On Fusogenicity of Positively Charged Phased-Separated Lipid Vesicles: Experiments and Computational Simulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Approach
2.1.1. Materials
2.1.2. Preparation and Characterization of SUVs for Fusion Experiments
2.1.3. Preparation of GUVs
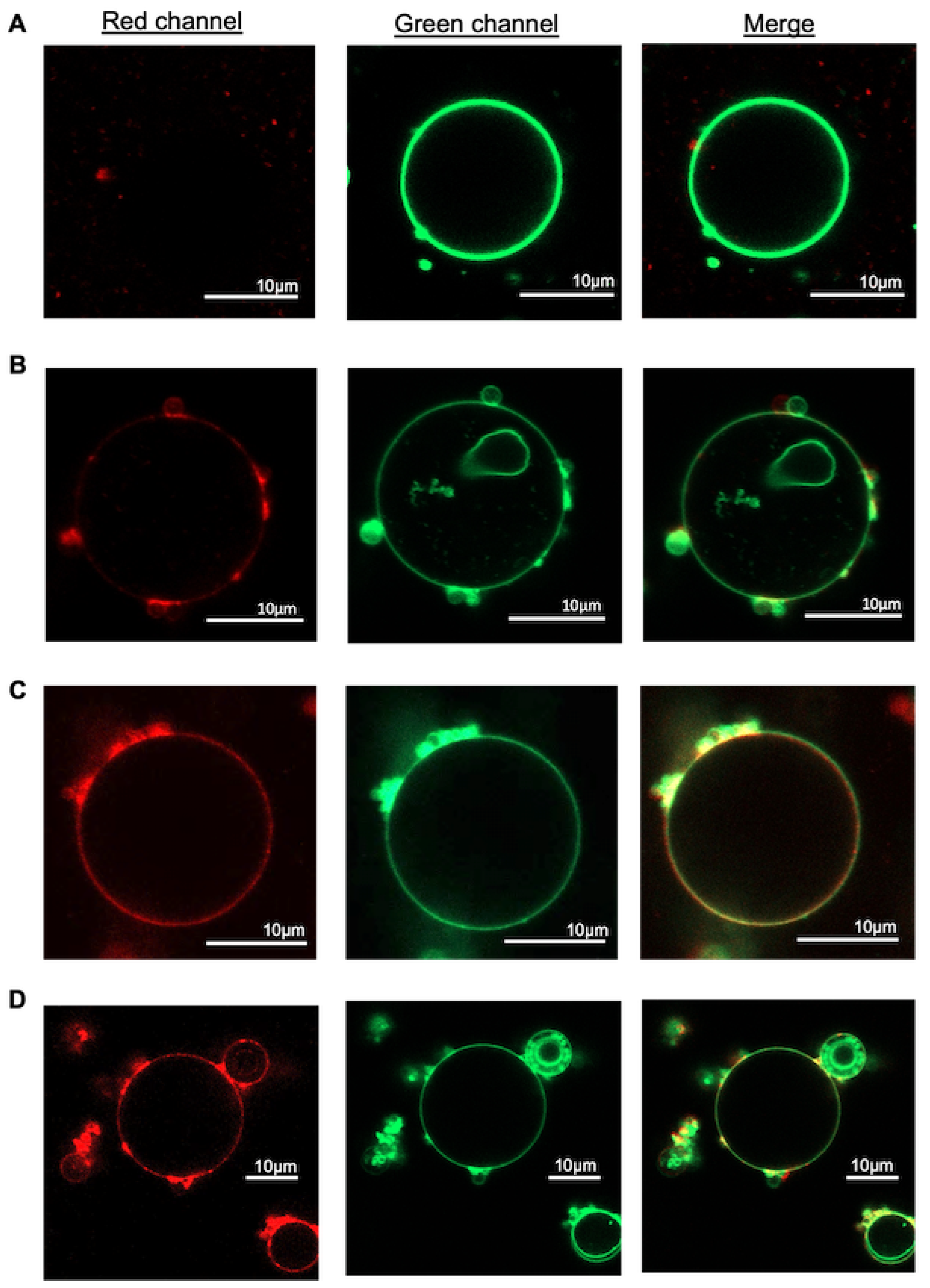
2.1.4. Imaging and Analysis
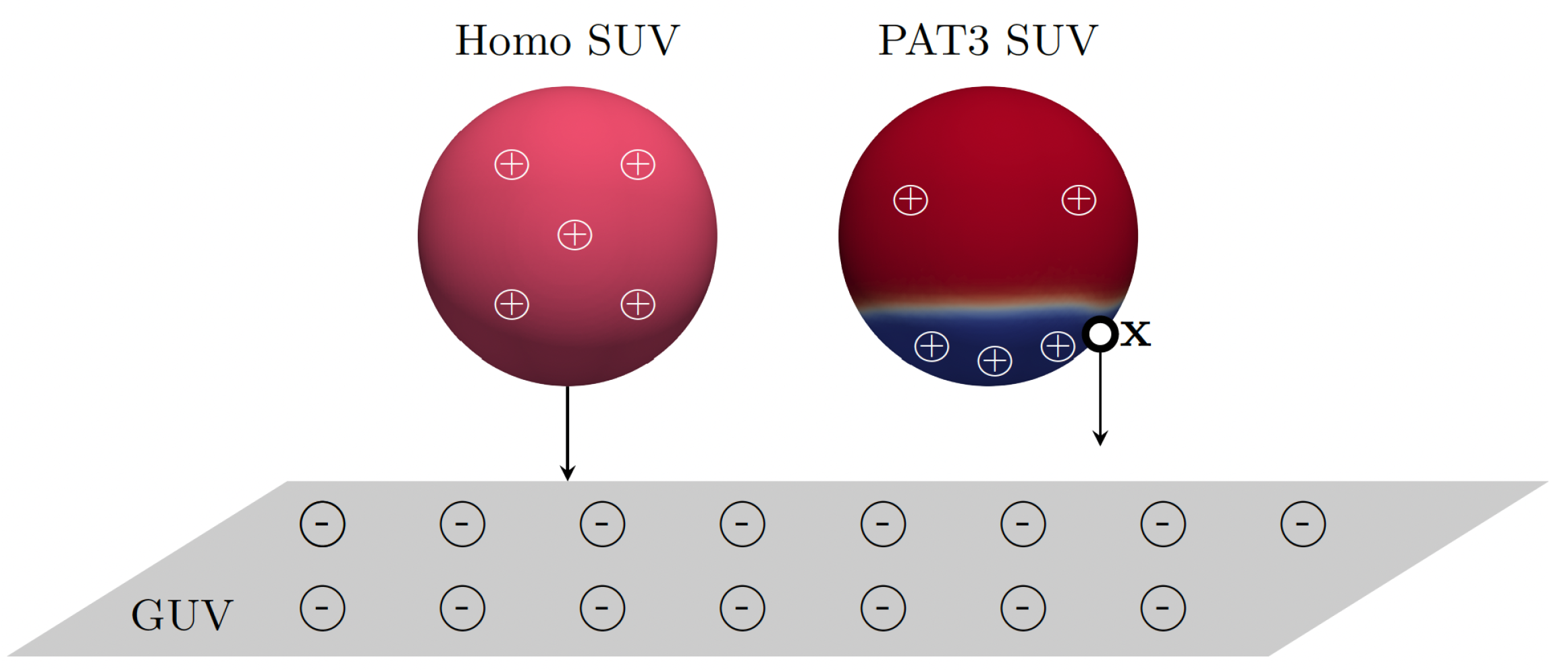
2.2. Computational Approach
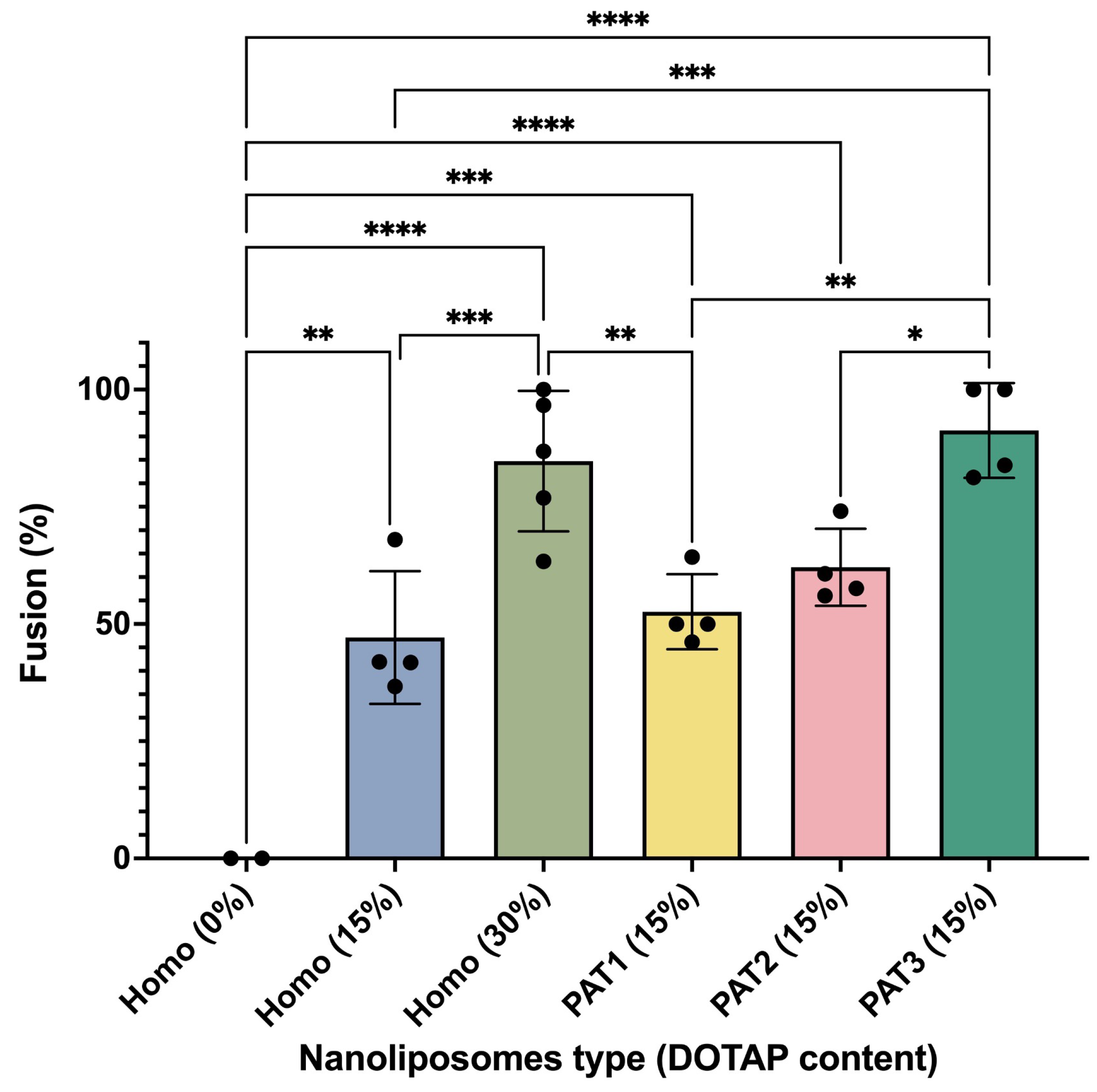
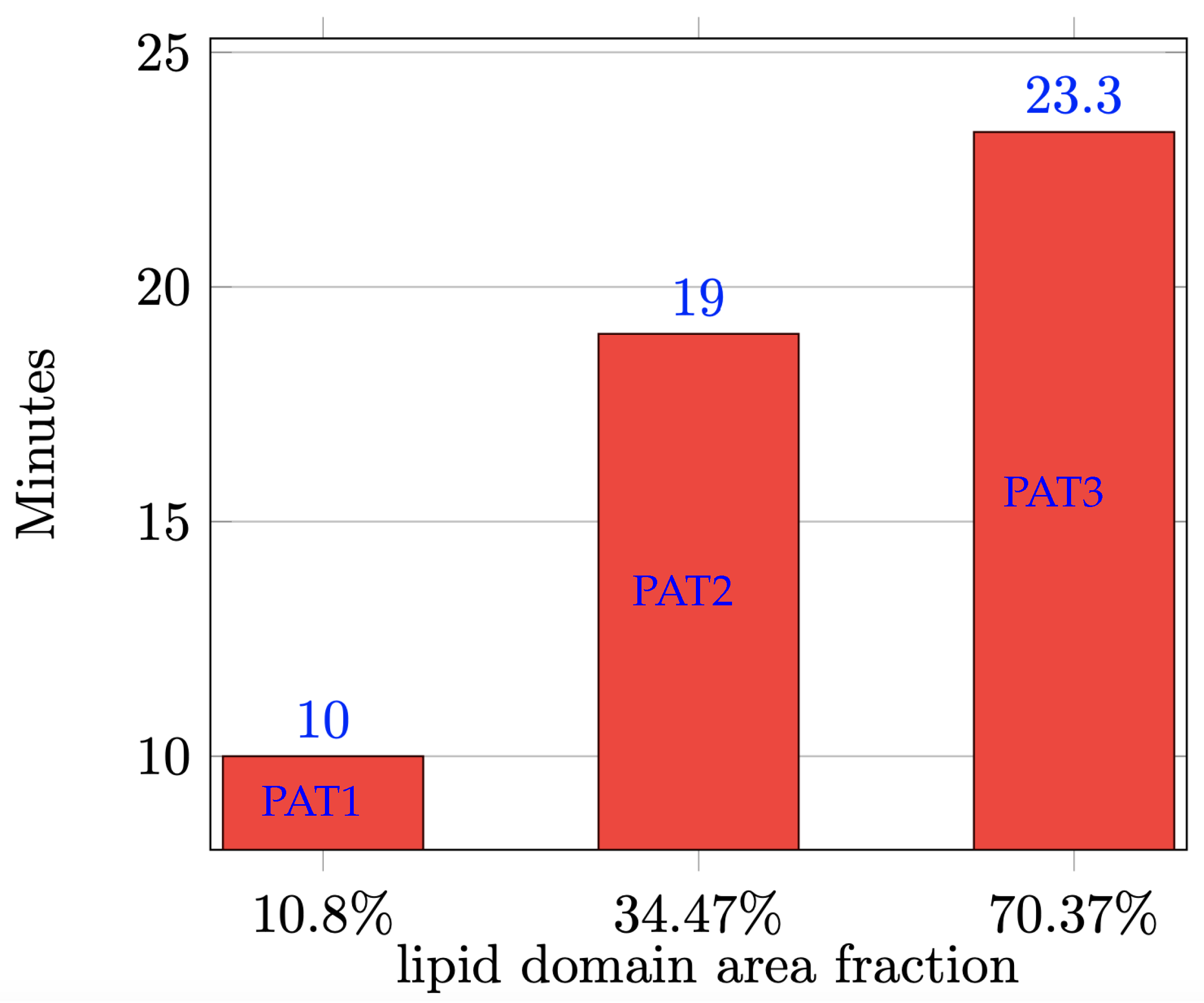
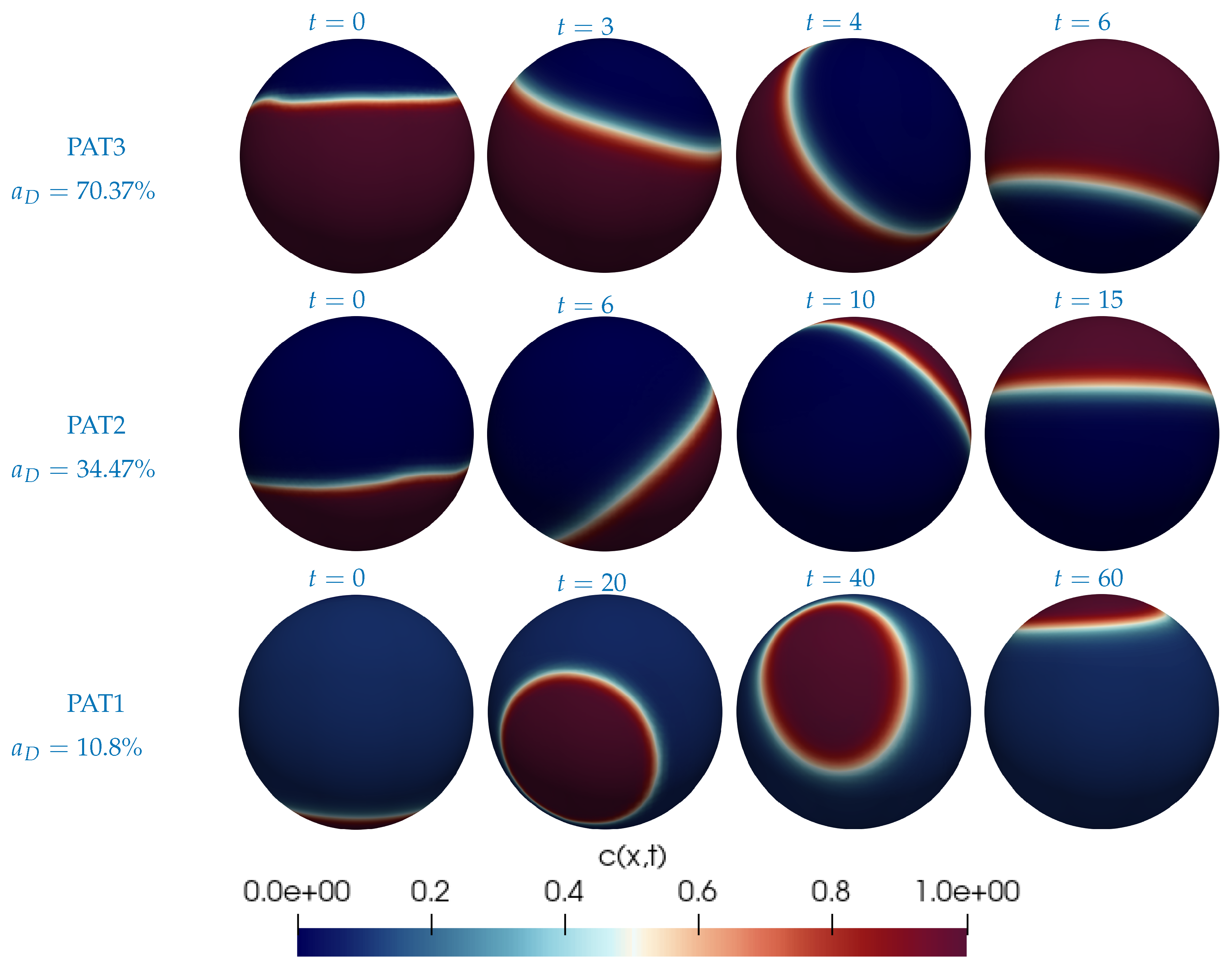
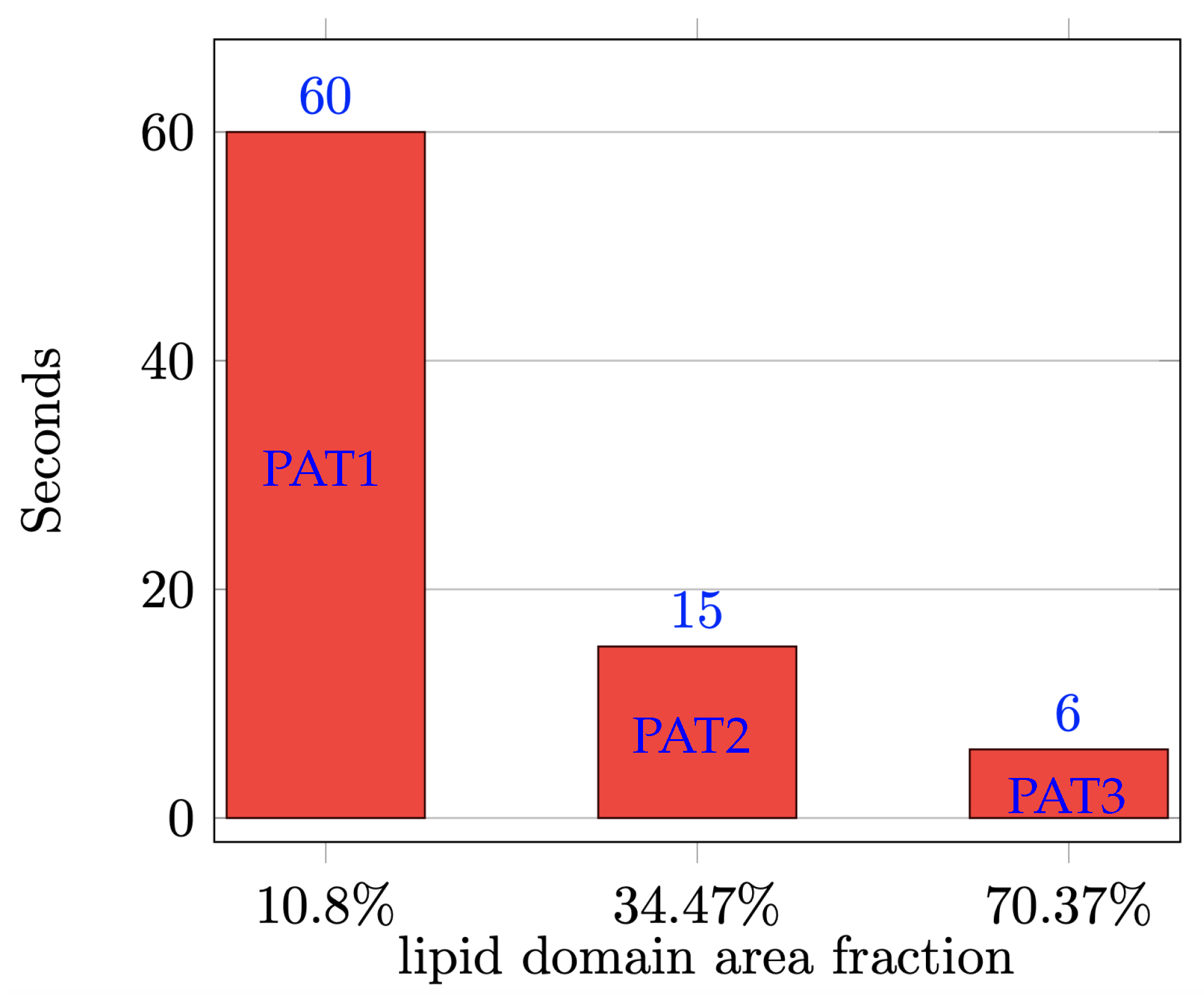
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, C.R.; Bondurant, B.; McLean, S.D.; McGovern, K.A.; O’Brien, D.F. Liposome- cell interactions in vitro: Effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 1998, 37, 12875–12883. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Fujisawa, M.; Yoshino, K.; Takeoka, S. Intracellular distribution of lipids and encapsulated model drugs from cationic liposomes with different uptake pathways. Int. J. Nanomed. 2020, 15, 8401–8409. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, D.; Ceña, V. Endocytosis: The nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Düzgüneş, N.; Nir, S. Mechanisms and kinetics of liposome–cell interactions. Adv. Drug Deliv. Rev. 1999, 40, 3–18. [Google Scholar] [CrossRef]
- Yang, J.; Bahreman, A.; Daudey, G.; Bussmann, J.; Olsthoorn, R.C.L.; Kros, A. Drug delivery via cell membrane fusion using lipopeptide modified liposomes. ACS Cent. Sci. 2016, 2, 621–630. [Google Scholar] [CrossRef]
- Hui, S.W.; Langner, M.; Zhao, Y.; Ross, P.; Hurley, E.; Chan, K. The role of helper lipids in cationic liposome-mediated gene transfer. Biophys. J. 1996, 71, 590–599. [Google Scholar] [CrossRef]
- Almofti, M.R.; Harashima, H.; Shinohara, Y.; Almofti, A.; Baba, Y.; Kiwada, H. Cationic liposome-mediated gene delivery: Biophysical study and mechanism of internalization. Arch. Biochem. Biophys. 2003, 410, 246–253. [Google Scholar] [CrossRef]
- Simberg, D.; Weisman, S.; Talmon, Y.; Barenholz, Y. DOTAP (and other cationic lipids): Chemistry, biophysics, and transfection. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21(4), 257–317. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and strategies of cationic liposomes for cancer gene therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hersch, N.; Gerlach, S.; Dreissen, G.; Springer, R.; Merkel, R.; Csiszár, A.; Hoffmann, B. Complex size and surface charge determine nucleic acid transfer by fusogenic liposomes. Int. J. Mol. Sci. 2020, 21, 2244. [Google Scholar] [CrossRef]
- Kolašinac, R.; Jaksch, S.; Dreissen, G.; Braeutigam, A.; Merkel, R.; Csiszár, A. Influence of environmental conditions on the fusion of cationic liposomes with living mammalian cells. Nanomaterials 2019, 9, 1025. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Heberle, F.A.; Feigenson, G.W. Phase separation in lipid membranes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004630. [Google Scholar] [CrossRef] [PubMed]
- Zhiliakov, A.; Wang, Y.; Quaini, A.; Olshanskii, M.; Majd, S. Experimental validation of a phase-field model to predict coarsening dynamics of lipid domains in multicomponent membranes. Biochim. Biophys. Acta BBA Biomembr. 2021, 1863, 183446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Palzhanov, Y.; Quaini, A.; Olshanskii, M.; Majd, S. Lipid domain coarsening and fluidity in multicomponent lipid vesicles: A continuum based model and its experimental validation. Biochim. Biophys. Acta BBA Biomembr. 2022, 1864, 183898. [Google Scholar] [CrossRef]
- Kang, Y.J.; Wostein, H.S.; Majd, S. A simple and versatile method for the formation of arrays of giant vesicles with controlled size and composition. Adv. Mater. 2013, 25, 6834–6838. [Google Scholar] [CrossRef]
- Palzhanov, Y.; Zhiliakov, A.; Quaini, A.; Olshanskii, M. A decoupled, stable, and linear fem for a phase-field model of variable density two-phase incompressible surface flow. Comput. Methods Appl. Mech. Eng. 2021, 387, 114167. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Statistical Physics; Pergamon: Oxford, UK, 1958. [Google Scholar]
- Sakuma, Y.; Kawakatsu, T.; Taniguchi, T.; Imai, M. Viscosity landscape of phase-separated lipid membrane estimated from fluid velocity field. Biophys. J. 2020, 118, 1576–1587. [Google Scholar] [CrossRef]
- Heftberger, P.; Kollmitzer, B.; Rieder, A.A.; Amenitsch, H.; Pabst, G. In situ determination of structure and fluctuations of coexisting fluid membrane domains. Biophys J. 2015, 108, 854–862. [Google Scholar] [CrossRef]
- Kollmitzer, B.; Heftberger, P.; Rappolt, M.; Pabst, G. Monolayer spontaneous curvature of raft-forming membrane lipids. Soft Matter. 2013, 9, 10877–10884. [Google Scholar] [CrossRef]
- Kuzmin, P.I.; Akimov, S.A.; Chizmadzhev, Y.A.; Zimmerberg, J.; Cohen, F.S. Line tension and interaction energies of membrane rafts calculated from lipid splay and tilt. Biophys. J. 2005, 88, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, A.; Sofou, S. Floret-shaped solid domains on giant fluid lipid vesicles induced by pH. Langmuir 2012, 28, 4113–4122. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.K.H.-J.; Graf, K. The Electric Double Layer; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; Chapter 4; pp. 42–56. [Google Scholar]
- Chibowski, E.; Szczes, A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef]
- Makino, K.; Yamada, T.; Kimura, M.; Oka, T.; Ohshima, H.; Kondo, T. Temperature- and ionic strength-induced conformational changes in the lipid head group region of liposomes as suggested by zeta potential data. Biophys. Chem. 1991, 41, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Neunert, G.; Tomaszewska-Gras, J.; Witkowski, S.; Polewski, K. Tocopheryl succinate-induced structural changes in DPPC liposomes: DSC and ANS fluorescence studies. Molecules 2020, 25, 2780. [Google Scholar] [CrossRef]
- Gómez, H.; Calo, V.M.; Bazilevs, Y.; Hughes, T.J.R. Isogeometric analysis of the Cahn–Hilliard phase-field model. Comput. Methods Appl. Mech. Eng. 2008, 197, 4333–4352. [Google Scholar] [CrossRef]
- Yushutin, V.; Quaini, A.; Majd, S.; Olshanskii, M. A computational study of lateral phase separation in biological membranes. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3181. [Google Scholar] [CrossRef]
- Veatch, S.L.; Soubias, O.; Keller, S.L.; Gawrisch, K. Critical fluctuations in domain-forming lipid mixtures. Proc. Natl. Acad. Sci. USA 2007, 104, 17650–17655. [Google Scholar] [CrossRef]
- Wang, Y.; Majd, S. Charged lipids modulate the phase separation in multicomponent membranes. Langmuir 2023, 39, 11371–11378. [Google Scholar] [CrossRef]
- Pardhy, N.P.; Budhlall, B.M. Pickering emulsion as a template to synthesize janus colloids with anisotropy in the surface potential. Langmuir 2010, 26, 13130–13141. [Google Scholar] [CrossRef]
- Veatch, S.L.; Keller, S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003, 85, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, J.; Veatch, S.L. Adhesion stabilizes robust lipid heterogeneity in supercritical membranes at physiological temperature. Biophys. J. 2013, 104, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Imam, Z.I.; Kenyon, L.E.; Ashby, G.; Nagib, F.; Mendicino, M.; Zhao, C.; Gadok, A.K.; Stachowiak, J.C. Phase-separated liposomes enhance the efficiency of macromolecular delivery to the cellular cytoplasm. Cell. Mol. Bioeng. 2017, 10, 387–403. [Google Scholar] [CrossRef] [PubMed]
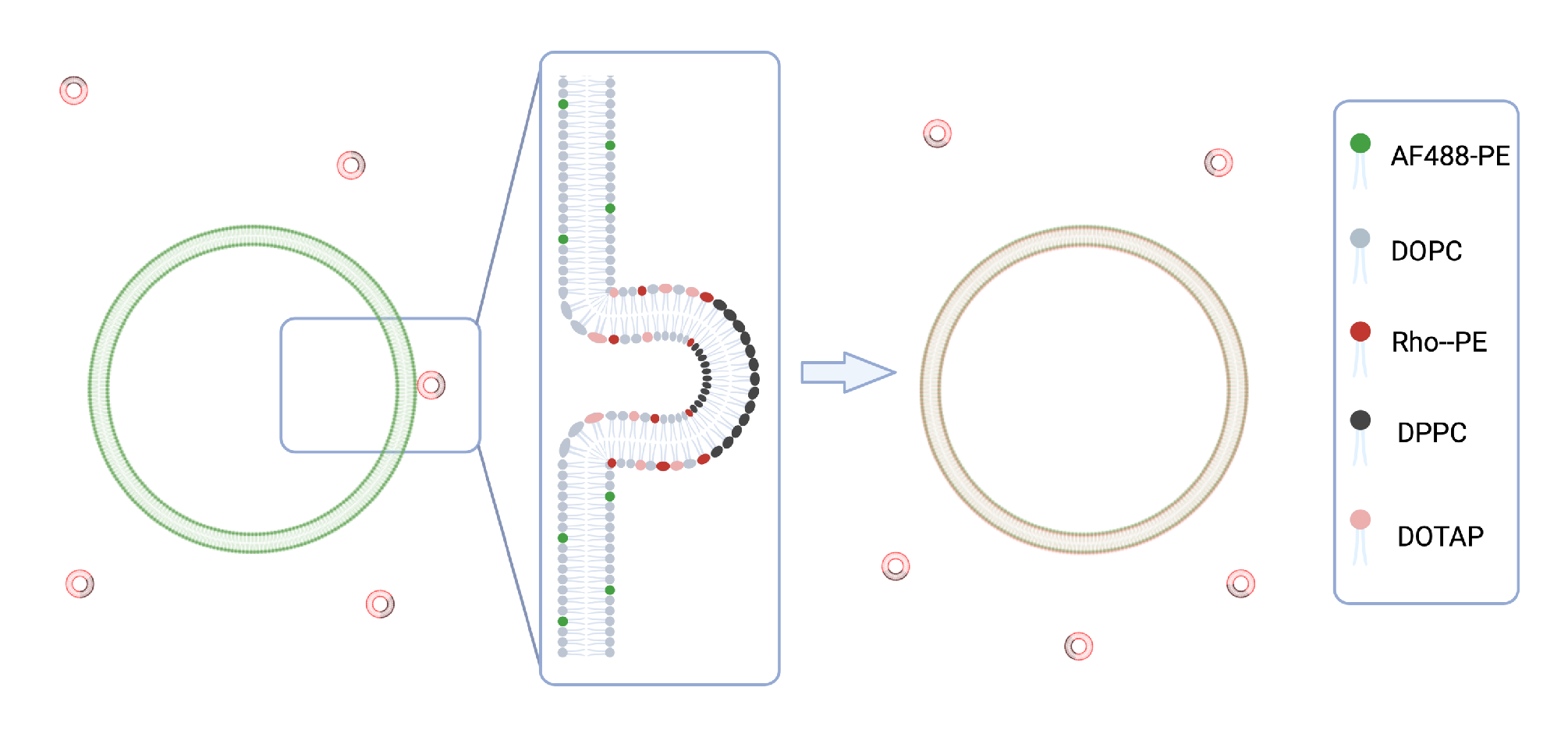
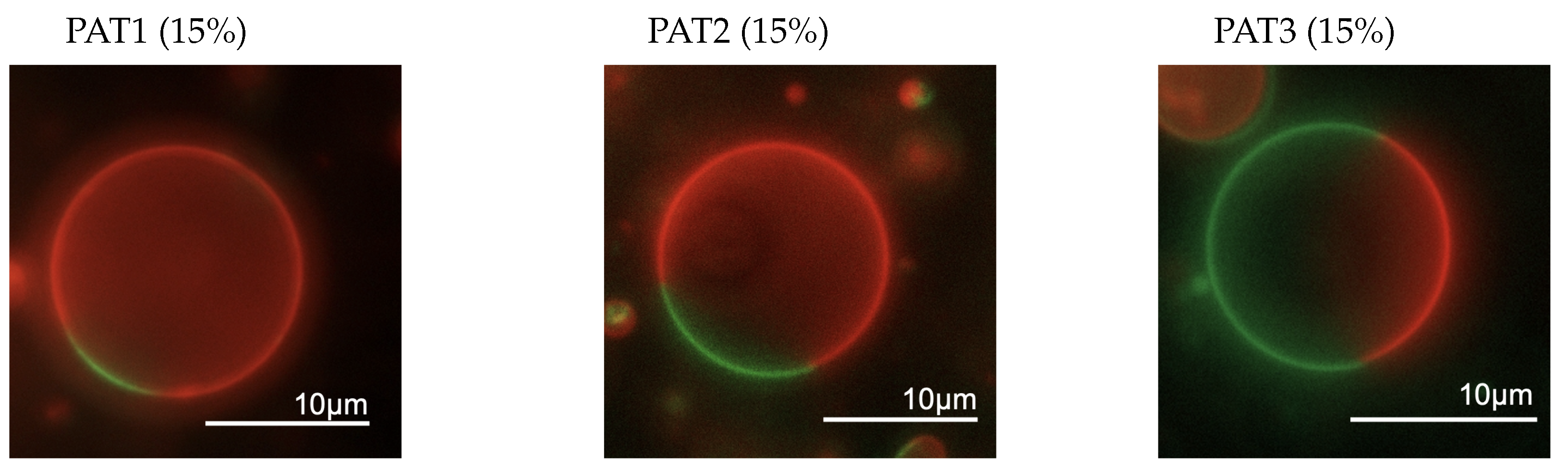
| Composition | DOPC | DPPC | Chol | Rho-PE |
|---|---|---|---|---|
| Homo | 99.4% | 0% | 0% | 0.6% |
| PAT1 | 59.4% | 20% | 20% | 0.6% |
| PAT2 | 41.9% | 42.5% | 15% | 0.6% |
| PAT3 | 24.4% | 50% | 25% | 0.6% |
| Composition | ||||||
|---|---|---|---|---|---|---|
| PAT1 | (15 °C) | 1401 | 1172 | 0.5–6 | 0.2–0.4 | 1.2–1.4 |
| PAT2 | (17.5 °C) | 1401 | 1172 | 0.43–5.7 | 0.2–0.4 | 1.2–1.6 |
| PAT3 | (15 °C) | 1435 | 1172 | 5–8 | 0.2–0.4 | 1.2–1.8 |
| Vesicle | Zeta Potential |
|---|---|
| GUV | − |
| PAT1 | |
| PAT2 | |
| PAT3 |
| Phase | Phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Composition | DOTAP | DOPC | DPPC | Chol | DOTAP | DOPC | DPPC | Chol |
| PAT1 (15%) | 16.67% | 49.33% | 16% | 18% | 5.56% | 16.44% | 43% | 35% |
| PAT2 (15%) | 22.91% | 41.09% | 29% | 7% | 4.65% | 8.35% | 61% | 26% |
| PAT3 (15%) | 41.80% | 26.20% | 24% | 8% | 8.61% | 5.39% | 57% | 29% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Palzhanov, Y.; Dang, D.T.; Quaini, A.; Olshanskii, M.; Majd, S. On Fusogenicity of Positively Charged Phased-Separated Lipid Vesicles: Experiments and Computational Simulations. Biomolecules 2023, 13, 1473. https://doi.org/10.3390/biom13101473
Wang Y, Palzhanov Y, Dang DT, Quaini A, Olshanskii M, Majd S. On Fusogenicity of Positively Charged Phased-Separated Lipid Vesicles: Experiments and Computational Simulations. Biomolecules. 2023; 13(10):1473. https://doi.org/10.3390/biom13101473
Chicago/Turabian StyleWang, Yifei, Yerbol Palzhanov, Dang T. Dang, Annalisa Quaini, Maxim Olshanskii, and Sheereen Majd. 2023. "On Fusogenicity of Positively Charged Phased-Separated Lipid Vesicles: Experiments and Computational Simulations" Biomolecules 13, no. 10: 1473. https://doi.org/10.3390/biom13101473
APA StyleWang, Y., Palzhanov, Y., Dang, D. T., Quaini, A., Olshanskii, M., & Majd, S. (2023). On Fusogenicity of Positively Charged Phased-Separated Lipid Vesicles: Experiments and Computational Simulations. Biomolecules, 13(10), 1473. https://doi.org/10.3390/biom13101473






