Circ-CPSF1 Worsens Radiation-Induced Oxidative Stress Injury in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. C. elegans Culture
2.2. Synchronization of C. elegans
2.3. Radiation Treatment
2.4. CircRNA Sequencing
2.5. Quantitative Real Time PCR
2.6. RNAi
2.7. Lifespan and Eggs Assay
2.8. Apoptosis Assay
2.9. Detection of Reactive Oxygen Species Level
2.10. Mitochondrial Membrane Potential Assay
2.11. Statistical Analysis
3. Results
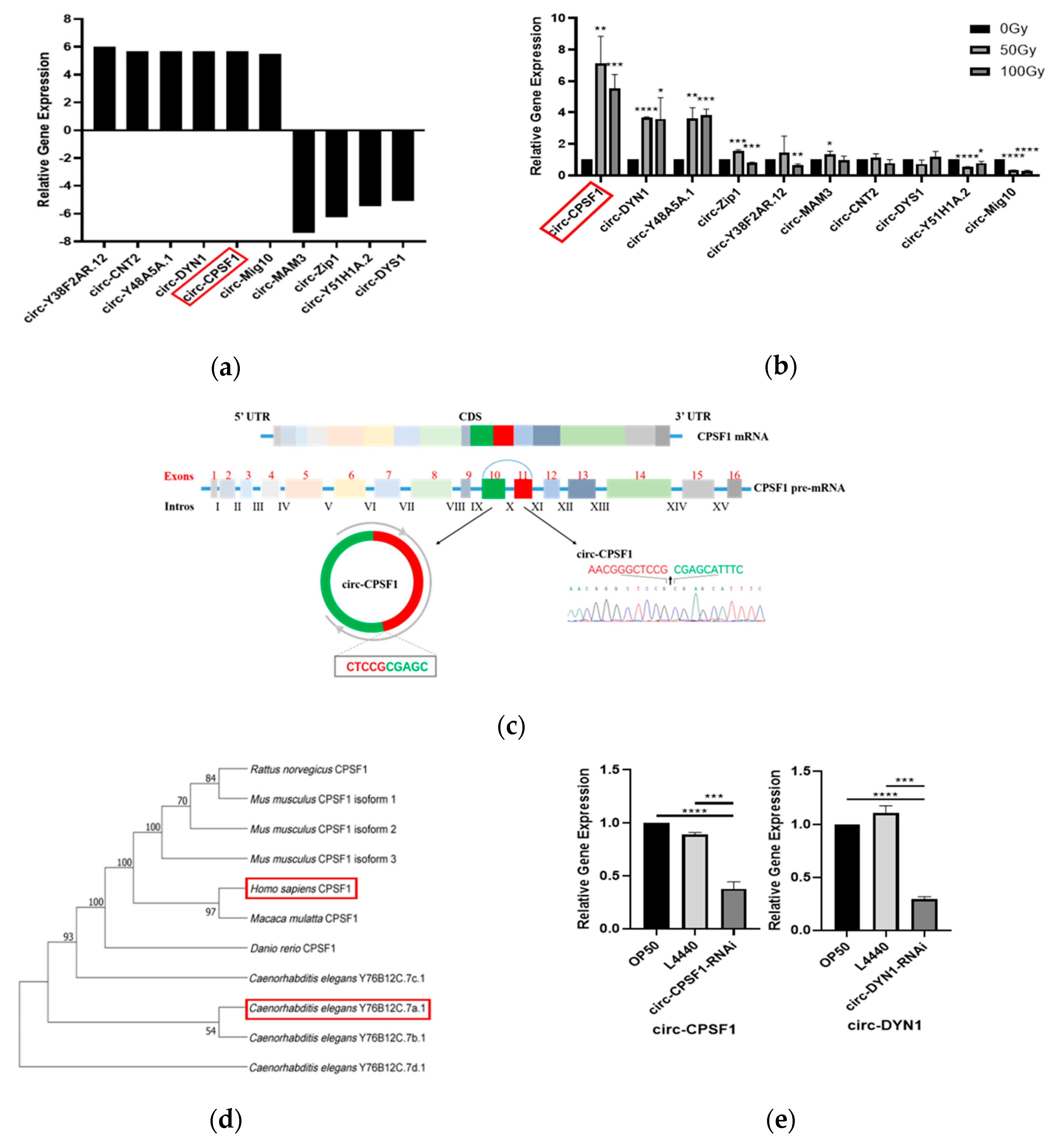
3.1. Characteristics of Differentially Expressed circRNAs Induced by Ionizing Radiation
3.2. Circ-CPSF1 Was Upregulated in C. elegans Exposed to X-ray Irradiation and RNAi of Circ-CPSF1
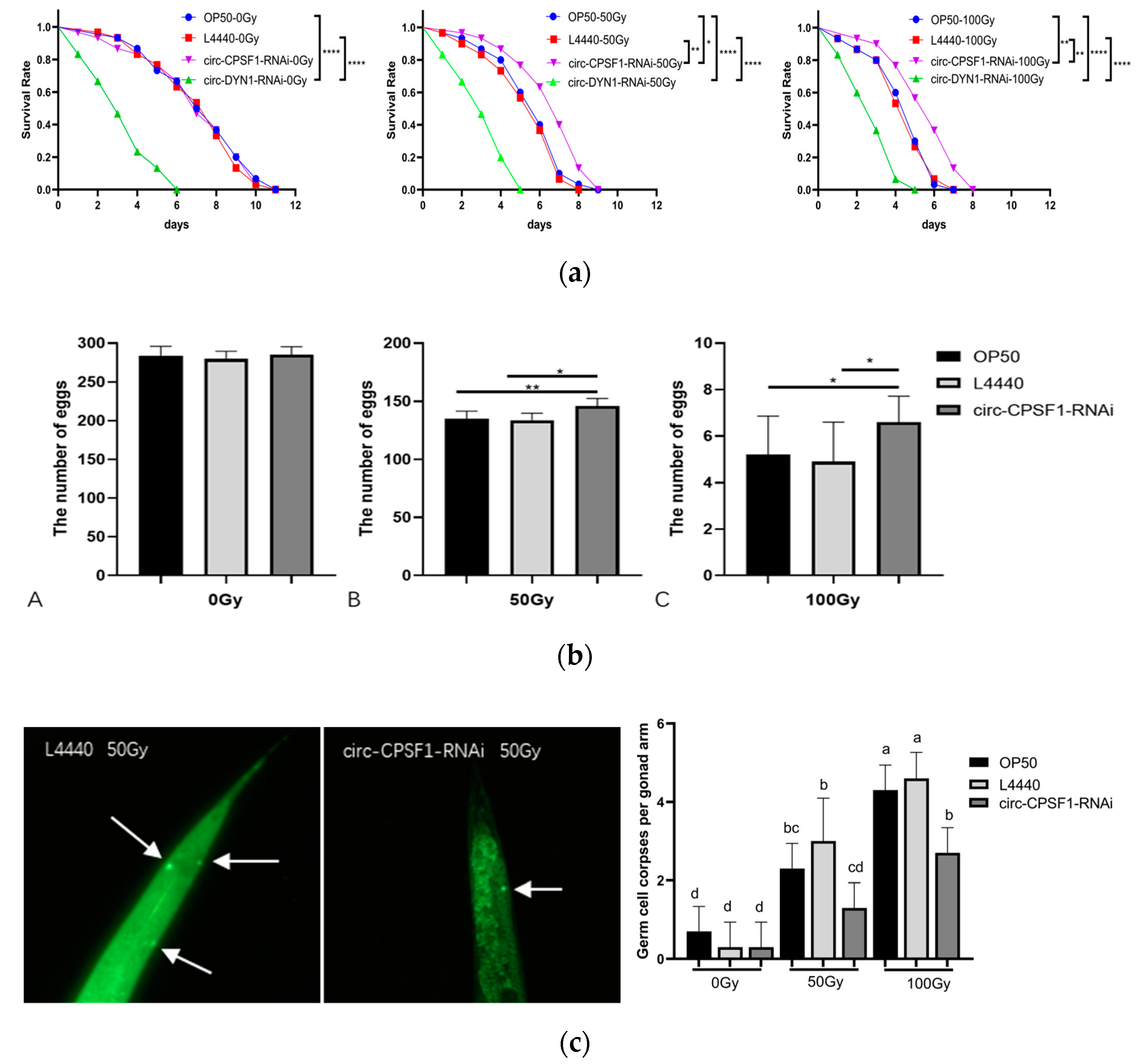
3.3. Circ-CPSF1 Aggravated Radiation Damage to C. elegans on the Lifespan, Eggs Production and Germ Cell Apoptosis
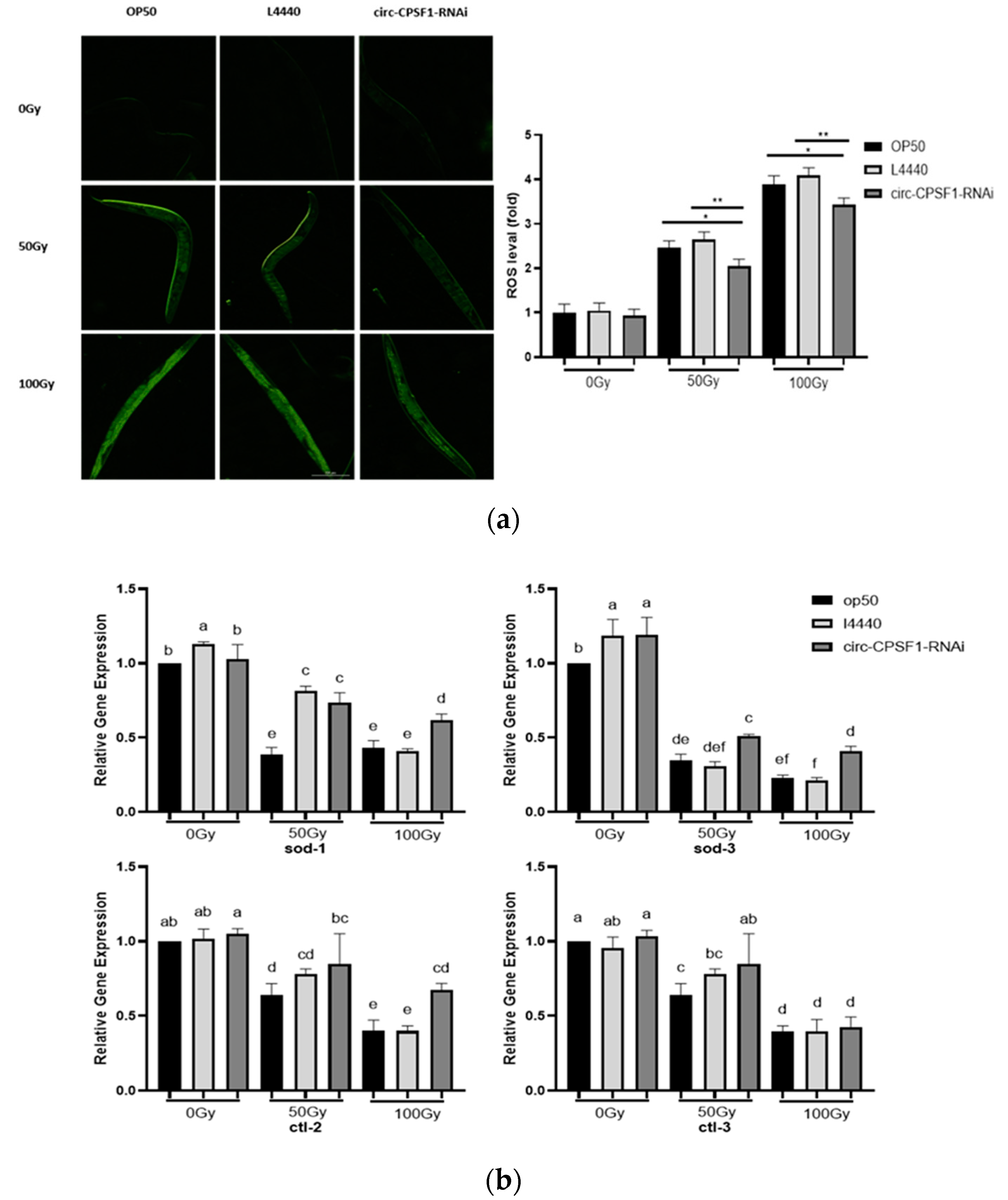
3.4. The Induction of Circ-CPSF1 in Radiation Mediated ROS
3.5. Function of Circ-CPSF1 in Mitochondrial Transmembrane Potential in C. elegans Exposed to X-ray Irradiation
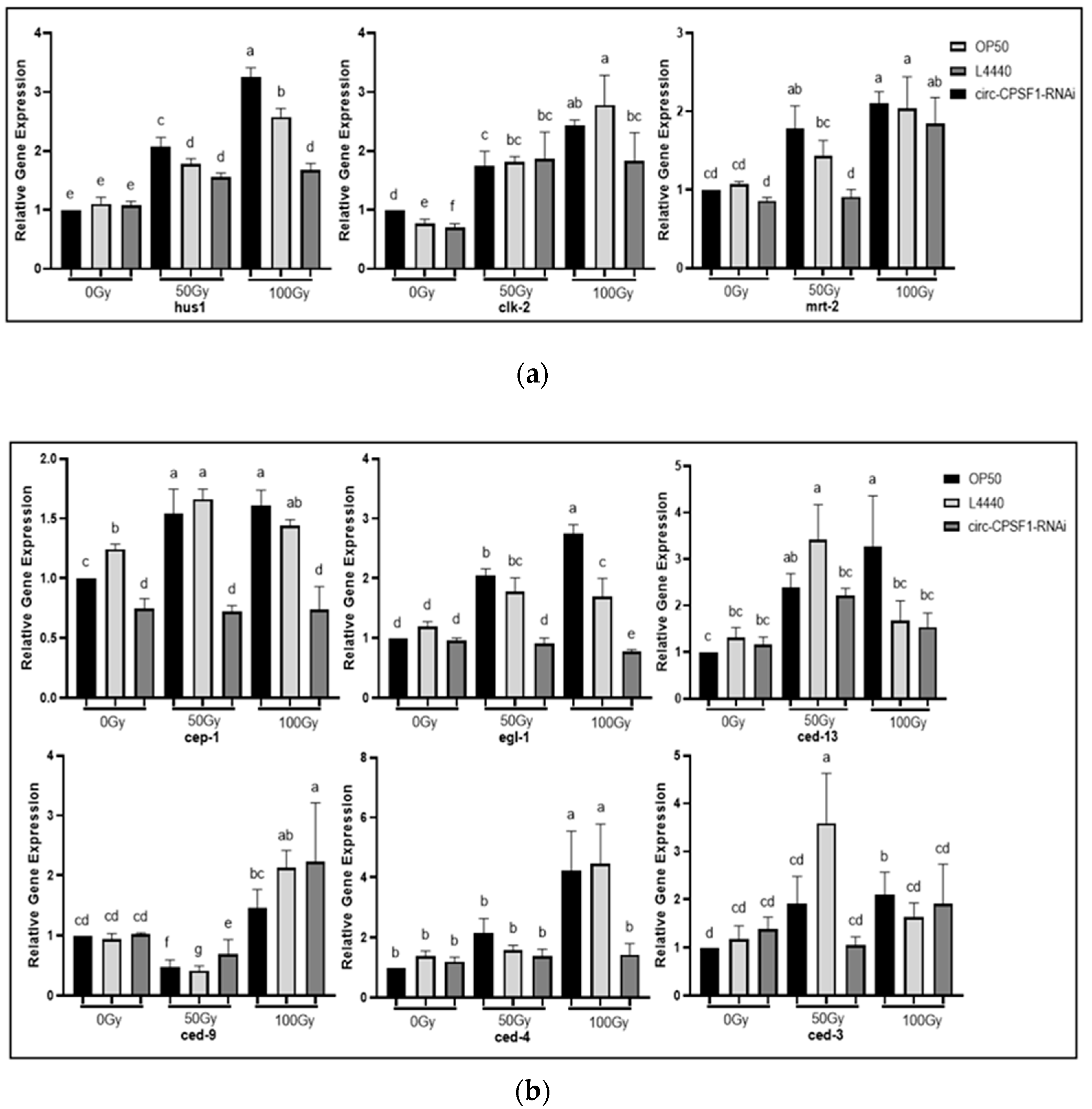
3.6. Involvement of Circ-CPSF1 in Radiation Mediated DNA Damage Pathway and Apoptosis
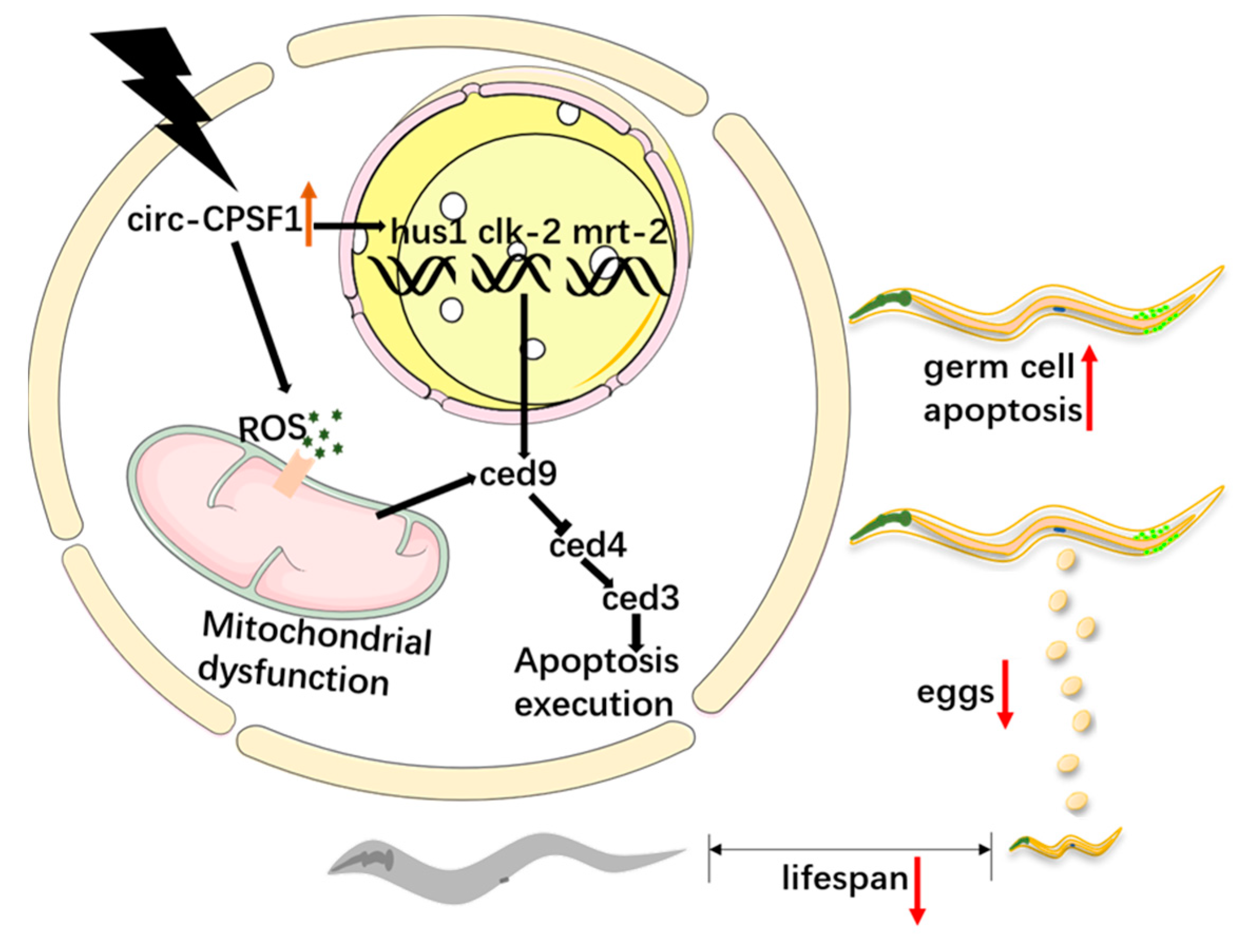
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- da Costa Dantas, R.; Navoni, J.A.; de Alencar, F.L.S.; da Costa Xavier, L.A.; do Amaral, V.S. Natural radioactivity in Brazil: A systematic review. Environ. Sci. Pollut. Res. Int. 2020, 27, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Bornman, J.F.; Barnes, P.W.; Robson, T.M.; Robinson, S.A.; Jansen, M.A.; Ballare, C.L.; Flint, S.D. Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems. Photochem. Photobiol. Sci. 2019, 18, 681–716. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, M.S.M.; Ramli, A.T.; Hashim, S.; Lee, M.H. Radiological hazard associated with amang processing industry in Peninsular Malaysia and its environmental impacts. Ecotoxicol. Environ. Saf. 2021, 208, 111727. [Google Scholar] [CrossRef] [PubMed]
- Daguenet, E.; Louati, S.; Wozny, A.S.; Vial, N.; Gras, M.; Guy, J.B.; Vallard, A.; Rodriguez-Lafrasse, C.; Magne, N. Radiation-induced bystander and abscopal effects: Important lessons from preclinical models. Br. J. Cancer 2020, 123, 339–348. [Google Scholar] [CrossRef]
- Cakmak, G.; Severcan, M.; Zorlu, F.; Severcan, F. Structural and functional damages of whole body ionizing radiation on rat brain homogenate membranes and protective effect of amifostine. Int. J. Radiat. Biol. 2016, 92, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Fucic, A.; Aghajanyan, A.; Druzhinin, V.; Minina, V.; Neronova, E. Follow-up studies on genome damage in children after Chernobyl nuclear power plant accident. Arch. Toxicol. 2016, 90, 2147–2159. [Google Scholar] [CrossRef]
- Aghajanyan, A.; Suskov, I. Transgenerational genomic instability in children of irradiated parents as a result of the Chernobyl Nuclear Accident. Mutat. Res. 2009, 671, 52–57. [Google Scholar] [CrossRef]
- Fucic, A.; Brunborg, G.; Lasan, R.; Jezek, D.; Knudsen, L.E.; Merlo, D.F. Genomic damage in children accidentally exposed to ionizing radiation: A review of the literature. Mutat. Res. 2008, 658, 111–123. [Google Scholar] [CrossRef]
- Santivasi, W.L.; Xia, F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid. Redox Signal. 2014, 21, 251–259. [Google Scholar] [CrossRef]
- Hertel-Aas, T.; Oughton, D.H.; Jaworska, A.; Bjerke, H.; Salbu, B.; Brunborg, G. Effects of chronic gamma irradiation on reproduction in the earthworm Eisenia fetida (Oligochaeta). Radiat. Res. 2007, 168, 515–526. [Google Scholar] [CrossRef]
- Gross, J.P.; Kim, S.Y.; Gondi, V.; Pankuch, M.; Wagner, S.; Grover, A.; Luan, Y.; Woodruff, T.K. Proton Radiotherapy to Preserve Fertility and Endocrine Function: A Translational Investigation. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, J.; Gong, Y.; Wei, S.; Wei, Y.; Yi, L. MicroRNA: A novel implication for damage and protection against ionizing radiation. Environ. Sci. Pollut. Res. Int. 2021, 28, 15584–15596. [Google Scholar] [CrossRef] [PubMed]
- Doody, M.M.; Freedman, D.M.; Alexander, B.H.; Hauptmann, M.; Miller, J.S.; Rao, R.S.; Mabuchi, K.; Ron, E.; Sigurdson, A.J.; Linet, M.S. Breast cancer incidence in U.S. radiologic technologists. Cancer 2006, 106, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Maremonti, E.; Eide, D.M.; Oughton, D.H.; Salbu, B.; Grammes, F.; Kassaye, Y.A.; Guédon, R.; Lecomte-Pradines, C.; Brede, D.A. Gamma radiation induces life stage-dependent reprotoxicity in Caenorhabditis elegans via impairment of spermatogenesis. Sci. Total Environ. 2019, 695, 133835. [Google Scholar] [CrossRef] [PubMed]
- Tjahjono, E.; Revtovich, A.V.; Kirienko, N.V. Box C/D small nucleolar ribonucleoproteins regulate mitochondrial surveillance and innate immunity. PLoS Genet. 2022, 18, e1010103. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xiong, Y.; Shan, S.; Zhu, Y.; Zeng, D.; Shi, Y.; Zhang, Y.; Lu, W. Eleutheroside E Enhances the Long-Term Memory of Radiation-Damaged C. elegans through G-Protein-Coupled Receptor and Neuropeptide Signaling Pathways. J. Nat. Prod. 2020, 83, 3315–3323. [Google Scholar] [CrossRef]
- Kaletsky, R.; Moore, R.S.; Vrla, G.D.; Parsons, L.R.; Gitai, Z.; Murphy, C.T. C. elegans interprets bacterial non-coding RNAs to learn pathogenic avoidance. Nature 2020, 586, 445–451. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct. Target. Ther. 2021, 6, 185. [Google Scholar] [CrossRef]
- Misir, S.; Wu, N.; Yang, B.B. Specific expression and functions of circular RNAs. Cell Death Differ. 2022, 29, 481–491. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Zhai, S.; Shi, M.; Peng, C.; Deng, X.; Fu, D.; Wang, J.; Shen, B. Hypoxia-induced exosomal circPDK1 promotes pancreatic cancer glycolysis via c-myc activation by modulating miR-628-3p/BPTF axis and degrading BIN1. J. Hematol. Oncol. 2022, 15, 128. [Google Scholar] [CrossRef]
- Han, D.; Wang, Y.; Wang, Y.; Dai, X.; Zhou, T.; Chen, J.; Tao, B.; Zhang, J.; Cao, F. The Tumor-Suppressive Human Circular RNA CircITCH Sponges miR-330-5p to Ameliorate Doxorubicin-Induced Cardiotoxicity Through Upregulating SIRT6, Survivin, and SERCA2a. Circ. Res. 2020, 127, e108–e125. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, N.; Guo, Y.; Niu, K.; Gao, L.; Zhang, S.; Gu, H.; Wang, X.; Zhao, D.; Fan, R. CircRNA_100367 regulated the radiation sensitivity of esophageal squamous cell carcinomas through miR-217/Wnt3 pathway. Aging (Albany NY) 2019, 11, 12412–12427. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, Y.; He, Y.; Zeng, B.; Yi, C.; Wang, C.; Zhang, X.; Zhao, W.; Yu, D. Upregulation of Circular RNA circATRNL1 to Sensitize Oral Squamous Cell Carcinoma to Irradiation. Mol. Ther. Nucleic Acids 2020, 19, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, T.; Wang, Q.; Li, C.; Zhou, H.; Deng, S.; Lv, Z.; He, Y.; Hou, B.; Zhu, G. Silencing circPVT1 enhances radiosensitivity in non-small cell lung cancer by sponging microRNA-1208. Cancer Biomark. 2021, 31, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Cortés-López, M.; Gruner, M.R.; Cooper, D.A.; Gruner, H.N.; Voda, A.; van der Linden, A.M.; Miura, P. Global accumulation of circRNAs during aging in Caenorhabditis elegans. BMC Genom. 2018, 19, 58. [Google Scholar] [CrossRef]
- Yang, Q.; Doublié, S. Structural biology of poly(A) site definition. Wiley Interdiscip. Rev. RNA 2011, 2, 732–747. [Google Scholar] [CrossRef]
- Wang, L.; Lang, G.T.; Xue, M.Z.; Yang, L.; Chen, L.; Yao, L.; Li, X.; Wang, P.; Hu, X.; Shao, Z. Dissecting the heterogeneity of the alternative polyadenylation profiles in triple-negative breast cancers. Theranostics 2020, 10, 10531–10547. [Google Scholar] [CrossRef]
- Zetka, M.; Rose, A. The genetics of meiosis in Caenorhabditis elegans. Trends Genet. 1995, 11, 27–31. [Google Scholar] [CrossRef]
- Lionaki, E.; Tavernarakis, N. Assessing Aging and Senescent Decline in Caenorhabditis elegans: Cohort Survival Analysis. Methods Mol. Biol. 2013, 965, 473–484. [Google Scholar]
- Xu, Y.; Chen, L.; Liu, M.; Lu, Y.; Yue, Y.; Liu, Y.; Chen, H.; Xie, F.; Zhang, C. High-throughput transcriptome sequencing reveals extremely high doses of ionizing radiation-response genes in Caenorhabditis elegans. Toxicol. Res. 2019, 8, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cao, X.; Lu, H.; Wen, P.; Qi, X.; Chen, S.; Wu, L.; Li, C.; Xu, A.; Zhao, G. N-(3-oxo-acyl) homoserine lactone induced germ cell apoptosis and suppressed the over-activated RAS/MAPK tumorigenesis via mitochondrial-dependent ROS in C. elegans. Apoptosis 2018, 23, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Si, J.; Xu, H.; Dong, J.; Zheng, D.; Lu, X.; Li, X. Resveratrol-loaded nanoparticles reduce oxidative stress induced by radiation or amyloid-beta in transgenic Caenorhabditis elegans. J. Biomed. Nanotechnol. 2014, 10, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xing, X.; Zhang, F.; Shao, M.; Jin, S.; Yang, H.; Wang, G.; Cui, J.; Cai, L.; Li, W.; et al. Low-dose radiation induces renal SOD1 expression and activity in type 1 diabetic mice. Int. J. Radiat. Biol. 2014, 90, 224–230. [Google Scholar] [CrossRef]
- Burke, T.A.; Cascio, W.E.; Costa, D.L.; Deener, K.; Fontaine, T.D.; Fulk, F.A.; Jackson, L.E.; Munns, W.R.; Orme-Zavaleta, J.; Slimak, M.W.; et al. Rethinking Environmental Protection: Meeting the Challenges of a Changing World. Environ. Health Perspect. 2017, 125, A43–A49. [Google Scholar] [CrossRef]
- Takanami, T.; Mori, A.; Takahashi, H.; Higashitani, A. Hyper-resistance of meiotic cells to radiation due to a strong expression of a single recA-like gene in Caenorhabditis elegans. Nucleic Acids Res. 2000, 28, 4232–4236. [Google Scholar] [CrossRef]
- Jones, M.W.; Hare, D.J.; James, S.A.; de Jonge, M.D.; McColl, G. Radiation Dose Limits for Bioanalytical X-ray Fluorescence Microscopy. Anal. Chem. 2017, 89, 12168–12175. [Google Scholar] [CrossRef]
- Cui, C.; Yang, J.; Li, X.; Liu, D.; Fu, L.; Wang, X. Functions and mechanisms of circular RNAs in cancer radiotherapy and chemotherapy resistance. Mol. Cancer 2020, 19, 58. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Liu, D.; Yang, L. Targeting cleavage and polyadenylation specific factor 1 via shRNA inhibits cell proliferation in human ovarian cancer. J. Biosci. 2017, 42, 417–425. [Google Scholar] [CrossRef]
- Kuzmic, M.; Galas, S.; Lecomte-Pradines, C.; Dubois, C.; Dubourg, N.; Frelon, S. Interplay between ionizing radiation effects and aging in C. elegans. Free Radic. Biol. Med. 2019, 134, 657–665. [Google Scholar] [CrossRef]
- Buisset-Goussen, A.; Goussen, B.; Della-Vedova, C.; Galas, S.; Adam-Guillermin, C.; Lecomte-Pradines, C. Effects of chronic gamma irradiation: A multigenerational study using Caenorhabditis elegans. J. Environ. Radioact. 2014, 137, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, G.; Xu, Y.; Cheng, X.; Xu, S.; Chen, S.; Wu, L. Ceramide mediates radiation-induced germ cell apoptosis via regulating mitochondria function and MAPK factors in Caenorhabditis elegans. Ecotox. Environ. Saf. 2021, 208, 111579. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. J. Cell Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, J.; Deng, H.; Ma, R.; Liao, J.Y.; Liang, H.; Hu, J.; Li, J.; Guo, Z.; Cai, J.; et al. Targeting Mitochondria-Located circRNA SCAR Alleviates NASH via Reducing mROS Output. Cell 2020, 183, 76–93 e22. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Li, X.; Cui, Y.; Zhang, T.; Xu, S.; Li, S. Hydrogen sulfide exposure induces pyroptosis in the trachea of broilers via the regulatory effect of circRNA-17828/miR-6631-5p/DUSP6 crosstalk on ROS production. J. Hazard. Mater. 2021, 418, 126172. [Google Scholar] [CrossRef]
- Harris, J.; Lowden, M.; Clejan, I.; Tzoneva, M.; Thomas, J.H.; Hodgkin, J.; Ahmed, S. Mutator phenotype of Caenorhabditis elegans DNA damage checkpoint mutants. Genetics 2006, 174, 601–616. [Google Scholar] [CrossRef]
- Ito, S.; Greiss, S.; Gartner, A.; Derry, W.B. Cell-nonautonomous regulation of C. elegans germ cell death by kri-1. Curr. Biol. 2010, 20, 333–338. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, J.; Jin, W.; Sun, Y.; Xu, Y.; Wang, Y.; Liang, X.; Su, D. CircNR3C2 promotes HRD1-mediated tumor-suppressive effect via sponging miR-513a-3p in triple-negative breast cancer. Mol. Cancer 2021, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, A.; Yan, A.; Yao, J.; Huang, H.; Gao, Z.; Han, T.; Gu, J.; Li, N.; Wu, H.; et al. Circular RNA MTCL1 promotes advanced laryngeal squamous cell carcinoma progression by inhibiting C1QBP ubiquitin degradation and mediating beta-catenin activation. Mol. Cancer 2022, 21, 92. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Lin, F.; Wu, Z.; Jiang, Z.; Wang, T.; Huo, S.; Lai, W.; Li, L.; Zhang, C. Circ-CPSF1 Worsens Radiation-Induced Oxidative Stress Injury in Caenorhabditis elegans. Biomolecules 2023, 13, 102. https://doi.org/10.3390/biom13010102
Yuan J, Lin F, Wu Z, Jiang Z, Wang T, Huo S, Lai W, Li L, Zhang C. Circ-CPSF1 Worsens Radiation-Induced Oxidative Stress Injury in Caenorhabditis elegans. Biomolecules. 2023; 13(1):102. https://doi.org/10.3390/biom13010102
Chicago/Turabian StyleYuan, Jing, Fei Lin, Zhiyong Wu, Zhilin Jiang, Ting Wang, Sitong Huo, Weinan Lai, Li Li, and Chao Zhang. 2023. "Circ-CPSF1 Worsens Radiation-Induced Oxidative Stress Injury in Caenorhabditis elegans" Biomolecules 13, no. 1: 102. https://doi.org/10.3390/biom13010102
APA StyleYuan, J., Lin, F., Wu, Z., Jiang, Z., Wang, T., Huo, S., Lai, W., Li, L., & Zhang, C. (2023). Circ-CPSF1 Worsens Radiation-Induced Oxidative Stress Injury in Caenorhabditis elegans. Biomolecules, 13(1), 102. https://doi.org/10.3390/biom13010102





