Age-Related Changes of the Synucleins Profile in the Mouse Retina
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Processing and Cryostat Sectioning
2.3. Immunohistochemistry
2.4. Confocal Microscopy and Fluorescence Image Analysis
2.5. Statistical Analysis
3. Results
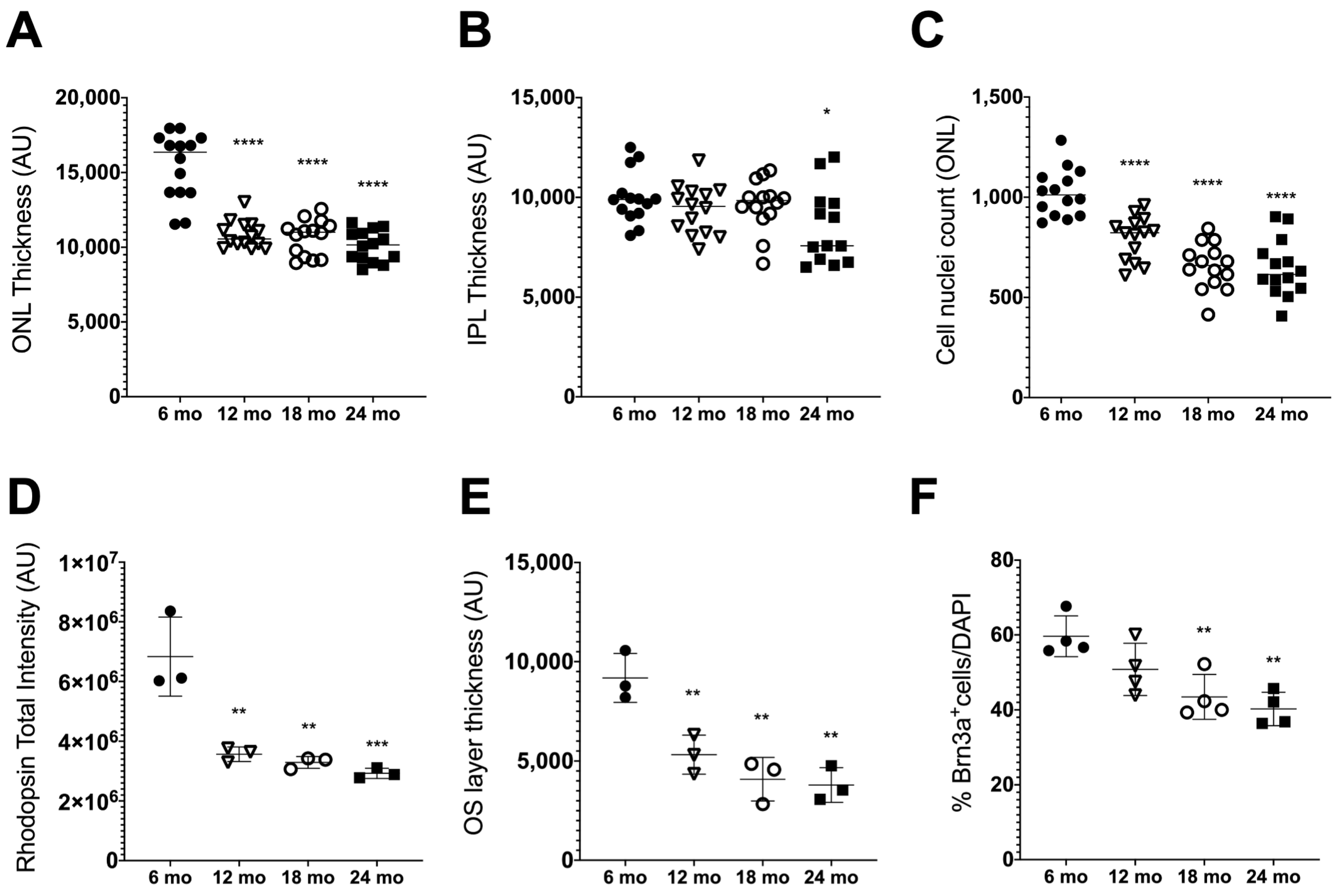
3.1. Age-Related Retinal Neurodegeneration
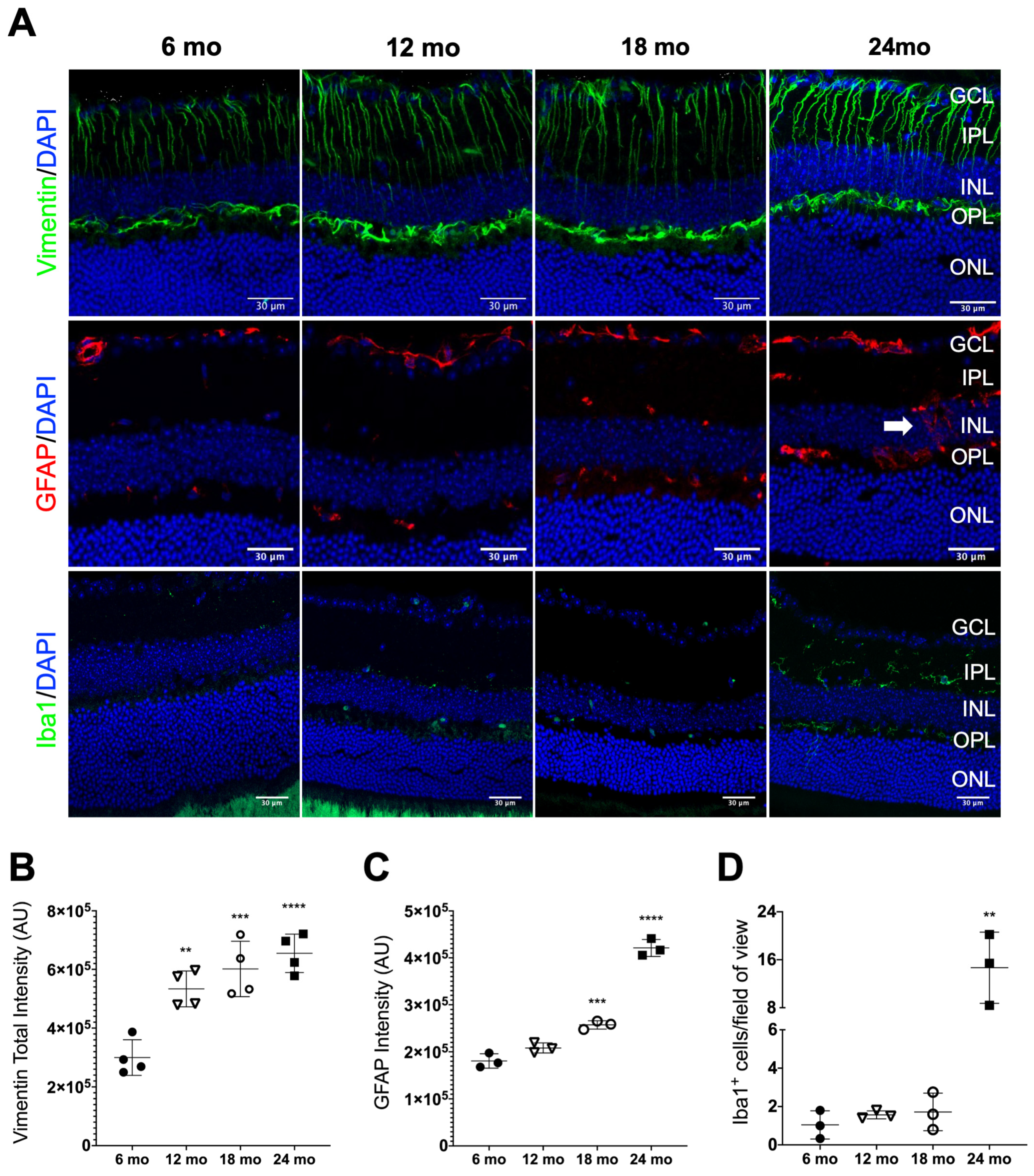
3.2. Age-Related Retinal Neuroinflamation
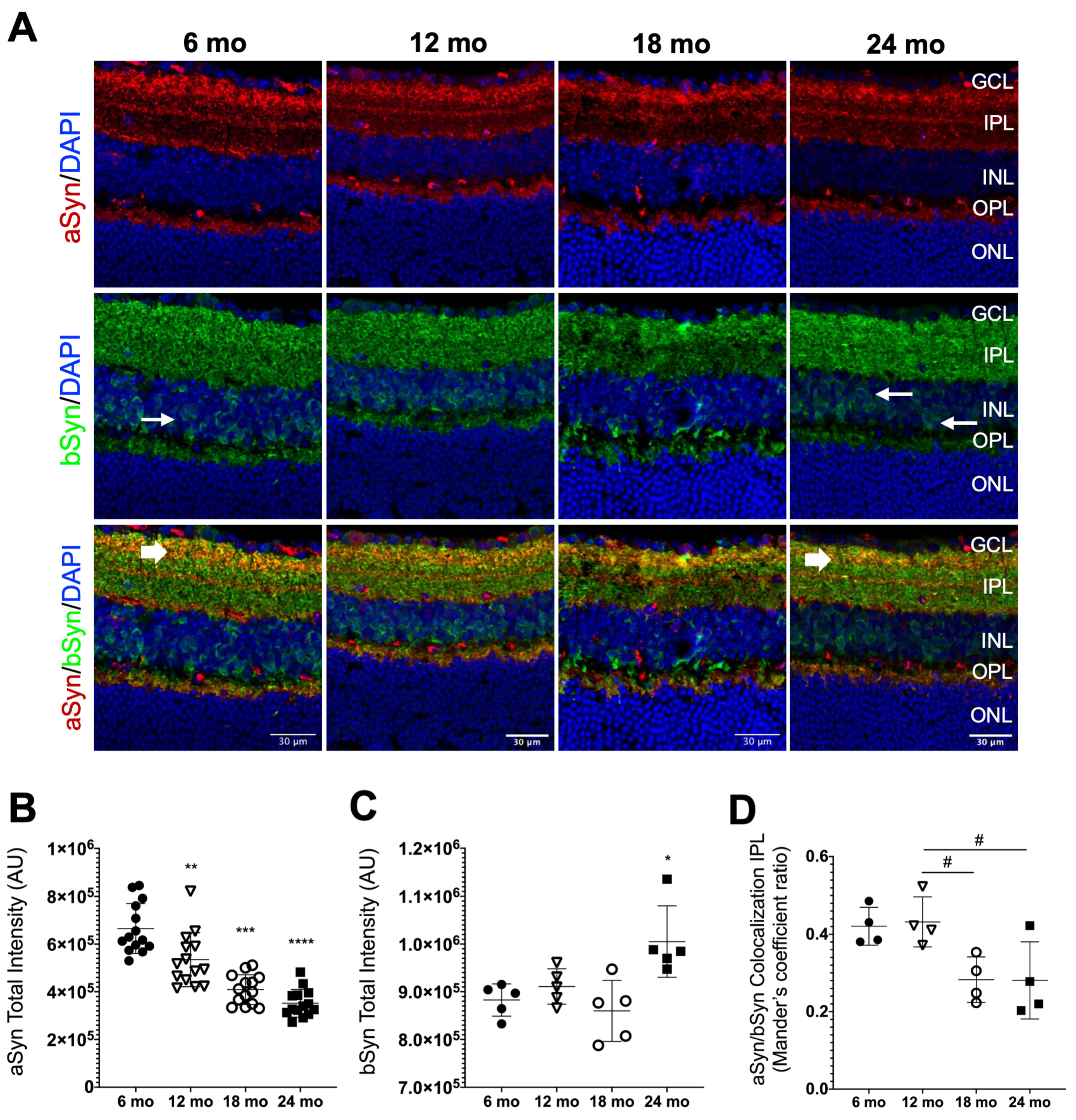
3.3. Synuclein Distribution in the Neural Retina of Naturally Aged Mice
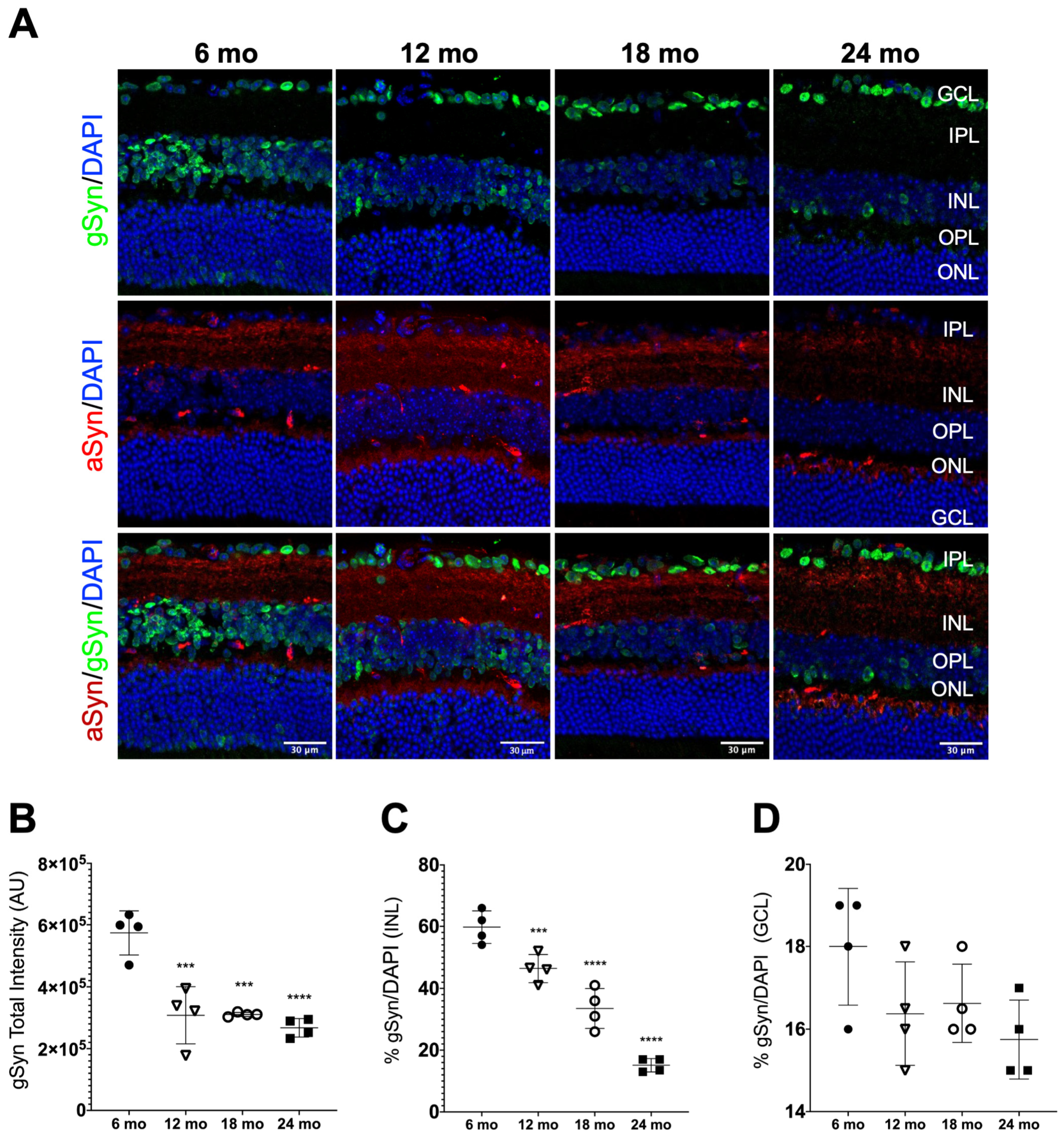
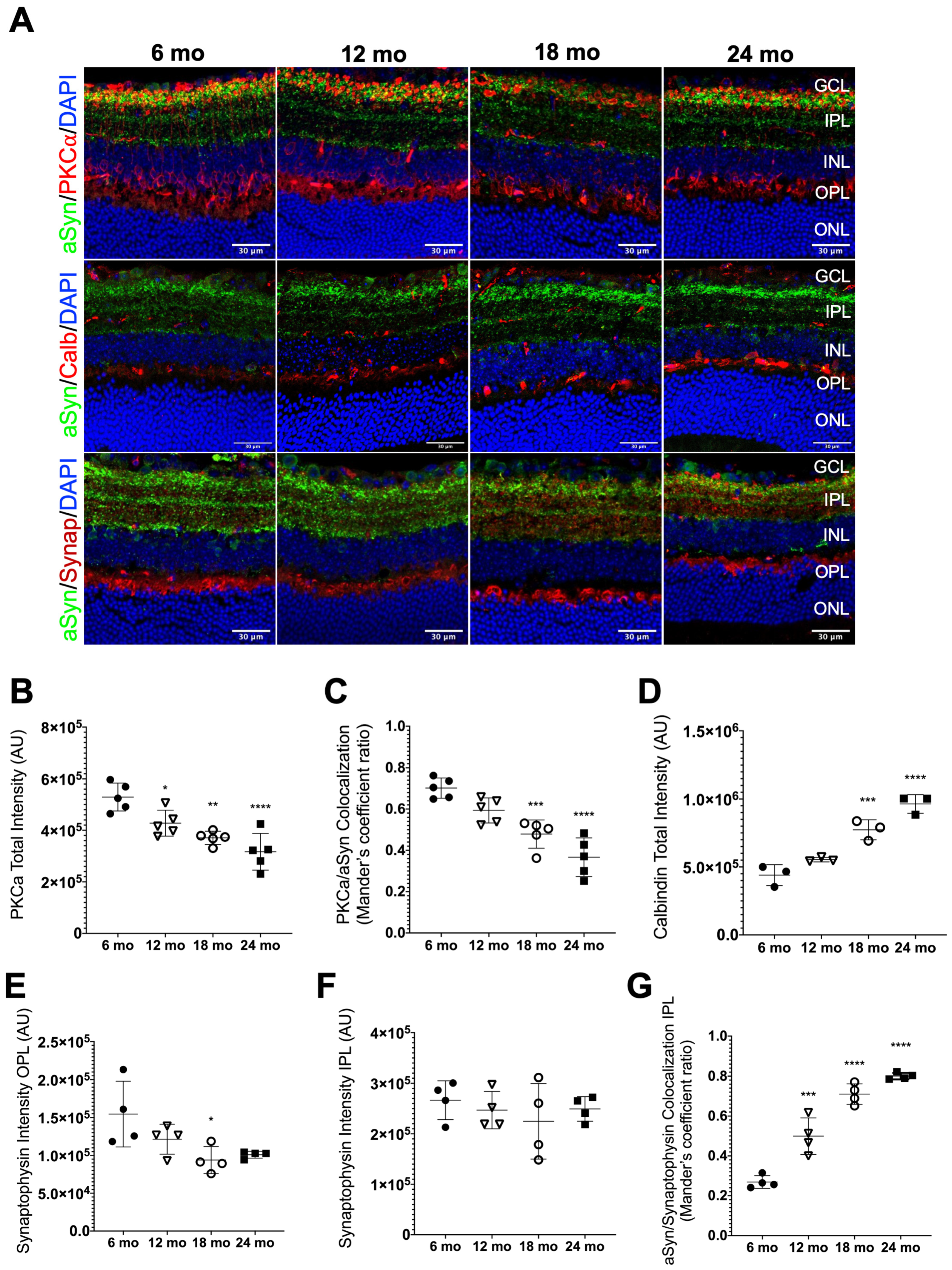
3.4. aSyn Distribution in Specific Retinal Neuronal Populations in Naturally Aged Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of alpha-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef]
- Wong, Y.C.; Krainc, D. alpha-synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat. Med. 2017, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Schmauck-Medina, T.; Moliere, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef]
- Lopes da Fonseca, T.; Villar-Pique, A.; Outeiro, T.F. The Interplay between Alpha-Synuclein Clearance and Spreading. Biomolecules 2015, 5, 435–471. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, H.; Limprasert, P.; Fan, Y.; Onodera, O.; Kakita, A.; Takahashi, H.; Bonner, L.T.; Tsuang, D.W.; Murray, I.V.; Lee, V.M.; et al. Beta-synuclein gene alterations in dementia with Lewy bodies. Neurology 2004, 63, 805–811. [Google Scholar] [CrossRef]
- Tenreiro, S.; Rosado-Ramos, R.; Gerhardt, E.; Favretto, F.; Magalhaes, F.; Popova, B.; Becker, S.; Zweckstetter, M.; Braus, G.H.; Outeiro, T.F. Yeast reveals similar molecular mechanisms underlying alpha- and beta-synuclein toxicity. Hum. Mol. Genet. 2016, 25, 275–290. [Google Scholar] [CrossRef]
- Taschenberger, G.; Toloe, J.; Tereshchenko, J.; Akerboom, J.; Wales, P.; Benz, R.; Becker, S.; Outeiro, T.F.; Looger, L.L.; Bahr, M.; et al. beta-synuclein aggregates and induces neurodegeneration in dopaminergic neurons. Ann. Neurol. 2013, 74, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Surgucheva, I.; McMahan, B.; Ahmed, F.; Tomarev, S.; Wax, M.B.; Surguchov, A. Synucleins in glaucoma: Implication of gamma-synuclein in glaucomatous alterations in the optic nerve. J. Neurosci. Res. 2002, 68, 97–106. [Google Scholar] [CrossRef]
- Galvin, J.E.; Giasson, B.; Hurtig, H.I.; Lee, V.M.; Trojanowski, J.Q. Neurodegeneration with brain iron accumulation, type 1 is characterized by alpha-, beta-, and gamma-synuclein neuropathology. Am. J. Pathol. 2000, 157, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E.; Uryu, K.; Lee, V.M.; Trojanowski, J.Q. Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc. Natl. Acad. Sci. USA 1999, 96, 13450–13455. [Google Scholar] [CrossRef] [PubMed]
- Peters, O.M.; Shelkovnikova, T.; Highley, J.R.; Cooper-Knock, J.; Hortobagyi, T.; Troakes, C.; Ninkina, N.; Buchman, V.L. Gamma-synuclein pathology in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2015, 2, 29–37. [Google Scholar] [CrossRef]
- Ninkina, N.; Peters, O.; Millership, S.; Salem, H.; van der Putten, H.; Buchman, V.L. Gamma-synucleinopathy: Neurodegeneration associated with overexpression of the mouse protein. Hum. Mol. Genet. 2009, 18, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Eisbach, S.E.; Outeiro, T.F. Alpha-synuclein and intracellular trafficking: Impact on the spreading of Parkinson’s disease pathology. J. Mol. Med. 2013, 91, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Burre, J.; Sharma, M.; Sudhof, T.C. alpha-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc. Natl. Acad. Sci. USA 2014, 111, E4274–E4283. [Google Scholar] [CrossRef] [PubMed]
- Vargas, K.J.; Schrod, N.; Davis, T.; Fernandez-Busnadiego, R.; Taguchi, Y.V.; Laugks, U.; Lucic, V.; Chandra, S.S. Synucleins Have Multiple Effects on Presynaptic Architecture. Cell Rep. 2017, 18, 161–173. [Google Scholar] [CrossRef]
- Logan, T.; Bendor, J.; Toupin, C.; Thorn, K.; Edwards, R.H. alpha-Synuclein promotes dilation of the exocytotic fusion pore. Nat. Neurosci. 2017, 20, 681–689. [Google Scholar] [CrossRef]
- Chandra, S.; Fornai, F.; Kwon, H.B.; Yazdani, U.; Atasoy, D.; Liu, X.; Hammer, R.E.; Battaglia, G.; German, D.C.; Castillo, P.E.; et al. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proc. Natl. Acad. Sci. USA 2004, 101, 14966–14971. [Google Scholar] [CrossRef]
- Chandra, S.; Gallardo, G.; Fernandez-Chacon, R.; Schluter, O.M.; Sudhof, T.C. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 2005, 123, 383–396. [Google Scholar] [CrossRef]
- Connor-Robson, N.; Peters, O.M.; Millership, S.; Ninkina, N.; Buchman, V.L. Combinational losses of synucleins reveal their differential requirements for compensating age-dependent alterations in motor behavior and dopamine metabolism. Neurobiol. Aging 2016, 46, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Vargas, K.J.; Makani, S.; Davis, T.; Westphal, C.H.; Castillo, P.E.; Chandra, S.S. Synucleins regulate the kinetics of synaptic vesicle endocytosis. J. Neurosci. 2014, 34, 9364–9376. [Google Scholar] [CrossRef] [PubMed]
- Ninkina, N.; Peters, O.M.; Connor-Robson, N.; Lytkina, O.; Sharfeddin, E.; Buchman, V.L. Contrasting effects of alpha-synuclein and gamma-synuclein on the phenotype of cysteine string protein alpha (CSPalpha) null mutant mice suggest distinct function of these proteins in neuronal synapses. J. Biol. Chem. 2012, 287, 44471–44477. [Google Scholar] [CrossRef] [PubMed]
- Abeliovich, A.; Schmitz, Y.; Farinas, I.; Choi-Lundberg, D.; Ho, W.H.; Castillo, P.E.; Shinsky, N.; Verdugo, J.M.; Armanini, M.; Ryan, A.; et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 2000, 25, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Haebig, K.; Bonin, M.; Ninkina, N.; Buchman, V.L.; Poths, S.; Riess, O. Whole genome expression analyses of single- and double-knock-out mice implicate partially overlapping functions of alpha- and gamma-synuclein. Neurogenetics 2007, 8, 71–81. [Google Scholar] [CrossRef]
- Robertson, D.C.; Schmidt, O.; Ninkina, N.; Jones, P.A.; Sharkey, J.; Buchman, V.L. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J. Neurochem. 2004, 89, 1126–1136. [Google Scholar] [CrossRef]
- Papachroni, K.; Ninkina, N.; Wanless, J.; Kalofoutis, A.T.; Gnuchev, N.V.; Buchman, V.L. Peripheral sensory neurons survive in the absence of alpha- and gamma-synucleins. J. Mol. Neurosci. 2005, 25, 157–164. [Google Scholar] [CrossRef]
- Ninkina, N.; Tarasova, T.V.; Chaprov, K.D.; Roman, A.Y.; Kukharsky, M.S.; Kolik, L.G.; Ovchinnikov, R.; Ustyugov, A.A.; Durnev, A.D.; Buchman, V.L. Alterations in the nigrostriatal system following conditional inactivation of alpha-synuclein in neurons of adult and aging mice. Neurobiol. Aging 2020, 91, 76–87. [Google Scholar] [CrossRef]
- Greten-Harrison, B.; Polydoro, M.; Morimoto-Tomita, M.; Diao, L.; Williams, A.M.; Nie, E.H.; Makani, S.; Tian, N.; Castillo, P.E.; Buchman, V.L.; et al. alphabetagamma-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2010, 107, 19573–19578. [Google Scholar] [CrossRef]
- Klaestrup, I.H.; Just, M.K.; Holm, K.L.; Alstrup, A.K.O.; Romero-Ramos, M.; Borghammer, P.; Van Den Berge, N. Impact of aging on animal models of Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 909273. [Google Scholar] [CrossRef]
- Yang, X.; Williams, J.K.; Yan, R.; Mouradian, M.M.; Baum, J. Increased Dynamics of alpha-Synuclein Fibrils by beta-Synuclein Leads to Reduced Seeding and Cytotoxicity. Sci. Rep. 2019, 9, 17579. [Google Scholar] [CrossRef]
- Hashimoto, M.; Rockenstein, E.; Mante, M.; Mallory, M.; Masliah, E. beta-Synuclein inhibits alpha-synuclein aggregation: A possible role as an anti-parkinsonian factor. Neuron 2001, 32, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Windisch, M.; Hutter-Paier, B.; Rockenstein, E.; Hashimoto, M.; Mallory, M.; Masliah, E. Development of a new treatment for Alzheimer’s disease and Parkinson’s disease using anti-aggregatory beta-synuclein-derived peptides. J. Mol. Neurosci. 2002, 19, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Surguchov, A.; McMahan, B.; Masliah, E.; Surgucheva, I. Synucleins in ocular tissues. J. Neurosci. Res. 2001, 65, 68–77. [Google Scholar] [CrossRef]
- Martinez-Navarrete, G.C.; Martin-Nieto, J.; Esteve-Rudd, J.; Angulo, A.; Cuenca, N. Alpha synuclein gene expression profile in the retina of vertebrates. Mol. Vis. 2007, 13, 949–961. [Google Scholar] [PubMed]
- Armstrong, R.A. Visual symptoms in Parkinson’s disease. Parkinsons Dis. 2011, 2011, 908306. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Visual Dysfunction in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 134, 921–946. [Google Scholar]
- Anderson, J.P.; Walker, D.E.; Goldstein, J.M.; de Laat, R.; Banducci, K.; Caccavello, R.J.; Barbour, R.; Huang, J.; Kling, K.; Lee, M.; et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 2006, 281, 29739–29752. [Google Scholar] [CrossRef]
- Beach, T.G.; Carew, J.; Serrano, G.; Adler, C.H.; Shill, H.A.; Sue, L.I.; Sabbagh, M.N.; Akiyama, H.; Cuenca, N.; Arizona Parkinson’s Disease, C. Phosphorylated alpha-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci. Lett. 2014, 571, 34–38. [Google Scholar] [CrossRef]
- Ortuno-Lizaran, I.; Beach, T.G.; Serrano, G.E.; Walker, D.G.; Adler, C.H.; Cuenca, N. Phosphorylated alpha-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov. Disord. 2018, 33, 1315–1324. [Google Scholar] [CrossRef]
- Bodis-Wollner, I.; Kozlowski, P.B.; Glazman, S.; Miri, S. alpha-synuclein in the inner retina in parkinson disease. Ann. Neurol. 2014, 75, 964–966. [Google Scholar] [CrossRef]
- Maurage, C.A.; Ruchoux, M.M.; de Vos, R.; Surguchov, A.; Destee, A. Retinal involvement in dementia with Lewy bodies: A clue to hallucinations? Ann. Neurol. 2003, 54, 542–547. [Google Scholar] [CrossRef]
- Leger, F.; Fernagut, P.O.; Canron, M.H.; Leoni, S.; Vital, C.; Tison, F.; Bezard, E.; Vital, A. Protein aggregation in the aging retina. J. Neuropathol. Exp. Neurol. 2011, 70, 63–68. [Google Scholar] [CrossRef]
- Nguyen, J.V.; Soto, I.; Kim, K.Y.; Bushong, E.A.; Oglesby, E.; Valiente-Soriano, F.J.; Yang, Z.; Davis, C.H.; Bedont, J.L.; Son, J.L.; et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc. Natl. Acad. Sci. USA 2011, 108, 1176–1181. [Google Scholar] [CrossRef]
- Bohm, M.R.; Mertsch, S.; Konig, S.; Spieker, T.; Thanos, S. Macula-less rat and macula-bearing monkey retinas exhibit common lifelong proteomic changes. Neurobiol. Aging 2013, 34, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Chorostecki, J.; Seraji-Bozorgzad, N.; Shah, A.; Bao, F.; Bao, G.; George, E.; Gorden, V.; Caon, C.; Frohman, E.; Bhatti, M.T.; et al. Characterization of retinal architecture in Parkinson’s disease. J. Neurol. Sci. 2015, 355, 44–48. [Google Scholar] [CrossRef]
- Ikeda, H.; Head, G.M.; Ellis, C.J. Electrophysiological signs of retinal dopamine deficiency in recently diagnosed Parkinson’s disease and a follow up study. Vis. Res. 1994, 34, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Price, D.L.; Rockenstein, E.; Mante, M.; Adame, A.; Overk, C.; Spencer, B.; Duong-Polk, K.X.; Bonhaus, D.; Lindsey, J.; Masliah, E. Longitudinal live imaging of retinal alpha-synuclein::GFP deposits in a transgenic mouse model of Parkinson’s Disease/Dementia with Lewy Bodies. Sci. Rep. 2016, 6, 29523. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, E.; Indrieri, A.; Esposito, F.; Tarallo, V.; Carboncino, A.; Alvino, F.G.; De Falco, S.; Franco, B.; De Risi, M.; De Leonibus, E. alpha-synuclein overexpression in the retina leads to vision impairment and degeneration of dopaminergic amacrine cells. Sci. Rep. 2020, 10, 9619. [Google Scholar] [CrossRef]
- Gresh, J.; Goletz, P.W.; Crouch, R.K.; Rohrer, B. Structure-function analysis of rods and cones in juvenile, adult, and aged C57bl/6 and Balb/c mice. Vis. Neurosci. 2003, 20, 211–220. [Google Scholar] [CrossRef]
- Curcio, C.A.; Millican, C.L.; Allen, K.A.; Kalina, R.E. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Invest. Ophthalmol. Vis. Sci. 1993, 34, 3278–3296. [Google Scholar]
- Bonnel, S.; Mohand-Said, S.; Sahel, J.A. The aging of the retina. Exp. Gerontol. 2003, 38, 825–831. [Google Scholar] [CrossRef]
- Liu, H.; Mercieca, K.; Anders, F.; Prokosch, V. Hydrogen Sulfide and beta-Synuclein Are Involved and Interlinked in the Aging Glaucomatous Retina. J. Ophthalmol. 2020, 2020, 8642135. [Google Scholar] [PubMed]
- Samuel, M.A.; Zhang, Y.; Meister, M.; Sanes, J.R. Age-related alterations in neurons of the mouse retina. J. Neurosci. 2011, 31, 16033–16044. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Nieto-Lopez, L.; Canovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, R.K.; Miller, L.J.; Singh, P.K.; Kanwar, M. Muller glia in retinal innate immunity: A perspective on their roles in endophthalmitis. Crit. Rev. Immunol. 2013, 33, 119–135. [Google Scholar] [CrossRef]
- Lewis, G.P.; Fisher, S.K. Up-regulation of glial fibrillary acidic protein in response to retinal injury: Its potential role in glial remodeling and a comparison to vimentin expression. Int. Rev. Cytol. 2003, 230, 263–290. [Google Scholar]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Veys, L.; Vandenabeele, M.; Ortuno-Lizaran, I.; Baekelandt, V.; Cuenca, N.; Moons, L.; De Groef, L. Retinal alpha-synuclein deposits in Parkinson’s disease patients and animal models. Acta Neuropathol. 2019, 137, 379–395. [Google Scholar] [CrossRef]
- Kosaka, J.; Suzuki, A.; Morii, E.; Nomura, S. Differential localization and expression of alpha and beta isoenzymes of protein kinase C in the rat retina. J. Neurosci. Res. 1998, 54, 655–663. [Google Scholar] [CrossRef]
- Kolb, H.; Zhang, L.; Dekorver, L. Differential staining of neurons in the human retina with antibodies to protein kinase C isozymes. Vis. Neurosci. 1993, 10, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, H.; Liu, W.L.; Massey, S.C.; Mills, S.L. ON inputs to the OFF layer: Bipolar cells that break the stratification rules of the retina. J. Neurosci. 2009, 29, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Luo, X.; Liu, S.; Shen, Y.; Nawy, S.; Shen, Y. Rod bipolar cells dysfunction occurs before ganglion cells loss in excitotoxin-damaged mouse retina. Cell Death Dis. 2019, 10, 905. [Google Scholar] [CrossRef]
- Kwon, S.E.; Chapman, E.R. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron 2011, 70, 847–854. [Google Scholar] [CrossRef]
- Von Kriegstein, K.; Schmitz, F.; Link, E.; Sudhof, T.C. Distribution of synaptic vesicle proteins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. Eur. J. Neurosci. 1999, 11, 1335–1348. [Google Scholar] [CrossRef]
- Brandstatter, J.H.; Lohrke, S.; Morgans, C.W.; Wassle, H. Distributions of two homologous synaptic vesicle proteins, synaptoporin and synaptophysin, in the mammalian retina. J. Comp. Neurol. 1996, 370, 1–10. [Google Scholar] [CrossRef]
- Gao, V.; Briano, J.A.; Komer, L.E.; Burre, J. Functional and Pathological Effects of alpha-Synuclein on Synaptic SNARE Complexes. J. Mol. Biol. 2022, 435, 167714. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Cano, J.; Machado, A.; Reinoso-Suarez, F. Morphological changes in the retina of ageing rats. Arch. Gerontol. Geriatr. 1986, 5, 41–50. [Google Scholar] [CrossRef]
- Feng, L.; Sun, Z.; Han, H.; Zhou, Y.; Zhang, M. No age-related cell loss in three retinal nuclear layers of the Long-Evans rat. Vis. Neurosci. 2007, 24, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Yamamoto, H.; Maeda, Y.; Furukawa, T. Influence of Aging on the Retina and Visual Motion Processing for Optokinetic Responses in Mice. Front. Neurosci. 2020, 14, 586013. [Google Scholar] [CrossRef]
- Eliasieh, K.; Liets, L.C.; Chalupa, L.M. Cellular reorganization in the human retina during normal aging. Invest. Ophthalmol. Vis. Sci. 2007, 48, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Parapuram, S.K.; Cojocaru, R.I.; Chang, J.R.; Khanna, R.; Brooks, M.; Othman, M.; Zareparsi, S.; Khan, N.W.; Gotoh, N.; Cogliati, T.; et al. Distinct signature of altered homeostasis in aging rod photoreceptors: Implications for retinal diseases. PLoS One 2010, 5, e13885. [Google Scholar] [CrossRef]
- Gao, H.; Hollyfield, J.G. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar] [PubMed]
- Harwerth, R.S.; Wheat, J.L.; Rangaswamy, N.V. Age-related losses of retinal ganglion cells and axons. Invest. Ophthalmol. Vis. Sci. 2008, 49, 4437–4443. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. The aging rat retina: From function to anatomy. Neurobiol. Aging 2018, 61, 146–168. [Google Scholar] [CrossRef]
- Verardo, M.R.; Lewis, G.P.; Takeda, M.; Linberg, K.A.; Byun, J.; Luna, G.; Wilhelmsson, U.; Pekny, M.; Chen, D.F.; Fisher, S.K. Abnormal reactivity of muller cells after retinal detachment in mice deficient in GFAP and vimentin. Invest. Ophthalmol. Vis. Sci. 2008, 49, 3659–3665. [Google Scholar] [CrossRef]
- Wassle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef]
- Li, W.; Lesuisse, C.; Xu, Y.; Troncoso, J.C.; Price, D.L.; Lee, M.K. Stabilization of alpha-synuclein protein with aging and familial parkinson’s disease-linked A53T mutation. J. Neurosci. 2004, 24, 7400–7409. [Google Scholar] [CrossRef]
- Carnazza, K.E.; Komer, L.E.; Xie, Y.X.; Pineda, A.; Briano, J.A.; Gao, V.; Na, Y.; Ramlall, T.; Buchman, V.L.; Eliezer, D.; et al. Synaptic vesicle binding of alpha-synuclein is modulated by beta- and gamma-synucleins. Cell Rep. 2022, 39, 110675. [Google Scholar] [CrossRef]
- Sambri, I.; D’Alessio, R.; Ezhova, Y.; Giuliano, T.; Sorrentino, N.C.; Cacace, V.; De Risi, M.; Cataldi, M.; Annunziato, L.; De Leonibus, E.; et al. Lysosomal dysfunction disrupts presynaptic maintenance and restoration of presynaptic function prevents neurodegeneration in lysosomal storage diseases. EMBO Mol. Med. 2017, 9, 112–132. [Google Scholar] [CrossRef] [PubMed]
- Ninkina, N.; Millership, S.J.; Peters, O.M.; Connor-Robson, N.; Chaprov, K.; Kopylov, A.T.; Montoya, A.; Kramer, H.; Withers, D.J.; Buchman, V.L. beta-synuclein potentiates synaptic vesicle dopamine uptake and rescues dopaminergic neurons from MPTP-induced death in the absence of other synucleins. J. Biol. Chem. 2021, 297, 101375. [Google Scholar] [CrossRef]
- Witkovsky, P. Dopamine and retinal function. Doc. Ophthalmol. 2004, 108, 17–40. [Google Scholar] [CrossRef]
- Brown, J.W.P.; Meisl, G.; Knowles, T.P.J.; Buell, A.K.; Dobson, C.M.; Galvagnion, C. Kinetic barriers to alpha-synuclein protofilament formation and conversion into mature fibrils. Chem. Commun. 2018, 54, 7854–7857. [Google Scholar] [CrossRef] [PubMed]
- Tsigelny, I.F.; Bar-On, P.; Sharikov, Y.; Crews, L.; Hashimoto, M.; Miller, M.A.; Keller, S.H.; Platoshyn, O.; Yuan, J.X.; Masliah, E. Dynamics of alpha-synuclein aggregation and inhibition of pore-like oligomer development by beta-synuclein. FEBS J. 2007, 274, 1862–1877. [Google Scholar] [CrossRef] [PubMed]
- Leitao, A.; Bhumkar, A.; Hunter, D.J.B.; Gambin, Y.; Sierecki, E. Unveiling a Selective Mechanism for the Inhibition of alpha-Synuclein Aggregation by beta-Synuclein. Int. J. Mol. Sci. 2018, 19, 334. [Google Scholar] [CrossRef]
- Moriarty, G.M.; Olson, M.P.; Atieh, T.B.; Janowska, M.K.; Khare, S.D.; Baum, J. A pH-dependent switch promotes beta-synuclein fibril formation via glutamate residues. J. Biol. Chem. 2017, 292, 16368–16379. [Google Scholar] [CrossRef] [PubMed]
- Surgucheva, I.; Weisman, A.D.; Goldberg, J.L.; Shnyra, A.; Surguchov, A. Gamma-synuclein as a marker of retinal ganglion cells. Mol. Vis. 2008, 14, 1540–1548. [Google Scholar]
- Al-Wandi, A.; Ninkina, N.; Millership, S.; Williamson, S.J.; Jones, P.A.; Buchman, V.L. Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol. Aging 2010, 31, 796–804. [Google Scholar] [CrossRef]
- Luk, K.C.; Lee, V.M. Modeling Lewy pathology propagation in Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20 (Suppl. 1), S85–S87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, S.B.; de Lemos, L.; Sousa, L.; Bitoque, D.B.; Silva, G.A.; Seabra, M.C.; Tenreiro, S. Age-Related Changes of the Synucleins Profile in the Mouse Retina. Biomolecules 2023, 13, 180. https://doi.org/10.3390/biom13010180
Dias SB, de Lemos L, Sousa L, Bitoque DB, Silva GA, Seabra MC, Tenreiro S. Age-Related Changes of the Synucleins Profile in the Mouse Retina. Biomolecules. 2023; 13(1):180. https://doi.org/10.3390/biom13010180
Chicago/Turabian StyleDias, Sarah Batista, Luísa de Lemos, Luís Sousa, Diogo B. Bitoque, Gabriela Araújo Silva, Miguel C. Seabra, and Sandra Tenreiro. 2023. "Age-Related Changes of the Synucleins Profile in the Mouse Retina" Biomolecules 13, no. 1: 180. https://doi.org/10.3390/biom13010180
APA StyleDias, S. B., de Lemos, L., Sousa, L., Bitoque, D. B., Silva, G. A., Seabra, M. C., & Tenreiro, S. (2023). Age-Related Changes of the Synucleins Profile in the Mouse Retina. Biomolecules, 13(1), 180. https://doi.org/10.3390/biom13010180







