Transcriptional Profiling Identifies Prognostic Gene Signatures for Conjunctival Extranodal Marginal Zone Lymphoma
Abstract
1. Introduction
2. Methods
2.1. Patients and Clinical Outcome
2.2. Formalin Fixation and Paraffin Embedding
2.3. RNA Isolation
2.4. RNA Sequencing
2.5. Bioinformatics
3. Results
3.1. Patient Characteristics
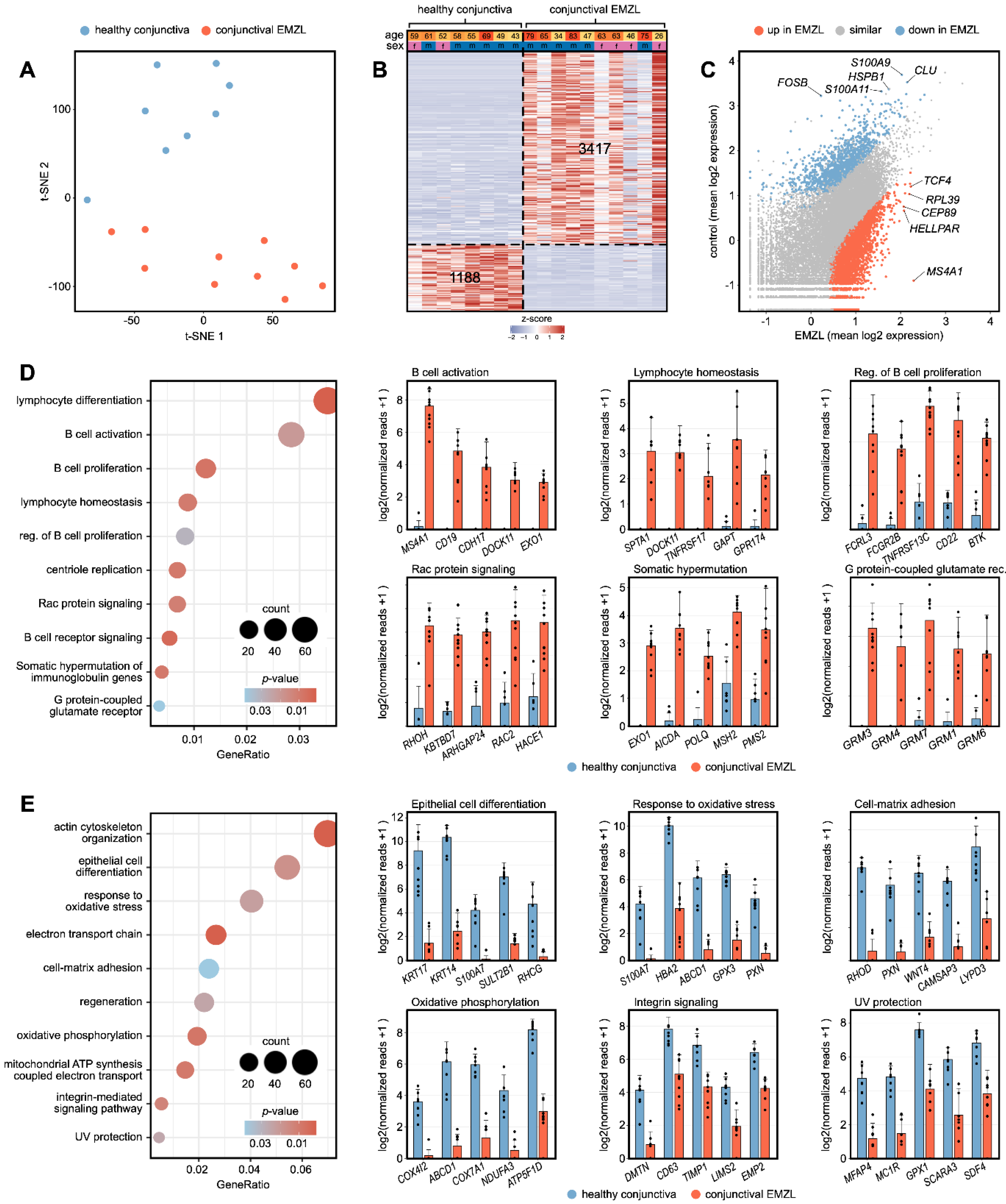
3.2. Transcriptional Characterization of Conjunctival EMZL
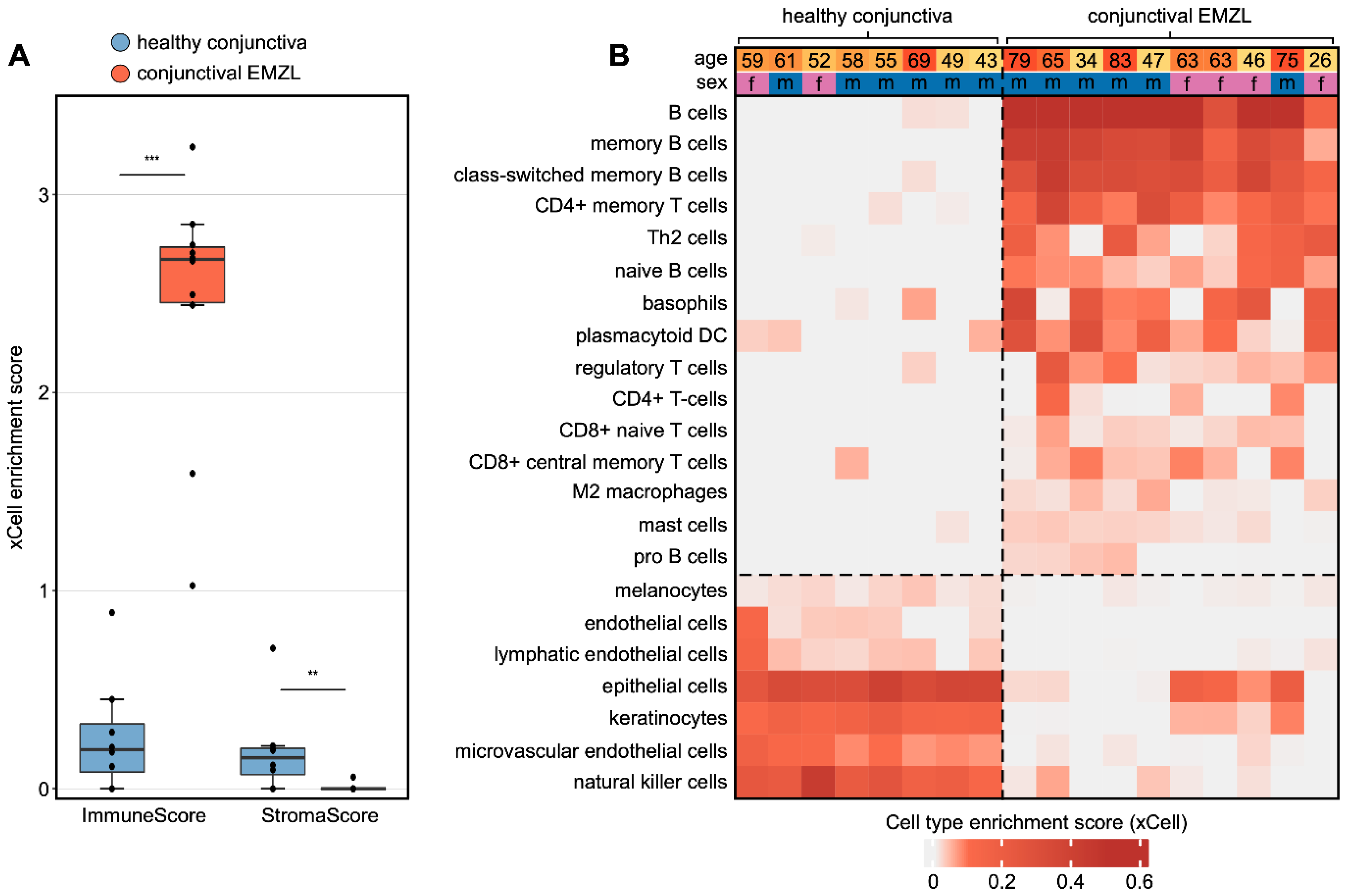
3.3. Cellular Tumor Microenvironment of Conjunctival EMZL
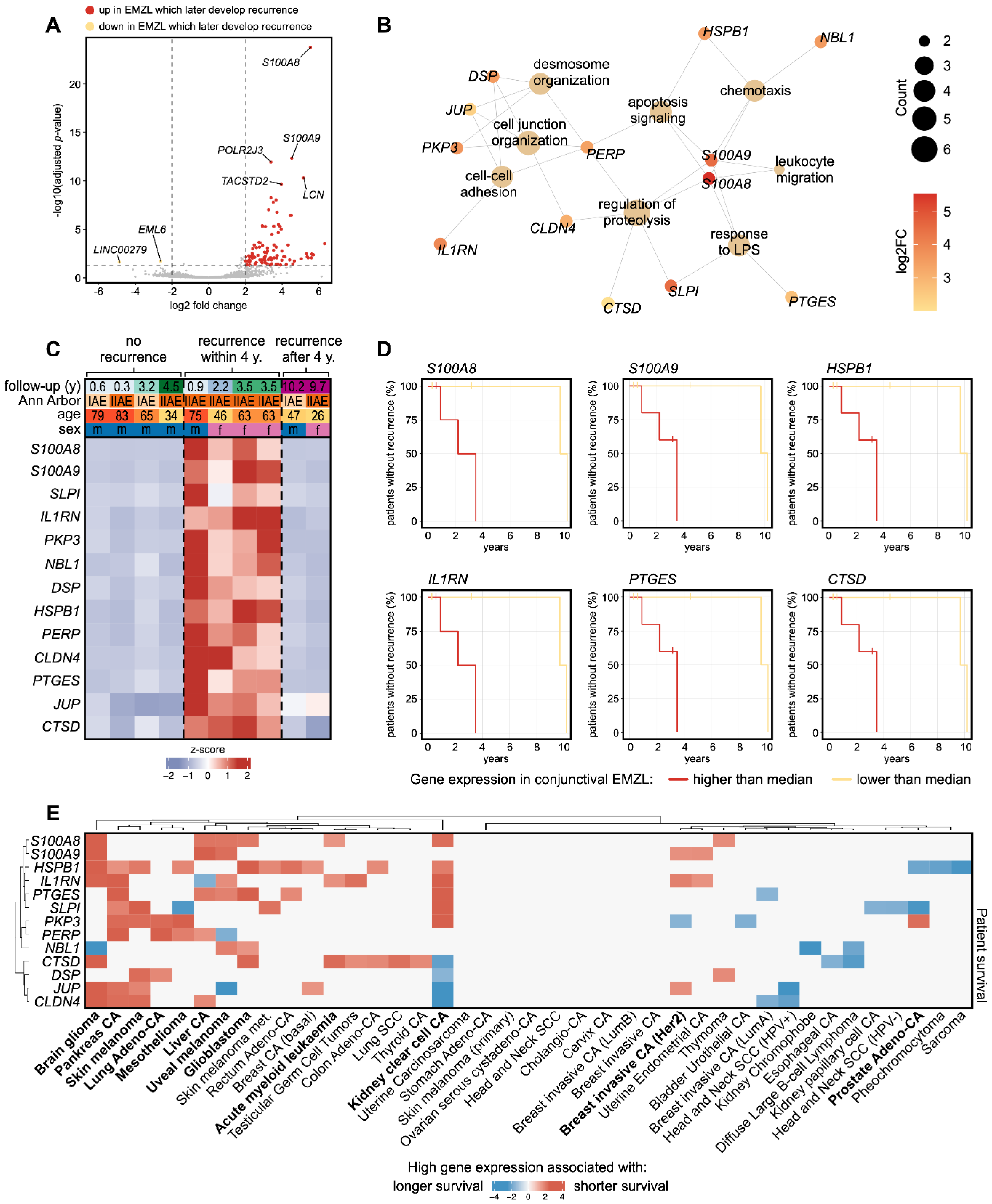
3.4. Biomarkers Associated with Recurrence of Conjunctival EMZL
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGrath, L.A.; Ryan, D.A.; Warrier, S.K.; Coupland, S.E.; Glasson, W.J. Conjunctival Lymphoma. Eye 2022. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, M.M.; Coupland, S.E.; Prause, J.U.; Heegaard, S. Malignant lymphoma of the conjunctiva. Surv. Ophthalmol. 2015, 60, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Sjo, L.D. Ophthalmic lymphoma: Epidemiology and pathogenesis. Acta Ophthalmol. 2009, 87, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, M.M.; Rasmussen, P.K.; Coupland, S.E.; Esmaeli, B.; Finger, P.T.; Graue, G.F.; Grossniklaus, H.E.; Honavar, S.G.; Khong, J.J.; McKelvie, P.A.; et al. Conjunctival Lymphoma--An International Multicenter Retrospective Study. JAMA Ophthalmol. 2016, 134, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Carvalho, C.; Rundle, P.; Smith, A.F. Conjunctival lymphoid tumors: Clinical analysis of 117 cases and relationship to systemic lymphoma. Ophthalmology 2001, 108, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Lee, J.S.; Lee, C.; Yoon, D.H.; Sa, H.S. Prognostic factors for relapse and survival among patients with ocular adnexal lymphoma: Validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM classification. Br. J. Ophthalmol. 2021, 105, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Beykin, G.; Pe’er, J.; Amir, G.; Frenkel, S. Paediatric and adolescent elevated conjunctival lesions in the plical area: Lymphoma or reactive lymphoid hyperplasia? Br. J. Ophthalmol. 2014, 98, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Chien, J.L.; Surakiatchanukul, T.; Sioufi, K.; Lally, S.E.; Shields, J.A. Conjunctival Tumors: Review of Clinical Features, Risks, Biomarkers, and Outcomes—The 2017 J. Donald M. Gass Lecture. Asia Pac. J. Ophthalmol. 2017, 6, 109–120. [Google Scholar] [CrossRef]
- Cani, A.K.; Soliman, M.; Hovelson, D.H.; Liu, C.J.; McDaniel, A.S.; Haller, M.J.; Bratley, J.V.; Rahrig, S.E.; Li, Q.; Briceno, C.A.; et al. Comprehensive genomic profiling of orbital and ocular adnexal lymphomas identifies frequent alterations in MYD88 and chromatin modifiers: New routes to targeted therapies. Mod. Pathol. 2016, 29, 685–697. [Google Scholar] [CrossRef]
- Vela, V.; Juskevicius, D.; Gerlach, M.M.; Meyer, P.; Graber, A.; Cathomas, G.; Dirnhofer, S.; Tzankov, A. High throughput sequencing reveals high specificity of TNFAIP3 mutations in ocular adnexal marginal zone B-cell lymphomas. Hematol. Oncol. 2020, 38, 284–292. [Google Scholar] [CrossRef]
- Auw-Haedrich, C.; Coupland, S.E.; Kapp, A.; Schmitt-Graff, A.; Buchen, R.; Witschel, H. Long term outcome of ocular adnexal lymphoma subtyped according to the REAL classification. Revised European and American Lymphoma. Br. J. Ophthalmol. 2001, 85, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Auw-Haedrich, C.; Schlecht, A.; Boneva, S.; Mittelviefhaus, H.; Lapp, T.; Agostini, H.; Reinhard, T.; Schlunck, G.; Lange, C.A.K. Transcriptional characterization of conjunctival melanoma identifies the cellular tumor microenvironment and prognostic gene signatures. Sci. Rep. 2020, 10, 17022. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Hajdu, R.I.; Boneva, S.; Schlecht, A.; Lapp, T.; Wacker, K.; Agostini, H.; Reinhard, T.; Auw-Hadrich, C.; Schlunck, G.; et al. Characterization of the Cellular Microenvironment and Novel Specific Biomarkers in Pterygia Using RNA Sequencing. Front. Med. 2021, 8, 714458. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.A.K.; Lehnert, P.; Boneva, S.K.; Zhang, P.; Ludwig, F.; Boeker, M.; Hoffmeier, K.; Horres, R.; Schlunck, G.; Reinhard, T.; et al. Increased expression of hypoxia-inducible factor-1 alpha and its impact on transcriptional changes and prognosis in malignant tumours of the ocular adnexa. Eye 2018, 32, 1772–1782. [Google Scholar] [CrossRef]
- Boneva, S.; Schlecht, A.; Zhang, P.; Boehringer, D.; Lapp, T.; Mittelviefhaus, H.; Reinhard, T.; Auw-Haedrich, C.; Schlunck, G.; Wolf, J.; et al. MACE RNA sequencing analysis of conjunctival squamous cell carcinoma and papilloma using formalin-fixed paraffin-embedded tumor tissue. Sci. Rep. 2020, 10, 21292. [Google Scholar] [CrossRef]
- Boneva, S.; Schlecht, A.; Bohringer, D.; Mittelviefhaus, H.; Reinhard, T.; Agostini, H.; Auw-Haedrich, C.; Schlunck, G.; Wolf, J.; Lange, C. 3’ MACE RNA-sequencing allows for transcriptome profiling in human tissue samples after long-term storage. Lab. Investig. 2020, 100, 1345–1355. [Google Scholar] [CrossRef]
- Galaxy, C. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2022 update. Nucleic Acids Res. 2022, 50, W345–W351. [Google Scholar] [CrossRef]
- Wolf, J.; Schlecht, A.; Rosmus, D.D.; Boneva, S.; Agostini, H.; Schlunck, G.; Wieghofer, P.; Lange, C. Comparative transcriptome analysis of human and murine choroidal neovascularization identifies fibroblast growth factor inducible-14 as phylogenetically conserved mediator of neovascular age-related macular degeneration. Biochim. Biophys Acta Mol. Basis Dis. 2022, 1868, 166340. [Google Scholar] [CrossRef]
- Wolf, J.; Boneva, S.; Schlecht, A.; Lapp, T.; Auw-Haedrich, C.; Lagreze, W.; Agostini, H.; Reinhard, T.; Schlunck, G.; Lange, C. The Human Eye Transcriptome Atlas: A searchable comparative transcriptome database for healthy and diseased human eye tissue. Genomics 2022, 114, 110286. [Google Scholar] [CrossRef]
- Zhang, P.; Schlecht, A.; Wolf, J.; Boneva, S.; Laich, Y.; Koch, J.; Ludwig, F.; Boeck, M.; Thien, A.; Hardtner, C.; et al. The role of interferon regulatory factor 8 for retinal tissue homeostasis and development of choroidal neovascularisation. J. Neuroinflam. 2021, 18, 215. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef]
- van der Maaten, L. Accelerating t-SNE using Tree-Based Algorithms. J. Mach. Learn. Res. 2014, 15, 3221–3245. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome. Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome. Biol. 2017, 18, 220. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. 2012, 131, 281–285. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Carbone, P.P.; Kaplan, H.S.; Musshoff, K.; Smithers, D.W.; Tubiana, M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971, 31, 1860–1861. [Google Scholar] [PubMed]
- Coupland, S.E.; Barnhill, R.; Conway, R. Conjunctival melanoma. Cancer Staging Man. 2017, 8, 803–812. [Google Scholar]
- Girard, L.; Rodriguez-Canales, J.; Behrens, C.; Thompson, D.M.; Botros, I.W.; Tang, H.; Xie, Y.; Rekhtman, N.; Travis, W.D.; Wistuba, I.I.; et al. An Expression Signature as an Aid to the Histologic Classification of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 4880–4889. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Ashton-Key, M.; Cogliatti, S.; Rondeau, S.; Schmitz, S.F.; Ghielmini, M.; Cragg, M.S.; Johnson, P. Expression of the inhibitory Fc gamma receptor IIB (FCGR2B, CD32B) on follicular lymphoma cells lowers the response rate to rituximab monotherapy (SAKK 35/98). Br. J. Haematol. 2015, 168, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhang, B.; Li, Z.; Lei, L.; Sun, G.; Jiang, X.; Guan, J.; Zhang, Y.; Xu, S.; Li, Q. KBTBD7 promotes non-small cell lung carcinoma progression by enhancing ubiquitin-dependent degradation of PTEN. Cancer Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fang, J.; Chen, S.; Wang, W.; Meng, S.; Liu, B. Nonconserved miR-608 suppresses prostate cancer progression through RAC2/PAK4/LIMK1 and BCL2L1/caspase-3 pathways by targeting the 3’-UTRs of RAC2/BCL2L1 and the coding region of PAK4. Cancer Med. 2019, 8, 5716–5734. [Google Scholar] [CrossRef]
- Godsmark, G.; LA, D.E.S.R.; Mowla, S. Activation-Induced Cytidine Deaminase Promotes Proliferation and Enhances Chemoresistance and Migration in B-cell Lymphoma. Anticancer. Res. 2021, 41, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Geng, L.; Black, A.; Talmon, G.; Berim, L.; Wang, J. The miR-487b-3p/GRM3/TGFbeta signaling axis is an important regulator of colon cancer tumorigenesis. Oncogene 2017, 36, 3477–3489. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The Warburg Effect 97 Years after Its Discovery. Cancers 2020, 12, 2819. [Google Scholar] [CrossRef]
- Wang, M.; Chen, B.; Zhang, W.; Zhang, F.; Qiu, Y.; Lin, Y.; Yang, S. Dematin inhibits glioblastoma malignancy through RhoA-mediated CDKs downregulation and cytoskeleton remodeling. Exp. Cell Res. 2022, 417, 113196. [Google Scholar] [CrossRef]
- Kasamatsu, S.; Hachiya, A.; Fujimura, T.; Sriwiriyanont, P.; Haketa, K.; Visscher, M.O.; Kitzmiller, W.J.; Bello, A.; Kitahara, T.; Kobinger, G.P.; et al. Essential role of microfibrillar-associated protein 4 in human cutaneous homeostasis and in its photoprotection. Sci. Rep. 2011, 1, 164. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Knorr, C.; Qin, Y.; Sebald, W.; Schimpl, A.; Banchereau, J.; Muller-Hermelink, H.K. Low-grade B cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signaling and Th2-type cytokines for in vitro growth and differentiation. Am. J. Pathol. 1997, 150, 1583–1593. [Google Scholar] [PubMed]
- Zangani, M.M.; Froyland, M.; Qiu, G.Y.; Meza-Zepeda, L.A.; Kutok, J.L.; Thompson, K.M.; Munthe, L.A.; Bogen, B. Lymphomas can develop from B cells chronically helped by idiotype-specific T cells. J. Exp. Med. 2007, 204, 1181–1191. [Google Scholar] [CrossRef]
- Rothermel, A.L.; Gilbert, K.M.; Weigle, W.O. Differential abilities of Th1 and Th2 to induce polyclonal B cell proliferation. Cell Immunol. 1991, 135, 1–15. [Google Scholar] [CrossRef]
- Xiong, X.; Xie, X.; Wang, Z.; Zhang, Y.; Wang, L. Tumor-associated macrophages in lymphoma: From mechanisms to therapy. Int. Immunopharmacol. 2022, 112, 109235. [Google Scholar] [CrossRef]
- Mitchell, D.; Chintala, S.; Dey, M. Plasmacytoid dendritic cell in immunity and cancer. J. Neuroimmunol. 2018, 322, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Dhodapkar, M.V. Natural Killer T Cells in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1178. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2020, 58, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, K.; Li, S.; Liu, J.; Zhang, Y.; Zhou, M.; Zhao, H.; Chen, H.; Wu, N.; Liu, Z.; et al. CFEA: A cell-free epigenome atlas in human diseases. Nucleic Acids Res. 2020, 48, D40–D44. [Google Scholar] [CrossRef]
- Yu, F.; Quan, F.; Xu, J.; Zhang, Y.; Xie, Y.; Zhang, J.; Lan, Y.; Yuan, H.; Zhang, H.; Cheng, S.; et al. Breast cancer prognosis signature: Linking risk stratification to disease subtypes. Brief Bioinform. 2019, 20, 2130–2140. [Google Scholar] [CrossRef]
- Sturm, G.; Finotello, F.; Petitprez, F.; Zhang, J.D.; Baumbach, J.; Fridman, W.H.; List, M.; Aneichyk, T. Comprehensive evaluation of transcriptome-based cell-type quantification methods for immuno-oncology. Bioinformatics 2019, 35, i436–i445. [Google Scholar] [CrossRef] [PubMed]
| Group | Conjunctival EMZL | Healthy Conjunctiva | p |
|---|---|---|---|
| n | 10 | 8 | - |
| Age at surgery (years) | 58.0 (25.9–82.9) | 55.7 (43.0–69.0) | 0.736 |
| Sex | 0.867 | ||
| Male | 6 (60.0%) | 6 (75.0%) | |
| Female | 4 (40.0%) | 2 (25.0%) | |
| Ann arbor staging | - | - | |
| IE | 3 (30.0%) | ||
| IIE | 7 (70.0%) | ||
| AJCC staging (8th edition) | - | - | |
| T1 | 7 (70.0%) | ||
| T2 | 1 (10.0%) | ||
| T3 | 2 (20.0%) | ||
| Recurrence | 6 (60.0%) | - | - |
| Ocular | 3 (30.0%) | ||
| Systemic disease | 3 (30.0%) | ||
| Time to event (years) | 5.0 (0.9–10.2) | - | - |
| Group | No Recurrence | Recurrence/ Systemic Disease | p-Value (Kaplan–Meier) |
|---|---|---|---|
| n | 4 | 6 | - |
| Age at surgery (years) | 65.1 (33.6–82.9) | 53.3 (25.9–74.9) | 0.254 * |
| Sex | 0.242 | ||
| Male | 4 (100.0%) | 2 (33.3%) | |
| Female | 0 (0.0%) | 4 (66.7 %) | |
| Ann arbor staging | 0.126 | ||
| IE | 2 (50.0%) | 1 (16.7%) | |
| IIE | 2 (50.0%) | 5 (83.3%) | |
| AJCC staging (8th edition) | 0.746 | ||
| T1 | 3 (75.0%) | 4 (66.7%) | |
| T2 | 1 (25.0%) | 0 (0.0%) | |
| T3 | 0 (0.0%) | 2 (33.3%) | |
| Transcriptome signature | 0.014 | ||
| no recurrence | 4 (100.0%) | 2 (33.3%) | |
| recurrence/systemic disease | 0 (0.0%) | 4 (66.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, J.; Reinhard, T.; Hajdu, R.I.; Schlunck, G.; Auw-Haedrich, C.; Lange, C. Transcriptional Profiling Identifies Prognostic Gene Signatures for Conjunctival Extranodal Marginal Zone Lymphoma. Biomolecules 2023, 13, 115. https://doi.org/10.3390/biom13010115
Wolf J, Reinhard T, Hajdu RI, Schlunck G, Auw-Haedrich C, Lange C. Transcriptional Profiling Identifies Prognostic Gene Signatures for Conjunctival Extranodal Marginal Zone Lymphoma. Biomolecules. 2023; 13(1):115. https://doi.org/10.3390/biom13010115
Chicago/Turabian StyleWolf, Julian, Thomas Reinhard, Rozina Ida Hajdu, Günther Schlunck, Claudia Auw-Haedrich, and Clemens Lange. 2023. "Transcriptional Profiling Identifies Prognostic Gene Signatures for Conjunctival Extranodal Marginal Zone Lymphoma" Biomolecules 13, no. 1: 115. https://doi.org/10.3390/biom13010115
APA StyleWolf, J., Reinhard, T., Hajdu, R. I., Schlunck, G., Auw-Haedrich, C., & Lange, C. (2023). Transcriptional Profiling Identifies Prognostic Gene Signatures for Conjunctival Extranodal Marginal Zone Lymphoma. Biomolecules, 13(1), 115. https://doi.org/10.3390/biom13010115







