Human Pulmonary Tuberculosis: Understanding the Immune Response in the Bronchoalveolar System
Abstract
1. Introduction
2. M. tuberculosis Complex and Its Influence on the Immune Response
3. Alveolar Epithelial Cells (AECs) during M. tuberculosis Infection
4. Cellular and Molecular Composition of the Bronchoalveolar Space of Patients with TB
5. Antioxidant Status of the Bronchoalveolar Space of Patients with TB
6. Alveolar Macrophages in the Bronchoalveolar Space of Patients with TB
7. Alveolar Dendritic Cells (DCs) in the Bronchoalveolar Space of Patients with TB
8. Alveolar Lymphocytes in the Bronchoalveolar Space of Patients with TB
9. Tissue-Resident T Cells and Lung Memory Immunity
10. The Pulmonary Immune Response and the Granuloma
11. Local Administration of Live Attenuated Vaccines against Pulmonary TB
12. Concluding Remarks
- The role of AECs in the local response to M. tuberculosis.
- The phenotypes of macrophages and DCs and the processing and presentation of mycobacterial antigens during different phases of the disease.
- The immune response associated with the characteristics of patients and their comorbidities.
- The relationship between lineages of the M. tuberculosis complex with the lung immune response during active TB.
- Assessing the local immune response at different times after vaccination and exploring the memory immune response induced by BCG sub-strains used for vaccination.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Carranza, C.; Herrera, M.T.; Guzmán-Beltrán, S.; Salgado-Cantú, M.G.; Salido-Guadarrama, I.; Santiago, E.; Chávez-Galán, L.; Gutiérrez-González, L.H.; González, Y. A Dual Marker for Monitoring MDR-TB Treatment: Host-Derived miRNAs and M. tuberculosis-Derived RNA Sequences in Serum. Front. Immunol. 2021, 12, 4615. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-González, L.H.; Juárez, E.; Carranza, C.; Carreto-Binaghi, L.E.; Alejandre, A.; Cabello-Gutiérrrez, C.; Gonzalez, Y. Immunological aspects of diagnosis and management of childhood tuberculosis. Infect. Drug Resist. 2021, 14, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Brites, D.; Gagneux, S. The nature and evolution of genomic diversity in the Mycobacterium tuberculosis complex. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1019. [Google Scholar]
- Gutierrez, C.; Somoskovi, A. Human Pathogenic Mycobacteria. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Coscolla, M.; Gagneux, S. Consequences of genomic diversity in Mycobacterium tuberculosis. Semin. Immunol. 2014, 26, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Malaga, W.; Constant, P.; Caws, M.; Thi Hoang Chau, T.; Salmons, J.; Thi Ngoc Lan, N.; Bang, N.D.; Daffé, M.; Young, D.B.; et al. Mycobacterium tuberculosis lineage influences innate immune response and virulence and is associated with distinct cell envelope lipid profiles. PLoS ONE 2011, 6, e23870. [Google Scholar] [CrossRef]
- Romagnoli, A.; Petruccioli, E.; Palucci, I.; Camassa, S.; Carata, E.; Petrone, L.; Mariano, S.; Sali, M.; Dini, L.; Girardi, E.; et al. Clinical isolates of the modern Mycobacterium tuberculosis lineage 4 evade host defense in human macrophages through eluding IL-1β-induced autophagy article. Cell Death Dis. 2018, 9, 624. [Google Scholar] [CrossRef]
- Hunter, L.; Hingley-Wilson, S.; Stewart, G.R.; Sharpe, S.A.; Salguero, F.J. Dynamics of Macrophage, T and B Cell Infiltration Within Pulmonary Granulomas Induced by Mycobacterium tuberculosis in Two Non-Human Primate Models of Aerosol Infection. Front. Immunol. 2022, 12, 776913. [Google Scholar] [CrossRef]
- Parker, D.; Prince, A. Innate immunity in the respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 2011, 45, 189–201. [Google Scholar] [CrossRef]
- Knowles, M.R.; Boucher, R.C. Innate defenses in the lung. J. Clin. Investig. 2002, 109, 571–577. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Marsland, B.J. Lung Homeostasis: Influence of Age, Microbes, and the Immune System. Immunity 2017, 46, 549–561. [Google Scholar] [CrossRef]
- Bals, R. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 2000, 1, 5. [Google Scholar] [CrossRef]
- Middleton, A.M.; Chadwick, M.V.; Nicholson, A.G.; Wilson, R.; Thornton, D.J.; Kirkham, S.; Sheehan, J.K. Interaction between mycobacteria and mucus on a human respiratory tissue organ culture model with an air interface. Exp. Lung Res. 2004, 30, 17–29. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef]
- Tecle, T.; Tripathi, S.; Hartshorn, K.L. Defensins and cathelicidins in lung immunity. Innate Immun. 2010, 16, 151–159. [Google Scholar] [CrossRef]
- Ryndak, M.B.; Laal, S. Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells. Front. Cell. Infect. Microbiol. 2019, 9, 299. [Google Scholar] [CrossRef]
- Heron, M.; Grutters, J.C.; Ten Dam-Molenkamp, K.M.; Hijdra, D.; Van Heugten-Roeling, A.; Claessen, A.M.E.; Ruven, H.J.T.; Van den Bosch, J.M.M.; Van Velzen-Blad, H. Bronchoalveolar lavage cell pattern from healthy human lung. Clin. Exp. Immunol. 2012, 167, 523–531. [Google Scholar] [CrossRef]
- Fehrenbach, H. Alveolar epithelial type II cell: Defender of the alveolus revisited. Respir. Res. 2001, 2, 33–46. [Google Scholar] [CrossRef]
- Chuquimia, O.D.; Petursdottir, D.H.; Periolo, N.; Ferńndez, C. Alveolar epithelial cells are critical in protection of the respiratory tract by secretion of factors able to modulate the activity of pulmonary macrophages and directly control bacterial growth. Infect. Immun. 2013, 81, 381–389. [Google Scholar] [CrossRef]
- Gold, J.A.; Hoshino, Y.; Tanaka, N.; Rom, W.N.; Raju, B.; Condos, R.; Weiden, M.D. Surfactant protein A modulates the inflammatory response in macrophages during tuberculosis. Infect. Immun. 2004, 72, 645–650. [Google Scholar] [CrossRef][Green Version]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; Du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef]
- Torres, M.; Carranza, C.; Sarkar, S.; Gonzalez, Y.; Osornio Vargas, A.; Black, K.; Meng, Q.; Quintana-Belmares, R.; Hernandez, M.; Angeles Garcia, J.J.F.; et al. Urban airborne particle exposure impairs human lung and blood Mycobacterium tuberculosis immunity. Thorax 2019, 74, 675–683. [Google Scholar] [CrossRef]
- Jarvela, J.; Moyer, M.; Leahy, P.; Bonfield, T.; Fletcher, D.; Mkono, W.N.; Aung, H.; Canaday, D.H.; Dazard, J.-E.; Silver, R.F. Mycobacterium tuberculosis-Induced Bronchoalveolar Lavage Gene Expression Signature in Latent Tuberculosis Infection Is Dominated by Pleiotropic Effects of CD4 + T Cell-Dependent IFN-γ Production despite the Presence of Polyfunctional T Cells within the Airways. J. Immunol. 2019, 203, 2194–2209. [Google Scholar] [CrossRef] [PubMed]
- Thiel, B.A.; Worodria, W.; Nalukwago, S.; Nsereko, M.; Sanyu, I.; Rejani, L.; Zawedde, J.; Canaday, D.H.; Stein, C.M.; Chervenak, K.A.; et al. Immune cells in bronchoalveolar lavage fluid of Ugandan adults who resist versus those who develop latent Mycobacterium tuberculosis infection. PLoS ONE 2021, 16, e0249477. [Google Scholar] [CrossRef] [PubMed]
- Aubert-Pivert, E.M.; Chedevergne, F.M.; Lopez-Ramirez, G.M.; Colle, J.H.; Scheinmann, P.L.; Gicquel, B.M.; De Blic, J.M. Cytokine transcripts in pediatric tuberculosis: A study with bronchoalveolar cells. Tuber. Lung Dis. 2000, 80, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, G.M.; Solomon, J.A.; Bateman, E.D. Lymphocyte and lymphocyte subset numbers in blood and in bronchoalveolar lavage and pleural fluid in various forms of human pulmonary tuberculosis at presentation and during recovery. Thorax 1992, 47, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Law, K.F.; Jagirdar, J.; Weiden, M.D.; Bodkin, M.; Rom, W.N. Tuberculosis in HIV-positive patients: Cellular response and immune activation in the lung. Am. J. Respir. Crit. Care Med. 1996, 153, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Schwander, S.K.; Sada, E.; Torres, M.; Escobedo, D.; Sierra, J.G.; Alt, S.; Rich, E.A. T lymphocytic and immature macrophage alveolitis in active pulmonary tuberculosis. J. Infect. Dis. 1996, 173, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, K.; Mukaida, N.; Fujimura, M.; Yasui, M.; Nakazumi, Y.; Matsuda, T.; Matsushima, K. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am. J. Respir. Crit. Care Med. 1997, 155, 1474–1477. [Google Scholar] [CrossRef]
- Law, K.; Weiden, M.; Harkin, T.; Tchou-Wong, K.; Chi, C.; Rom, W.N. Increased release of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 1996, 153, 799–804. [Google Scholar] [CrossRef]
- Thillai, M.; Eberhardt, C.; Lewin, A.M.; Potiphar, L.; Hingley-Wilson, S.; Sridhar, S.; Macintyre, J.; Kon, O.M.; Wickremasinghe, M.; Wells, A.; et al. Sarcoidosis and tuberculosis cytokine profiles: Indistinguishable in bronchoalveolar lavage but different in blood. PLoS ONE 2012, 7, e38083. [Google Scholar] [CrossRef]
- Zhu, F.; Ou, Q.; Zheng, J.; Zhou, M.; Chen, H.; Jiang, X. Role of bronchoalveolar lavage fluid and serum interleukin-27 in the diagnosis of smear-negative pulmonary tuberculosis. Medicine 2021, 100, e25821. [Google Scholar] [CrossRef]
- Scriba, T.J.; Kalsdorf, B.; Abrahams, D.-A.; Isaacs, F.; Hofmeister, J.; Black, G.; Hassan, H.Y.; Wilkinson, R.J.; Walzl, G.; Gelderbloem, S.J.; et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 2008, 180, 1962–1970. [Google Scholar] [CrossRef]
- Ashenafi, S.; Aderaye, G.; Bekele, A.; Zewdie, M.; Aseffa, G.; Hoang, A.T.N.; Carow, B.; Habtamu, M.; Wijkander, M.; Rottenberg, M.; et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin. Immunol. 2014, 151, 84–99. [Google Scholar] [CrossRef]
- Nolan, A.; Condos, R.; Huie, M.L.; Dawson, R.; Dheda, K.; Bateman, E.; Rom, W.N.; Weiden, M.D. Elevated IP-10 and IL-6 from bronchoalveolar lavage cells are biomarkers of non-cavitary tuberculosis. Int. J. Tuberc. Lung Dis. 2013, 17, 922–927. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Mortaz, E.; Tabarsi, P.; Jamaati, H.; Maghsoomi, Z.; Khosravi, A.; Garssen, J.; Masjedi, M.R.; Velayati, A.A.; Folkerts, G.; et al. Elevated CXCL-8 expression in bronchoalveolar lavage correlates with disease severity in patients with acute respiratory distress syndrome resulting from tuberculosis. J. Inflamm. 2014, 11, 21. [Google Scholar] [CrossRef]
- Elkington, P.; Shiomi, T.; Breen, R.; Nuttall, R.K.; Ugarte-Gil, C.A.; Walker, N.F.; Saraiva, L.; Pedersen, B.; Mauri, F.; Lipman, M.; et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J. Clin. Investig. 2011, 121, 1827–1833. [Google Scholar] [CrossRef]
- Singh, S.; Kubler, A.; Singh, U.K.; Singh, A.; Gardiner, H.; Prasad, R.; Elkington, P.T.; Friedland, J.S. Antimycobacterial drugs modulate immunopathogenic matrix metalloproteinases in a cellular model of pulmonary tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 4657–4665. [Google Scholar] [CrossRef]
- Lu, L.L.; Chung, A.W.; Rosebrock, T.R.; Ghebremichael, M.; Yu, W.H.; Grace, P.S.; Schoen, M.K.; Tafesse, F.; Martin, C.; Leung, V.; et al. A Functional Role for Antibodies in Tuberculosis. Cell 2016, 167, 433–443.e14. [Google Scholar] [CrossRef]
- Davids, M.; Pooran, A.; Hermann, C.; Mottay, L.; Thompson, F.; Cardenas, J.; Gu, J.; Koeuth, T.; Meldau, R.; Limberis, J.; et al. A human lung challenge model to evaluate the safety and immunogenicity of PPD and live bacillus calmette-guérin. Am. J. Respir. Crit. Care Med. 2020, 201, 1277–1291. [Google Scholar] [CrossRef]
- Demkow, U.; Białas-Chromiec, B.; Filewska, M.; Sobiecka, M.; Kuś, J.; Szturmowicz, M.; Zielonka, T.; Augustynowicz-Kopeć, E.; Zwolska, Z.; Wa̧sik, M.; et al. Humoral immune response against mycobacterial antigens in bronchoalveolar fluid from tuberculosis patients. J. Physiol. Pharmacol. 2005, 56, 79–84. [Google Scholar]
- Raja, A.; Baughman, R.P.; Daniel, T.M. The detection by immunoassay of antibody to mycobacterial antigens and mycobacterial antigens in bronchoalveolar lavage fluid from patients with tuberculosis and control subjects. Chest 1988, 94, 133–137. [Google Scholar] [CrossRef]
- Lamsal, M.; Gautam, N.; Bhatta, N.; Toora, B.D.; Bhattacharya, S.K.; Baral, N. Evaluation of lipid peroxidation product, nitrite and antioxidant levels in newly diagnosed and two months follow-up patients with pulmonary tuberculosis. Southeast Asian J. Trop. Med. Public Health 2007, 38, 695–703. [Google Scholar]
- Qi, C.; Wang, H.; Liu, Z.; Yang, H. Oxidative Stress and Trace Elements in Pulmonary Tuberculosis Patients During 6 Months Anti-Tuberculosis Treatment. Biol. Trace Elem. Res. 2021, 199, 1259–1267. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126. [Google Scholar] [CrossRef]
- Jack, C.I.A.; Jackson, M.J.; Hind, C.R.K. Circulating markers of free radical activity in patients with pulmonary tuberculosis. Tuber. Lung Dis. 1994, 75, 132–137. [Google Scholar] [CrossRef]
- Madebo, T.; Lindtjørn, B.; Aukrust, P.; Berge, R.K. Circulating antioxidants and lipid peroxidation products in untreated tuberculosis patients in Ethiopia. Am. J. Clin. Nutr. 2003, 78, 117–122. [Google Scholar] [CrossRef]
- Moses, A.O.; Emmanuel, O.O.; Ganiyu, A.O.; Fidelis, A.A.; Dickson, A.O. Assessment of antioxidants and nutritional status of pulmonary tuberculosis patients in Nigeria. Eur. J. Gen. Med. 2008, 5, 208–211. [Google Scholar] [CrossRef]
- Akpovi, D.C.; Gbaguidi, L.H.S.; Anago, E.; Affolabi, D.; Dougnon, T.V.; Faihun, F.; Anagonou, S. Tuberculosis treatment raises total cholesterol level and restores high density lipoprotein cholesterol (HDLC) in patients with pulmonary tuberculosis. African J. Biotechnol. 2016, 12, 6019–6024. [Google Scholar] [CrossRef][Green Version]
- Kubáň, P.; Foret, F. Exhaled breath condensate: Determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review. Anal. Chim. Acta 2013, 805, 1–18. [Google Scholar] [CrossRef]
- Konstantinidi, E.M.; Lappas, A.S.; Tzortzi, A.S.; Behrakis, P.K. Exhaled Breath Condensate: Technical and Diagnostic Aspects. Sci. World J. 2015, 2015, 435160. [Google Scholar] [CrossRef]
- Moloney, E.D.; Mumby, S.E.; Gajdocsi, R.; Cranshaw, J.H.; Kharitonov, S.A.; Quinlan, G.J.; Griffiths, M.J. Exhaled breath condensate detects markers of pulmonary inflammation after cardiothoracic surgery. Am. J. Respir. Crit. Care Med. 2004, 169, 64–69. [Google Scholar] [CrossRef]
- Guzmán-Beltrán, S.; Carreto-Binaghi, L.E.; Carranza, C.; Torres, M.; Gonzalez, Y.; Muñoz-Torrico, M.; Juárez, E. Oxidative stress and inflammatory mediators in exhaled breath condensate of patients with pulmonary tuberculosis. A pilot study with a biomarker perspective. Antioxidants 2021, 10, 1572. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Roberts, L.J. The isoprostanes: Unique bioactive products of lipid peroxidation. Prog. Lipid Res. 1997, 36, 1–21. [Google Scholar] [CrossRef]
- Ufimtseva, E.G.; Eremeeva, N.I.; Umpeleva, T.V.; Vakhrusheva, D.V.; Skornyakov, S.N. Mycobacterium tuberculosis load in host cells and the antibacterial activity of alveolar macrophages are linked and differentially regulated in various lung lesions of patients with pulmonary tuberculosis. Int. J. Mol. Sci. 2021, 22, 3452. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, S.; Szkudlarek, U.; Łuczyńska, M.; Nowak, D.; Zieba, M. Elevated exhalation of hydrogen peroxide and circulating IL-18 in patients with pulmonary tuberculosis. Respir. Med. 2007, 101, 574–580. [Google Scholar] [CrossRef]
- Kharitonov, S.A.; Barnes, P.J. Clinical aspects of exhaled nitric oxide. Eur. Respir. J. 2000, 16, 781–792. [Google Scholar] [CrossRef]
- Van Beek, S.C.; Nhung, N.V.; Sy, D.N.; Sterk, P.J.; Tiemersma, E.W.; Cobelens, F.G.J. Measurement of exhaled nitric oxide as a potential screening tool for pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2011, 15, 185–191. [Google Scholar]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Shastri, M.D.; Shukla, S.D.; Chong, W.C.; Dua, K.; Peterson, G.M.; Patel, R.P.; Hansbro, P.M.; Eri, R.; O’Toole, R.F. Role of Oxidative Stress in the Pathology and Management of Human Tuberculosis. Oxid. Med. Cell. Longev. 2018, 2018, 7695364. [Google Scholar] [CrossRef]
- Palanisamy, G.S.; Kirk, N.M.; Ackart, D.F.; Shanley, C.A.; Orme, I.M.; Basaraba, R.J. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis. PLoS ONE 2011, 6, e26254. [Google Scholar] [CrossRef]
- Venketaraman, V.; Millman, A.; Salman, M.; Swaminathan, S.; Goetz, M.; Lardizabal, A.; Hom, D.; Connell, N.D. Glutathione levels and immune responses in tuberculosis patients. Microb. Pathog. 2008, 44, 255–261. [Google Scholar] [CrossRef]
- Baniasadi, S.; Eftekhari, P.; Tabarsi, P.; Fahimi, F.; Raoufy, M.R.; Masjedi, M.R.; Velayati, A.A. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1235–1238. [Google Scholar] [CrossRef]
- Safe, I.P.; Amaral, E.P.; Araújo-Pereira, M.; Lacerda, M.V.G.; Printes, V.S.; Souza, A.B.; Beraldi-Magalhães, F.; Monteiro, W.M.; Sampaio, V.S.; Barreto-Duarte, B.; et al. Adjunct N-Acetylcysteine Treatment in Hospitalized Patients with HIV-Associated Tuberculosis Dampens the Oxidative Stress in Peripheral Blood: Results from the RIPENACTB Study Trial. Front. Immunol. 2021, 11, 3791. [Google Scholar] [CrossRef]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Russell, D.G. TB comes to a sticky beginning. Nat. Med. 2001, 7, 894–895. [Google Scholar] [CrossRef]
- Steigbigel, T.; Lambert, L.H.; Remington, J.S. Phagocytic and bacterial properties of normal human monocytes. J. Clin. Investig. 1974, 53, 131–142. [Google Scholar] [CrossRef][Green Version]
- Guirado, E.; Schlesinger, L.S. Modeling the Mycobacterium tuberculosis Granuloma—The Critical Battlefield in Host Immunity and Disease. Front. Immunol. 2013, 4, 98. [Google Scholar] [CrossRef]
- Rocha, N.; Neefjes, J. MHC class II molecules on the move for successful antigen presentation. EMBO J. 2008, 27, 1–5. [Google Scholar] [CrossRef]
- Cohen, S.B.; Gern, B.H.; Delahaye, J.L.; Adams, K.N.; Plumlee, C.R.; Winkler, J.K.; Sherman, D.R.; Gerner, M.Y.; Urdahl, K.B. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host Microbe 2018, 24, 439–446.e4. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Sangari, F.J.; Kolonoski, P.; Petrofsky, M.; Goodman, J. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect. Immun. 2002, 70, 140–146. [Google Scholar] [CrossRef]
- Keane, J.; Balcewicz-Sablinska, M.K.; Remold, H.G.; Chupp, G.L.; Meek, B.B.; Fenton, M.J.; Kornfeld, H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 1997, 65, 298–304. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, T.; Liu, Z.; Zhang, G.; Wang, J.; Feng, S.; Liang, J. Inhibition of autophagy by MiR-30A induced by Mycobacteria tuberculosis as a possible mechanism of immune escape in human macrophages. Jpn. J. Infect. Dis. 2015, 68, 420–424. [Google Scholar] [CrossRef]
- Schüller, S.; Neefjes, J.; Ottenhoff, T.; Thole, J.; Young, D. Coronin is involved in uptake of Mycobacterium bovis BCG in human macrophages but not in phagosome maintenance. Cell. Microbiol. 2001, 3, 785–793. [Google Scholar] [CrossRef]
- Armstrong, J.A.; Hart, D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 1971, 134, 713–740. [Google Scholar] [CrossRef]
- Mwandumba, H.C.; Russell, D.G.; Nyirenda, M.H.; Anderson, J.; White, S.A.; Molyneux, M.E.; Squire, S.B. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J. Immunol. 2004, 172, 4592–4598. [Google Scholar] [CrossRef]
- O’Leary, S.; O’Sullivan, M.P.; Keane, J. IL-10 blocks phagosome maturation in Mycobacterium tuberculosis-infected human macrophages. Am. J. Respir. Cell Mol. Biol. 2011, 45, 172–180. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, Q.; Guo, Y.; Chen, J.; Xiong, G.; Peng, Y.; Ye, J.; Li, J. Mycobacterium tuberculosis-induced polarization of human macrophage orchestrates the formation and development of tuberculous granulomas in vitro. PLoS ONE 2015, 10, e0129744. [Google Scholar] [CrossRef]
- Wolf, A.J.; Desvignes, L.; Linas, B.; Banaiee, N.; Tamura, T.; Takatsu, K.; Ernst, J.D. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 2008, 205, 105–115. [Google Scholar] [CrossRef]
- Axelrod, S.; Oschkinat, H.; Enders, J.; Schlegel, B.; Brinkmann, V.; Kaufmann, S.H.E.; Haas, A.; Schaible, U.E. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell. Microbiol. 2008, 10, 1530–1545. [Google Scholar] [CrossRef] [PubMed]
- Scordo, J.M.; Arcos, J.; Kelley, H.V.; Diangelo, L.; Sasindran, S.J.; Youngmin, E.; Wewers, M.D.; Wang, S.H.; Balada-Llasat, J.M.; Torrelles, J.B. Mycobacterium tuberculosis Cell Wall Fragments Released upon Bacterial Contact with the Human Lung Mucosa Alter the Neutrophil Response to Infection. Front. Immunol. 2017, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Arcos, J.; Sasindran, S.J.; Fujiwara, N.; Turner, J.; Schlesinger, L.S.; Torrelles, J.B. Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J. Immunol. 2011, 187, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Condon, T.V.; Sawyer, R.T.; Fenton, M.J.; Riches, D.W.H. Lung dendritic cells at the innate-adaptive immune interface. J. Leukoc. Biol. 2011, 90, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Hanekom, W.A.; Mendillo, M.; Manca, C.; Haslett, P.A.J.; Siddiqui, M.R.; Barry, C.; Kaplan, G. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J. Infect. Dis. 2003, 188, 257–266. [Google Scholar] [CrossRef]
- Sallusto, F.; Schaerli, P.; Loetscher, P.; Schaniel, C.; Lenig, D.; Mackay, C.R.; Qin, S.; Lanzavecchia, A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998, 28, 2760–2769. [Google Scholar] [CrossRef]
- Holt, P.G.; Strickland, D.H.; Wikström, M.E.; Jahnsen, F.L. Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 2008, 8, 142–152. [Google Scholar] [CrossRef]
- Donnenberg, V.S.; Donnenberg, A.D. Identification, rare-event detection and analysis of dendritic cell subsets in broncho-alveolar lavage fluid and peripheral blood by flow cytometry. Front. Biosci. 2003, 8, s1175–s1180. [Google Scholar] [CrossRef]
- Masten, B.J.; Olson, G.K.; Tarleton, C.A.; Rund, C.; Schuyler, M.; Mehran, R.; Archibeque, T.; Lipscomb, M.F. Characterization of myeloid and plasmacytoid dendritic cells in human lung. J. Immunol. 2006, 177, 7784–7793. [Google Scholar] [CrossRef]
- Yu, Y.R.A.; Hotten, D.F.; Malakhau, Y.; Volker, E.; Ghio, A.J.; Noble, P.W.; Kraft, M.; Hollingsworth, J.W.; Gunn, M.D.; Tighe, R.M. Flow cytometric analysis of myeloid cells in human blood, bronchoalveolar lavage, and lung tissues. Am. J. Respir. Cell Mol. Biol. 2016, 54, 13–24. [Google Scholar] [CrossRef]
- Khan, N.; Vidyarthi, A.; Pahari, S.; Agrewala, J.N. Distinct Strategies Employed by Dendritic Cells and Macrophages in Restricting Mycobacterium tuberculosis Infection: Different Philosophies but Same Desire. Int. Rev. Immunol. 2016, 35, 386–398. [Google Scholar] [CrossRef]
- Giacomini, E.; Iona, E.; Ferroni, L.; Miettinen, M.; Fattorini, L.; Orefici, G.; Julkunen, I.; Coccia, E.M. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 2001, 166, 7033–7041. [Google Scholar] [CrossRef]
- Tailleux, L.; Waddel, S.J.; Pelizzola, M.; Mortellaro, A.; Withers, M.; Tanne, A.; Castagnoli, P.R.; Gicquel, B.; Stoker, N.G.; Butcher, P.D.; et al. Probing host pathogen cross-talk by transcriptional profiling of both Mycobacterium tuberculosis and infected human dendritic cells and macrophages. PLoS ONE 2008, 3, e1403. [Google Scholar] [CrossRef]
- Baharom, F.; Thomas, S.; Rankin, G.; Lepzien, R.; Pourazar, J.; Behndig, A.F.; Ahlm, C.; Blomberg, A.; Smed-Sörensen, A. Dendritic Cells and Monocytes with Distinct Inflammatory Responses Reside in Lung Mucosa of Healthy Humans. J. Immunol. 2016, 196, 4498–4509. [Google Scholar] [CrossRef]
- Tallieux, L.; Schwartz, O.; Herrmann, J.L.; Pivert, E.; Jackson, M.; Amara, A.; Legres, L.; Dreher, D.; Nicod, L.P.; Gluckman, J.C.; et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 2003, 197, 121–127. [Google Scholar] [CrossRef]
- Tailleux, L.; Pham-Thi, N.; Bergeron-Lafaurie, A.; Herrmann, J.L.; Charles, P.; Schwartz, O.; Scheinmann, P.; Lagrange, P.H.; De Blic, J.; Tazi, A.; et al. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2005, 2, e381. [Google Scholar] [CrossRef]
- Mendelson, M.; Hanekom, W.A.; Ntutela, S.; Vogt, M.; Steyn, L.; Maartens, G.; Kaplan, G. Quantitative and functional differences between peripheral blood myeloid dendritic cells from patients with pleural and parenchymal lung tuberculosis. Clin. Vaccine Immunol. 2006, 13, 1299–1306. [Google Scholar] [CrossRef][Green Version]
- Uehira, K.; Amakawa, R.; Ito, T.; Tajima, K.; Naitoh, S.; Ozaki, Y.; Shimizu, T.; Yamaguchi, K.; Uemura, Y.; Kitajima, H.; et al. Dendritic cells are decreased in blood and accumulated in granuloma in tuberculosis. Clin. Immunol. 2002, 105, 296–303. [Google Scholar] [CrossRef]
- Winau, F.; Weber, S.; Sad, S.; De Diego, J.; Hoops, S.L.; Breiden, B.; Sandhoff, K.; Brinkmann, V.; Kaufmann, S.H.E.; Schaible, U.E. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 2006, 24, 105–117. [Google Scholar] [CrossRef]
- Behar, S.M.; Briken, V. Apoptosis inhibition by intracellular bacteria and its consequence on host immunity. Curr. Opin. Immunol. 2019, 60, 103–110. [Google Scholar] [CrossRef]
- Kuczkowska, K.; Copland, A.; Øverland, L.; Mathiesen, G.; Tran, A.C.; Paul, M.J.; Eijsink, V.G.H.; Reljic, R. Inactivated Lactobacillus plantarum Carrying a Surface-Displayed Ag85B-ESAT-6 Fusion Antigen as a Booster Vaccine Against Mycobacterium tuberculosis Infection. Front. Immunol. 2019, 10, 105–117. [Google Scholar] [CrossRef]
- McCormick, S.; Shaler, C.R.; Xing, Z. Pulmonary mucosal dendritic cells in T-cell activation: Implications for TB therapy. Expert Rev. Respir. Med. 2011, 5, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Granucci, F.; Zanoni, I.; Feau, S.; Ricciardi-Castagnoli, P. Dendritic cell regulation of immune responses: A new role for interleukin 2 at the intersection of innate and adaptive immunity. EMBO J. 2003, 22, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Bustamante, J. Mendelian susceptibility to mycobacterial disease: Recent discoveries. Hum. Genet. 2020, 139, 993–1000. [Google Scholar] [CrossRef]
- Rosain, J.; Kong, X.-F.; Martinez-Barricarte, R.; Oleaga-Quintas, C.; Ramirez-Alejo, N.; Markle, J.; Okada, S.; Boisson-Dupuis, S.; Casanova, J.-L.; Bustamante, J. Mendelian susceptibility to mycobacterial disease: 2014–2018 update. Immunol. Cell Biol. 2019, 97, 360–367. [Google Scholar] [CrossRef]
- Herrera, M.T.; Torres, M.; Nevels, D.; Perez-Redondo, C.N.; Ellner, J.J.; Sada, E.; Schwander, S.K. Compartmentalized bronchoalveolar IFN-γ and IL-12 response in human pulmonary tuberculosis. Tuberculosis 2009, 89, 38–47. [Google Scholar] [CrossRef]
- Schwander, S.K.; Torres, M.; Sada, E.; Carranza, C.; Ramos, E.; Tary-Lehmann, M.; Wallis, R.S.; Sierra, J.; Rich, E.A. Enhanced responses to Mycobacterium tuberculosis antigens by human alveolar lymphocytes during active pulmonary tuberculosis. J. Infect. Dis. 1998, 178, 1434–1445. [Google Scholar] [CrossRef]
- Lo, C.Y.; Huang, Y.C.; Huang, H.Y.; Chung, F.T.; Lin, C.W.; Chung, K.F.; Wang, C.H. Increased Th1 cells with disease resolution of active pulmonary tuberculosis in non-atopic patients. Biomedicines 2021, 9, 724. [Google Scholar] [CrossRef]
- Tambunan, B.A.; Priyanto, H.; Nugraha, J. Soedarsono CD4+ AND CD8+ T-Cells expressing interferon gamma in active pulmonary tuberculosis patients. African J. Infect. Dis. 2018, 12, 49–53. [Google Scholar] [CrossRef]
- Nikitina, I.Y.; Panteleev, A.V.; Kosmiadi, G.A.; Serdyuk, Y.V.; Nenasheva, T.A.; Nikolaev, A.A.; Gorelova, L.A.; Radaeva, T.V.; Kiseleva, Y.Y.; Bozhenko, V.K.; et al. Th1, Th17, and Th1Th17 Lymphocytes during Tuberculosis: Th1 Lymphocytes Predominate and Appear as Low-Differentiated CXCR3 + CCR6 + Cells in the Blood and Highly Differentiated CXCR3 +/− CCR6 − Cells in the Lungs. J. Immunol. 2018, 200, 2090–2103. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Maniakis-Grivas, G.; Singh, U.K.; Asher, R.M.; Mauri, F.; Elkington, P.T.; Friedland, J.S. Interleukin-17 regulates matrix metalloproteinase activity in human pulmonary tuberculosis. J. Pathol. 2018, 244, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Semple, P.L.; Binder, A.B.; Davids, M.; Maredza, A.; Van Zyl-Smit, R.N.; Dheda, K. Regulatory T cells attenuate mycobacterial stasis in alveolar and blood-derived macrophages from patients with tuberculosis. Am. J. Respir. Crit. Care Med. 2013, 187, 1249–1258. [Google Scholar] [CrossRef]
- Herzmann, C.; Ernst, M.; Ehlers, S.; Stenger, S.; Maertzdorf, J.; Sotgiu, G.; Lange, C. Increased frequencies of pulmonary regulatory T-cells in latent Mycobacterium tuberculosis infection. Eur. Respir. J. 2012, 40, 1450–1457. [Google Scholar] [CrossRef]
- Mazzarella, G.; Bianco, A.; Perna, F.; D’Auria, D.; Grella, E.; Moscariello, E.; Sanduzzi, A. T lymphocyte phenotypic profile in lung segments affected by cavitary and non-cavitary tuberculosis. Clin. Exp. Immunol. 2003, 132, 283–288. [Google Scholar] [CrossRef]
- Snyder, M.E.; Farber, D.L. Human lung tissue resident memory T cells in health and disease. Curr. Opin. Immunol. 2019, 59, 101–108. [Google Scholar] [CrossRef]
- Ogongo, P.; Zachary Porterfield, J.; Leslie, A. Lung Tissue Resident Memory T-Cells in the Immune Response to Mycobacterium tuberculosis. Front. Immunol. 2019, 10, 992. [Google Scholar] [CrossRef]
- Snyder, M.E.; Finlayson, M.O.; Connors, T.J.; Dogra, P.; Senda, T.; Bush, E.; Carpenter, D.; Marboe, C.; Benvenuto, L.; Shah, L.; et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol. 2019, 4, aav5581. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Masopust, D. Tissue-resident memory T cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef]
- Morrison, H.; McShane, H. Local Pulmonary Immunological Biomarkers in Tuberculosis. Front. Immunol. 2021, 12, 640916. [Google Scholar] [CrossRef]
- Gonzalez, Y.; Herrera, M.T.; Juárez, E.; Salazar-Lezama, M.A.; Bobadilla, K.; Torres, M. CD161 Expression Defines a Th1/Th17 Polyfunctional Subset of Resident Memory T Lymphocytes in Bronchoalveolar Cells. PLoS ONE 2015, 10, e0123591. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, G.; Chen, X. Th1 cytokines, true functional signatures for protective immunity against TB? Cell. Mol. Immunol. 2018, 15, 206–215. [Google Scholar] [CrossRef]
- Yu, S.; Lao, S.; Yang, B.; Wu, C. Tissue-Resident Memory-Like CD8 + T Cells Exhibit Heterogeneous Characteristics in Tuberculous Pleural Effusion. J. Immunol. Res. 2021, 2021, 6643808. [Google Scholar] [CrossRef]
- Bull, N.C.; Kaveh, D.A.; Garcia-Pelayo, M.C.; Stylianou, E.; McShane, H.; Hogarth, P.J. Induction and maintenance of a phenotypically heterogeneous lung tissue-resident CD4 + T cell population following BCG immunisation. Vaccine 2018, 36, 5625–5635. [Google Scholar] [CrossRef]
- Lange, J.; Rivera-Ballesteros, O.; Buggert, M. Human mucosal tissue-resident memory T cells in health and disease. Mucosal Immunol. 2021, 15, 389–397. [Google Scholar] [CrossRef]
- Perdomo, C.; Zedler, U.; Kühl, A.A.; Lozza, L.; Saikali, P.; Sander, L.E.; Vogelzang, A.; Kaufmann, S.H.E.; Kupz, A. Mucosal BCG Vaccination Induces Protective Lung-Resident Memory T Cell Populations against Tuberculosis. mBio 2016, 7, e01686-16. [Google Scholar] [CrossRef]
- Wu, Q.; Kang, S.; Huang, J.; Wan, S.; Yang, B.; Wu, C. Antigen-Specific Tissue-Resident Memory T Cells in the Respiratory System Were Generated following Intranasal Vaccination of Mice with BCG. J. Immunol. Res. 2021, 2021, 6660379. [Google Scholar] [CrossRef]
- Beverley, P.C.L.; Sridhar, S.; Lalvani, A.; Tchilian, E.Z. Harnessing local and systemic immunity for vaccines against tuberculosis. Mucosal Immunol. 2014, 7, 20–26. [Google Scholar] [CrossRef]
- Beura, L.K.; Masopust, D. SnapShot: Resident memory T cells. Cell 2014, 157, 1488.e1. [Google Scholar] [CrossRef]
- Silva Miranda, M.; Breiman, A.; Allain, S.; Deknuydt, F.; Altare, F. The tuberculous granuloma: An unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin. Dev. Immunol. 2012, 2012, 139127. [Google Scholar] [CrossRef]
- Sholeye, A.R.; Williams, A.A.; Loots, D.T.; Tutu van Furth, A.M.; van der Kuip, M.; Mason, S. Tuberculous Granuloma: Emerging Insights From Proteomics and Metabolomics. Front. Neurol. 2022, 13, 804838. [Google Scholar] [CrossRef] [PubMed]
- Pagán, A.J.; Ramakrishnan, L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb. Perspect. Med. 2015, 5, a018499. [Google Scholar] [CrossRef] [PubMed]
- Lagranderie, M.; Ravisse, P.; Marchal, G.; Gheorghiu, M.; Balasubramanian, V.; Weigeshaus, E.H.; Smith, D.W. BCG-induced protection in guinea pigs vaccinated and challenged via the respiratory route. Tuber. Lung Dis. 1993, 74, 38–46. [Google Scholar] [CrossRef]
- Aguilo, N.; Alvarez-Arguedas, S.; Uranga, S.; Marinova, D.; Monzón, M.; Badiola, J.; Martin, C. Pulmonary but Not Subcutaneous Delivery of BCG Vaccine Confers Protection to Tuberculosis-Susceptible Mice by an Interleukin 17–Dependent Mechanism. J. Infect. Dis. 2016, 213, 831–839. [Google Scholar] [CrossRef]
- Kaushal, D.; Foreman, T.W.; Gautam, U.S.; Alvarez, X.; Adekambi, T.; Rangel-Moreno, J.; Golden, N.A.; Johnson, A.M.F.; Phillips, B.L.; Ahsan, M.H.; et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat. Commun. 2015, 6, 104305. [Google Scholar] [CrossRef]
- Verreck, F.A.W.; Tchilian, E.Z.; Vervenne, R.A.W.; Sombroek, C.C.; Kondova, I.; Eissen, O.A.; Sommandas, V.; van der Werff, N.M.; Verschoor, E.; Braskamp, G.; et al. Variable BCG efficacy in rhesus populations: Pulmonary BCG provides protection where standard intra-dermal vaccination fails. Tuberculosis 2017, 104, 46–57. [Google Scholar] [CrossRef]
- Dijkman, K.; Sombroek, C.C.; Vervenne, R.A.W.; Hofman, S.O.; Boot, C.; Remarque, E.J.; Kocken, C.H.M.; Ottenhoff, T.H.M.; Kondova, I.; Khayum, M.A.; et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 2019, 25, 255–262. [Google Scholar] [CrossRef]
- Manjaly Thomas, Z.R.; Satti, I.; Marshall, J.L.; Harris, S.A.; Ramon, R.L.; Hamidi, A.; Minhinnick, A.; Riste, M.; Stockdale, L.; Lawrie, A.M.; et al. Alternate aerosol and systemic immunisation with a recombinant viral vector for tuberculosis, MVA85A: A phase I randomised controlled trial. PLoS Med. 2019, 16, 1002790. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Fritz, D.K.; Afkhami, S.; Aguirre, E.; Howie, K.J.; Zganiacz, A.; Dvorkin-Gheva, A.; Thompson, M.R.; Silver, R.F.; Cusack, R.P.; et al. Aerosol delivery, but not intramuscular injection, of adenovirus-vectored tuberculosis vaccine induces respiratory-mucosal immunity in humans. JCI Insight 2022, 7, e155655. [Google Scholar] [CrossRef]
- Moliva, J.I.; Hossfeld, A.P.; Sidiki, S.; Canan, C.H.; Dwivedi, V.; Beamer, G.; Turner, J.; Torrelles, J.B. Selective delipidation of Mycobacterium bovis BCG enables direct pulmonary vaccination and enhances protection against Mycobacterium tuberculosis. Mucosal Immunol. 2019, 12, 805–815. [Google Scholar] [CrossRef]
- Redmann, R.K.; Kaushal, D.; Golden, N.; Threeton, B.; Killeen, S.Z.; Kuehl, P.J.; Roy, C.J. Particle Dynamics and Bioaerosol Viability of Aerosolized Bacillus Calmette-Guérin Vaccine Using Jet and Vibrating Mesh Clinical Nebulizers. J. Aerosol Med. Pulm. Drug Deliv. 2022, 35, 50–56. [Google Scholar] [CrossRef]
- Gomez, M.; McCollum, J.; Wang, H.; Bachchhav, S.; Tetreau, I.; Gerhardt, A.; Press, C.; Kramer, R.M.; Fox, C.B.; Vehring, R. Evaluation of the stability of a spray-dried tuberculosis vaccine candidate designed for dry powder respiratory delivery. Vaccine 2021, 39, 5025–5036. [Google Scholar] [CrossRef]
- White, A.D.; Sarfas, C.; Sibley, L.S.; Gullick, J.; Clark, S.; Rayner, E.; Gleeson, F.; Català, M.; Nogueira, I.; Cardona, P.J.; et al. Protective Efficacy of Inhaled BCG Vaccination Against Ultra-Low Dose Aerosol M. tuberculosis Challenge in Rhesus Macaques. Pharmaceutics 2020, 12, 394. [Google Scholar] [CrossRef]
- Flores-Valdez, M.A.; Segura-Cerda, C.A. Preclinical evaluation of tuberculosis vaccine candidates: Is it time to harmonize study design and readouts for prioritizing their development? Vaccine 2021, 39, 173–175. [Google Scholar] [CrossRef]
- Angelidou, A.; Conti, M.G.; Diray-Arce, J.; Benn, C.S.; Shann, F.; Netea, M.G.; Liu, M.; Potluri, L.P.; Sanchez-Schmitz, G.; Husson, R.; et al. Licensed Bacille Calmette-Guérin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine 2020, 38, 2229–2240. [Google Scholar] [CrossRef]

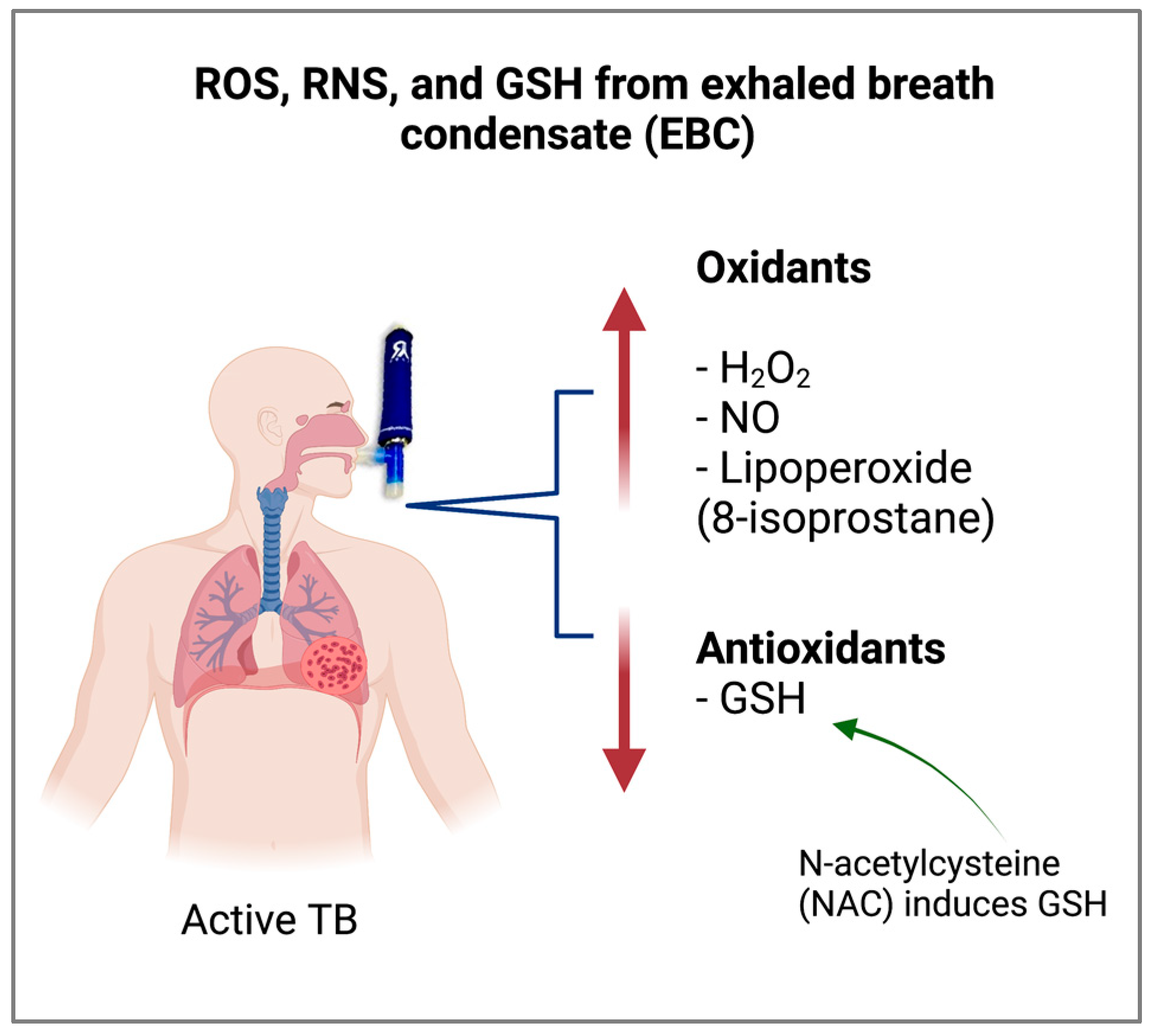

| Cell Type | Percentage | Condition | Reference |
|---|---|---|---|
| Alveolar macrophages | >85% | Healthy * | [21,22] |
| Lymphocytes | 10–15% | Healthy * | [21,22] |
| CD4:CD8 ratio | 0.9–2.5 | Healthy * | [21,22] |
| Neutrophils | <3% | Healthy * | [21,22] |
| Eosinophils | <1% | Healthy * | [21,22] |
| Epithelial cells ** | <5% | Healthy * | [21,22] |
| Alveolar macrophages | 80.5–91.14% | Latent TB | [23,24] |
| Lymphocytes | 2.99–13.5% | Latent TB | [23,24] |
| CD4:CD8 ratio | 3.14 | Latent TB | [23,24] |
| Neutrophils | 2–5.71% | Latent TB | [23,24] |
| Eosinophils | 0.15–1% | Latent TB | [23,24] |
| Alveolar macrophages | 70.60% | Children with TB *** | [25] |
| Lymphocytes | 20.20% | Children with TB *** | [25] |
| Neutrophils | 9.20% | Children with TB *** | [25] |
| Alveolar macrophages | 52.4–66.7% | Adult with TB | [26,27,28] |
| Lymphocytes | 12.2–33% | Adult with TB | [26,27,28] |
| Neutrophils | 5.30% | Adult with TB | [26,27,28] |
| Eosinophils | 2.90% | Adult with TB | [26,27,28] |
| DCs | Phenotype | Location |
|---|---|---|
| Myeloid conventional DC1 | CD11c+ CD14− MHC-II+ CD1c+ | Alveoli/interstitium |
| Myeloid conventional DC2 | CD11c+ CD14− MHC-II+ CD141+ | Alveoli/interstitium |
| Plasmacytoid DC | CD11c− CD14− MHC-II+ CD123+ | Interstitium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, M.T.; Guzmán-Beltrán, S.; Bobadilla, K.; Santos-Mendoza, T.; Flores-Valdez, M.A.; Gutiérrez-González, L.H.; González, Y. Human Pulmonary Tuberculosis: Understanding the Immune Response in the Bronchoalveolar System. Biomolecules 2022, 12, 1148. https://doi.org/10.3390/biom12081148
Herrera MT, Guzmán-Beltrán S, Bobadilla K, Santos-Mendoza T, Flores-Valdez MA, Gutiérrez-González LH, González Y. Human Pulmonary Tuberculosis: Understanding the Immune Response in the Bronchoalveolar System. Biomolecules. 2022; 12(8):1148. https://doi.org/10.3390/biom12081148
Chicago/Turabian StyleHerrera, María Teresa, Silvia Guzmán-Beltrán, Karen Bobadilla, Teresa Santos-Mendoza, Mario Alberto Flores-Valdez, Luis Horacio Gutiérrez-González, and Yolanda González. 2022. "Human Pulmonary Tuberculosis: Understanding the Immune Response in the Bronchoalveolar System" Biomolecules 12, no. 8: 1148. https://doi.org/10.3390/biom12081148
APA StyleHerrera, M. T., Guzmán-Beltrán, S., Bobadilla, K., Santos-Mendoza, T., Flores-Valdez, M. A., Gutiérrez-González, L. H., & González, Y. (2022). Human Pulmonary Tuberculosis: Understanding the Immune Response in the Bronchoalveolar System. Biomolecules, 12(8), 1148. https://doi.org/10.3390/biom12081148






