platelet receptors (α2β1 integrin), such as EMS16 from Echis multisquamatus [164] and rhodocetin from Calloselasma rhodostoma [165]. Recently, vipegitide, developed from CTL derived from Vipera palaestinae venom and targeting the GPIa/IIa receptor, was shown to be effective in inhibiting platelet adhesion to collagen, but this work is still in the preclinical stage [14,92]. Overall, despite the supportive role that CTLs play in inhibiting platelet adhesion, there is relatively poor drug development targeting snake-venom CTLs with respect to arterial thrombosis [14]. Indeed, due to their interaction with various integrins, CTLs have been mainly studied in angiogenic pathologies such as cancer and ocular neovascularization [19,166,167]. This is the case for lebecetin which has, in addition to its antiplatelet properties [46], anti-integrin (αvβ3, αvβ5 and α5β1) effects [166], and which is currently patented for the treatment of neovascular disorders such as age-related macular degeneration (AMD) and diabetic retinopathy [19].
6. Three-Finger Toxins
Three-finger toxins (3FTx) represent one of the most abundant families of snake-venom toxins. They contain 57–82 amino acid residues, characterized by three β-structural loops extending from a compact hydrophobic core that is stabilized by 4–5 disulfide bridges [168]. The pharmacological effects of 3FTx are extremely varied due to the wide range of molecular targets they recognize, such as L-type calcium channels and nicotinic and muscarinic acetylcholine receptors, which leads to different biological properties, particularly in the CVS [51,169]. It has been found that 3FTx may induce cardiac arrest [170] and changes in BP [52,171] and HR [48], inhibit platelet aggregation [172], blood coagulation [173,174], or cell adhesion [175]. Currently there are increasingly new sequences of 3FTx being available in public databases, which should provide new opportunities for the development of therapeutics or research probes targeting the CVS [51].
6.1. Three-Finger Toxins and Cardioprotection in Ischemia–Reperfusion Injury
Several 3FTx may be of interest in reducing IR injury following MI [51]. Some of them exert antiplatelet effects, such as the compound KT-6.9, purified from the monocled cobra (Naja kaouthia) and acting via the ADP receptors located at the surface of the platelets [176]. Other 3FTx include muscarinic toxins (MTα from Dendroaspis polylepis [177]), β-adrenergic toxins (β-cardiotoxin from the king cobra (Ophiophagus hannah) [48]), cholinergic toxins [178] or acid-sensing ion channel (ASIC)-inhibiting toxins (mambalgins from mambas (Dendroaspis) [179]) that may be potentially useful in the treatment of chronic HF and BP disorders, although there is no strong evidence of their applicability in IHD. Certain 3FTx from Naja naja siamensis with high toxicity for heart and vessels, have been found to induce a positive inotropic response [49]. This group of toxins could be potentially useful in cardiac conditions with decreased pumping function of the heart, particularly because there is only one pharmacological class of cardiotonic drugs, i.e., cardiac glycosides, used in the chronic HF with reduced left ventricular ejection function [180].
6.2. Three-Finger Toxins in Hypertension Therapy
Some 3FTx family members such as calciseptine [52], FS2 [171], C10S2C2 [181] and S4C8 toxins [182], all purified from mamba snakes, have the property of acting on various ion channels such as the L-type Ca2+-channel blockade, which leads to a vasorelaxant effect on smooth muscles, causing vasodilation and a reduction in BP [52,53] (Figure 2). In humans, L-type Ca2+-channel blockers are used for the treatment of hypertension, angina and arrhythmia [183]. However, they lack selectivity and are associated with serious adverse events. In this context, synthetic analogues based on calciseptine and FS2 have been generated via the identification of the amino acid residues of the toxin acting as a potential attachment region at the Ca2+-channel-receptor site. These analogues have been proven to have conformational and molecular properties similar to those of nifedipine, a 1,4-dihydropyridine L-type Ca2+-channel blocker currently used as antihypertensive treatment [184].
6.3. Three-Finger Toxins in Atherosclerosis Therapy
Platelets, which are major players in the atherosclerosis process, express receptors that are molecular targets of many snake toxins, including 3FTx. Several 3FTx that inhibit platelet aggregation, such as dendroaspin (also named mambin) [54] and S5C1 [55] from Dendroaspis jamesoni kaimosae, thrombostatin from Dendroaspis angusticeps [56] and γ-bungarotoxin from the venom of Bungarus multicinctus, have an RGD tripeptide sequence in their structure, which is crucial for binding to platelet integrin αIIbβ3 and blocking platelet aggregation from binding to fibrinogen at a nanomolar level (Figure 3). Changes in the optimal conformation of the RGD tripeptide have been shown to modify the antiplatelet activity of 3FTx-related proteins [185]. Recently, other 3FTx, such as TA-bm16 and NTL2 identified from Bungarus multicinctus [51], have also been shown to potentially display a similar function.
7. Vascular Endothelial Growth Factor
VEGF-like proteins derived from snake venom, svVEGFs or VEGF-F, are a subgroup of the VEGF family that includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E (viral VEGF) and placental growth factor (PlGF) [24]. VEGF binds to endothelial tyrosine kinase cell receptors known as VEGFR-1 and -2 to mediate its angiogenic, mitogenic and anti-apoptotic activities [186]. Receptor-binding assays demonstrated that svVEGFs are highly specific ligands [57], selectively interacting with VEGFR-2, as is the case with vammin from the venom of the sand viper Vipera ammodytes; or with VEGFR-1, as for Tf-svVEGF and Pm-VEGF from the venoms of Trimeresurus flavoviridis and Protobothrops mucrosquamatus, respectively [187]. After an acute MI, VEGF induces angiogenesis by initiating the reactive oxygen species–endoplasmic reticulum stress–autophagy axis in the vascular endothelial cells [188]. Therapeutic angiogenesis has shown promising results in preclinical studies in acute MI models by inducing safe, effective and sustained myocardial angiogenesis, increasing perfusion of the infarct border zone and the ventricular ejection fraction [189]. Since after MI, endogenous angiogenesis cannot maintain normal capillary density [190]. Thus, VEGF-based treatment strategies may help restore adequate vasculogenesis and be useful as a pro-angiogenic therapy in IHD. In mice IR experiments, svVEGF purified from Macrovipera lebetina venom and called Increasing Capillary Permeability Protein (ICPP), was found to exert cardioprotective effects by reducing the infarct size through the activation of VEGFR-2 receptors and the ERK pathway, and the subsequent inhibition of mPTP opening at the onset of reperfusion [16]. This protein and others, such as vammin and VR-1 [57], also induce endothelium-dependent vasorelaxation via the release of NO and PGI2 [191] thus leading to hypotension, higher than that induced by natural VEGF (Figure 2) [16,57]. Other VEGF-like proteins such as heparin-binding dimeric hypotensive factor (HF) purified from Aspic viper (Vipera aspis aspis) venom have also been demonstrated to induce hypotensive effects [192].
8. Sarafotoxins
Sarafotoxins (SRTXs) are a family of small 2.5 kDa peptides that are overproduced in Atractaspis venoms. They have a common structural pattern consisting of 21 amino acids and two invariant disulfide bridges between cysteines 1 and 15 and cysteines 3 and 11. A longer isoform, SRTX-m of 24 amino acids with three additional C-terminal residues, has, however, been isolated from the venom of Atractaspis microlepidota microlepidota and shows high sequence homology to SRTX-b [193]. SRTXs are potent vasoconstrictor peptides with approximately 60% sequence homology and functional identity to the mammalian vasoconstrictor hormones endothelins [194,195]. Several isoforms of SRTXs can coexist within the same venom with different effects, such as SRTX-a, -b, and -c that are all purified from the snake venom Atractaspis engaddensis and that present different vasoactive effects linked to their primary structure [196]. SRTXs also exhibit opposite cardioprotective effects. For example, unlike other SRTX isoforms, SRTX-6c, a specific agonist of the ETB endothelin receptor, exerts vasorelaxant and vasodilatory effects [60,61]. Administration of SRTX-6c prior to coronary occlusion has been shown to significantly reduce infarct size and the incidence of arrhythmias in several experimental models [58,59,60,61] through the selective activation of ETB receptors, NO release and subsequent cardiomyocyte mitochondrial KATP-channel opening during IR [59]. Conversely, SRTX-b, whose effects are mainly associated with extracellular calcium input via L-type Ca2+ channels, causes cardiac arrest and death in mice within minutes of intravenous administration [197]. Despite their overall detrimental effects on the CVS related to their vasoconstrictor and arrhythmogenic properties, SRTXs are used in basic research for endothelin receptor labeling and in the development of vasospasm models [198].
9. Cysteine-Rich Secretory Proteins
Snake venom cysteine-rich secretory proteins (CRISPs) are a family of 23–25 kDa proteins containing eight disulfide bonds and are characterized by a single polypeptide protein with a molecular weight between 20 and 30 kDa [199]. CRISPs are present in the venoms of viperids, elapids and colubrids and absent in the venoms of atractaspidids and those of certain elapids such as coral snakes [22]. The first CRISP group to have been characterized includes ablomin from Gloydius blomhoffii, triflin from Protobothrops flavoviridis, latisemin from Laticauda semifasciata and tigrin from Rhabdophis tigrinus, which have the non-enzymatic inhibitory activity of various membrane channels involved in the regulation of vascular tone, such as voltage-gated L-type Ca2+ channels and high-conductance calcium-activated potassium (BKCa) [63,64]. Other members of the CRISP family, such as natrin, block the skeletal isoform of the ryanodine receptor [67] and KV1.3 voltage-gated potassium channels [65]. Other CRISP toxins have been shown to act on other cellular targets, such as inhibiting angiogenesis [200,201] and increasing blood vascular permeability in vivo and in vitro [202]; however in most cases, these proteins have not been experimentally isolated or characterized, and their targets and biological roles remain poorly understood.
10. Fibrinolytic Enzymes
Apart from antiplatelet compounds that have antithrombotic activity, snake venom also includes important thrombolysis molecules such as fibrinolytic enzymes involved in fibrinolysis, which is the process of dissolving blood clots (Figure 3). These enzymes break down fibrin-rich clots and help prevent additional clot formation by their action on fibrinogen (Figure 3). Given that these enzymes should not be inactivated by mammalian blood inhibitors, a significant number of studies have focused on their identification and characterization from snake venom, particularly for the development of thrombolytic enzymes to treat occlusive thrombi [203]. Two classes of venom fibrinolytic enzymes have been identified, the metalloproteinases and serine proteinases, with the same biological effects but different mechanisms of action by targeting different amino acid sequences of fibrinogen. Several fibrinolytic enzymes have been isolated and purified from snake venom and show potential use in thrombolytic therapy, including fibrolase, a fibrinolytic metalloproteinase isolated from Agkistrodon contortrix venom. Fibrolase possesses three disulfide bonds, is non-glycosylated and binds an intrinsic zinc atom that is essential for activity and structural integrity. It acts directly on fibrinogen/fibrin in both arteries and veins by cleaving the α- and β-chains of fibrinogen but not the γ-chain [87,88,89]. The recombinant fibrolase alfimeprase has been shown to have the same in vivo and in vitro activities as native fibrolase and to be much more potent than plasminogen activators [83]. Hence, treatment with alfimeprase would be more beneficial, as systemic plasminogen activation can lead to serious side effects, such as intra-cranial hemorrhaging [204]. Since then, other fibrinolytic enzymes have been identified and purified from other species such as lebetase, a serine beta-fibrinolytic proteinase from Macrovipera lebetina [205], basiliscus fibrases from the venom of the Mexican West Coast rattlesnake (Crotalus basiliscus) [72] and graminelysin I from Trimeresurus gramineus [205].
10.1. Fibrinolytic Enzymes and Cardioprotection in Ischemia–Reperfusion Injury
Following MI, thrombolytic therapy is the primary approach used for reperfusion to restore blood flow in the thrombus-obstructed coronary artery. The fibrinolytic enzyme-based drug for the treatment of thrombotic diseases is Defibrase, which has thrombin-like activity and is developed from batroxobin, derived from the venom of the Brazilian lancehead snake (Bothrops moojeni) [85]. It exhibits anticoagulant properties by converting fibrinogen into fibrin through the cleavage of the α-chain [86]. In clinical trials in MI patients, Defibrase was effective in inducing significant clot thrombolysis and coronary artery recanalization, with a low risk of recurrence and bleeding complications [69]. In preclinical studies, batroxobin was shown to reduce the mortality rate after IR, improve cardiac contractility and decrease myocardial injury and levels of ACS biomarkers [68]. Since Defibrase, other snake defibrinogenating agents have been identified. Reptilase is another trade name for a thrombin-like serine protease that was similarly developed from the common lancehead (Bothrops atrox), tested in animals, and used in patients to achieve coronary reperfusion [206]. In contrast, fibrolase (Alfimeprase) and ancrod (Viprinex) reached phase III clinical trials but failed to have marketing approval, since they did not meet the expected therapeutic endpoints, which led to the termination of their development [90]. However, experimental studies carried out previously on these molecules have shown the beneficial effects of fibrolase in the canine model of carotid artery thrombosis [88]. In this model, the occlusive thrombus was lysed within 6 min of initiating fibrolase infusion. Treatment with ancrod, however, had no effect on infarct size in the rabbit coronary ligation model [207] and did not protect the myocardium from reperfusion injury after acute MI in dogs [208].
10.2. Fibrinolytic Enzymes in Atherosclerosis Therapy
Snake-venom-derived fibrinolytic enzymes such as batroxobin and the batroxobin based-drug Defibrase may have an effect on atherosclerotic lesions. The first was found to possess the action of stabilizing the atherosclerotic plaque in a rabbit model of atherosclerosis [70]. From these results, the atherosclerotic plaque in the batroxobin-treated groups tended to be static four weeks after treatment. For the batroxobin-based drug Defibrase, experimental data have shown that treatment with Defibrase can significantly inhibit atherosclerosis in rabbits [71]. In this study, Defibrase decreased the mean aortic plaque area in the thoracic and abdominal aorta as well as the total cholesterol content compared with control animals. In addition, Defibrase treatment reduced the number of plaques at the small artery openings in the thoracic aorta and the abdominal aorta. Cross sections performed at the upper third of all hearts showed that Defibrase administration reduced the degree of stenosis of coronary artery branches in the heart wall compared with untreated animals.
11. Phospholipases A2
PLA2s are the most abundant proteins found in Viperidae snake venom and one of the major toxic components with a broad spectrum of pharmacological effects [209]. There are at least 15 distinct PLA2s groups, clustered into four major enzyme types: cytosolic PLA2s (cPLA2), Ca2+-independent PLA2s (iPLA2), secreted PLA2s (sPLA2) and platelet-activating factor acetylhydrolases (PAF-AH) [210]. Snake venom phospholipases belong to the sPLA2 family and exhibit diverse effects, including neurotoxicity, cardiotoxicity, myotoxicity, inhibition of blood coagulation, modulation of platelet function, as well as antiangiogenic activity independent of their enzymatic function [211]. In the CVS, snake venom PLA2 can cause myocardial structural and functional alterations [73,74,75,76]. This cardiotoxic activity can differ considerably between PLA2s of different venoms, e.g., PLA2s from Ophiophagus hannah and Naja nigricollis cause myocardial damage [74,76,77], in contrast to the PLA2 from the venom of Naja atra, which lacks cardiotoxicity [77]. PLA2s from certain snake species, such as the Elapinae and Viperinae subfamilies can also decrease BP through the hydrolysis of membrane glycerophospholipids and the subsequent release of arachidonic acid [212], a precursor of cyclooxygenase metabolites (prostaglandins or prostacyclin (PGI2)) (Figure 2). PLA2 fractions from Vipera russelli venom injected intravenously into animals induced marked vasodilation and a decrease in the mean arterial pressure and renal vascular resistance, partly due to increased plasma PGI2 and thromboxane A2 levels and a decrease in plasma renin activity [78,79]. The hypotensive and vascular relaxing effects of PLA2s have also been reported with toxins from the Australasian elapid Papuan taipan (Oxyuranus scutellatus) venom, such as the OSC3 peptide, whose antihypertensive activity is mediated by cyclooxygenase metabolites, BK and H1-receptors [93]. Some reports also suggested anti-atherogenic activity of snake PLA2s such as crotoxin from the South American rattlesnake (Crotalus durissus terrificus) [80]. In human umbilical vein endothelial cells (HUVEC), crotoxin has been found to downregulate the increased levels of adhesion molecules, inflammatory cytokines and oxidative stress, which are characteristic features of the early stages of atherosclerosis [80]. Another work also reported that Vipera palaestinae venom could potentially have anti-atherosclerotic action, as it rapidly decreases serum cholesterol levels in humans, with changes in lipoprotein transport and metabolism likely caused by the PLA2 component of this snake [81] (Figure 3).
Overall, due to their cytotoxic and inflammatory potential [79], pharmacological applications of snake venom PLA2s remain limited and are instead used in basic research to help elucidate mechanisms of action of PLA2s. However, the growing interest in the design of therapeutic drugs based on low-mass peptides has encouraged the design of small synthetic peptides from PLA2s. In particular, the identification of regions of PLA2, mainly in the C-terminal domain, with toxic or therapeutic functions, has contributed to the development of PLA2 analogues that may have high therapeutic potential [213]. Other work has also demonstrated that small subunits of venom PLA2s, such as Hemilipin2, derived from the Hemiscorpius lepturus scorpion, can mediate the biological effects of the entire protein without inducing cytotoxic effects [214]. These findings could serve as a starting point for the design of a new generation of low-molecular-mass PLA2s for the treatment of IHD without the adverse effects associated with high-molecular-mass native molecules.
12. Discussion
Despite the significant decrease in coronary mortality over the past 20 years, IHD is still the leading cause of death, reaching 9 million deaths worldwide [1,2,215]. This mortality decrease is mainly linked to considerable progress in medical therapies, far ahead of the effects of revascularization [215]; this, in particular, is thanks to the use of four major therapeutic classes with clinically proven benefits: platelet aggregation inhibitors, beta blockers, ACE inhibitors and statins. To further reduce the high mortality rate induced by IHD, new therapeutic alternatives are still needed, particularly through the exploration of snake venom, which is one of the main natural sources of therapeutic peptides and proteins. The advent of new technologies in the field of biomedical research, such as “omics” (genomics, transcriptomics, proteomics, metabolomics) and bioinformatics [17], has demonstrated promising new prospects for the use of snake venom toxins as a basis for drug discovery. This has led to the marketing of several approved drugs designed or inspired by the chemical structure of snake venom bioactive molecules, as well as a plethora of other toxins of pharmacological, biomedical and biotechnological interest (Table 2). However, despite these promising venomic advances, there are still challenges as well as limitations in the development of therapeutic drugs from snake venom toxins, due to the significant gap between the increased number of venom compounds with relevant pharmacological properties and the few compounds approved and used in human therapeutics. The main limitation lies in the development of drugs from high-molecular-weight toxins generally being considered more immunogenic than their low-molecular-weight counterparts and more difficult to produce and synthesize [216]. In this respect, in recent years, research has increasingly focused on peptide-based drugs as therapeutics for the management of several diseases.
Technological advances in venom toxin purification and characterization as well as new chemical synthesis methodologies have opened up new opportunities in the discovery of low-mass venom components that could not previously be detected or identified, and in the development of peptidomimetics for therapeutic purposes [217]. Small toxins can be easily produced and engineered to develop peptidomimetics through the introduction of structural modifications that may improve their therapeutic potency or confer resistance to proteolytic degradation, key factors that need to be optimized prior to clinical trials [218]. In turn, the use of synthetic peptides has challenged the design and development of small peptidomimetics from high-molecular-weight proteins [213]. Considering that large molecules represent the majority of the snake venom protein content (i.e., 90 to 95%) [219], it is therefore worth developing low-mass peptides to overcome the deleterious effects of native proteins while preserving their beneficial pharmacological properties [213].
13. Conclusions
Despite the complex challenges in the field of toxinology, snake-venom-derived molecules remain a highly promising source of lead compounds and novel drugs due to their high selectivity toward cellular targets, stability and efficacy compared with their human counterparts. Since the successful discovery in 1981 of the snake venom drug, captopril, several examples from venomic research, as reported in this review, have proven the benefit of using venom molecules rather than their human counterparts. In certain cases, venom molecules are developed to counter unexpected adverse effects found with human components such as the venom DNP-based cenderitide developed to overcome the deleterious hypotensive effects of nesiritide, the recombinant human BNP used as a drug for the treatment of HF. Another example is that of L2, a snake venom BNP-like peptide that has been shown to be more potent than the human counterpart, with additional effects and a novel mechanism of action not observed with human NPs [15,17,18,20]. The venom-derived CTL, lebecetin, approved in preclinical studies, has been patented for its high selectivity and potent ability, compared with other synthetic family members, to simultaneously induce multiple cellular targets for the treatment of neovascular eye diseases [19]. Other toxins have the ability to escape human enzyme systems, thus being useful as diagnostic tools, e.g., for the assay of coagulation factors and for the study of hemostasis [220].
Funding
My work at the Pasteur Institute in Tunis, Tunisia, is supported by grants/funds from the LBVAT (LR20IPT01), the Ministry of Higher Education and Scientific Research (PHC-Utique 13G0824 and 21G819), and the Institut Pasteur International Network (Doctoral Grants Calmette and Yersin).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
I thank all the members of the Laboratoire des Biomolécules, Venins et Applications Théranostiques (LBVAT, LR20IPT01) for their insights and help in my research. I am grateful to Naziha Marrakchi, Mohamed Elayeb, Najet Srairi-Abid, Jed Jebali, Amor Mosbah, Christine Richer-Giudicelli, François Alhenc-Gelas, Paul Mulder, Elise Belaidi, Mohamed Amiche, Christophe Piesse, Ali Ladram, Pierre Sicard and Hechmi Louzir for helpful discussions and continuous support. I thank Salvatore Tinè for reading the article and providing editorial suggestions.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ACS | Acute coronary syndrome |
| Ang I | Angiotensin I |
| Ang II | Angiotensin II |
| BK | Bradykinin |
| BP | Blood pressure |
| BPPs | Bradykinin-potentiating peptides |
| CTLs | C-type lectins |
| CVDs | Cardiovascular diseases |
| CVS | Cardiovascular system |
| HF | Heart failure |
| IHD | Ischemic heart disease |
| IR | Ischemia–reperfusion |
| MI | Myocardial infarction |
| mPTP | Mitochondria permeability transition pore |
| NO | Nitric oxide |
| NPs | Natriuretic peptides |
| NPRs | Natriuretic peptide receptors |
| PLA2s | Phospholipases A2 |
| SRTXs | Sarafotoxins |
| 3FTx | Three-finger toxins |
| VEGF | Vascular endothelial growth factor |
References
- WHO. The Top 10 Causes of Death; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Wang, F.; Yu, Y.; Mubarik, S. Global burden of ischemic heart disease and attributable risk factors, 1990–2017: A secondary analysis based on the global burden of disease study 2017. Clin. Epidemiol. 2021, 13, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, B.R.; Cannata, F.; Stefanini, G.G. Myocardial ischemia and coronary disease in heart failure. Heart Fail. Rev. 2020, 25, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Pinar, J.i.S. Hypertension and ischemic heart disease. Cor Vasa 2012, 54, e433–e438. [Google Scholar]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef]
- Verma, S.; Fedak, P.W.; Weisel, R.D.; Butany, J.; Rao, V.; Maitland, A.; Li, R.K.; Dhillon, B.; Yau, T.M. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation 2002, 105, 2332–2336. [Google Scholar] [CrossRef]
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake venom: From deadly toxins to life-saving therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef] [PubMed]
- Messadi-Laribi, E.; Griol-Charhbili, V.; Pizard, A.; Vincent, M.P.; Heudes, D.; Meneton, P.; Alhenc-Gelas, F.; Richer, C. Tissue kallikrein is involved in the cardioprotective effect of AT1-receptor blockade in acute myocardial ischemia. J. Pharmacol. Exp. Ther. 2007, 323, 210–216. [Google Scholar] [CrossRef]
- Griol-Charhbili, V.; Messadi-Laribi, E.; Bascands, J.L.; Heudes, D.; Meneton, P.; Giudicelli, J.F.; Alhenc-Gelas, F.; Richer, C. Role of tissue kallikrein in the cardioprotective effects of ischemic and pharmacological preconditioning in myocardial ischemia. FASEB J. 2005, 19, 1172–1174. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Viegas, M.F. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar]
- Kakumanu, R.; Kemp-Harper, B.K.; Silva, A.; Kuruppu, S.; Isbister, G.K.; Hodgson, W.C. An in vivo examination of the differences between rapid cardiovascular collapse and prolonged hypotension induced by snake venom. Sci. Rep. 2019, 9, 20231. [Google Scholar] [CrossRef]
- Hering, C. The guiding symptoms of our materia medica. Am. Homoeopath. Publ. Soc. 1879, 6, 559–650. [Google Scholar]
- Ferreira, S.H.; Bartelt, D.C.; Greene, L.J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry 1970, 9, 2583–2593. [Google Scholar] [CrossRef]
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. From snake venom’s disintegrins and c-type lectins to anti-platelet drugs. Toxins 2019, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Tourki, B.; Mateo, P.; Morand, J.; Elayeb, M.; Godin-Ribuot, D.; Marrakchi, N.; Belaidi, E.; Messadi, E. Lebetin 2, a snake venom-derived natriuretic peptide, attenuates acute myocardial ischemic injury through the modulation of mitochondrial permeability transition pore at the time of reperfusion. PLoS ONE 2016, 11, e0162632. [Google Scholar]
- Messadi, E.; Aloui, Z.; Belaidi, E.; Vincent, M.P.; Couture-Lepetit, E.; Waeckel, L.; Decorps, J.; Bouby, N.; Gasmi, A.; Karoui, H.; et al. Cardioprotective effect of VEGF and venom VEGF-like protein in acute myocardial ischemia in mice: Effect on mitochondrial function. J. Cardiovasc. Pharmacol. 2014, 63, 274–281. [Google Scholar] [CrossRef]
- Allaoui, H.; Rached, N.; Marrakchi, N.; Cherif, A.; Mosbah, A.; Messadi, E. In silico study of the mechanisms underlying the action of the snake natriuretic-like peptide lebetin 2 during cardiac ischemia. Toxins 2022, 14, 787. [Google Scholar] [CrossRef]
- Tourki, B.; Dumesnil, A.; Belaidi, E.; Ghrir, S.; Godin-Ribuot, D.; Marrakchi, N.; Richard, V.; Mulder, P.; Messadi, E. Lebetin 2, a snake venom-derived B-type natriuretic peptide, provides immediate and prolonged protection against myocardial ischemia-reperfusion injury via modulation of post-ischemic inflammatory response. Toxins 2019, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Montassar, F.; Darche, M.; Blaizot, A.; Augustin, S.; Conart, J.B.; Millet, A.; Elayeb, M.; Sahel, J.A.; Reaux-Le Goazigo, A.; Sennlaub, F.; et al. Lebecetin, a C-type lectin, inhibits choroidal and retinal neovascularization. FASEB J. 2017, 31, 1107–1119. [Google Scholar] [CrossRef]
- Bouzazi, D.; Mami, W.; Mosbah, A. Natriuretic-like peptide lebetin 2 mediates m2 macrophage polarization in lps-activated raw264.7 cells in an il-10-dependent manner. Toxins 2023, 15, 298. [Google Scholar] [CrossRef]
- Mehta, S.R.; Sashindran, V.K. Clinical features and management of snake bite. Med. J. Armed Forces India 2002, 58, 247–249. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G.K. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Peterfi, O.; Boda, F.; Szabo, Z.; Ferencz, E.; Baba, L. Hypotensive Snake Venom Components-A Mini-Review. Molecules 2019, 24, 2778. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.G.; Pucca, M.B.; Oliveira, I.S.; Cerni, F.A.; Jacob, B.; Arantes, E.C. Snake venom vascular endothelial growth factors (svVEGFs): Unravelling their molecular structure, functions, and research potential. Cytokine Growth Factor Rev. 2021, 60, 133–143. [Google Scholar] [PubMed]
- Hartman, J.C.; Wall, T.M.; Hullinger, T.G.; Shebuski, R.J. Reduction of myocardial infarct size in rabbits by ramiprilat: Reversal by the bradykinin antagonist HOE 140. J. Cardiovasc. Pharmacol. 1993, 21, 996–1003. [Google Scholar] [CrossRef]
- Weidenbach, R.; Schulz, R.; Gres, P.; Behrends, M.; Post, H.; Heusch, G. Enhanced reduction of myocardial infarct size by combined ACE inhibition and AT(1)-receptor antagonism. Br. J. Pharmacol. 2000, 131, 138–144. [Google Scholar] [CrossRef]
- Cavasin, M.A.; Yang, X.P.; Liu, Y.H.; Mehta, D.; Karumanchi, R.; Bulagannawar, M.; Carretero, O.A. Effects of ACE inhibitor, AT1 antagonist, and combined treatment in mice with heart failure. J. Cardiovasc. Pharmacol. 2000, 36, 472–480. [Google Scholar] [CrossRef]
- Salah, E.M.; Bastacky, S.I.; Jackson, E.K.; Tofovic, S.P. Captopril Attenuates Cardiovascular and Renal Disease in a Rat Model of Heart Failure With Preserved Ejection Fraction. J. Cardiovasc. Pharmacol. 2018, 71, 205–214. [Google Scholar] [CrossRef]
- Brivet, F.; Delfraissy, J.F.; Giudicelli, J.F.; Richer, C.; Legrand, A.; Dormont, J. Immediate effects of captopril in acute left ventricular failure secondary to myocardial infarction. Eur. J. Clin. Investig. 1981, 11, 369–373. [Google Scholar] [CrossRef]
- Ohtsubo, T.; Shibata, R.; Kai, H.; Okamoto, R.; Kumagai, E.; Kawano, H.; Fujiwara, A.; Kitazono, T.; Murohara, T.; Arima, H. Angiotensin-converting enzyme inhibitors versus angiotensin receptor blockers in hypertensive patients with myocardial infarction or heart failure: A systematic review and meta-analysis. Hypertens. Res. 2019, 42, 641–649. [Google Scholar] [CrossRef]
- Hansen, M.L.; Gislason, G.H.; Kober, L.; Schramm, T.K.; Folke, F.; Buch, P.; Abildstrom, S.Z.; Madsen, M.; Rasmussen, S.; Torp-Pedersen, C. Different angiotensin-converting enzyme inhibitors have similar clinical efficacy after myocardial infarction. Br. J. Clin. Pharmacol. 2008, 65, 217–223. [Google Scholar] [CrossRef]
- McMurray, J.; Solomon, S.; Pieper, K.; Reed, S.; Rouleau, J.; Velazquez, E.; White, H.; Howlett, J.; Swedberg, K.; Maggioni, A.; et al. The effect of valsartan, captopril, or both on atherosclerotic events after acute myocardial infarction: An analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT). J. Am. Coll. Cardiol. 2006, 47, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Hayek, T.; Attias, J.; Smith, J.; Breslow, J.L.; Keidar, S. Antiatherosclerotic and antioxidative effects of captopril in apolipoprotein E-deficient mice. J. Cardiovasc. Pharmacol. 1998, 31, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.B.; Henry, G.C.; Macaya, C.; O’Neill, B.J.; Pucillo, A.L.; Carere, R.G.; Wargovich, T.J.; Mudra, H.; Luscher, T.F.; Klibaner, M.I.; et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation 1996, 94, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Farhy, R.D.; Carretero, O.A.; Ho, K.L.; Scicli, A.G. Role of kinins and nitric oxide in the effects of angiotensin converting enzyme inhibitors on neointima formation. Circ. Res. 1993, 72, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, T.; Tiu, J.; Panjrath, G.; Solomon, A. The effect of angiotensin-converting enzyme inhibitors on clinical outcomes in patients with ischemic cardiomyopathy and midrange ejection fraction: A post hoc subgroup analysis from the PEACE trial. Ther. Adv. Cardiovasc. Dis. 2018, 12, 351–359. [Google Scholar] [CrossRef]
- Kawakami, R.; Lee, C.Y.W.; Scott, C.; Bailey, K.R.; Schirger, J.A.; Chen, H.H.; Benike, S.L.; Cannone, V.; Martin, F.L.; Sangaralingham, S.J.; et al. A human study to evaluate safety, tolerability, and cyclic GMP activating properties of cenderitide in subjects with stable chronic heart failure. Clin. Pharmacol. Ther. 2018, 104, 546–552. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chen, H.H.; Lisy, O.; Swan, S.; Cannon, C.; Lieu, H.D.; Burnett, J.C., Jr. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J. Clin. Pharmacol. 2009, 49, 668–673. [Google Scholar] [CrossRef]
- Da Silva, S.L.; Almeida, J.R.; Resende, L.M.; Martins, W.; Henriques, F.A.F.A.; Baldasso, P.A.; Soares, A.M.; Taranto, A.G.; Resende, R.R.; Marangoni, S.; et al. Isolation and characterization of a natriuretic peptide from crotalus oreganus abyssus (grand canyon rattlesnake) and its effects on systemic blood pressure and nitrite levels. Int. J. Pept. Res. Ther. 2011, 17, 165–173. [Google Scholar] [CrossRef]
- Ziegler, M.; Wang, X.; Peter, K. Platelets in cardiac ischaemia/reperfusion injury: A promising therapeutic target. Cardiovasc. Res. 2019, 115, 1178–1188. [Google Scholar]
- Zhou, X.; Wu, X.; Sun, H.; Li, J. Efficacy and safety of eptifibatide versus tirofiban in acute coronary syndrome patients: A systematic review and meta-analysis. J. Evid. Based Med. 2017, 10, 136–144. [Google Scholar] [CrossRef]
- Day, J.R.; Malik, I.S.; Weerasinghe, A.; Poullis, M.; Nadra, I.; Haskard, D.O.; Taylor, K.M.; Landis, R.C. Distinct yet complementary mechanisms of heparin and glycoprotein IIb/IIIa inhibitors on platelet activation and aggregation: Implications for restenosis during percutaneous coronary intervention. Heart 2004, 90, 794–799. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oh, M.; Lee, C.W.; Lee, H.S.; Chang, M.; Ahn, J.M.; Park, D.W.; Kang, S.J.; Lee, S.W.; Kim, Y.H.; Moon, D.H.; et al. Similar impact of clopidogrel or ticagrelor on carotid atherosclerotic plaque inflammation. Clin. Cardiol. 2016, 39, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, T.; Yao, L.; Yue, H.; Zhang, Z. Effects of tirofiban on stent thrombosis, Hs-CRP, IL-6 and sICAM-1 after PCI of acute myocardial infarction. Exp. Ther. Med. 2018, 16, 3383–3388. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jia, H.Z.; Li, M.C.; Zhu, W. Comparison of the effect of ticagrelor combined with tirofiban versus clopidogrel combined with tirofiban on inflammation response and prognosis of patients with unstable angina pectoris in long term follow-up. Kaohsiung J. Med. Sci. 2021, 37, 1010–1015. [Google Scholar] [CrossRef]
- Sarray, S.; Srairi, N.; Luis, J.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N. Lebecetin, a C-lectin protein from the venom of Macrovipera lebetina that inhibits platelet aggregation and adhesion of cancerous cells. Haemostasis 2001, 31, 173–176. [Google Scholar] [CrossRef]
- Sarray, S.; Luis, J.; El Ayeb, M.; Marrakchi, N. Snake venoms C-type lectins and their receptors on platelets and cancerous cells. Arch. Inst. Pasteur Tunis 2008, 85, 69–80. [Google Scholar]
- Rajagopalan, N.; Pung, Y.F.; Zhu, Y.Z.; Wong, P.T.; Kumar, P.P.; Kini, R.M. Beta-cardiotoxin: A new three-finger toxin from Ophiophagus hannah (king cobra) venom with beta-blocker activity. FASEB J. 2007, 21, 3685–3695. [Google Scholar] [CrossRef]
- Harvey, A.L.; Marshall, R.J.; Karlsson, E. Effects of purified cardiotoxins from the Thailand cobra (Naja naja siamensis) on isolated skeletal and cardiac muscle preparations. Toxicon 1982, 20, 379–396. [Google Scholar] [CrossRef]
- Averin, A.S.; Astashev, M.E.; Andreeva, T.V.; Tsetlin, V.I.; Utkin, Y.N. Cardiotoxins from cobra naja oxiana change the force of contraction and the character of rhythmoinotropic phenomena in the rat myocardium. Dokl. Biochem. Biophys. 2019, 487, 282–286. [Google Scholar] [CrossRef]
- Kini, R.M.; Koh, C.Y. Snake venom three-finger toxins and their potential in drug development targeting cardiovascular diseases. Biochem. Pharmacol. 2020, 181, 114105. [Google Scholar] [CrossRef]
- de Weille, J.R.; Schweitz, H.; Maes, P.; Tartar, A.; Lazdunski, M. Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc. Natl. Acad. Sci. USA 1991, 88, 2437–2440. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.X.; Itahara, Y.; Kuroda, H.; Chen, Y.N.; Kimura, T.; Sakakibara, S. Smooth muscle relaxing and hypotensive activities of synthetic calciseptine and the homologous snake venom peptide FS2. Jpn. J. Pharmacol. 1995, 68, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, M.J.; Jaseja, M.; Hyde, E.I.; Lu, X.; Williams, J.A. Three-dimensional structure of the RGD-containing neurotoxin homologue dendroaspin. Nat. Struct. Biol. 1994, 1, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Joubert, F.J.; Taljaard, N. Some properties and the complete primary structures of two reduced and S-carboxymethylated polypeptides (S5C1 and S5C10) from Dendroaspis jamesoni kaimosae (Jameson’s mamba) venom. Biochim. Biophys. Acta 1979, 579, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Carsi-Gabrenas, J.M. Purification of Toxins from Green Mamba Venom with Distinct Receptor Selectivities. Ph.D. Thesis, University of Miami, Coral Gables, FL, USA, 1997; pp. 1–310. [Google Scholar]
- Yamazaki, Y.; Takani, K.; Atoda, H.; Morita, T. Snake venom vascular endothelial growth factors (VEGFs) exhibit potent activity through their specific recognition of KDR (VEGF receptor 2). J. Biol. Chem. 2003, 278, 51985–51988. [Google Scholar] [CrossRef]
- Crockett, T.R.; Gray, G.A.; Kane, K.A.; Wainwright, C.L. Sarafotoxin 6c (S6c) reduces infarct size and preserves mRNA for the ETB receptor in the ischemic/reperfused myocardium of anesthetized rats. J. Cardiovasc. Pharmacol. 2004, 44, 148–154. [Google Scholar] [CrossRef]
- Das, B.; Sarkar, C.; Shankar, P.R. Pretreatment with sarafotoxin 6c prior to coronary occlusion protects against infarction and arrhythmias via cardiomyocyte mitochondrial K(ATP) channel activation in the intact rabbit heart during ischemia/reperfusion. Cardiovasc. Drugs Ther. 2007, 21, 243–251. [Google Scholar] [CrossRef]
- Seo, B.; Oemar, B.S.; Siebenmann, R.; von Segesser, L.; Luscher, T.F. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation 1994, 89, 1203–1208. [Google Scholar] [CrossRef]
- Thompson, M.; Westwick, J.; Woodward, B. Responses to endothelins-1, -2, and -3 and sarafotoxin 6c after ischemia/reperfusion in isolated perfused rat heart: Role of vasodilator loss. J. Cardiovasc. Pharmacol. 1995, 25, 156–162. [Google Scholar] [CrossRef]
- Monteiro, D.A.; Kalinin, A.L.; Selistre-de-Araujo, H.S.; Nogueira, L.A.N.; Beletti, M.E.; Fernandes, M.N.; Rantin, F.T. Cardioprotective effects of alternagin-C (ALT-C), a disintegrin-like protein from Rhinocerophis alternatus snake venom, on hypoxia-reoxygenation-induced injury in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 215, 67–75. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Koike, H.; Sugiyama, Y.; Motoyoshi, K.; Wada, T.; Hishinuma, S.; Mita, M.; Morita, T. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. Eur. J. Biochem. 2002, 269, 2708–2715. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hyodo, F.; Morita, T. Wide distribution of cysteine-rich secretory proteins in snake venoms: Isolation and cloning of novel snake venom cysteine-rich secretory proteins. Arch. Biochem. Biophys. 2003, 412, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, H.; Liu, M.N.; Song, H.; Han, H.M.; Wang, Q.L.; Yin, C.C.; Zhou, Y.C.; Qi, Z.; Shu, Y.Y.; et al. Structural and functional analysis of natrin, a venom protein that targets various ion channels. Biochem. Biophys. Res. Commun. 2006, 351, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wu, W.G. Amphiphilic beta-sheet cobra cardiotoxin targets mitochondria and disrupts its network. FEBS Lett. 2005, 579, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, Q.L.; Meng, X.; Shu, Y.; Jiang, T.; Wagenknecht, T.; Yin, C.C.; Sui, S.F.; Liu, Z. Structural and functional characterization of ryanodine receptor-natrin toxin interaction. Biophys. J. 2008, 95, 4289–4299. [Google Scholar] [CrossRef]
- Jiang, Z.S.; Xia, C.F.; Tian, Q.P.; Fu, M.G.; Wang, X.H.; Pang, Y.Z.; Tang, C.S.; Liu, N.K. Effect of batroxobin against dog heart ischemia/reperfusion injury. Acta Pharmacol. Sin. 2000, 21, 70–74. [Google Scholar]
- Huang, D.X.; Gai, L.Y.; Wang, S.R.; Li, T.D.; Yang, T.S.; Zhi, G.; Du, L.S.; Li, L.H. Defibrase, a purified fibrinolytic protease from snake venom in acute myocardial infarction. Acta Cardiol. 1992, 47, 445–458. [Google Scholar]
- Huang, J.; Zhou, D.; Tian, L.; Wu, H.; Zhang, J.; Zhang, S.; Chen, H. The effect of batroxobin on atherosclerosis. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2003, 20, 197–201. [Google Scholar]
- Tang, Y.; Liu, C.; Li, C.; Li, W. The effect of defibrase in prevention of experimental atherosclerosis in rabbits. Zhonghua Bing Li Xue Za Zhi 1999, 28, 112–114. [Google Scholar]
- Swenson, S.; Bush, L.R.; Markland, F.S. Chimeric derivative of fibrolase, a fibrinolytic enzyme from southern copperhead venom, possesses inhibitory activity on platelet aggregation. Arch. Biochem. Biophys. 2000, 384, 227–237. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Dias, L.; Renno, A.L.; Sousa, N.C.; Smaal, A.; da Silva, D.A.; Hyslop, S. Rat atrial responses to Bothrops jararacussu (jararacucu) snake venom. Toxicology 2014, 323, 109–124. [Google Scholar] [CrossRef]
- Huang, M.Z.; Gopalakrishnakone, P. Pathological changes induced by an acidic phospholipase A2 from Ophiophagus hannah venom on heart and skeletal muscle of mice after systemic injection. Toxicon 1996, 34, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, I.L.; Martins, A.M.; Nascimento, N.R.; Havt, A.; Evangelista, J.S.; de Noroes, T.B.; Toyama, M.H.; Diz-Filho, E.B.; Toyama Dde, O.; Fonteles, M.C.; et al. Renal and cardiovascular effects of Bothrops marajoensis venom and phospholipase A2. Toxicon 2010, 55, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Z.; Wang, Q.C.; Liu, G.F. Effects of an acidic phospholipase A2 purified from Ophiophagus hannah (king cobra) venom on rat heart. Toxicon 1993, 31, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lin, W.W.; Chen, Y.M.; Lee, S.Y. Is direct cardiotoxicity the primary cause of death following i.v. injection of the basic phospholipase A2 from Naja nigricollis venom? Acta Physiol. Pharmacol. Latinoam. 1989, 39, 383–391. [Google Scholar] [PubMed]
- Huang, H.C. Effects of phospholipases A2 from Vipera russelli snake venom on blood pressure, plasma prostacyclin level and renin activity in rats. Toxicon 1984, 22, 253–264. [Google Scholar] [CrossRef]
- Mitrmoonpitak, C.; Chulasugandha, P.; Khow, O.; Noiprom, J.; Chaiyabutr, N.; Sitprija, V. Effects of phospholipase A2 and metalloprotease fractions of Russell’s viper venom on cytokines and renal hemodynamics in dogs. Toxicon 2013, 61, 47–53. [Google Scholar] [CrossRef]
- de Andrade, C.M.; Rey, F.M.; Cintra, A.C.O.; Sampaio, S.V.; Torqueti, M.R. Effects of crotoxin, a neurotoxin from Crotalus durissus terrificus snake venom, on human endothelial cells. Int. J. Biol. Macromol. 2019, 134, 613–621. [Google Scholar] [CrossRef]
- Winkler, E.; Chovers, M.; Almog, S.; Pri-Chen, S.; Rotenberg, M.; Tirosh, M.; Ezra, D.; Halkin, H. Decreased serum cholesterol level after snake bite (Vipera palaestinae) as a marker of severity of envenomation. J. Lab. Clin. Med. 1993, 121, 774–778. [Google Scholar]
- Capricor Therapeutics Provides Update on Natriuretic Peptide Program. Available online: https://www.prnewswire.com/news-releases/capricor-therapeutics-provides-update-on-natriuretic-peptide-program-300409174.html (accessed on 2 January 2018).
- Alfimeprase. Drugs R D 2008, 9, 185–190. [CrossRef]
- Camargo, A.C.; Ianzer, D.; Guerreiro, J.R.; Serrano, S.M. Bradykinin-potentiating peptides: Beyond captopril. Toxicon 2012, 59, 516–523. [Google Scholar] [CrossRef]
- Holleman, W.H.; Weiss, L.J. The thrombin-like enzyme from Bothrops atrox snake venom. Properties of the enzyme purified by affinity chromatography on p-aminobenzamidine-substituted agarose. J. Biol. Chem. 1976, 251, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Amorim, F.G.; Menaldo, D.L. New insights on moojase, a thrombin-like serine protease from Bothrops moojeni snake venom. Toxins 2018, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Trikha, M.; Schmitmeier, S.; Markland, F.S. Purification and characterization of fibrolase isoforms from venom of individual southern copperhead (Agkistrodon contortrix Contortrix) snakes. Toxicon 1994, 32, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S.; Friedrichs, G.S.; Pewitt, S.R.; Lucchesi, B.R. Thrombolytic effects of recombinant fibrolase or APSAC in a canine model of carotid artery thrombosis. Circulation 1994, 90, 2448–2456. [Google Scholar] [CrossRef]
- Markland, F.S. Fibrolase, an active thrombolytic enzyme in arterial and venous thrombosis model systems. Adv. Exp. Med. Biol. 1996, 391, 427–438. [Google Scholar]
- Koh, C.Y.; Kini, R.M. From snake venom toxins to therapeutics—Cardiovascular examples. Toxicon 2012, 59, 497–506. [Google Scholar]
- Dos Santos, P.K.; Altei, W.F.; Danilucci, T.M.; Lino, R.L.B.; Pachane, B.C.; Nunes, A.C.C.; Selistre-de-Araujo, H.S. Alternagin-C (ALT-C), a disintegrin-like protein, attenuates alpha2beta1 integrin and VEGF receptor 2 signaling resulting in angiogenesis inhibition. Biochimie 2020, 174, 144–158. [Google Scholar] [CrossRef]
- Momic, T.; Katzhendler, J.; Shai, E.; Noy, E.; Senderowitz, H.; Eble, J.A.; Marcinkiewicz, C.; Varon, D.; Lazarovici, P. Vipegitide: A folded peptidomimetic partial antagonist of α2β1 integrin with antiplatelet aggregation activity. Drug Des. Devel. Ther. 2015, 9, 291–304. [Google Scholar]
- Chaisakul, J.; Isbister, G.K.; Tare, M.; Parkington, H.C.; Hodgson, W.C. Hypotensive and vascular relaxant effects of phospholipase A2 toxins from Papuan taipan (Oxyuranus scutellatus) venom. Eur. J. Pharmacol. 2014, 723, 227–233. [Google Scholar] [CrossRef]
- Golias, C.; Charalabopoulos, A.; Stagikas, D.; Charalabopoulos, K.; Batistatou, A. The kinin system–bradykinin: Biological effects and clinical implications. Multiple role of the kinin system–Bradykinin. Hippokratia 2007, 11, 124–128. [Google Scholar] [PubMed]
- Sciani, J.M.; Pimenta, D.C. The modular nature of bradykinin-potentiating peptides isolated from snake venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.M.; Ferreira, S.H.; Greene, L.J. Bradykinin potentiating peptide PCA-Lys-Trp-Ala-Pro. An inhibitor of the pulmonary inactivation of bradykinin and conversion of angiotensin I to II. Biochem. Pharmacol. 1971, 20, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Masuyer, G.; Schwager, S.L.; Sturrock, E.D.; Isaac, R.E.; Acharya, K.R. Molecular recognition and regulation of human angiotensin-I converting enzyme (ACE) activity by natural inhibitory peptides. Sci. Rep. 2012, 2, 717. [Google Scholar] [CrossRef]
- Chi, C.W.; Wang, S.Z.; Xu, L.G.; Wang, M.Y.; Lo, S.S.; Huang, W.D. Structure-function studies on the bradykinin potentiating peptide from Chinese snake venom (Agkistrodon halys Pallas). Peptides 1985, 6 (Suppl. S3), 339–342. [Google Scholar]
- Sato, M.; Engelman, R.M.; Otani, H.; Maulik, N.; Rousou, J.A.; Flack, J.E., 3rd; Deaton, D.W.; Das, D.K. Myocardial protection by preconditioning of heart with losartan, an angiotensin II type 1-receptor blocker: Implication of bradykinin-dependent and bradykinin-independent mechanisms. Circulation 2000, 102, III346–III351. [Google Scholar] [CrossRef]
- Dong, R.; Xu, X.; Li, G.; Feng, W.; Zhao, G.; Zhao, J.; Wang, D.W.; Tu, L. Bradykinin inhibits oxidative stress-induced cardiomyocytes senescence via regulating redox state. PLoS ONE 2013, 8, e77034. [Google Scholar]
- DiBianco, R. Angiotensin converting enzyme inhibition. Unique and effective therapy for hypertension and congestive heart failure. Postgrad. Med. 1985, 78, 229–241, 244, 247–248. [Google Scholar] [CrossRef]
- Brown, N.J.; Vaughan, D.E. Angiotensin-converting enzyme inhibitors. Circulation 1998, 97, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- McCleary, R.J.; Kini, R.M. Non-enzymatic proteins from snake venoms: A gold mine of pharmacological tools and drug leads. Toxicon 2013, 62, 56–74. [Google Scholar]
- Wei, L.; Alhenc-Gelas, F.; Corvol, P.; Clauser, E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J. Biol. Chem. 1991, 266, 9002–9008. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Nussberger, J. Vasopeptidase inhibition and angio-oedema. Lancet 2000, 356, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, D.; Beau, F.; Czarny, B.; Cotton, J.; Yiotakis, A.; Dive, V. Roles of the two active sites of somatic angiotensin-converting enzyme in the cleavage of angiotensin I and bradykinin: Insights from selective inhibitors. Circ. Res. 2003, 93, 148–154. [Google Scholar] [CrossRef] [PubMed]
- van Esch, J.H.; Tom, B.; Dive, V.; Batenburg, W.W.; Georgiadis, D.; Yiotakis, A.; van Gool, J.M.; de Bruijn, R.J.; de Vries, R.; Danser, A.H. Selective angiotensin-converting enzyme C-domain inhibition is sufficient to prevent angiotensin I-induced vasoconstriction. Hypertension 2005, 45, 120–125. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Ong, F.S.; Blackwell, W.L.; Shah, K.H.; Giani, J.F.; Gonzalez-Villalobos, R.A.; Shen, X.Z.; Fuchs, S.; Touyz, R.M. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol. Rev. 2013, 65, 1–46. [Google Scholar]
- Babenko, V.V.; Ziganshin, R.H.; Weise, C.; Dyachenko, I.; Shaykhutdinova, E.; Murashev, A.N.; Zhmak, M.; Starkov, V.; Hoang, A.N.; Tsetlin, V.; et al. Novel bradykinin-potentiating peptides and three-finger toxins from viper venom: Combined ngs venom gland transcriptomics and quantitative venom proteomics of the azemiops feae viper. Biomedicines 2020, 8, 249. [Google Scholar] [CrossRef]
- Cotton, J.; Hayashi, M.A.; Cuniasse, P.; Vazeux, G.; Ianzer, D.; De Camargo, A.C.; Dive, V. Selective inhibition of the C-domain of angiotensin I converting enzyme by bradykinin potentiating peptides. Biochemistry 2002, 41, 6065–6071. [Google Scholar] [CrossRef]
- Hayashi, M.A.; Murbach, A.F.; Ianzer, D.; Portaro, F.C.; Prezoto, B.C.; Fernandes, B.L.; Silveira, P.F.; Silva, C.A.; Pires, R.S.; Britto, L.R.; et al. The C-type natriuretic peptide precursor of snake brain contains highly specific inhibitors of the angiotensin-converting enzyme. J. Neurochem. 2003, 85, 969–977. [Google Scholar] [CrossRef]
- Barreto, S.A.; Chaguri, L.C.; Prezoto, B.C.; Lebrun, I. Characterization of two vasoactive peptides isolated from the plasma of the snake Crotalus durissus terrificus. Biomed. Pharmacother. 2012, 66, 256–265. [Google Scholar] [CrossRef]
- Kodama, R.T.; Cajado-Carvalho, D.; Kuniyoshi, A.K.; Kitano, E.S.; Tashima, A.K.; Barna, B.F.; Takakura, A.C.; Serrano, S.M.; Dias-Da-Silva, W.; Tambourgi, D.V.; et al. New proline-rich oligopeptides from the venom of African adders: Insights into the hypotensive effect of the venoms. Biochim. Biophys. Acta 2015, 1850, 1180–1187. [Google Scholar] [CrossRef]
- Gomes, C.L.; Konno, K.; Conceicao, I.M.; Ianzer, D.; Yamanouye, N.; Prezoto, B.C.; Assakura, M.T.; Radis-Baptista, G.; Yamane, T.; Santos, R.A.; et al. Identification of novel bradykinin-potentiating peptides (BPPs) in the venom gland of a rattlesnake allowed the evaluation of the structure-function relationship of BPPs. Biochem. Pharmacol. 2007, 74, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.R.; Oliveira-Carvalho, A.L.; Wermelinger, L.S.; Zingali, R.B.; Ho, P.L.; Junqueira-de-Azevedo, I.L.; Diniz, M.R. Identification of novel bradykinin-potentiating peptides and C-type natriuretic peptide from Lachesis muta venom. Toxicon 2005, 46, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.; Galle, A.; Raida, M.; Schrader, M.; Lebrun, I.; Habermehl, G. Isolation: Analysis and properties of three bradykinin-potentiating peptides (BPP-II, BPP-III, and BPP-V) from Bothrops neuwiedi venom. J. Protein Chem. 1998, 17, 285–289. [Google Scholar] [CrossRef]
- Murayama, N.; Hayashi, M.A.; Ohi, H.; Ferreira, L.A.; Hermann, V.V.; Saito, H.; Fujita, Y.; Higuchi, S.; Fernandes, B.L.; Yamane, T.; et al. Cloning and sequence analysis of a Bothrops jararaca cDNA encoding a precursor of seven bradykinin-potentiating peptides and a C-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 1997, 94, 1189–1193. [Google Scholar] [CrossRef]
- Hayashi, M.A.; Camargo, A.C. The Bradykinin-potentiating peptides from venom gland and brain of Bothrops jararaca contain highly site specific inhibitors of the somatic angiotensin-converting enzyme. Toxicon 2005, 45, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, E.; Bacchelli, S.; Degli Esposti, D.; Borghi, C. ACE-inhibitors and atherosclerosis. Eur. J. Epidemiol. 1992, 8 (Suppl. S1), 129–133. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yang, X.P.; Sharov, V.G.; Sigmon, D.H.; Sabbath, H.N.; Carretero, O.A. Paracrine systems in the cardioprotective effect of angiotensin-converting enzyme inhibitors on myocardial ischemia/reperfusion injury in rats. Hypertension 1996, 27, 7–13. [Google Scholar] [CrossRef]
- Martorana, P.A.; Kettenbach, B.; Breipohl, G.; Linz, W.; Scholkens, B.A. Reduction of infarct size by local angiotensin-converting enzyme inhibition is abolished by a bradykinin antagonist. Eur. J. Pharmacol. 1990, 182, 395–396. [Google Scholar] [CrossRef]
- Querobino, S.M.; Ribeiro, C.A.J.; Alberto-Silva, C. Bradykinin-potentiating PEPTIDE-10C, an argininosuccinate synthetase activator, protects against H(2)O(2)-induced oxidative stress in SH-SY5Y neuroblastoma cells. Peptides 2018, 103, 90–97. [Google Scholar]
- Guerreiro, J.R.; Lameu, C.; Oliveira, E.F.; Klitzke, C.F.; Melo, R.L.; Linares, E.; Augusto, O.; Fox, J.W.; Lebrun, I.; Serrano, S.M.; et al. Argininosuccinate synthetase is a functional target for a snake venom anti-hypertensive peptide: Role in arginine and nitric oxide production. J. Biol. Chem. 2009, 284, 20022–20033. [Google Scholar] [CrossRef]
- Ianzer, D.; Xavier, C.H.; Fraga, F.C.; Lautner, R.Q.; Guerreiro, J.R.; Machado, L.T.; Mendes, E.P.; de Camargo, A.C.; Santos, R.A. BPP-5a produces a potent and long-lasting NO-dependent antihypertensive effect. Ther. Adv. Cardiovasc. Dis. 2011, 5, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Morais, K.L.; Hayashi, M.A.; Bruni, F.M.; Lopes-Ferreira, M.; Camargo, A.C.; Ulrich, H.; Lameu, C. Bj-PRO-5a, a natural angiotensin-converting enzyme inhibitor, promotes vasodilatation mediated by both bradykinin B(2)and M1 muscarinic acetylcholine receptors. Biochem. Pharmacol. 2011, 81, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Denadai, A.M.; Ianzer, D.; Alcantara, A.F.; Santoro, M.M.; Santos, C.F.; Lula, I.S.; de Camargo, A.C.; Faljoni-Alario, A.; dos Santos, R.A.; Sinisterra, R.D. Novel pharmaceutical composition of bradykinin potentiating penta peptide with beta-cyclodextrin: Physical-chemical characterization and anti-hypertensive evaluation. Int. J. Pharm. 2007, 336, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.D.C.; Alves, P.H.; Mendes, E.P.; Ianzer, D.; Castro, C.H. BJ-PRO-7A and BJ-PRO-10C induce vasodilatation and inotropic effects in normotensive and hypertensive rats: Role of nitric oxide and muscarinic receptors. Peptides 2018, 110, 1–9. [Google Scholar] [CrossRef]
- Rioli, V.; Prezoto, B.C.; Konno, K.; Melo, R.L.; Klitzke, C.F.; Ferro, E.S.; Ferreira-Lopes, M.; Camargo, A.C.; Portaro, F.C. A novel bradykinin potentiating peptide isolated from Bothrops jararacussu venom using catallytically inactive oligopeptidase EP24.15. FEBS J. 2008, 275, 2442–2454. [Google Scholar] [CrossRef]
- Munawar, A.; Zahid, A.; Negm, A.; Akrem, A.; Spencer, P.; Betzel, C. Isolation and characterization of Bradykinin potentiating peptides from Agkistrodon bilineatus venom. Proteome Sci. 2016, 14, 1. [Google Scholar] [CrossRef]
- Pinheiro-Júnior, E.L.; Boldrini-França, J.; de Campos Araújo, L.M.P.; Santos-Filho, N.A.; Bendhack, L.M.; Cilli, E.M.; Arantes, E.C. LmrBPP9: A synthetic bradykinin-potentiating peptide from Lachesis muta rhombeata venom that inhibits the angiotensin-converting enzyme activity in vitro and reduces the blood pressure of hypertensive rats. Peptides 2018, 102, 1–7. [Google Scholar] [CrossRef]
- Lopes, D.M.; Junior, N.E.; Costa, P.P.; Martins, P.L.; Santos, C.F.; Carvalho, E.D.; Carvalho, M.D.; Pimenta, D.C.; Cardi, B.A.; Fonteles, M.C.; et al. A new structurally atypical bradykinin-potentiating peptide isolated from Crotalus durissus cascavella venom (South American rattlesnake). Toxicon 2014, 90, 36–44. [Google Scholar] [CrossRef]
- Cea, L.B. Natriuretic peptide family: New aspects. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2005, 3, 87–98. [Google Scholar] [CrossRef]
- Rubattu, S.; Volpe, M. Natriuretic peptides in the cardiovascular system: Multifaceted roles in physiology, pathology and therapeutics. Int. J. Mol. Sci. 2019, 20, 3991. [Google Scholar] [CrossRef]
- Barbouche, R.; Marrakchi, N.; Mansuelle, P.; Krifi, M.; Fenouillet, E.; Rochat, H.; el Ayeb, M. Novel anti-platelet aggregation polypeptides from Vipera lebetina venom: Isolation and characterization. FEBS Lett. 1996, 392, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, J.S.; Martins, A.M.; Nascimento, N.R.; Sousa, C.M.; Alves, R.S.; Toyama, D.O.; Toyama, M.H.; Evangelista, J.J.; Menezes, D.B.; Fonteles, M.C.; et al. Renal and vascular effects of the natriuretic peptide isolated from Crotalus durissus cascavella venom. Toxicon 2008, 52, 737–744. [Google Scholar] [CrossRef] [PubMed]
- St Pierre, L.; Flight, S.; Masci, P.P.; Hanchard, K.J.; Lewis, R.J.; Alewood, P.F.; de Jersey, J.; Lavin, M.F. Cloning and characterisation of natriuretic peptides from the venom glands of Australian elapids. Biochimie 2006, 88, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Amininasab, M.; Elmi, M.M.; Endlich, N.; Endlich, K.; Parekh, N.; Naderi-Manesh, H.; Schaller, J.; Mostafavi, H.; Sattler, M.; Sarbolouki, M.N.; et al. Functional and structural characterization of a novel member of the natriuretic family of peptides from the venom of Pseudocerastes persicus. FEBS Lett. 2004, 557, 104–108. [Google Scholar] [CrossRef]
- Fry, B.G.; Wickramaratana, J.C.; Lemme, S.; Beuve, A.; Garbers, D.; Hodgson, W.C.; Alewood, P. Novel natriuretic peptides from the venom of the inland taipan (Oxyuranus microlepidotus): Isolation, chemical and biological characterisation. Biochem. Biophys. Res. Commun. 2005, 327, 1011–1015. [Google Scholar] [CrossRef]
- Siang, A.S.; Doley, R.; Vonk, F.J.; Kini, R.M. Transcriptomic analysis of the venom gland of the red-headed krait (Bungarus flaviceps) using expressed sequence tags. BMC Mol. Biol. 2010, 11, 24. [Google Scholar]
- Sridharan, S.; Kini, R.M.; Richards, A.M. Venom natriuretic peptides guide the design of heart failure therapeutics. Pharmacol. Res. 2020, 155, 104687. [Google Scholar] [CrossRef]
- Ichiki, T.; Dzhoyashvili, N.; Burnett, J.C., Jr. Natriuretic peptide based therapeutics for heart failure: Cenderitide: A novel first-in-class designer natriuretic peptide. Int. J. Cardiol. 2019, 281, 166–171. [Google Scholar] [CrossRef]
- Docherty, K.F.; Vaduganathan, M.; Solomon, S.D.; McMurray, J.J.V. Sacubitril/valsartan: Neprilysin inhibition 5 years after PARADIGM-HF. JACC Heart Fail. 2020, 8, 800–810. [Google Scholar] [CrossRef]
- Colucci, W.S. Nesiritide for the treatment of decompensated heart failure. J. Card. Fail. 2001, 7, 92–100. [Google Scholar] [CrossRef]
- Da Silva, S.L.; Dias-Junior, C.A.; Baldasso, P.A.; Damico, D.C.; Carvalho, B.M.; Garanto, A.; Acosta, G.; Oliveira, E.; Albericio, F.; Soares, A.M.; et al. Vascular effects and electrolyte homeostasis of the natriuretic peptide isolated from Crotalus oreganus abyssus (North American Grand Canyon rattlesnake) venom. Peptides 2012, 36, 206–212. [Google Scholar] [CrossRef]
- Vink, S.; Jin, A.H.; Poth, K.J.; Head, G.A.; Alewood, P.F. Natriuretic peptide drug leads from snake venom. Toxicon 2012, 59, 434–445. [Google Scholar] [PubMed]
- Nakanishi, M.; Saito, Y.; Kishimoto, I.; Harada, M.; Kuwahara, K.; Takahashi, N.; Kawakami, R.; Nakagawa, Y.; Tanimoto, K.; Yasuno, S.; et al. Role of natriuretic peptide receptor guanylyl cyclase-A in myocardial infarction evaluated using genetically engineered mice. Hypertension 2005, 46, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.J.; Huang, T.F.; Rucinski, B.; Strzyzewski, M.; Tuma, R.F.; Williams, J.A.; Niewiarowski, S. Inhibition of platelet hemostatic plug formation by trigramin, a novel RGD-peptide. Am. J. Physiol. 1989, 256, H1038–H1043. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, C. Functional characteristic of snake venom disintegrins: Potential therapeutic implication. Curr. Pharm. Des. 2005, 11, 815–827. [Google Scholar] [CrossRef]
- Huang, T.F.; Holt, J.C.; Lukasiewicz, H.; Niewiarowski, S. Trigramin. A low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb-IIIa complex. J. Biol. Chem. 1987, 262, 16157–16163. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Wu, Y.J.; Ouyang, C. Characterization of a potent platelet aggregation inhibitor from Agkistrodon rhodostoma snake venom. Biochim. Biophys. Acta 1987, 925, 248–257. [Google Scholar] [CrossRef]
- Huang, T.F.; Liu, C.Z.; Ouyang, C.H.; Teng, C.M. Halysin, an antiplatelet Arg-Gly-Asp-containing snake venom peptide, as fibrinogen receptor antagonist. Biochem. Pharmacol. 1991, 42, 1209–1219. [Google Scholar]
- Pinto, A.; Angulo, Y.; Jimenez, R.; Lomonte, B. Isolation of bothrasperin, a disintegrin with potent platelet aggregation inhibitory activity, from the venom of the snake Bothrops asper. Rev. Biol. Trop. 2003, 51, 253–259. [Google Scholar]
- Scarborough, R.M.; Naughton, M.A.; Teng, W.; Rose, J.W.; Phillips, D.R.; Nannizzi, L.; Arfsten, A.; Campbell, A.M.; Charo, I.F. Design of potent and specific integrin antagonists. Peptide antagonists with high specificity for glycoprotein IIb-IIIa. J. Biol. Chem. 1993, 268, 1066–1073. [Google Scholar] [CrossRef]
- Ben-Mabrouk, H.; Zouari-Kessentini, R.; Montassar, F.; Koubaa, Z.A.; Messaadi, E.; Guillonneau, X.; ElAyeb, M.; Srairi-Abid, N.; Luis, J.; Micheau, O.; et al. CC5 and CC8, two homologous disintegrins from Cerastes cerastes venom, inhibit in vitro and ex vivo angiogenesis. Int. J. Biol. Macromol. 2016, 86, 670–680. [Google Scholar]
- Crea, F.; Liuzzo, G. Pathogenesis of acute coronary syndromes. J. Am. Coll. Cardiol. 2013, 61, 1–11. [Google Scholar]
- Ozawa, K.; Packwood, W.; Varlamov, O.; Muller, M.; Xie, A.; Wu, M.D.; Abraham-Fan, R.J.; López, J.A. Elevated ldl cholesterol increases microvascular endothelial vwf and thromboinflammation after myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1041–1053. [Google Scholar] [CrossRef]
- Badimon, L.; Padró, T.; Vilahur, G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 60–74. [Google Scholar] [CrossRef]
- Sarray, S.; Srairi, N.; Hatmi, M.; Luis, J.; Louzir, H.; Regaya, I.; Slema, H.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N. Lebecetin, a potent antiplatelet C-type lectin from Macrovipera lebetina venom. Biochim. Biophys. Acta 2003, 1651, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Kroll, M.H.; Ward, C.M.; Rose, J.W.; Scarborough, R.M.; Smith, A.I.; López, J.A.; Berndt, M.C. Binding of a novel 50-kilodalton alboaggregin from Trimeresurus albolabris and related viper venom proteins to the platelet membrane glycoprotein Ib-IX-V complex. Effect on platelet aggregation and glycoprotein Ib-mediated platelet activation. Biochemistry 1996, 35, 12629–12639. [Google Scholar] [CrossRef] [PubMed]
- Polgár, J.; Magnenat, E.M.; Peitsch, M.C.; Wells, T.N.; Saqi, M.S.; Clemetson, K.J. Amino acid sequence of the alpha subunit and computer modelling of the alpha and beta subunits of echicetin from the venom of Echis carinatus (saw-scaled viper). Biochem. J. 1997, 323 Pt 2, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Zhang, Y. Molecular cloning and characterization of a platelet glycoprotein Ib-binding protein from the venom of Trimeresurus stejnegeri. Toxicon 2003, 41, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Taniuchi, Y.; Hisamichi, N.; Fujimura, Y.; Suzuki, M.; Titani, K.; Sakai, Y.; Kaku, S.; Satoh, N.; Takenaka, T.; et al. Tokaracetin, a new platelet antagonist that binds to platelet glycoprotein ib and inhibits von Willebrand factor-dependent shear-induced platelet aggregation. Biochem. J. 1995, 308 Pt 3, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Chang, M.C.; Peng, H.C.; Huang, T.F. Pharmacological characterization and antithrombotic effect of agkistin, a platelet glycoprotein Ib antagonist. Br. J. Pharmacol. 2001, 132, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Horii, K.; Okuda, D.; Morita, T.; Mizuno, H. Structural characterization of EMS16, an antagonist of collagen receptor (GPIa/IIa) from the venom of Echis multisquamatus. Biochemistry 2003, 42, 12497–12502. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Tuckwell, D.S. The alpha2beta1 integrin inhibitor rhodocetin binds to the A-domain of the integrin alpha2 subunit proximal to the collagen-binding site. Biochem. J. 2003, 376, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Sarray, S.; Delamarre, E.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N.; Luis, J. Lebectin and lebecetin, two C-type lectins from snake venom, inhibit alpha5beta1 and alphaV-containing integrins. Matrix Biol. 2007, 26, 306–313. [Google Scholar] [CrossRef]
- Pilorget, A.; Conesa, M.; Sarray, S.; Michaud-Levesque, J.; Daoud, S.; Kim, K.S.; Demeule, M.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N.; et al. Lebectin, a Macrovipera lebetina venom-derived C-type lectin, inhibits angiogenesis both in vitro and in vivo. J. Cell. Physiol. 2007, 211, 307–315. [Google Scholar] [CrossRef]
- Girish, V.M.; Kumar, S.; Joseph, L.; Jobichen, C.; Kini, R.M.; Sivaraman, J. Identification and structural characterization of a new three-finger toxin hemachatoxin from Hemachatus haemachatus venom. PLoS ONE 2012, 7, e48112. [Google Scholar]
- Ferraz, C. Multifunctional toxins in snake venoms and therapeutic implications: From pain to hemorrhage and necrosis. Front. Ecol. Evol. 2019, 7, 218. [Google Scholar]
- Dufton, M.J.; Hider, R.C. Conformational properties of the neurotoxins and cytotoxins isolated from Elapid snake venoms. CRC Crit. Rev. Biochem. 1983, 14, 113–171. [Google Scholar]
- Yasuda, O.; Morimoto, S.; Jiang, B.; Kuroda, H.; Kimura, T.; Sakakibara, S.; Fukuo, K.; Chen, S.; Tamatani, M.; Ogihara, T. FS2. a mamba venom toxin, is a specific blocker of the L-type calcium channels. Artery 1994, 21, 287–302. [Google Scholar]
- McDowell, R.S.; Dennis, M.S.; Louie, A.; Shuster, M.; Mulkerrin, M.G.; Lazarus, R.A. Mambin, a potent glycoprotein IIb-IIIa antagonist and platelet aggregation inhibitor structurally related to the short neurotoxins. Biochemistry 1992, 31, 4766–4772. [Google Scholar] [CrossRef]
- Girish, V.M.; Kini, R.M. Exactin: A specific inhibitor of Factor X activation by extrinsic tenase complex from the venom of Hemachatus haemachatus. Sci. Rep. 2016, 6, 32036. [Google Scholar] [CrossRef]
- Barnwal, B.; Jobichen, C.; Girish, V.M.; Foo, C.S.; Sivaraman, J.; Kini, R.M. Ringhalexin from Hemachatus haemachatus: A novel inhibitor of extrinsic tenase complex. Sci. Rep. 2016, 6, 25935. [Google Scholar] [PubMed]
- Wu, P.L.; Lee, S.C.; Chuang, C.C.; Mori, S.; Akakura, N.; Wu, W.G.; Takada, Y. Non-cytotoxic cobra cardiotoxin A5 binds to alpha(v)beta3 integrin and inhibits bone resorption. Identification of cardiotoxins as non-RGD integrin-binding proteins of the Ly-6 family. J. Biol. Chem. 2006, 281, 7937–7945. [Google Scholar] [CrossRef] [PubMed]
- Chanda, C.; Sarkar, A.; Sistla, S.; Chakrabarty, D. Anti-platelet activity of a three-finger toxin (3FTx) from Indian monocled cobra (Naja kaouthia) venom. Biochem. Biophys. Res. Commun. 2013, 441, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Koivula, K.; Rondinelli, S.; Näsman, J. The three-finger toxin MTalpha is a selective alpha(2B)-adrenoceptor antagonist. Toxicon 2010, 56, 440–447. [Google Scholar] [CrossRef]
- Intachai, K.; Chattipakorn, S.C.; Chattipakorn, N.; Shinlapawittayatorn, K. Revisiting the cardioprotective effects of acetylcholine receptor activation against myocardial ischemia/reperfusion injury. Int. J. Mol. Sci. 2018, 19, 2466. [Google Scholar] [CrossRef]
- Baron, A.; Diochot, S.; Salinas, M.; Deval, E.; Noel, J.; Lingueglia, E. Venom toxins in the exploration of molecular, physiological and pathophysiological functions of acid-sensing ion channels. Toxicon 2013, 75, 187–204. [Google Scholar]
- Tariq, S.; Aronow, W.S. Use of inotropic agents in treatment of systolic heart failure. Int. J. Mol. Sci. 2015, 16, 29060–29068. [Google Scholar] [CrossRef]
- Joubert, F.J.; Taljaard, N. The complete primary structures of two reduced and S-carboxymethylated Angusticeps-type toxins from Dendroaspis angusticeps (green mamba) venom. Biochim. Biophys. Acta 1980, 623, 449–456. [Google Scholar] [CrossRef]
- Joubert, F.J.; Taljaard, N. The primary structure of a short neurotoxin homologue (S4C8) from Dendroaspis jamesoni kaimosae (Jameson’s mamba) venom. Int. J. Biochem. 1980, 12, 567–574. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar]
- Schleifer, K.J. Comparative molecular modelling study of the calcium channel blockers nifedipine and black mamba toxin FS2. J. Comput. Aided Mol. Des. 1997, 11, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Shiu, J.H.; Chen, C.Y.; Chang, L.S.; Chen, Y.C.; Chen, Y.C.; Lo, Y.H.; Liu, Y.C.; Chuang, W.J. Solution structure of gamma-bungarotoxin: The functional significance of amino acid residues flanking the RGD motif in integrin binding. Proteins 2004, 57, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor (vegf) and its receptor (vegfr) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Matsunaga, Y.; Tokunaga, Y.; Obayashi, S.; Saito, M.; Morita, T. Snake venom Vascular Endothelial Growth Factors (VEGF-Fs) exclusively vary their structures and functions among species. J. Biol. Chem. 2009, 284, 9885–9891. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Fei, Q.; Xiao, H.; Wang, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X.; Wang, K. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J. Cell. Physiol. 2019, 234, 17690–17703. [Google Scholar] [CrossRef] [PubMed]
- Pajula, J.; Lähteenvuo, J.; Lähteenvuo, M.; Honkonen, K.; Halonen, P.; Hätinen, O.P.; Kuivanen, A.; Heikkilä, M.; Nurro, J.; Hartikainen, J.; et al. Adenoviral VEGF-D(ΔN ΔC) gene therapy for myocardial ischemia. Front. Bioeng. Biotechnol. 2022, 10, 999226. [Google Scholar] [CrossRef]
- Albrecht-Schgoer, K.; Schgoer, W.; Holfeld, J.; Theurl, M.; Wiedemann, D.; Steger, C.; Gupta, R.; Semsroth, S.; Fischer-Colbrie, R.; Beer, A.G.; et al. The angiogenic factor secretoneurin induces coronary angiogenesis in a model of myocardial infarction by stimulation of vascular endothelial growth factor signaling in endothelial cells. Circulation 2012, 126, 2491–2501. [Google Scholar] [CrossRef]
- Wei, W.; Chen, Z.W.; Yang, Q.; Jin, H.; Furnary, A.; Yao, X.Q.; Yim, A.P.; He, G.W. Vasorelaxation induced by vascular endothelial growth factor in the human internal mammary artery and radial artery. Vascul. Pharmacol. 2007, 46, 253–259. [Google Scholar] [CrossRef]
- Komori, Y.; Nikai, T.; Taniguchi, K.; Masuda, K.; Sugihara, H. Vascular Endothelial Growth Factor VEGF-like Heparin-Binding Protein from the Venom of Vipera aspis aspis (Aspic Viper). Biochemistry 1999, 38, 11796–11803. [Google Scholar] [CrossRef]
- Mahjoub, Y.; Malaquin, S.; Mourier, G.; Lorne, E.; Abou Arab, O.; Massy, Z.A.; Dupont, H.; Ducancel, F. Short-versus Long-Sarafotoxins: Two Structurally Related Snake Toxins with Very Different in vivo Haemodynamic Effects. PLoS ONE 2015, 10, e0132864. [Google Scholar]
- Patocka, J.; Merka, V.; Hrdina, V.; Hrdina, R. Endothelins and sarafotoxins: Peptides of similar structure and different function. Acta Medica (Hradec Kral.) 2004, 47, 157–162. [Google Scholar] [CrossRef]
- Kähler, J.; Köster, R.; Paul, M.; Hamm, C.W.; Meinertz, T. Endothelins in cardiovascular diseases. Z. Kardiol. 1997, 86, 406–416. [Google Scholar] [PubMed]
- Wollberg, Z.; Bdolah, A.; Kochva, E. Vasoconstrictor effects of sarafotoxins in rabbit aorta: Structure-function relationships. Biochem. Biophys. Res. Commun. 1989, 162, 371–376. [Google Scholar] [CrossRef]
- Watanabe, C.; Hirano, K.; Kanaide, H. Role of extracellular and intracellular sources of Ca2+ in sarafotoxin S6b-induced contraction of strips of the rat aorta. Br. J. Pharmacol. 1993, 108, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Skovsted, G.F.; Kruse, L.S.; Berchtold, L.A.; Grell, A.S.; Warfvinge, K.; Edvinsson, L. Myocardial ischemia-reperfusion enhances transcriptional expression of endothelin-1 and vasoconstrictor ETB receptors via the protein kinase MEK-ERK1/2 signaling pathway in rat. PLoS ONE 2017, 12, e0174119. [Google Scholar]
- Matsunaga, Y.; Yamazaki, Y.; Hyodo, F.; Sugiyama, Y.; Nozaki, M.; Morita, T. Structural divergence of cysteine-rich secretory proteins in snake venoms. J. Biochem. 2009, 145, 365–375. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Morita, T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef]
- Mackessy, S.P. Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Suntravat, M.; Cromer, W.E.; Marquez, J.; Galan, J.A.; Zawieja, D.C.; Davies, P.; Salazar, E.; Sanchez, E.E. The isolation and characterization of a new snake venom cysteine-rich secretory protein (svCRiSP) from the venom of the Southern Pacific rattlesnake and its effect on vascular permeability. Toxicon 2019, 165, 22–30. [Google Scholar] [CrossRef]
- Swenson, S.; Markland, F.S., Jr. Snake venom fibrin(ogen)olytic enzymes. Toxicon 2005, 45, 1021–1039. [Google Scholar] [CrossRef]
- Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial infarction. Results of the TIMI IIIB Trial. Thrombolysis in Myocardial Ischemia. Circulation 1994, 89, 1545–1556. [CrossRef]
- Siigur, J.; Samel, M.; Tonismagi, K.; Subbi, J.; Siigur, E.; Tu, A.T. Biochemical characterization of lebetase, a direct-acting fibrinolytic enzyme from Vipera lebetina snake venom. Thromb. Res. 1998, 90, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Egberg, N.; Nordström, S. Effects of Reptilase-Induced Intravascular Coagulation in Dogs. Acta Physiol. Scand. 1970, 79, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Erlich, J.H.; Boyle, E.M.; Labriola, J.; Kovacich, J.C.; Santucci, R.A.; Fearns, C.; Morgan, E.N.; Yun, W.; Luther, T.; Kojikawa, O.; et al. Inhibition of the tissue factor-thrombin pathway limits infarct size after myocardial ischemia-reperfusion injury by reducing inflammation. Am. J. Pathol. 2000, 157, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Simpson, P.J.; Schelm, J.A.; Smith, G.F. Therapeutic defibrination with ancrod does not protect canine myocardium from reperfusion injury. J. Pharmacol. Exp. Ther. 1991, 256, 780–786. [Google Scholar]
- Xiao, H.; Pan, H.; Liao, K.; Yang, M.; Huang, C. Snake venom PLA(2), a promising target for broad-spectrum antivenom drug development. BioMed Res. Int. 2017, 2017, 6592820. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef]
- Zouari-Kessentini, R.; Srairi-Abid, N.; Bazaa, A.; El Ayeb, M.; Luis, J.; Marrakchi, N. Antitumoral potential of Tunisian snake venoms secreted phospholipases A2. BioMed Res. Int. 2013, 2013, 391389. [Google Scholar] [CrossRef][Green Version]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 biochemistry. Cardiovasc. Drugs Ther. 2009, 23, 49–59. [Google Scholar]
- Araya, C.; Lomonte, B. Antitumor effects of cationic synthetic peptides derived from Lys49 phospholipase A2 homologues of snake venoms. Cell Biol. Int. 2007, 31, 263–268. [Google Scholar] [CrossRef]
- Jridi, I.; Catacchio, I.; Majdoub, H.; Shahbazzadeh, D.; El Ayeb, M.; Frassanito, M.A.; Solimando, A.G.; Ribatti, D.; Vacca, A.; Borchani, L. The small subunit of Hemilipin2, a new heterodimeric phospholipase A2 from Hemiscorpius lepturus scorpion venom, mediates the antiangiogenic effect of the whole protein. Toxicon 2017, 126, 38–46. [Google Scholar] [CrossRef]
- Philippe, F. Cardiopathie ischémique: Le point sur le traitement pharmacologique en 2008. Médecine des maladies Métaboliques 2008, 2, 241–247. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Resende, L.; Watanabe, R.; Carregari, V.; Huancahuire-Vega, S.; Caldeira, C.d.S.; Coutinho-Neto, A.; Soares, A.; Vale, N.; Gomes, P.d.C.; et al. Snake Venom Peptides and Low Mass Proteins: Molecular Tools and Therapeutic Agents. Curr. Med. Chem. 2017, 24, 3254–3282. [Google Scholar] [CrossRef] [PubMed]
- Gentilucci, L.; Tolomelli, A.; Squassabia, F. Peptides and peptidomimetics in medicine, surgery and biotechnology. Curr. Med. Chem. 2006, 13, 2449–2466. [Google Scholar] [CrossRef]
- Koh, D.C.; Armugam, A.; Jeyaseelan, K. Snake venom components and their applications in biomedicine. Cell. Mol. Life Sci. 2006, 63, 3030–3041. [Google Scholar] [CrossRef]
- Marsh, N.A. Diagnostic uses of snake venom. Haemostasis 2001, 31, 211–217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).