The Placentas of Women Who Suffer an Episode of Psychosis during Pregnancy Have Increased Lipid Peroxidation with Evidence of Ferroptosis
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Sample Collection and Processing
2.3. Histological and Immunohistochemical Techniques
2.4. Lipid Peroxidation Assay
2.5. Gene Expression Study
2.6. Histological and Statistical Analysis
3. Results
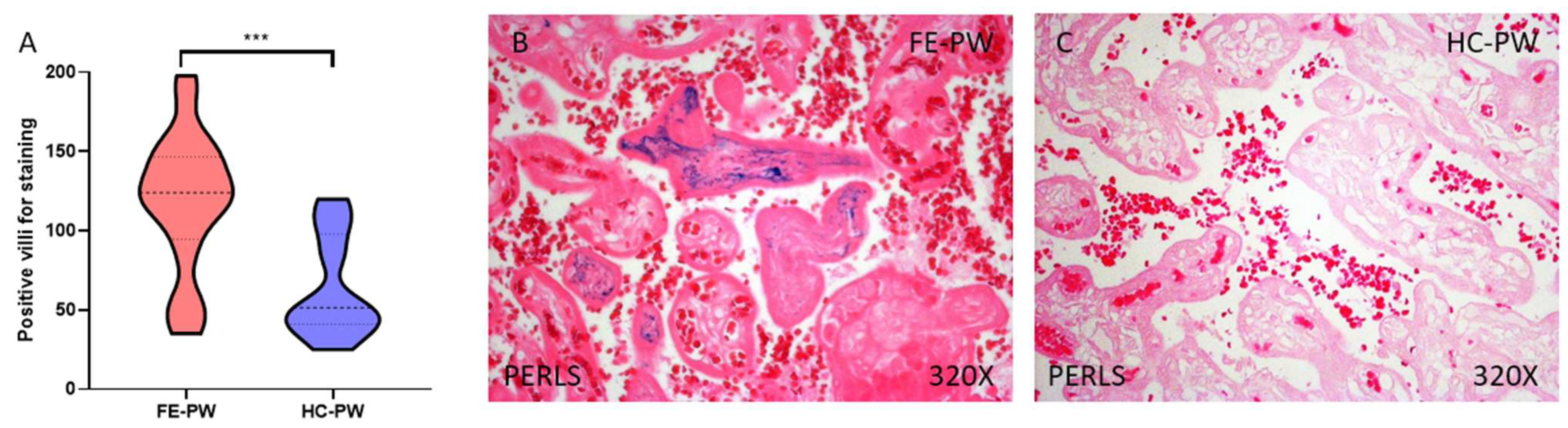
3.1. The Placental Tissue of Women with a First Episode of Psychosis in Pregnancy Show Evidence of Further Iron Deposits Together with an Enhanced Expression of the Transferrin Receptor Gene and Protein
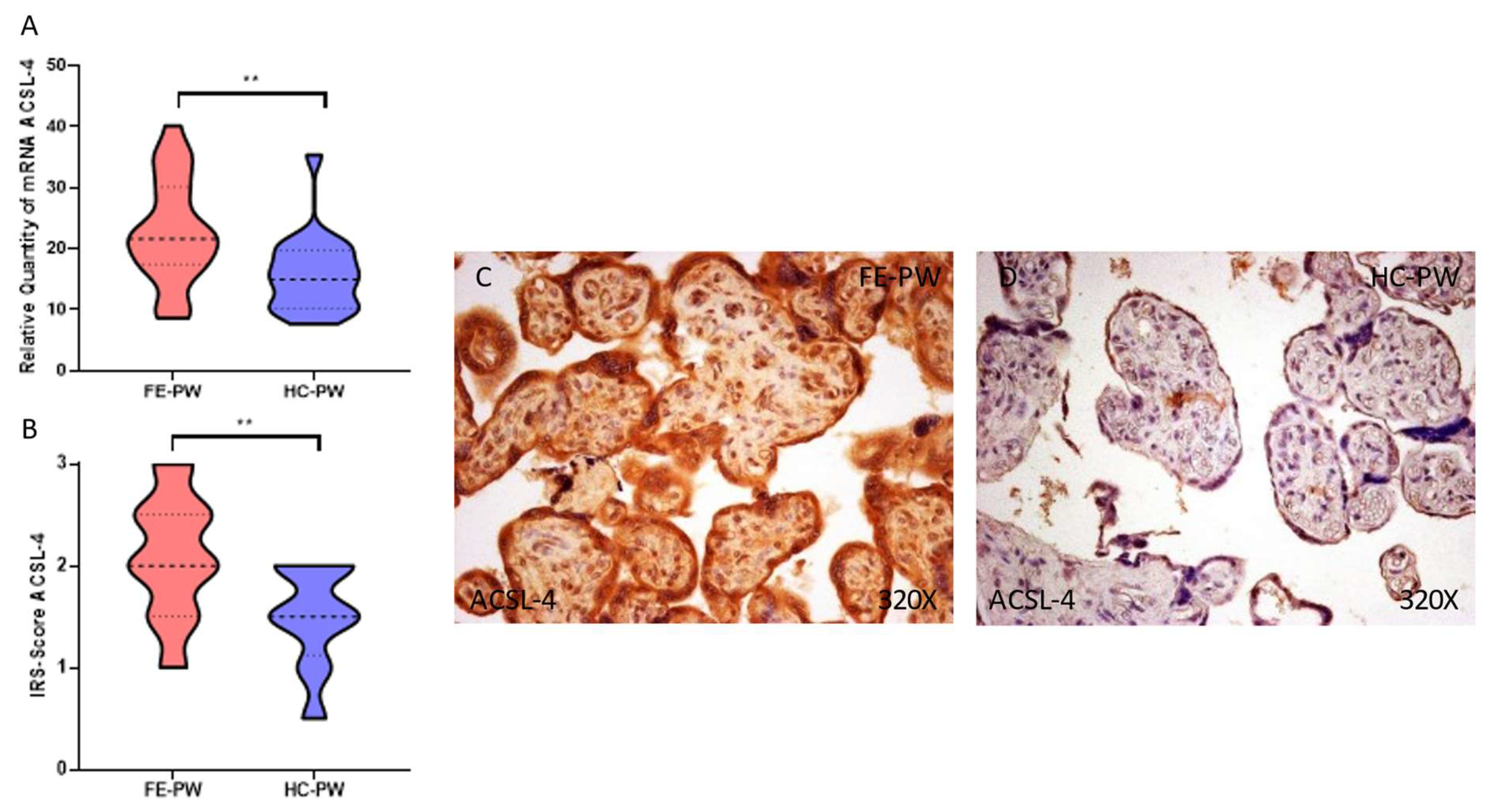
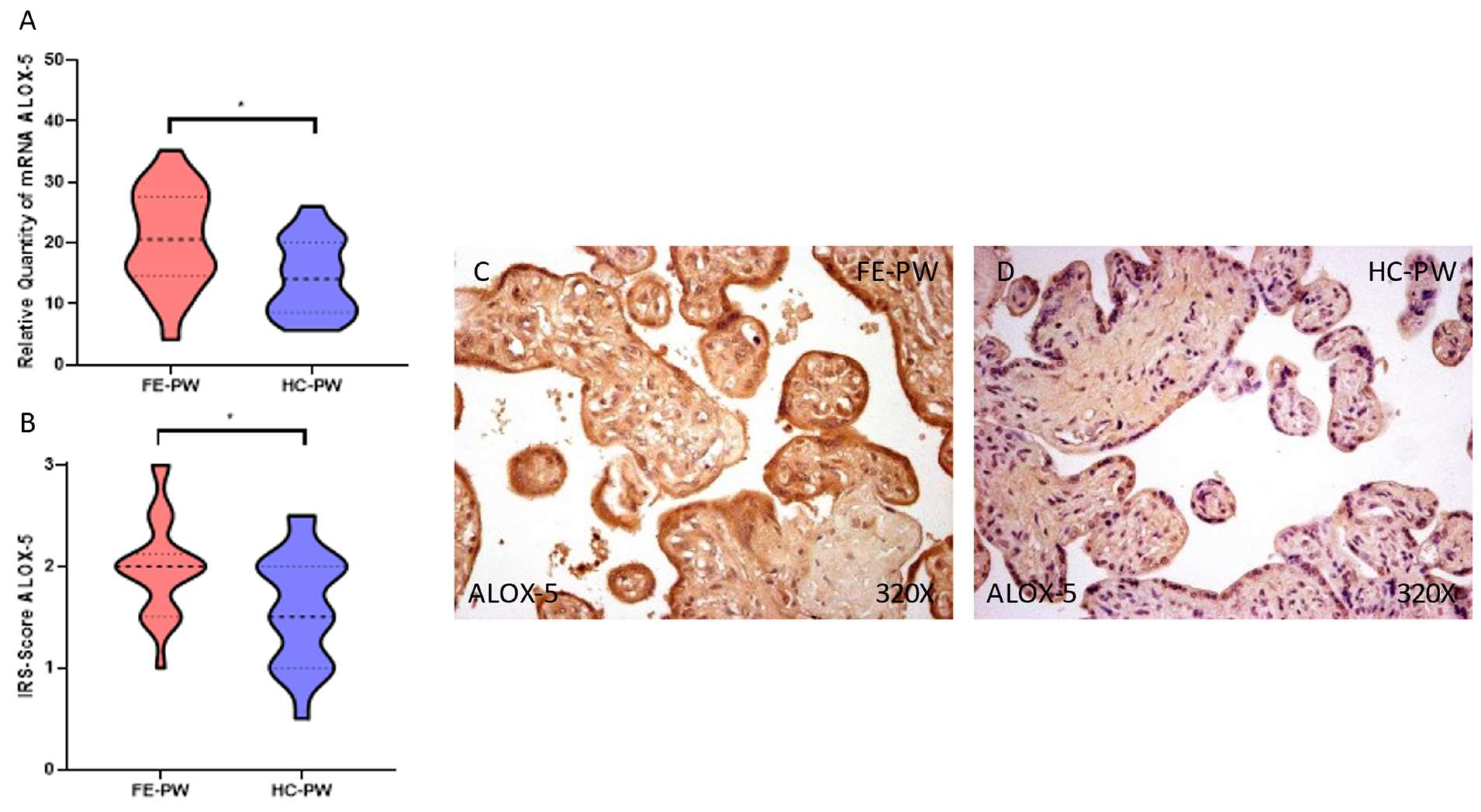
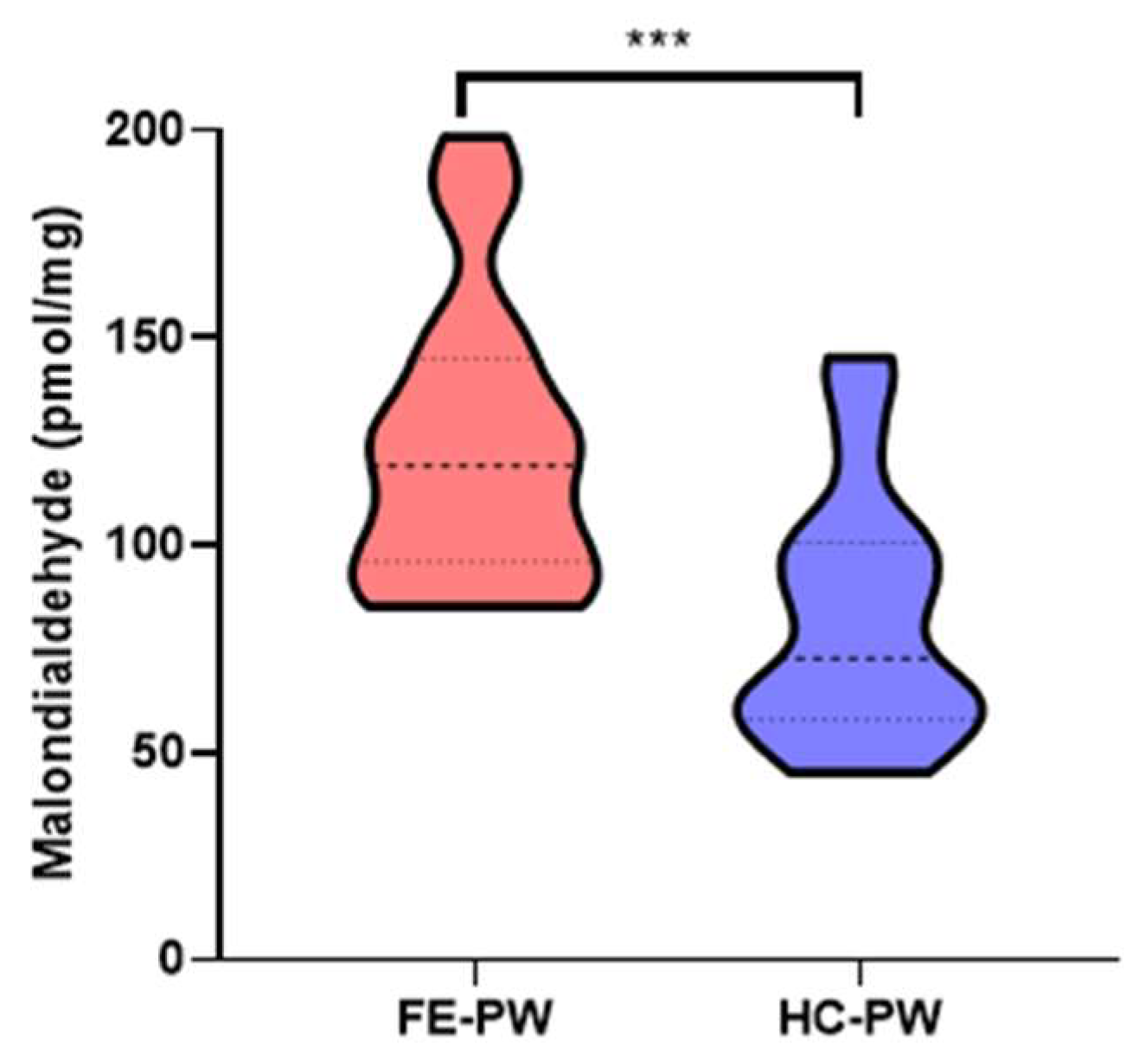
3.2. The Placentas of Women with a First Episode of Psychosis in Pregnancy Display a Significant Augmentation of Lipid Peroxidation and Ferroptosis Markers
3.3. Placental Tissue of Women with a First Episode of Psychosis during Pregnancy Present a Gene and Protein Overexpression of Glutathione Peroxidase 4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watkins, M.E.; Newport, D.J. Psychosis in Pregnancy. Obstet. Gynecol. 2009, 113, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, J.; Khalili, Y. Al Psychosis; StatPearls: Tampa, FL, USA, 2022. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Biedermann, F.; Fleischhacker, W.W. Psychotic Disorders in DSM-5 and ICD-11. CNS Spectr. 2016, 21, 349–354. [Google Scholar] [CrossRef]
- Moreno-Küstner, B.; Martín, C.; Pastor, L. Prevalence of Psychotic Disorders and Its Association with Methodological Issues. A Systematic Review and Meta-Analyses. PLoS ONE 2018, 13, e0195687. [Google Scholar] [CrossRef]
- Zhong, Q.Y.; Gelaye, B.; Fricchione, G.L.; Avillach, P.; Karlson, E.W.; Williams, M.A. Adverse Obstetric and Neonatal Outcomes Complicated by Psychosis among Pregnant Women in the United States. BMC Pregnancy Childbirth 2018, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Cott, A.D.; Wisner, K.L. Psychiatric Disorders during Pregnancy. Int. Rev. Psychiatry 2003, 15, 217–230. [Google Scholar] [CrossRef]
- Karakasi, M.V.; Markopoulou, M.; Tentes, I.K.; Tsikouras, P.N.; Vasilikos, E.; Pavlidis, P. Prepartum Psychosis and Neonaticide: Rare Case Study and Forensic-Psychiatric Synthesis of Literature. J. Forensic Sci. 2017, 62, 1097–1106. [Google Scholar] [CrossRef]
- Friedman, S.H.; Hall, R.C.W.; Sorrentino, R.M. Involuntary Treatment of Psychosis in Pregnancy. J. Am. Acad. Psychiatry Law 2018, 46, 217–223. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells 2022, 11, 568. [Google Scholar] [CrossRef]
- Garcia-Martin, I.; Penketh, R.J.A.; Garay, S.M.; Jones, R.E.; Grimstead, J.W.; Baird, D.M.; John, R.M. Symptoms of Prenatal Depression Associated with Shorter Telomeres in Female Placenta. Int. J. Mol. Sci. 2021, 22, 7458. [Google Scholar] [CrossRef]
- Lahti-Pulkkinen, M.; Cudmore, M.J.; Haeussner, E.; Schmitz, C.; Pesonen, A.K.; Hämäläinen, E.; Villa, P.M.; Mehtälä, S.; Kajantie, E.; Laivuori, H.; et al. Placental Morphology Is Associated with Maternal Depressive Symptoms during Pregnancy and Toddler Psychiatric Problems. Sci. Rep. 2018, 8, 791. [Google Scholar] [CrossRef]
- Dahlerup, B.R.; Egsmose, E.L.; Siersma, V.; Mortensen, E.L.; Hedegaard, M.; Knudsen, L.E.; Mathiesen, L. Maternal Stress and Placental Function, a Study Using Questionnaires and Biomarkers at Birth. PLoS ONE 2018, 13, e0207184. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.J.; Jensen, A.B.; Freeman, L.; Khalife, N.; O’Connor, T.G.; Glover, V. Maternal Prenatal Anxiety and Downregulation of Placental 11β-HSD2. Psychoneuroendocrinology 2012, 37, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Mégier, C.; Peoc’h, K.; Puy, V.; Cordier, A.G. Iron Metabolism in Normal and Pathological Pregnancies and Fetal Consequences. Metabolites 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Jin, L. Iron Metabolism and Ferroptosis in Physiological and Pathological Pregnancy. Int. J. Mol. Sci. 2022, 23, 9395. [Google Scholar] [CrossRef]
- Beharier, O.; Kajiwara, K.; Sadovsky, Y. Ferroptosis, Trophoblast Lipotoxic Damage, and Adverse Pregnancy Outcome. Placenta 2021, 108, 32–38. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, X.; Han, X.; Shi, M.; Sun, L.; Liu, M.; Zhang, P.; Huang, Z.; Yang, X.; Li, R. Ferroptosis-Related Gene Expression in the Pathogenesis of Preeclampsia. Front. Genet. 2022, 13, 2043. [Google Scholar] [CrossRef]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 30. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and Transferrin Receptors Update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2020, 31, 107–125. [Google Scholar] [CrossRef]
- First, M.; Williams, J.; Karg, R.; Spitzer, R. Structured Clinical Interview for DSM-5 Disorders—Research Version (SCID-5-RV); American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Ortega, M.A.; Sáez, M.A.; Fraile-Martínez, O.; Álvarez-Mon, M.A.; García-Montero, C.; Guijarro, L.G.; Asúnsolo, Á.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; et al. Overexpression of Glycolysis Markers in Placental Tissue of Pregnant Women with Chronic Venous Disease: A Histological Study. Int. J. Med. Sci. 2022, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Asúnsolo, Á.; Fraile-Martínez, O.; Sainz, F.; Saez, M.A.; Bravo, C.; de León-Luis, J.A.; Alvarez-Mon, M.A.; Coca, S.; Álvarez-Mon, M.; et al. An Increase in Elastogenic Components in the Placental Villi of Women with Chronic Venous Disease during Pregnancy Is Associated with Decreased EGFL7 Expression Level. Mol. Med. Rep. 2021, 24, 556. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Romero, B.; Asúnsolo, Á.; Martínez-Vivero, C.; Sainz, F.; Bravo, C.; De León-Luis, J.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Pregnancy-Associated Venous Insufficiency Course with Placental and Systemic Oxidative Stress. J. Cell. Mol. Med. 2020, 24, 4157–4170. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. The Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction: Twenty-Something Years On. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Vallone, P.M.; Butler, J.M. AutoDimer: A Screening Tool for Primer-Dimer and Hairpin Structures. Biotechniques 2004, 37, 226–231. [Google Scholar] [CrossRef]
- Jang, S.J.; Jeon, R.H.; Kim, H.D.; Hwang, J.C.; Lee, H.J.; Bae, S.G.; Lee, S.L.; Rho, G.J.; Kim, S.J.; Lee, W.J. TATA Box Binding Protein and Ribosomal Protein 4 Are Suitable Reference Genes for Normalization during Quantitative Polymerase Chain Reaction Study in Bovine Mesenchymal Stem Cells. Asian-Australasian J. Anim. Sci. 2020, 33, 2021. [Google Scholar] [CrossRef]
- Ortega, M.A.; Saez, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Pekarek, L.; Bravo, C.; Coca, S.; Sainz, F.; Álvarez-Mon, M.; Buján, J.; et al. Increased Angiogenesis and Lymphangiogenesis in the Placental Villi of Women with Chronic Venous Disease during Pregnancy. Int. J. Mol. Sci. 2020, 21, 2487. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dixon, S.J. Mechanisms of Ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology, and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266. [Google Scholar] [CrossRef]
- Barke, T.L.; Goldstein, J.A.; Sundermann, A.C.; Reddy, A.P.; Linder, J.E.; Correa, H.; Velez-Edwards, D.R.; Aronoff, D.M. Gestational Diabetes Mellitus Is Associated with Increased CD163 Expression and Iron Storage in the Placenta. Am. J. Reprod. Immunol. 2018, 80, e13020. [Google Scholar] [CrossRef]
- Hackney, D.N.; Tirumala, R.; Salamone, L.J.; Miller, R.K.; Katzman, P.J. Do Placental Histologic Findings of Chorion-Decidual Hemorrhage or Inflammation in Spontaneous Preterm Birth Influence Outcomes in the Subsequent Pregnancy? Placenta 2014, 35, 58–63. [Google Scholar] [CrossRef]
- Bastin, J.; Drakesmith, H.; Rees, M.; Sargent, I.; Townsend, A. Localisation of Proteins of Iron Metabolism in the Human Placenta and Liver. Br. J. Haematol. 2006, 134, 532–543. [Google Scholar] [CrossRef]
- Ponka, P.; Lok, C.N. The Transferrin Receptor: Role in Health and Disease. Int. J. Biochem. Cell Biol. 1999, 31, 1111–1137. [Google Scholar] [CrossRef]
- Mandò, C.; Tabano, S.; Colapietro, P.; Pileri, P.; Colleoni, F.; Avagliano, L.; Doi, P.; Bulfamante, G.; Miozzo, M.; Cetin, I. Transferrin Receptor Gene and Protein Expression and Localization in Human IUGR and Normal Term Placentas. Placenta 2011, 32, 44–50. [Google Scholar] [CrossRef]
- Khatun, R.; Wu, Y.; Kanenishi, K.; Ueno, M.; Tanaka, S.; Hata, T.; Sakamoto, H. Immunohistochemical Study of Transferrin Receptor Expression in the Placenta of Pre-Eclamptic Pregnancy. Placenta 2003, 24, 870–876. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Berry, S.A.; Wobken, J.D.; Leibold, E.A. Increased Placental Iron Regulatory Protein-1 Expression in Diabetic Pregnancies Complicated by Fetal Iron Deficiency. Placenta 1999, 20, 87–93. [Google Scholar] [CrossRef]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020, 30, 3411. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of Iron and Hydrogen Peroxide: The Fenton Reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, W.-B.; Yang, P.; Turan, S. SARS-CoV-2 Infection Induces Activation of Ferroptosis in Human Placenta. Front. Cell Dev. Biol. 2022, 10, 1022747. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.Y.; Zhou, H.H.; Mao, X.Y. Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxidative Med. Cell. Longev. 2019, 2019, 2749173. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, F.; Xu, Q.; Huang, W.; Wang, S.; Sun, R.; Ye, D.; Zhang, D. Lipoxin A4 Suppresses Angiotensin II Type 1 Receptor Autoantibody in Preeclampsia via Modulating Caspase-1. Cell Death Dis. 2020, 11, 78. [Google Scholar] [CrossRef]
- Liu, H.; Huang, W.; Chen, L.; Xu, Q.; Ye, D.; Zhang, D. Glucocorticoid Exposure Induces Preeclampsia via DampeningLipoxin A4, an Endogenous Anti-Inflammatory and Proresolving Mediator. Front. Pharmacol. 2020, 11, 1131. [Google Scholar] [CrossRef]
- Hubbard, D.B.; Miller, B.J. Meta-Analysis of Blood Cortisol Levels in Individuals with First-Episode Psychosis. Psychoneuroendocrinology 2019, 104, 269–275. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ortega, M.A.; Romero, B.; Asúnsolo, Á.; Sola, M.; Álavrez-Rocha, M.J.; Sainz, F.; Álavrez-Mon, M.; Buján, J.; García-Honduvilla, N. Patients with Incompetent Valves in Chronic Venous Insufficiency Show Increased Systematic Lipid Peroxidation and Cellular Oxidative Stress Markers. Oxidative Med. Cell. Longev. 2019, 2019, 5164576. [Google Scholar] [CrossRef]
- Ortega, M.A.; Sánchez-Trujillo, L.; Bravo, C.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Alvarez-Mon, M.A.; Sainz, F.; Alvarez-Mon, M.; Bujan, J.; et al. Newborns of Mothers with Venous Disease during Pregnancy Show Increased Levels of Lipid Peroxidation and Markers of Oxidative Stress and Hypoxia in the Umbilical Cord. Antioxidants 2021, 10, 980. [Google Scholar] [CrossRef]
- Weaver, K.; Skouta, R. The Selenoprotein Glutathione Peroxidase 4: From Molecular Mechanisms to Novel Therapeutic Opportunities. Biomedicines 2022, 10, 891. [Google Scholar] [CrossRef]
- Maiorino, M.; Conrad, M.; Ursini, F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid. Redox Signal. 2018, 29, 61–74. [Google Scholar] [CrossRef]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in Ferroptosis and Its Pharmacological Implication. Free Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid Peroxidation and Ferroptosis: The Role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Janssen, A.B.; Kertes, D.A.; McNamara, G.I.; Braithwaite, E.C.; Creeth, H.D.J.; Glover, V.I.; John, R.M. A Role for the Placenta in Programming Maternal Mood and Childhood Behavioural Disorders. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]



| FE-PW (n = 22) | HC-PW (n = 20) | |
|---|---|---|
| Median age (IQR), years | 33.5 (21–42) | 33.5 (25–39) |
| Median gestational age (IQR), weeks | 40 (38–41) | 40 (39–42) |
| C-section delivery, n (%) | 3 (13.6) | 2 (10.0) |
| Previous pregnancies, n (%) | 8 (36.4) | 9 (45.0) |
| Previous abortions, n (%) | 1 (4.5) | 2 (10.0) |
| Regular menstrual cycles, n (%) | 17 (77.3) | 16 (80.0) |
| PANSS mean (SD) | Positive 18.8 (6.3) | ----- |
| Negative 25.7 (7.9) |
| Antigen | Species | Dilution | Provider | Protocol Specifications |
|---|---|---|---|---|
| TFRC | Rabbit Monoclonal | 1:500 | Abcam (ab185550) | EDTA pH = 9, before incubation with blocking solution |
| ACSL-4 | Rabbit Monoclonal | 1:100 | Abcam (ab155282) | 100% Triton, 0.1% in PBS for 10 min, before incubation with blocking solution |
| ALOX-5 | Rabbit Monoclonal | 1:250 | Abcam (ab169755) | 100% Triton 0.1% in PBS for 10 min, before incubation with blocking solution |
| GPX4 | Rabbit Monoclonal | 1:100 | Abcam (ab125066) | 10 mM Sodium citrate pH = 6, before incubation with blocking solution |
| IgG (Rabbit) | Mouse | 1:1000 | Sigma-Aldrich (RG-96/B5283) | ------ |
| GENE | SEQUENCE Fwd (5′→3′) | SEQUENCE Rev (5′→3′) | Temp |
|---|---|---|---|
| TBP | TGCACAGGAGCCAAGAGTGAA | CACATCACAGCTCCCCACCA | 60 °C |
| TFRC | GAACTACACCGACCCTCGTG | GTGCTGTCCAGTTTCTCCGA | 60 °C |
| ACSL-4 | GCTGGGACAGTTACTGAAGGT | AGAGATACATACTCTCCTGCTTGT | 58 °C |
| ALOX-5 | TGGCGCGGTGGATTCATAC | AGGGGTCTGTTTTGTTGGCA | 60 °C |
| GPX4 | ATTGGTCGGCTGGACGAG | CCGAACTGGTTACACGGGAA | 59 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Funes Moñux, R.M.; Rodriguez-Martín, S.; Bravo, C.; De Leon-Luis, J.A.; Saz, J.V.; Saez, M.A.; Guijarro, L.G.; et al. The Placentas of Women Who Suffer an Episode of Psychosis during Pregnancy Have Increased Lipid Peroxidation with Evidence of Ferroptosis. Biomolecules 2023, 13, 120. https://doi.org/10.3390/biom13010120
Ortega MA, Fraile-Martinez O, García-Montero C, Funes Moñux RM, Rodriguez-Martín S, Bravo C, De Leon-Luis JA, Saz JV, Saez MA, Guijarro LG, et al. The Placentas of Women Who Suffer an Episode of Psychosis during Pregnancy Have Increased Lipid Peroxidation with Evidence of Ferroptosis. Biomolecules. 2023; 13(1):120. https://doi.org/10.3390/biom13010120
Chicago/Turabian StyleOrtega, Miguel A., Oscar Fraile-Martinez, Cielo García-Montero, Rosa M. Funes Moñux, Sonia Rodriguez-Martín, Coral Bravo, Juan A. De Leon-Luis, Jose V. Saz, Miguel A. Saez, Luis G. Guijarro, and et al. 2023. "The Placentas of Women Who Suffer an Episode of Psychosis during Pregnancy Have Increased Lipid Peroxidation with Evidence of Ferroptosis" Biomolecules 13, no. 1: 120. https://doi.org/10.3390/biom13010120
APA StyleOrtega, M. A., Fraile-Martinez, O., García-Montero, C., Funes Moñux, R. M., Rodriguez-Martín, S., Bravo, C., De Leon-Luis, J. A., Saz, J. V., Saez, M. A., Guijarro, L. G., Lahera, G., Mora, F., Fernandez-Rojo, S., Quintero, J., Monserrat, J., García-Honduvilla, N., Bujan, J., Alvarez-Mon, M., & Alvarez-Mon, M. A. (2023). The Placentas of Women Who Suffer an Episode of Psychosis during Pregnancy Have Increased Lipid Peroxidation with Evidence of Ferroptosis. Biomolecules, 13(1), 120. https://doi.org/10.3390/biom13010120















