Chitosan as an Adjuvant to Enhance the Control Efficacy of Low-Dosage Pyraclostrobin against Powdery Mildew of Rosa roxburghii and Improve Its Photosynthesis, Yield, and Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungicide, Atomizer and Chemical
2.2. Field Orchard
2.3. Field Control Experiment of Powdery Mildew
2.4. Determination of Disease Resistance and Photosynthesis Parameters of R. roxburghii
2.5. Determination of Yield, Quality and Amino Acids of R. roxburghii
2.6. Statistical Analyses
3. Results
3.1. Field Control Effect of Pyraclostrobin and Chitosan against Powdery Mildew
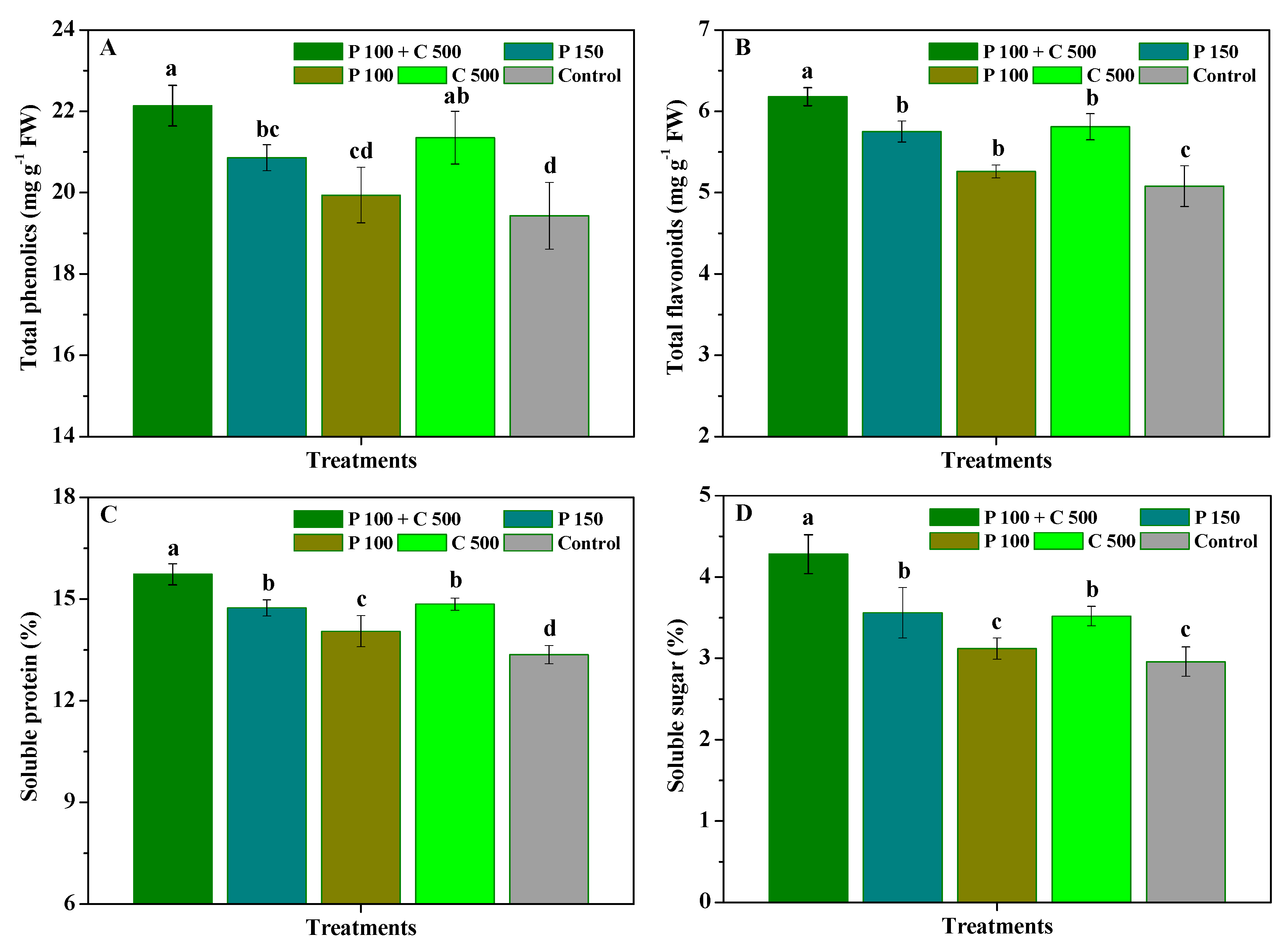
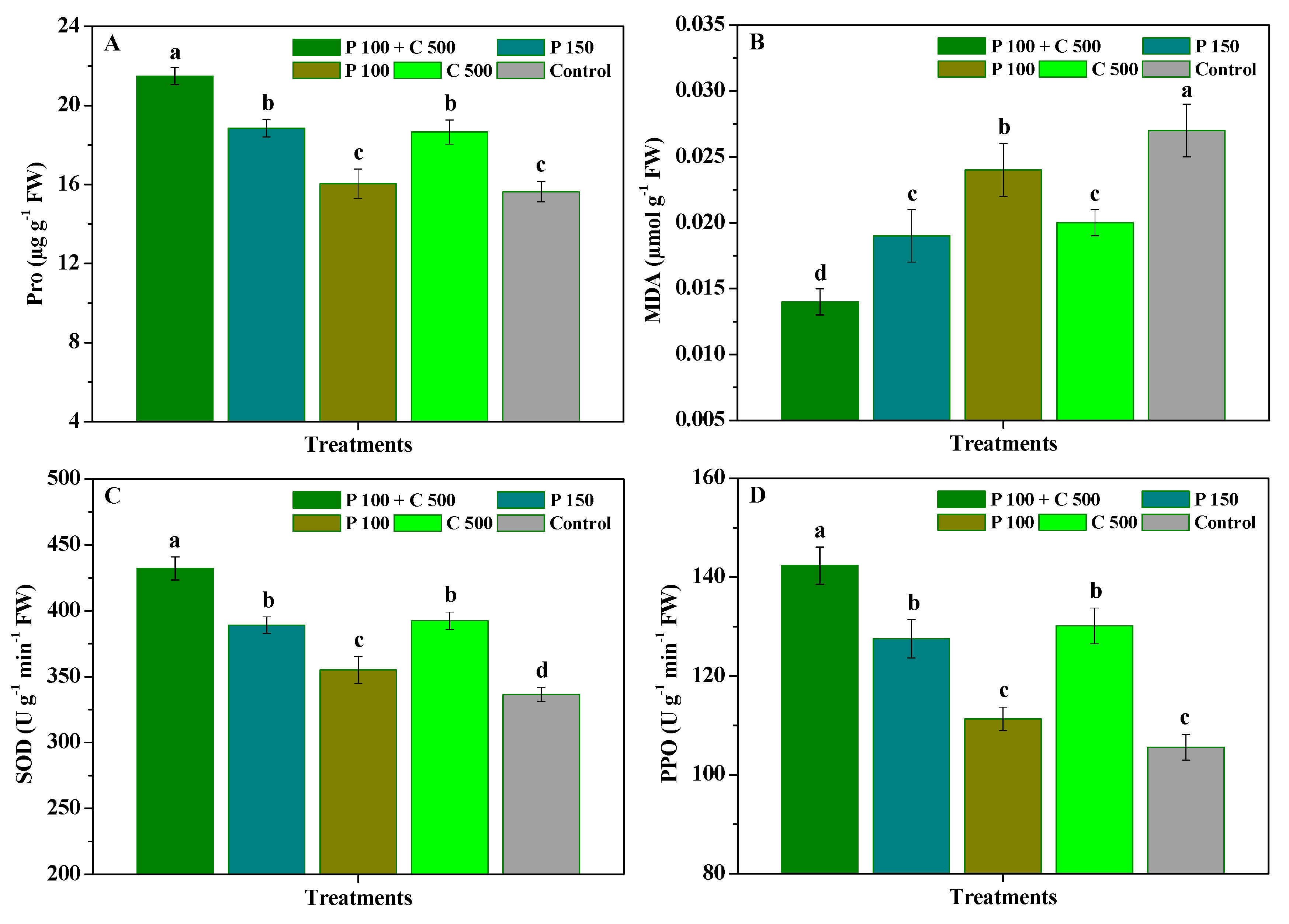
3.2. Influence of Pyraclostrobin and Chitosan on Disease Resistance of R. roxburghii Leaves
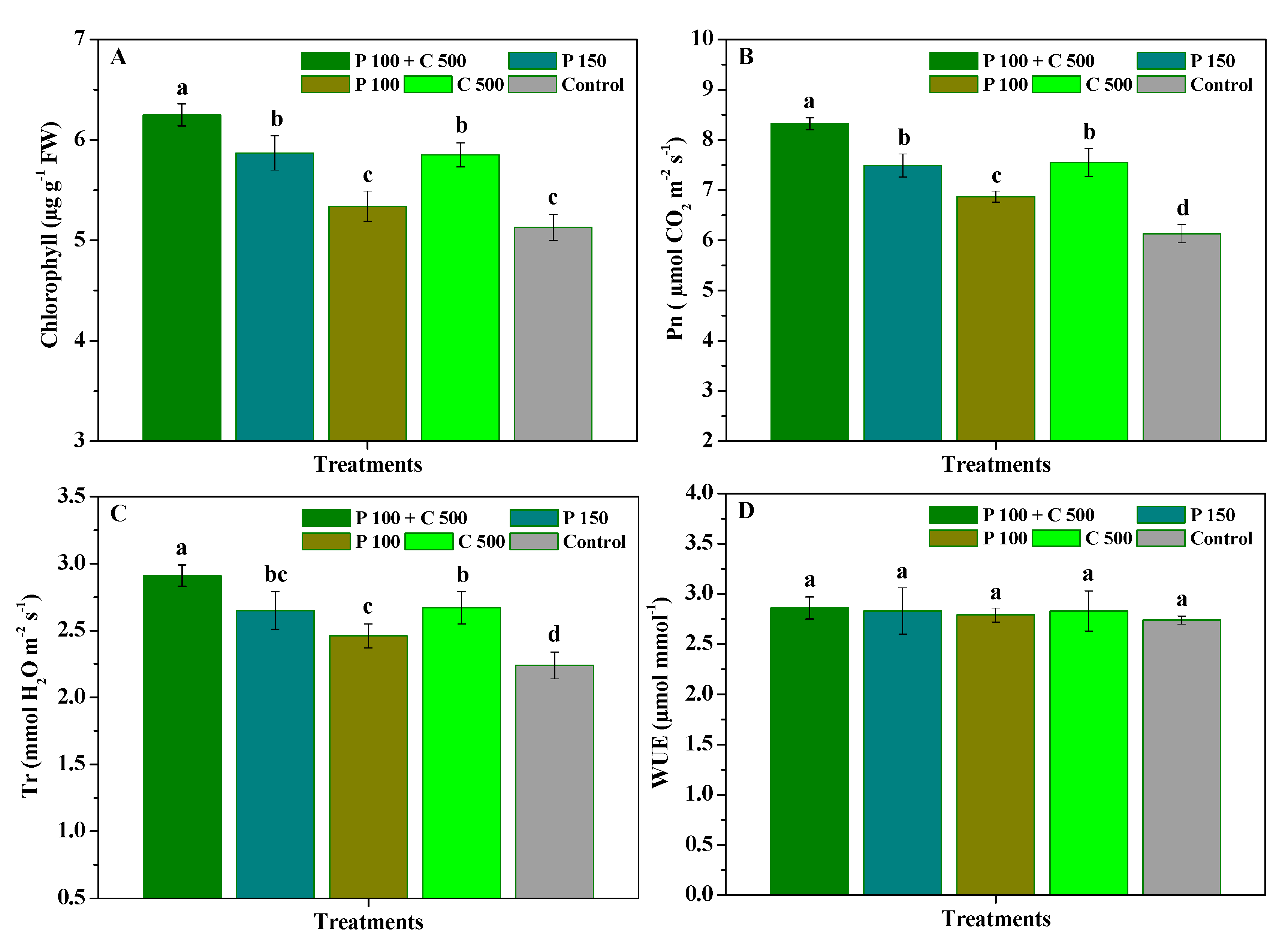
3.3. Influence of Pyraclostrobin and Chitosan on Photosynthetic Capacity of R. roxburghii Leaves
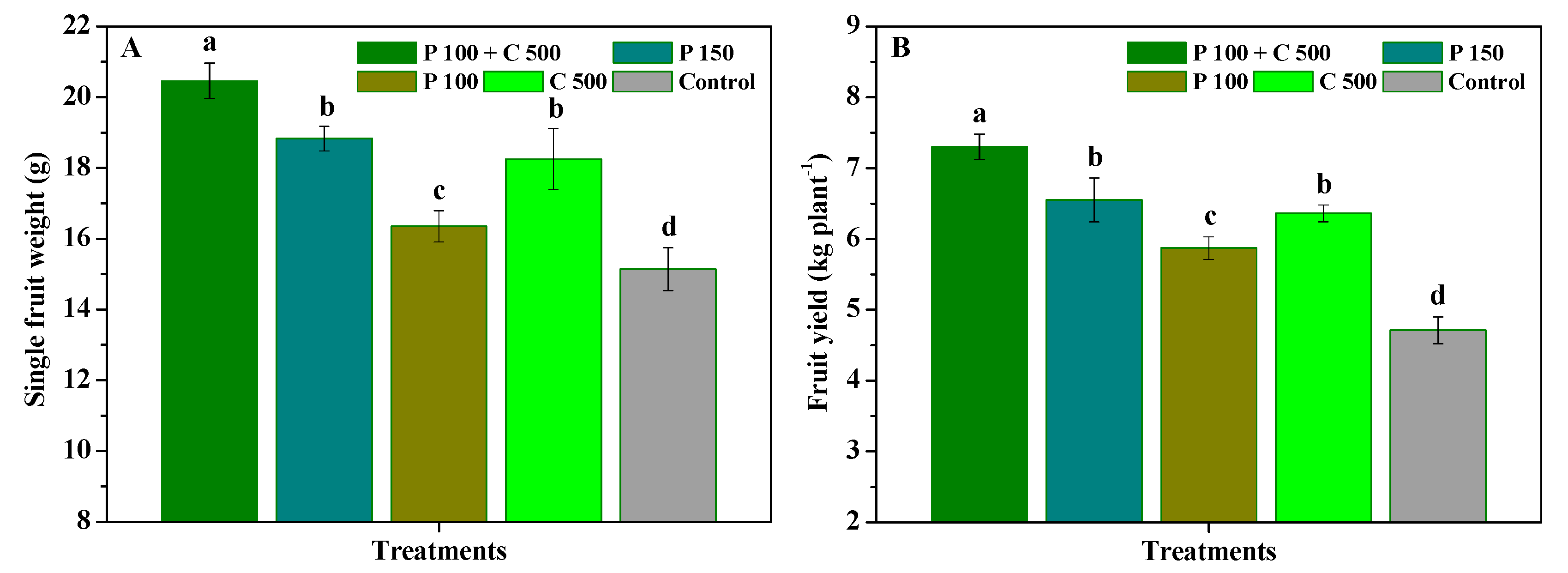
3.4. Influence of Pyraclostrobin and Chitosan on Yield and Quality of R. roxburghii
3.5. Influence of Pyraclostrobin and Chitosan on Amino Acids of R. roxburghii
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.-T.; Lv, M.-J.; An, J.-Y.; Fan, X.-H.; Dong, M.-Z.; Zhang, S.-D.; Wang, J.-D.; Wang, Y.-Q.; Cai, Z.-H.; Fu, Y.-J. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.L.; Zhou, R.L. The Healthcare Function and Development Trend of Toxburgh Rose. Food Res. Dev. 2016, 37, 212–214. [Google Scholar]
- Wang, D.; Lu, M.; Ludlow, R.A.; Zeng, J.; Ma, W.; An, H. Comparative ultrastructure of trichomes on various organs of Rosa roxburghii. Microsc. Res. Tech. 2021, 84, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Zhao, H.B.; Li, Y.F.; Yu, Z.H.; Liu, X.H.; Huang, M.Z. Identification and Oenological Properties Analysis of a Strain of Hanseniaspora uvarum from Rosa roxburghii. Food Ferment. Ind. 2020, 46, 97–104. [Google Scholar]
- Huang, X.; Yan, H.; Zhai, L.; Yang, Z.; Yi, Y. Characterization of the Rosa roxburghii Tratt transcriptome and analysis of MYB genes. PLoS ONE 2019, 14, e0203014. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.G.; Pan, X.J.; Chen, H.; Yang, H.R.; Gong, F.F.; Guan, J.Y.; Wang, M.L.; Mu, R. Effects of Oxalic Acid on the Nutrient of Calcareous Cultivated Soil and Leaf, Fruit Yield and Quality of Rosa roxburghii Tratt. J. Fruit Sci. 2021, 38, 1113–1122. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Luo, Y.; Wu, X.; An, H. Chitosan Can Induce Rosa roxburghii Tratt. against Sphaerotheca sp. and Enhance Its Resistance, Photosynthesis, Yield, and Quality. Horticulturae 2021, 7, 289. [Google Scholar] [CrossRef]
- Han, L.; Liu, X.D.; Huang, W.Y.; Wu, X.M. Occurrence and control technology of powdery mildew in Rose roxburgh Tratt. China Fruit 2021, 1, 6–10. [Google Scholar] [CrossRef]
- Yan, K.; Wang, J.L.; Zhou, Y.; Fu, D.P.; Huang, R.M. Efficacy of Five Fungicides in Rosa roxburghii Tratt against Sphaerotheca sp. Agrochemicals 2018, 57, 609–610. [Google Scholar]
- Yan, K.; Luo, Z.; Hu, F.; Wu, T.; Huang, R.M.; Yan, J. 6% Ascorbic Acid Aqueous Solutions Inducing Rosa roxburghii Tratt against Sphaerotheca sp. Agrochemicals 2017, 56, 528–530. [Google Scholar]
- Balba, H. Review of Strobilurin Fungicide Chemicals. J. Environ. Sci. Health Part B 2007, 42, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Musso, L.; Fabbrini, A.; Dallavalle, S. Natural Compound-derived Cytochrome bc1 Complex Inhibitors as Antifungal Agents. Molecules 2020, 25, 4582. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Liu, X.; Fan, Y.; Luan, S.; Huang, Q. Effect of Membrane Integrity on Survival Competition of Botrytis cinerea upon QoI Fungicide Pyraclostrobin. J. Phytopathol. 2020, 168, 601–608. [Google Scholar] [CrossRef]
- Esteve-Turrillas, F.A.; Mercader, J.V.; Agulló, C.; Abad-Somovilla, A.; Abad-Fuentes, A. Development of Immunoaffinity Columns for Pyraclostrobin Extraction from Fruit Juices and Analysis by Liquid Chromatography with UV Detection. J. Chromatogr. A 2011, 1218, 4902–4909. [Google Scholar] [CrossRef] [PubMed]
- Hunte, C.; Palsdottir, H.; Trumpower, B.L. Protonmotive Pathways and Mechanisms in the Cytochrome bc1Complex. FEBS Lett. 2003, 545, 39–46. [Google Scholar] [CrossRef]
- Bahia, K.H.; Pascal, L.; Dong-Woo, L.; Elisabeth, D.; Fevzi, D. Recent Advances in Cytochrome bc 1: Inter Monomer Electronic Communication? FEBS Lett. 2011, 586, 617–621. [Google Scholar]
- Pedersen, M.; Wegner, C.; Phansak, P.; Sarath, G.; Gaussoin, R.; Schlegel, V. Monitoring Wheat Mitochondrial Compositional and Respiratory Changes using Fourier transform Mid-infrared Spectroscopy in Response to Agrochemical Treatments. Spectrochim. Acta A 2017, 173, 727–732. [Google Scholar] [CrossRef]
- Wu, Q.; Lei, Q.; Li, Z.; Wang, X.; Luo, Y.; An, H.; Wu, X. Field Effects of Different Fungicides on Powdery Mildew of Rosa roxburghii Tratt. China Plant Prot. 2022, 42, 91–93. [Google Scholar]
- Li, D.; Liu, M.; Yang, Y.; Shi, H.; Zhou, J.; He, D. Strong lethality and teratogenicity of strobilurins on Xenopus tropicalis embryos: Basing on ten agricultural fungicides. Environ. Pollut. 2015, 208, 868–874. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Zhang, S.; Zhu, L.; Wang, J. Acute and Subchronic Toxicity of Pyraclostrobin in Zebrafish (Danio rerio). Chemosphere 2017, 188, 510–516. [Google Scholar] [CrossRef]
- Domingues, C.E.D.C.; Inoue, L.V.B.; Silva-Zacarin, E.C.M.; Malaspina, O. Fungicide Pyraclostrobin Affects Midgut Morphophysiology and Reduces Survival of Brazilian Native Stingless bee Melipona scutellaris. Ecotox. Environ. Safe 2020, 206, 111395. [Google Scholar] [CrossRef]
- Huang, X.; Yang, S.; Li, B.; Wang, A.; Mu, W. Comparative Toxicity of Multiple Exposure Routes of Pyraclostrobin in Adult Zebrafish (Danio rerio). Sci. Total Environ. 2021, 777, 145957. [Google Scholar] [CrossRef]
- Cameron, A.; Sarojini, V. Pseudomonas syringae pv. actinidiae: Chemical Control, Resistance Mechanisms and Possible Alternatives. Plant. Pathol. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Jones, E.E.; Casonato, S.; Monk, J.; Ridgway, H.J. Biological Control of Pseudomonas syringae pv. actinidiae (Psa), the Causal Agent of Bacterial Canker of Kiwifruit, Using Endophytic Bacteria Recovered from a Medicinal Plant. Biol. Control 2018, 116, 103–112. [Google Scholar] [CrossRef]
- Wang, Q.; Long, Y.; Ai, Q.; Su, Y.; Lei, Y. Oligosaccharins Used Together with Tebuconazole Enhances Resistance of Kiwifruit against Soft Rot Disease and Improves Its Yield and Quality. Horticulturae 2022, 8, 624. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent Developments in Antibacterial and Antifungal Chitosan and Its Derivatives. Carbohyd. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, M.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Mechanism of Plant Growth Promotion and Disease Suppression by Chitosan Biopolymer. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- Torres-Rodriguez, J.A.; Reyes-Pérez, J.J.; Castellanos, T.; Angulo, C.; Hernandez-Montiel, L.G. A Biopomyler with Antimicrobial Properties and Plant Resistance Inducer Against Phytopathogens: Chitosan. Not. Sci. Biol. 2021, 49, 12231. [Google Scholar] [CrossRef]
- Rahman, M.; Mukta, J.A.; Sabir, A.A.; Gupta, D.R.; Mohi-ud-din, M.; Hasanuzzaman, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Chitosan Biopolymer Promotes Yield and Stimulates Accumulation of Antioxidants in Strawberry Fruit. PLoS ONE 2018, 13, e0203769. [Google Scholar] [CrossRef]
- Coutinho, T.C.; Ferreira, M.C.; Rosa, L.H.; de Oliveira, A.M.; de Oliveira Júnior, E.N. Penicillium citrinum and Penicillium mallochii: New Phytopathogens of Orange Fruit and Their Control Using Chitosan. Carbohyd. Polym. 2020, 234, 115918. [Google Scholar] [CrossRef]
- El Amerany, F.; Meddich, A.; Wahbi, S.; Porzel, A.; Taourirte, M.; Rhazi, M.; Hause, B. Foliar Application of Chitosan Increases Tomato Growth and Influences Mycorrhization and Expression of Endo-Chitinase-Encoding Genes. Int. J. Mol. Sci. 2020, 21, 535. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, R.; Zhang, C.; Guo, Z.; Wu, X.; An, H. Co-Application of Allicin and Chitosan Increases Resistance of Rosa roxburghii against Powdery Mildew and Enhances Its Yield and Quality. Antibiotics 2021, 10, 1449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, H.; Lei, Y.; Su, Y.; Long, Y. Chitosan as an Adjuvant to Improve Isopyrazam Azoxystrobin against Leaf Spot Disease of Kiwifruit and Enhance Its Photosynthesis, Quality, and Amino Acids. Agriculture 2022, 12, 373. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Wu, X.; Su, Y.; Long, Y. Co-Application of Tetramycin and Chitosan in Controlling Leaf Spot Disease of Kiwifruit and Enhancing Its Resistance, Photosynthesis, Quality and Amino Acids. Biomolecules 2022, 12, 500. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Long, Y.; Wu, X.; Su, Y.; Lei, Y.; Ai, Q. Bioactivity and Control Efficacy of the Novel Antibiotic Tetramycin against Various Kiwifruit Diseases. Antibiotics 2021, 10, 289. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.-H.; Wang, Q.-P.; Li, J.-H.; Wu, X.-M.; Li, M. The effect of preharvest 28.6% chitosan composite film sprays for controlling the soft rot on kiwifruit. Hortic. Sci. 2019, 46, 180–194. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.; Li, J.; Li, M.; Xing, D.; An, H.; Wu, X.; Wu, Y. A Chitosan Composite Film Sprayed before Pathogen Infection Effectively Controls Postharvest Soft Rot in Kiwifruit. Agronomy 2020, 10, 265. [Google Scholar] [CrossRef]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef]
- Dzung, N.A.; Khanh, V.T.P.; Dzung, T.T. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar] [CrossRef]
- Zhu, S.T.; Wu, K. Nutritional evaluation of protein—ratio coefficient of amino acid. Acta Nutr. Sin. 1988, 10, 187–190. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]

| Indices | Content (g kg−1) | Indices | Content (mg kg−1) | Indices | Content (mg kg−1) |
|---|---|---|---|---|---|
| Organic matter | 13.56 | Available nitrogen | 56.84 | Available iron | 6.59 |
| Total nitrogen | 1.42 | Available phosphorus | 4.66 | Available boron | 0.15 |
| Total phosphorus | 1.68 | Available potassium | 27.51 | Exchangeable magnesium | 308.37 |
| Total potassium | 1.24 | Available zinc | 0.71 | Available manganese | 15.46 |
| Treatments | After 7 d of Spraying | After 14 d of Spraying | ||
|---|---|---|---|---|
| Disease Index | Control Effect (%) | Disease Index | Control Effect (%) | |
| P 100 + C 500 | 1.06 ± 0.20 eE | 89.30 ± 1.50 aA | 0.82 ± 0.22 eE | 94.58 ± 1.51 aA |
| P 150 | 1.79 ± 0.18 dD | 81.77 ± 2.85 bB | 1.70 ± 0.26 dD | 88.76 ± 1.77 bB |
| P 100 | 3.10 ± 0.11 cC | 68.47 ± 2.56 cC | 2.97 ± 0.19 cC | 80.39 ± 1.16 cC |
| C 500 | 4.05 ± 0.12 bB | 58.73 ± 4.02 dD | 3.96 ± 0.24 bB | 73.87 ± 1.26 dD |
| Control | 9.87 ± 0.71 aA | — | 15.16 ± 0.22 aA | — |
| Treatments | Vitamin C (mg g−1) | Soluble Solid (%) | Soluble Protein (%) | Soluble Sugar (%) | Total Acidity (%) | Total Flavonoids (mg g−1) | Total Triterpenes (mg g−1) | SOD Activity (U g−1 FW) |
|---|---|---|---|---|---|---|---|---|
| P 100 + C 500 | 23.59 ± 0.55 a | 12.44 ± 0.32 a | 15.89 ± 0.51 a | 4.16 ± 0.18 a | 1.52 ± 0.06 a | 6.32 ± 0.31 a | 20.71 ± 0.35 a | 711.54 ± 21.38 a |
| P 150 | 21.16 ± 0.69 b | 11.7 7± 0.74 ab | 14.65 ± 0.59 b | 3.83 ± 0.16 a | 1.41 ± 0.07 ab | 5.86 ± 0.23 b | 17.95 ± 0.65 bc | 668.15 ± 26.21 a |
| P 100 | 19.73 ± 0.6 c | 10.89 ± 0.59 bc | 14.12 ± 0.72 bc | 3.19 ± 0.17 b | 1.36 ± 0.07 b | 5.32 ± 0.12 c | 17.20 ± 0.68 c | 608.93 ± 28.44 b |
| C 500 | 21.05 ± 0.38 b | 11.85 ± 0.63 ab | 14.76 ± 0.63 b | 3.87 ± 0.19 a | 1.44 ± 0.09 ab | 5.93 ± 0.27 ab | 18.67 ± 0.55 b | 673.48 ± 17.68 a |
| Control | 18.04 ± 0.61 d | 10.53 ± 0.41 c | 13.41 ± 0.32 c | 3.08 ± 0.17 b | 1.23 ± 0.05 c | 5.16 ± 0.17 c | 15.16 ± 0.32 d | 556.89 ± 26.83 c |
| Treatments | EAA (mg kg−1) | NAA (mg kg−1) | TAA(mg kg−1) | The Percentage of EAA in TAA (%) | EAA/NAA |
|---|---|---|---|---|---|
| P 100 + C 500 | 88.47 ± 3.46 a | 339.22 ± 8.00 a | 461.76 ± 26.95 a | 19.17 ± 0.38 a | 0.26 ± 0.01 a |
| P 150 | 75.29 ± 6.55 bc | 318.47 ± 10.41 abc | 414.28 ± 41.72 ab | 18.19 ± 0.26 b | 0.24 ± 0.01 ab |
| P 100 | 68.86 ± 1.94 cd | 314.88 ± 14.11 bc | 401.33 ± 13.06 b | 17.16 ± 0.17 c | 0.22 ± 0.01 b |
| C 500 | 78.46 ± 2.12 b | 321.95 ± 9.02 ab | 424.85 ± 12.33 ab | 18.47 ± 0.05 b | 0.24 ± 0.00 ab |
| Control | 64.82 ± 5.52 d | 296.68 ± 16.25 c | 381.84 ± 32.65 b | 16.98 ± 0.07 c | 0.22 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, Q.; Li, J.; Su, Y.; Wu, X. Chitosan as an Adjuvant to Enhance the Control Efficacy of Low-Dosage Pyraclostrobin against Powdery Mildew of Rosa roxburghii and Improve Its Photosynthesis, Yield, and Quality. Biomolecules 2022, 12, 1304. https://doi.org/10.3390/biom12091304
Zhang C, Li Q, Li J, Su Y, Wu X. Chitosan as an Adjuvant to Enhance the Control Efficacy of Low-Dosage Pyraclostrobin against Powdery Mildew of Rosa roxburghii and Improve Its Photosynthesis, Yield, and Quality. Biomolecules. 2022; 12(9):1304. https://doi.org/10.3390/biom12091304
Chicago/Turabian StyleZhang, Cheng, Qinju Li, Jiaohong Li, Yue Su, and Xiaomao Wu. 2022. "Chitosan as an Adjuvant to Enhance the Control Efficacy of Low-Dosage Pyraclostrobin against Powdery Mildew of Rosa roxburghii and Improve Its Photosynthesis, Yield, and Quality" Biomolecules 12, no. 9: 1304. https://doi.org/10.3390/biom12091304
APA StyleZhang, C., Li, Q., Li, J., Su, Y., & Wu, X. (2022). Chitosan as an Adjuvant to Enhance the Control Efficacy of Low-Dosage Pyraclostrobin against Powdery Mildew of Rosa roxburghii and Improve Its Photosynthesis, Yield, and Quality. Biomolecules, 12(9), 1304. https://doi.org/10.3390/biom12091304






