Charge Transport across Proteins inside Proteins: Tunneling across Encapsulin Protein Cages and the Effect of Cargo Proteins
Abstract
1. Introduction
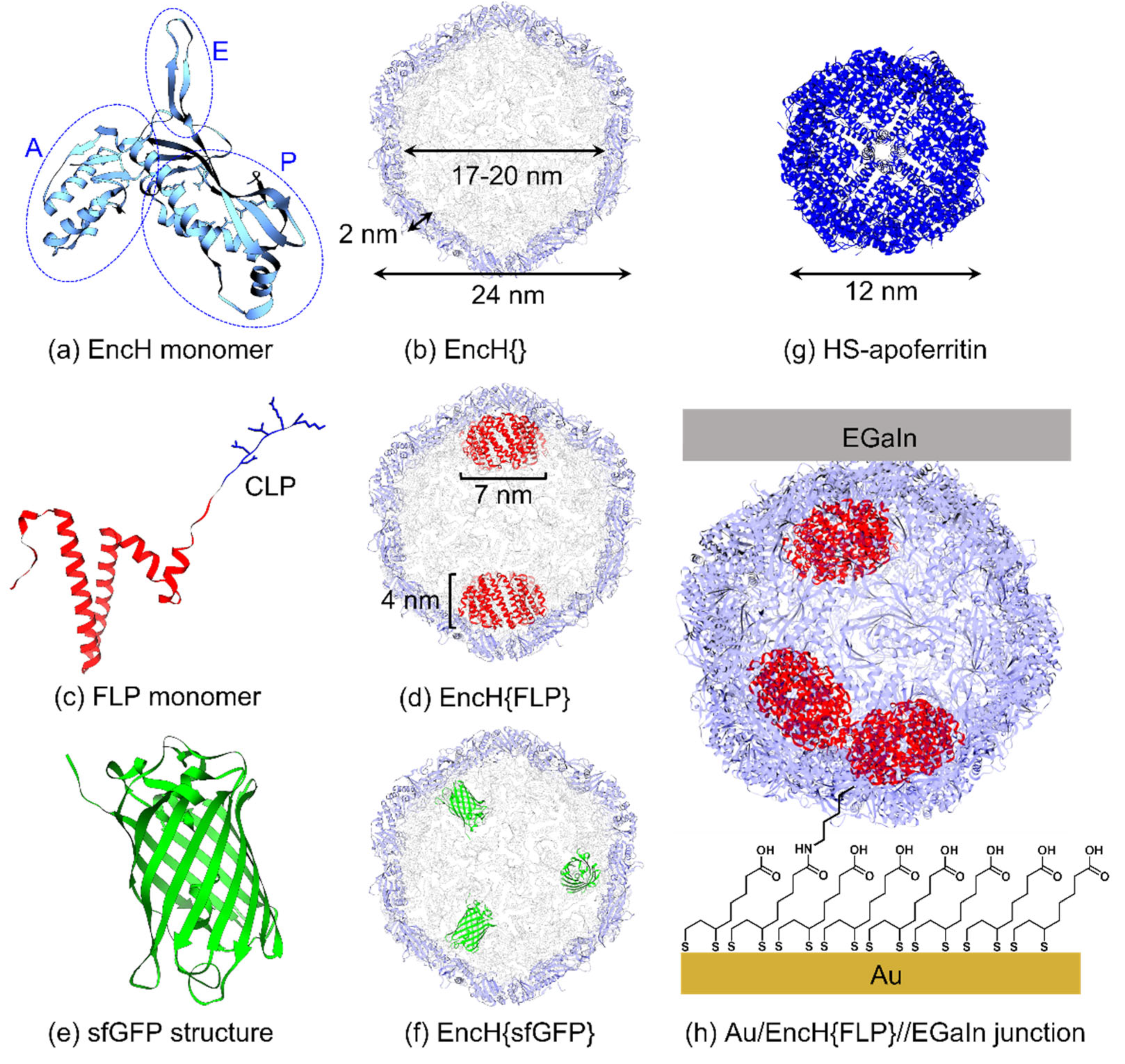
2. Materials and Methods
2.1. Protein Production and Purification
2.1.1. Cloning and Production
2.1.2. Purification
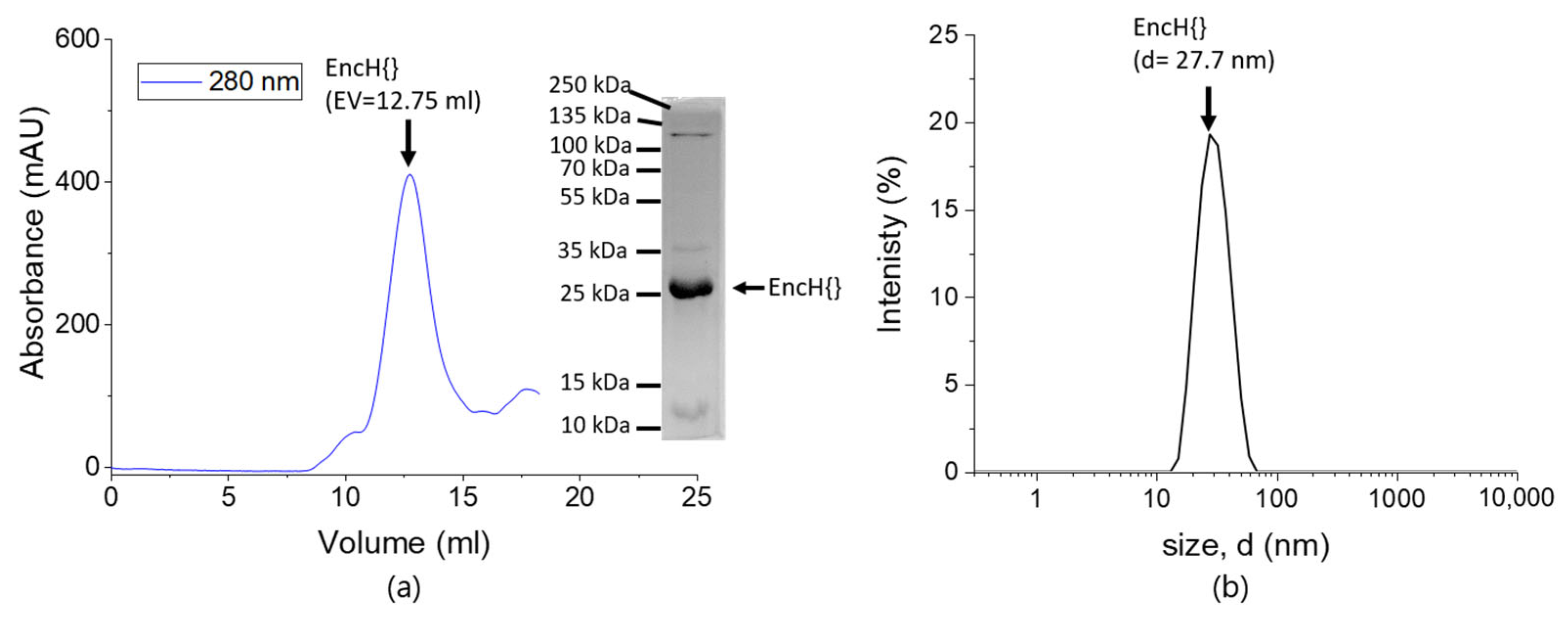
2.2. Sodium Dodecyl Sulfate PolyAcrylamide Gel Electrophoresis (SDS-PAGE)
2.3. Dynamic Light Scattering (DLS)
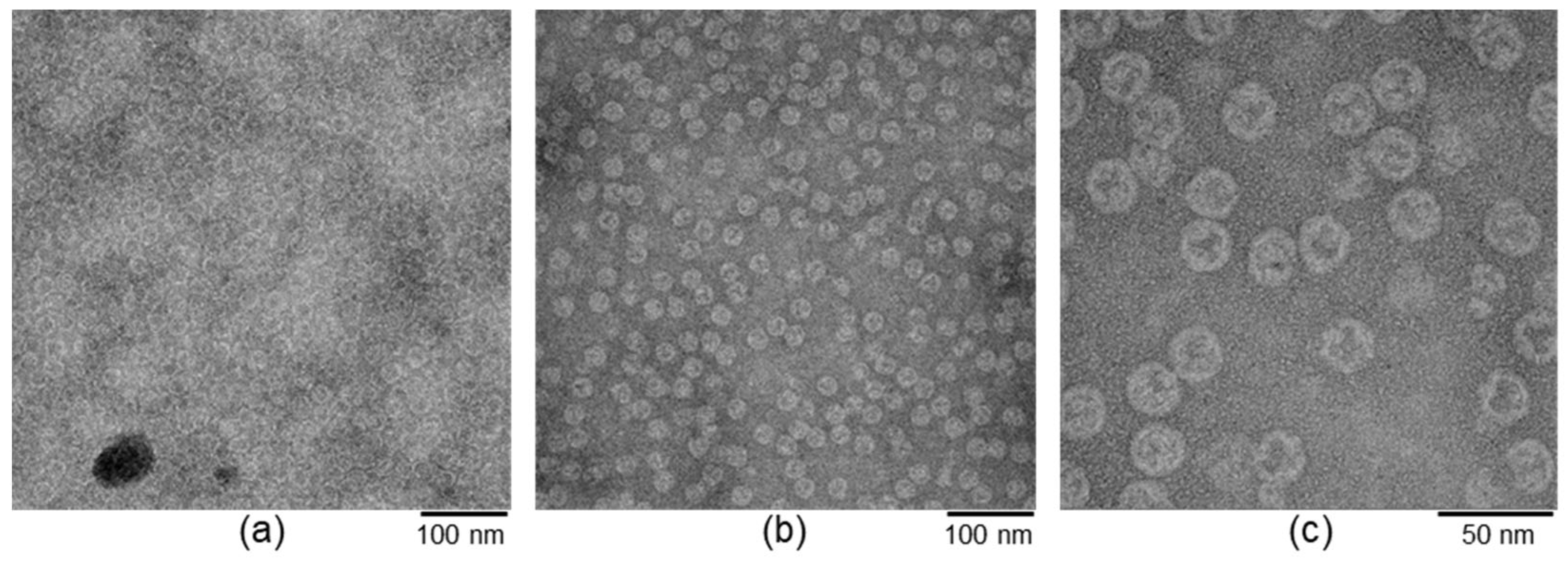
2.4. Transmission Electron Microscopy (TEM)
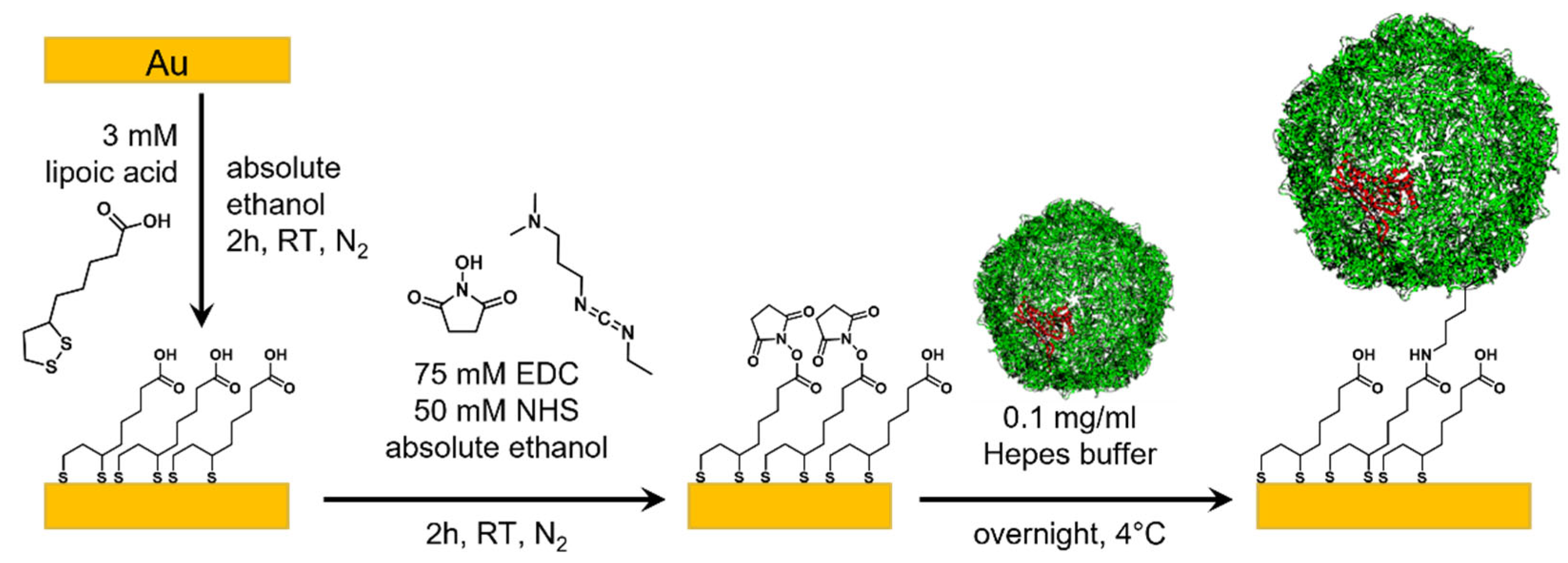
2.5. Template-Stripped Au
2.6. AFM Measurements
2.7. Current-Voltage Measurements
3. Results and Discussion
3.1. Experimental Design
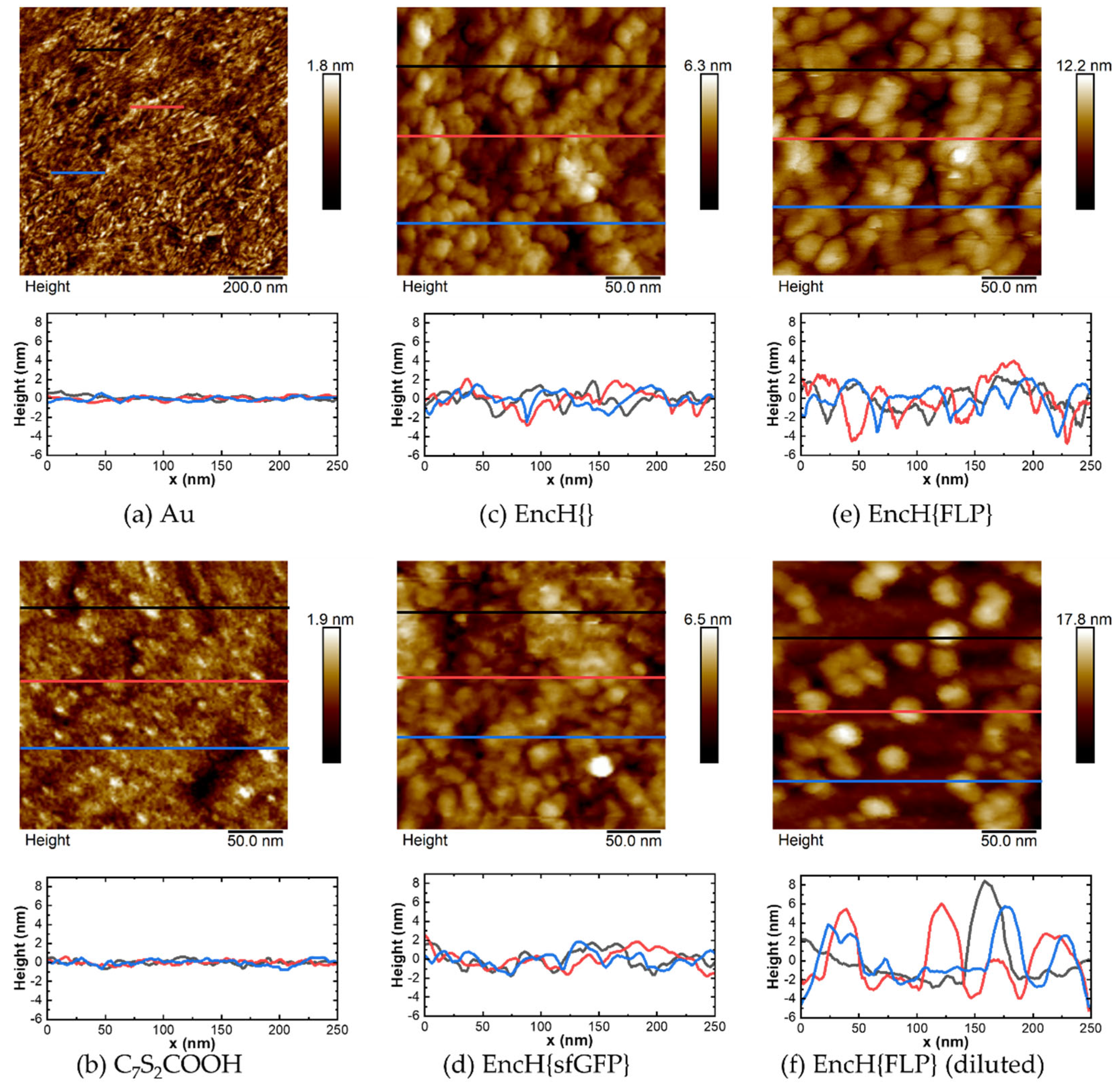
3.2. Surface Characterization of EncH Self-Assembled Monolayers
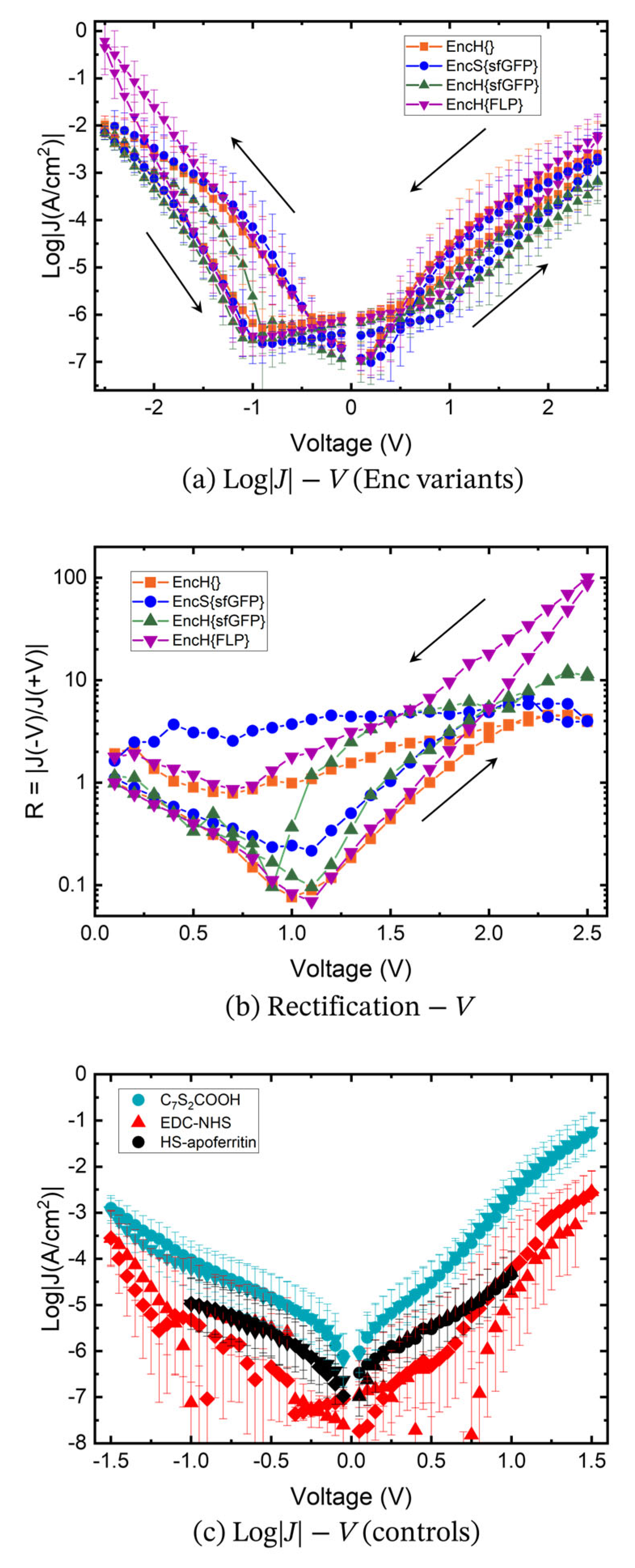
3.3. Charge Transport Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, D.T.; Gabrielsson, E.O.; Tybrandt, K.; Berggren, M. Organic Bioelectronics: Bridging the Signaling Gap between Biology and Technology. Chem. Rev. 2016, 116, 13009–13041. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Liang, Z.; Cao, Y.; Han, Z.; Feng, X. Flexible Inorganic Bioelectronics. NPJ Flex. Electron. 2020, 4, 2. [Google Scholar] [CrossRef]
- Kumar, A.; Osgood, R.M.; Dinneen, S.R.; Koker, B.D.; Pang, R.; Deravi, L.F. Natural Light-Scattering Nanoparticles Enable Visible through Short-Wave Infrared Color Modulation. Adv. Opt. Mater. 2018, 6, 1701369. [Google Scholar] [CrossRef]
- Jutz, G.; van Rijn, P.; Santos Miranda, B.; Böker, A. Ferritin: A Versatile Building Block for Bionanotechnology. Chem. Rev. 2015, 115, 1653–1701. [Google Scholar] [CrossRef] [PubMed]
- Cahen, D.; Pecht, I.; Sheves, M. What Can We Learn from Protein-Based Electron Transport Junctions? J. Phys. Chem. Lett. 2021, 12, 11598–11603. [Google Scholar] [CrossRef]
- Zhuravel, R.; Huang, H.; Polycarpou, G.; Polydorides, S.; Motamarri, P.; Katrivas, L.; Rotem, D.; Sperling, J.; Zotti, L.A.; Kotlyar, A.B.; et al. Backbone Charge Transport in Double-Stranded DNA. Nat. Nanotechnol. 2020, 15, 836–840. [Google Scholar] [CrossRef]
- Li, Y.; Artés, J.M.; Demir, B.; Gokce, S.; Mohammad, H.M.; Alangari, M.; Anantram, M.P.; Oren, E.E.; Hihath, J. Detection and Identification of Genetic Material via Single-Molecule Conductance. Nat. Nanotechnol. 2018, 13, 1167–1173. [Google Scholar] [CrossRef]
- Ha, T.Q.; Planje, I.J.; White, J.R.G.; Aragonès, A.C.; Díez-Pérez, I. Charge Transport at the Protein–Electrode Interface in the Emerging Field of BioMolecular Electronics. Curr. Opin. Electrochem. 2021, 28, 100734. [Google Scholar] [CrossRef]
- Cox-Pridmore, D.M.; Castro, F.A.; Silva, S.R.P.; Camelliti, P.; Zhao, Y. Emerging Bioelectronic Strategies for Cardiovascular Tissue Engineering and Implantation. Small 2022, 18, 2105281. [Google Scholar] [CrossRef]
- Jonsson, A.; Inal, S.; Uguz, I.; Williamson, A.J.; Kergoat, L.; Rivnay, J.; Khodagholy, D.; Berggren, M.; Bernard, C.; Malliaras, G.G.; et al. Bioelectronic Neural Pixel: Chemical Stimulation and Electrical Sensing at the Same Site. Proc. Natl. Acad. Sci. USA 2016, 113, 9440–9445. [Google Scholar] [CrossRef]
- Giordano, G.; Carlotti, M.; Mazzolai, B. A Perspective on Cephalopods Mimicry and Bioinspired Technologies toward Proprioceptive Autonomous Soft Robots. Adv. Mater. Technol. 2021, 6, 2100437. [Google Scholar] [CrossRef]
- Bostick, C.D.; Mukhopadhyay, S.; Pecht, I.; Sheves, M.; Cahen, D.; Lederman, D. Protein Bioelectronics: A Review of What We Do and Do Not Know. Rep. Prog. Phys. 2018, 81, 026601. [Google Scholar] [CrossRef]
- Sepunaru, L.; Friedman, N.; Pecht, I.; Sheves, M.; Cahen, D. Temperature-Dependent Solid-State Electron Transport through Bacteriorhodopsin: Experimental Evidence for Multiple Transport Paths through Proteins. J. Am. Chem. Soc. 2012, 134, 4169–4176. [Google Scholar] [CrossRef]
- Alami, F.A.; Soni, S.; Borrini, A.; Nijhuis, C.A. Perspective—Temperature Dependencies and Charge Transport Mechanisms in Molecular Tunneling Junctions Induced by Redox-Reactions. ECS J. Solid State Sci. Technol. 2022, 11, 055005. [Google Scholar] [CrossRef]
- Gupta, N.K.; Karuppannan, S.K.; Pasula, R.R.; Vilan, A.; Martin, J.; Xu, W.; May, E.M.; Pike, A.R.; Astier, H.P.A.G.; Salim, T.; et al. Temperature-Dependent Coherent Tunneling across Graphene–Ferritin Biomolecular Junctions. ACS Appl. Mater. Interfaces 2022, 14, 44665–44675. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Dutta, S.; Pecht, I.; Sheves, M.; Cahen, D. Conjugated Cofactor Enables Efficient Temperature-Independent Electronic Transport Across ∼6 Nm Long Halorhodopsin. J. Am. Chem. Soc. 2015, 137, 11226–11229. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, X.; Soni, S.; Chiechi, R.C. Charge Transport through Molecular Ensembles: Recent Progress in Molecular Electronics. Chem. Phys. Rev. 2021, 2, 021303. [Google Scholar] [CrossRef]
- Amdursky, N.; Marchak, D.; Sepunaru, L.; Pecht, I.; Sheves, M.; Cahen, D. Electronic Transport via Proteins. Adv. Mater. 2014, 26, 7142–7161. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Karuppannan, S.K.; Guo, C.; Fereiro, J.A.; Bergren, A.; Mukundan, V.; Qiu, X.; Castañeda Ocampo, O.E.; Chen, X.; Chiechi, R.C.; et al. Solid-State Protein Junctions: Cross-Laboratory Study Shows Preservation of Mechanism at Varying Electronic Coupling. iScience 2020, 23, 101099. [Google Scholar] [CrossRef]
- Gupta, N.K.; Pasula, R.R.; Karuppannan, S.K.; Ziyu, Z.; Tadich, A.; Cowie, B.; Qi, D.-C.; Bencok, P.; Lim, S.; Nijhuis, C.A. Switching of the Mechanism of Charge Transport Induced by Phase Transitions in Tunnel Junctions with Large Biomolecular Cages. J. Mater. Chem. C Mater. 2021, 9, 10768–10776. [Google Scholar] [CrossRef]
- Beratan, D.N. Why Are DNA and Protein Electron Transfer So Different? Annu. Rev. Phys. Chem. 2019, 70, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Fourmond, V.; Léger, C. Protein Electrochemistry: Questions and Answers. In Biophotoelectrochemistry: From Bioelectrochemistry to Biophotovoltaics; Springer: Cham, Switzerland, 2016; Volume 158, pp. 1–41. [Google Scholar]
- Paleček, E.; Tkáč, J.; Bartošík, M.; Bertók, T.; Ostatná, V.; Paleček, J. Electrochemistry of Nonconjugated Proteins and Glycoproteins. Toward Sensors for Biomedicine and Glycomics. Chem. Rev. 2015, 115, 2045–2108. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Wilkinson, E.A.; Karuppannan, S.K.; Bailey, L.; Vilan, A.; Zhang, Z.; Qi, D.C.; Tadich, A.; Tuite, E.M.; Pike, A.R.; et al. Role of Order in the Mechanism of Charge Transport across Single-Stranded and Double-Stranded DNA Monolayers in Tunnel Junctions. J. Am. Chem. Soc. 2021, 143, 20309–20319. [Google Scholar] [CrossRef] [PubMed]
- Ron, I.; Pecht, I.; Sheves, M.; Cahen, D. Proteins as Solid-State Electronic Conductors. Acc. Chem. Res. 2010, 43, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Okamoto, N.; Karuppannan, S.K.; Pasula, R.R.; Ziyu, Z.; Qi, D.-C.; Lim, S.; Nakamura, M.; Nijhuis, C.A. The Role of Structural Order in the Mechanism of Charge Transport across Tunnel Junctions with Various Iron-Storing Proteins. Small 2022, 18, 2203338. [Google Scholar] [CrossRef]
- Kumar, K.S.; Pasula, R.R.; Lim, S.; Nijhuis, C.A. Long-Range Tunneling Processes across Ferritin-Based Junctions. Adv. Mater. 2016, 28, 1824–1830. [Google Scholar] [CrossRef]
- Jin, Y.; Friedman, N.; Sheves, M.; He, T.; Cahen, D. Bacteriorhodopsin (BR) as an Electronic Conduction Medium: Current Transport through BR-Containing Monolayers. Proc. Natl. Acad. Sci. USA 2006, 103, 8601–8606. [Google Scholar] [CrossRef]
- Castañeda Ocampo, O.E.; Gordiichuk, P.; Catarci, S.; Gautier, D.A.; Herrmann, A.; Chiechi, R.C. Mechanism of Orientation-Dependent Asymmetric Charge Transport in Tunneling Junctions Comprising Photosystem I. J. Am. Chem. Soc. 2015, 137, 8419–8427. [Google Scholar] [CrossRef]
- Qiu, X.; Castañeda Ocampo, O.; de Vries, H.W.; van Putten, M.; Loznik, M.; Herrmann, A.; Chiechi, R.C. Self-Regenerating Soft Biophotovoltaic Devices. ACS Appl. Mater. Interfaces 2018, 10, 37625–37633. [Google Scholar] [CrossRef]
- Futera, Z.; Ide, I.; Kayser, B.; Garg, K.; Jiang, X.; van Wonderen, J.H.; Butt, J.N.; Ishii, H.; Pecht, I.; Sheves, M.; et al. Coherent Electron Transport across a 3 Nm Bioelectronic Junction Made of Multi-Heme Proteins. J. Phys. Chem. Lett. 2020, 11, 9766–9774. [Google Scholar] [CrossRef]
- Garg, K.; Ghosh, M.; Eliash, T.; van Wonderen, J.H.; Butt, J.N.; Shi, L.; Jiang, X.; Zdenek, F.; Blumberger, J.; Pecht, I.; et al. Direct Evidence for Heme-Assisted Solid-State Electronic Conduction in Multi-Heme c -Type Cytochromes. Chem. Sci. 2018, 9, 7304–7310. [Google Scholar] [CrossRef]
- Li, Y.; Artés, J.M.; Hihath, J. Long-Range Charge Transport in Adenine-Stacked RNA:DNA Hybrids. Small 2016, 12, 432–437. [Google Scholar] [CrossRef]
- Bixon, M.; Giese, B.; Wessely, S.; Langenbacher, T.; Michel-Beyerle, M.E.; Jortner, J. Long-Range Charge Hopping in DNA. Proc. Natl. Acad. Sci. USA 1999, 96, 11713–11716. [Google Scholar] [CrossRef]
- Bixon, M.; Jortner, J. Long-Range and Very Long-Range Charge Transport in DNA. Chem. Phys. 2002, 281, 393–408. [Google Scholar] [CrossRef]
- Chi, Q.; Zhang, J.; Andersen, J.E.T.; Ulstrup, J. Ordered Assembly and Controlled Electron Transfer of the Blue Copper Protein Azurin at Gold (111) Single-Crystal Substrates. J. Phys. Chem. B 2001, 105, 4669–4679. [Google Scholar] [CrossRef]
- Fereiro, J.A.; Yu, X.; Pecht, I.; Sheves, M.; Cuevas, J.C.; Cahen, D. Tunneling Explains Efficient Electron Transport via Protein Junctions. Proc. Natl. Acad. Sci. USA 2018, 115, E4577–E4583. [Google Scholar] [CrossRef]
- Qiu, X.; Chiechi, R.C. Printable Logic Circuits Comprising Self-Assembled Protein Complexes. Nat. Commun. 2022, 13, 2312. [Google Scholar] [CrossRef]
- Yan, H.; Bergren, A.J.; McCreery, R.; della Rocca, M.L.; Martin, P.; Lafarge, P.; Lacroix, J.C. Activationless Charge Transport across 4.5 to 22 Nm in Molecular Electronic Junctions. Proc. Natl. Acad. Sci. USA 2013, 110, 5326–5330. [Google Scholar] [CrossRef]
- Michel-Souzy, S.; Hamelmann, N.M.; Zarzuela-Pura, S.; Paulusse, J.M.J.; Cornelissen, J.J.L.M. Introduction of Surface Loops as a Tool for Encapsulin Functionalization. Biomacromolecules 2021, 22, 5234–5242. [Google Scholar] [CrossRef]
- LaFrance, B.J.; Cassidy-Amstutz, C.; Nichols, R.J.; Oltrogge, L.M.; Nogales, E.; Savage, D.F. The Encapsulin from Thermotoga Maritima Is a Flavoprotein with a Symmetry Matched Ferritin-like Cargo Protein. Sci. Rep. 2021, 11, 22810. [Google Scholar] [CrossRef]
- Sutter, M.; Boehringer, D.; Gutmann, S.; Günther, S.; Prangishvili, D.; Loessner, M.J.; Stetter, K.O.; Weber-Ban, E.; Ban, N. Structural Basis of Enzyme Encapsulation into a Bacterial Nanocompartment. Nat. Struct. Mol. Biol. 2008, 15, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Putri, R.M.; Allende-Ballestero, C.; Luque, D.; Klem, R.; Rousou, K.A.; Liu, A.; Traulsen, C.H.H.; Rurup, W.F.; Koay, M.S.T.; Castón, J.R.; et al. Structural Characterization of Native and Modified Encapsulins as Nanoplatforms for in Vitro Catalysis and Cellular Uptake. ACS Nano 2017, 11, 12796–12804. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; White, D.; Demirel, Y.; Kelly, R.; Noll, K.; Blum, P. Uncoupling Fermentative Synthesis of Molecular Hydrogen from Biomass Formation in Thermotoga Maritima. Appl. Environ. Microbiol. 2018, 84, e00998-18. [Google Scholar] [CrossRef] [PubMed]
- Nesbø, C.L.; Dlutek, M.; Doolittle, W.F. Recombination in Thermotoga: Implications for Species Concepts and Biogeography. Genetics 2006, 172, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.E.; Clayton, R.A.; Gill, S.R.; Gwinn, M.L.; Dodson, R.J.; Haft, D.H.; Hickey, E.K.; Peterson, J.D.; Nelson, W.C.; Ketchum, K.A.; et al. Evidence for Lateral Gene Transfer between Archaea and Bacteria from Genome Sequence of Thermotoga Maritima. Nature 1999, 399, 323–329. [Google Scholar] [CrossRef]
- Rurup, W.F.; Snijder, J.; Koay, M.S.T.; Heck, A.J.R.; Cornelissen, J.J.L.M. Self-Sorting of Foreign Proteins in a Bacterial Nanocompartment. J. Am. Chem. Soc. 2014, 136, 3828–3832. [Google Scholar] [CrossRef]
- Sangeeth, C.S.S.; Wan, A.; Nijhuis, C.A. Equivalent Circuits of a Self-Assembled Monolayer-Based Tunnel Junction Determined by Impedance Spectroscopy. J. Am. Chem. Soc. 2014, 136, 11134–11144. [Google Scholar] [CrossRef]
- Chiechi, R.C.; Weiss, E.A.; Dickey, M.D.; Whitesides, G.M. Eutectic Gallium–Indium (EGaIn): A Moldable Liquid Metal for Electrical Characterization of Self-Assembled Monolayers. Angew. Chem. Int. Ed. 2008, 47, 142–144. [Google Scholar] [CrossRef]
- Cademartiri, L.; Thuo, M.M.; Nijhuis, C.A.; Reus, W.F.; Tricard, S.; Barber, J.R.; Sodhi, R.N.S.; Brodersen, P.; Kim, C.; Chiechi, R.C.; et al. 0.7nm Thick: Electrical Resistance of Ag TS-S(CH 2) n -1CH 3//Ga 2O 3/EGaIn Tunneling Junctions. J. Phys. Chem. C 2012, 116, 10848–10860. [Google Scholar] [CrossRef]
- Amini, S.; Chen, X.; Chua, J.Q.I.; Tee, J.S.; Nijhuis, C.A.; Miserez, A. Interplay between Interfacial Energy, Contact Mechanics, and Capillary Forces in EGaIn Droplets. ACS Appl. Mater. Interfaces 2022, 14, 28074–28084. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Yim, H.-S.; Ryu, J.-Y.; Lee, H.S.; Lee, J.-H.; Seen, D.-S.; Kang, S.G. One-Step Sequence- and Ligation-Independent Cloning as a Rapid and Versatile Cloning Method for Functional Genomics Studies. Appl. Environ. Microbiol. 2012, 78, 5440–5443. [Google Scholar] [CrossRef]
- Chen, X.; Hu, H.; Trasobares, J.; Nijhuis, C.A. Rectification Ratio and Tunneling Decay Coefficient Depend on the Contact Geometry Revealed by in Situ Imaging of the Formation of EGaIn Junctions. ACS Appl. Mater. Interfaces 2019, 11, 21018–21029. [Google Scholar] [CrossRef]
- Reus, W.F.; Thuo, M.M.; Shapiro, N.D.; Nijhuis, C.A.; Whitesides, G.M. The SAM, Not the Electrodes, Dominates Charge Transport in Metal-Monolayer//Ga 2O 3/Gallium-Indium Eutectic Junctions. ACS Nano 2012, 6, 4806–4822. [Google Scholar] [CrossRef]
- Chiechi, R.C. GitHub—Rchiechi/GaussFit: Scientific Python for Processing EGaIn and CP-AFM Data. Available online: https://github.com/rchiechi/gaussfit (accessed on 15 December 2022).
- Zhang, Y.; Soni, S.; Krijger, T.L.; Gordiichuk, P.; Qiu, X.; Ye, G.; Jonkman, H.T.; Herrmann, A.; Zojer, K.; Zojer, E.; et al. Tunneling Probability Increases with Distance in Junctions Comprising Self-Assembled Monolayers of Oligothiophenes. J. Am. Chem. Soc. 2018, 140, 15048–15055. [Google Scholar] [CrossRef]
- Grabarek, Z.; Gergely, J. Zero-Length Crosslinking Procedure with the Use of Active Esters. Anal. Biochem. 1990, 185, 131–135. [Google Scholar] [CrossRef]
- Han, Y.; Maglione, M.S.; Diez Cabanes, V.; Casado-Montenegro, J.; Yu, X.; Karuppannan, S.K.; Zhang, Z.; Crivillers, N.; Mas-Torrent, M.; Rovira, C.; et al. Reversal of the Direction of Rectification Induced by Fermi Level Pinning at Molecule–Electrode Interfaces in Redox-Active Tunneling Junctions. ACS Appl. Mater. Interfaces 2020, 12, 55044–55055. [Google Scholar] [CrossRef]
- Weiss, E.A.; Kaufman, G.K.; Kriebel, J.K.; Li, Z.; Schalek, R.; Whitesides, G.M. Si/SiO2-Templated Formation of Ultraflat Metal Surfaces on Glass, Polymer, and Solder Supports: Their Use as Substrates for Self-Assembled Monolayers. Langmuir 2007, 23, 9686–9694. [Google Scholar] [CrossRef]
- Qiu, X.; Ivasyshyn, V.; Qiu, L.; Enache, M.; Dong, J.; Rousseva, S.; Portale, G.; Stöhr, M.; Hummelen, J.C.; Chiechi, R.C. Thiol-Free Self-Assembled Oligoethylene Glycols Enable Robust Air-Stable Molecular Electronics. Nat. Mater. 2020, 19, 330–337. [Google Scholar] [CrossRef]
| Oligo | Sequence 5′-3′ | Characteristics |
|---|---|---|
| OSMS3 | AAGGAGATATACATATGGTGAACATGGAATTTCTG | PCR Tmenc forward for NdeI site in pDuet MCS2 |
| OKB23 | GCGTGGCCGGCCGATATCTCACTTTTCGAACTGCGGGTGGCTCCAACCACCACCACCACCGAACTTTAGAAGAATCAA | PCR Tmenc reverse for EcoRV site in pDuet MCS2 |
| OSMS36 | AGGAGATATACCATGGCAGATCAGTACCAC | PCR flp forward for NcoI site in pDuet MCS1 |
| OSMS37 | CCGCAAGCTTGTCGACTCAGAGCTTCCTTATG | PCR flp reverse for SalI site in pDuet MCS1 |
| Plasmid | Characteristics | Origin |
|---|---|---|
| pCDFDuet-Tmenc127H10 | SmR, PT7, Expression of Tm encapsulin with a 10 Histidine tag after residue 127 (EncH) | Michel-Souzy et al. [40] |
| pRSFDuet-TmencCstrep | KmR, PT7, Expression of Tm encapsulin with a Strep-tagII after the C terminus (EncS) | this study |
| pETDuet-sfGFPEflp | ApR, PT7, Expression of the sfGFP fuserazmakd with the CLP of Flp (cargo) | Michel-Souzy et al. [40] |
| pETDuet-Flp | ApR, PT7, Ferredoxin-like protein (physiological cargo of Tm encapsulin) | this study |
| SAM | AFM Roughness a | EGaIn Junction Parameters | ||||
|---|---|---|---|---|---|---|
| 250 × 250 nm | 1 × 1 um | Voltage Range (V) c | Non-Shorting Junctions c | Total No. of Scans c | Yield (%) c | |
| Bare Au | n.a. | 0.25 | n.a. | n.a. | n.a. | n.a. |
| C7S2COOH | 0.25 | 0.28 | 1.5 | 22 | 440 | 76 |
| EDC-NHS | n.a. | 0.75 | 1.5 | 16 | 257 | 73 |
| HP-apoferritin | n.a. | n.a. | 1 | 17 | 340 | 74 |
| EncH{} | 0.89 | 1.19 | 1.5 (2.5) | 15 (15) | 278 (300) | 79 (83) |
| EncH{sfGFP} | 0.92 | 1.18 | 1.5 (2.5) | 15 (15) | 290 (300) | 88 (100) |
| EncS{sfGFP} | n.a. | n.a. | 1.5 (2.5) | 19 (17) | 380 (340) | 73 (74) |
| EncH{FLP} | 1.80 [2.72] b | 2.02 [3.08] b | 1.5 (2.5) | 12 (18) | 240 (360) | 67 (90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinelli, R.; Soni, S.; Cornelissen, J.J.L.M.; Michel-Souzy, S.; Nijhuis, C.A. Charge Transport across Proteins inside Proteins: Tunneling across Encapsulin Protein Cages and the Effect of Cargo Proteins. Biomolecules 2023, 13, 174. https://doi.org/10.3390/biom13010174
Zinelli R, Soni S, Cornelissen JJLM, Michel-Souzy S, Nijhuis CA. Charge Transport across Proteins inside Proteins: Tunneling across Encapsulin Protein Cages and the Effect of Cargo Proteins. Biomolecules. 2023; 13(1):174. https://doi.org/10.3390/biom13010174
Chicago/Turabian StyleZinelli, Riccardo, Saurabh Soni, Jeroen J. L. M. Cornelissen, Sandra Michel-Souzy, and Christian A. Nijhuis. 2023. "Charge Transport across Proteins inside Proteins: Tunneling across Encapsulin Protein Cages and the Effect of Cargo Proteins" Biomolecules 13, no. 1: 174. https://doi.org/10.3390/biom13010174
APA StyleZinelli, R., Soni, S., Cornelissen, J. J. L. M., Michel-Souzy, S., & Nijhuis, C. A. (2023). Charge Transport across Proteins inside Proteins: Tunneling across Encapsulin Protein Cages and the Effect of Cargo Proteins. Biomolecules, 13(1), 174. https://doi.org/10.3390/biom13010174








