Limb Remote Ischemic Conditioning Promotes Neurogenesis after Cerebral Ischemia by Modulating miR-449b/Notch1 Pathway in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Model Building and Hypoxia/Ischemic Conditioning
2.2. Two-Dimensional Laser Speckle Imaging
2.3. Neurological Function Assay
2.4. Generation of the Notch1 shRNA Lentivirus and Lateral Ventricle Implantation
2.5. Cell Culture, miR-449b shRNA or/and Notch1 shRNA Infection
2.6. Immunofluorescence Analysis
2.7. Western Blotting
2.8. Real Time RT-PCR
2.9. Luciferase Assay
2.10. Statistical Analysis
3. Results
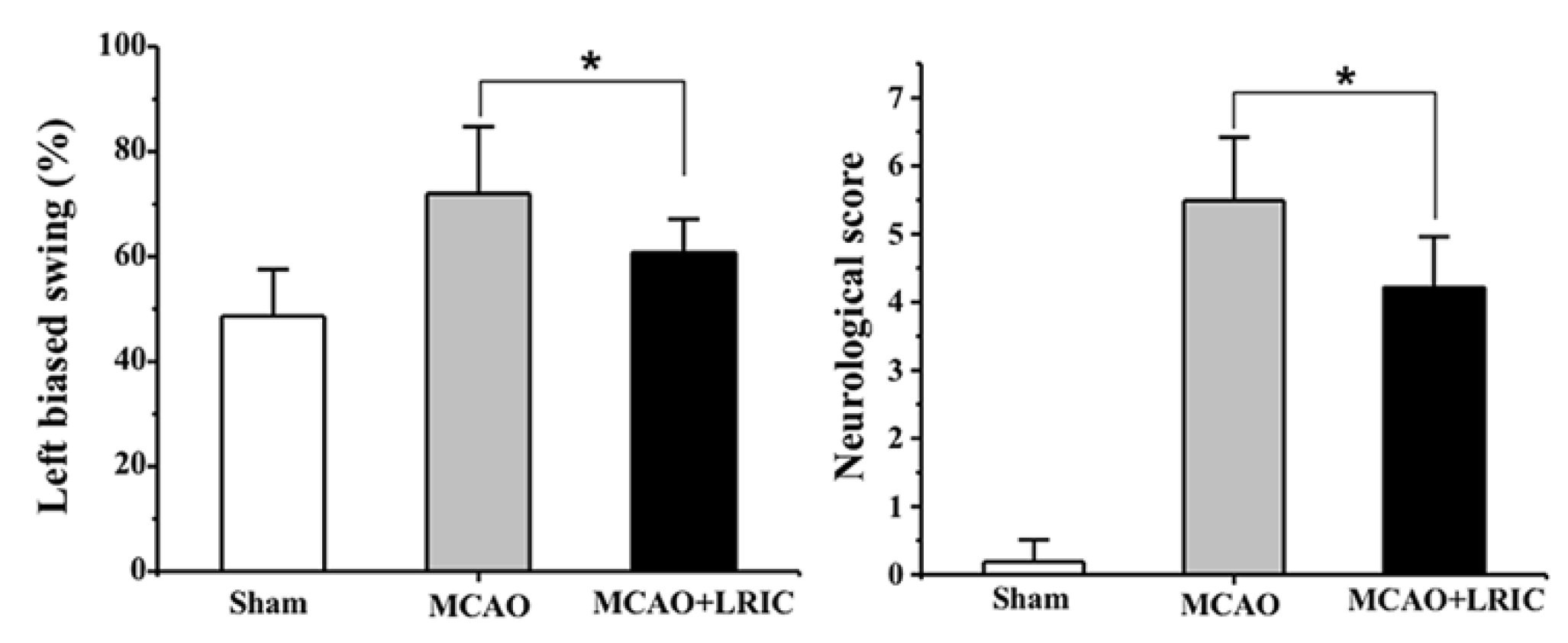
3.1. LRIC Improved Neurological Function at 28 Day after MCAO
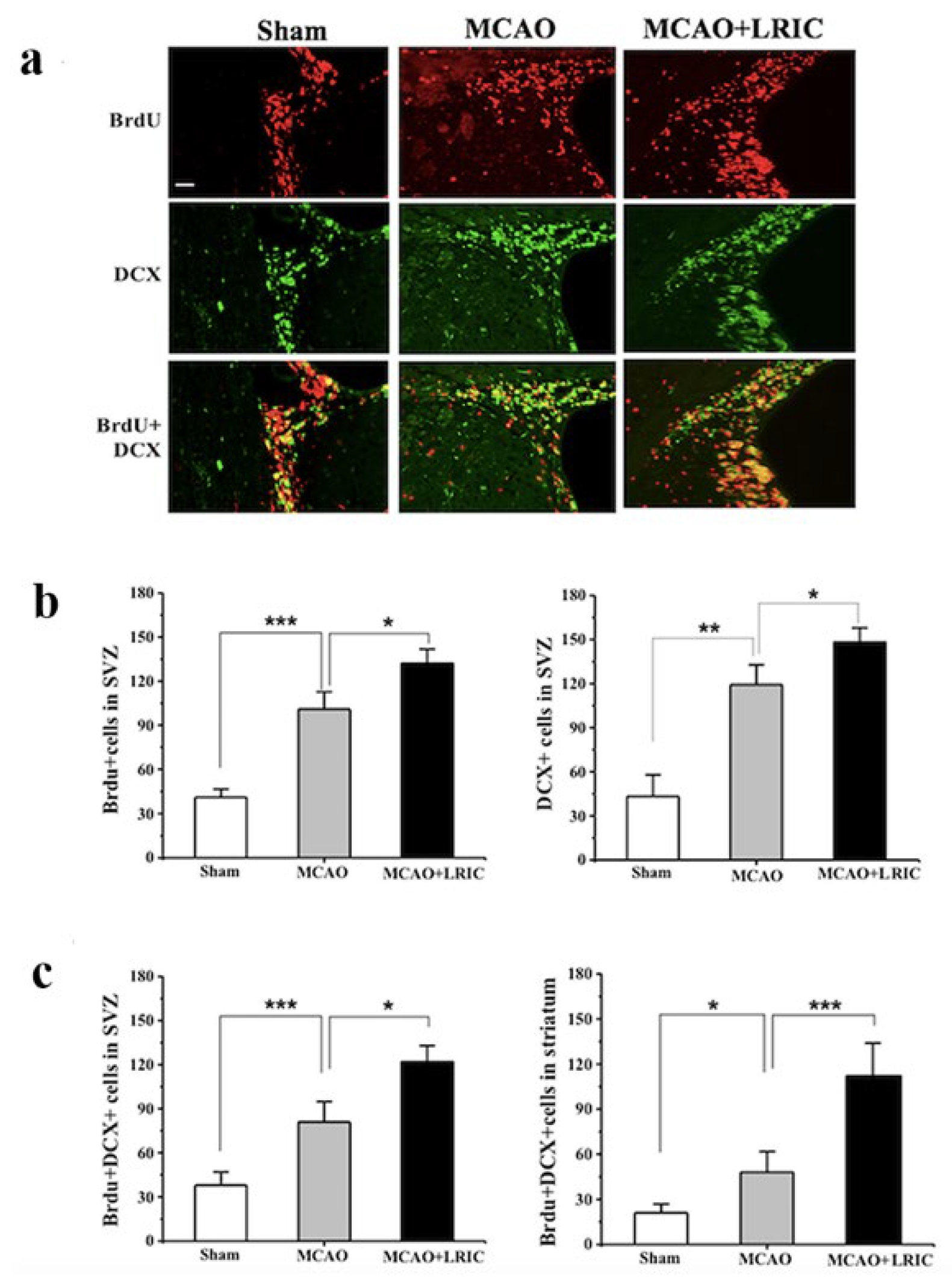
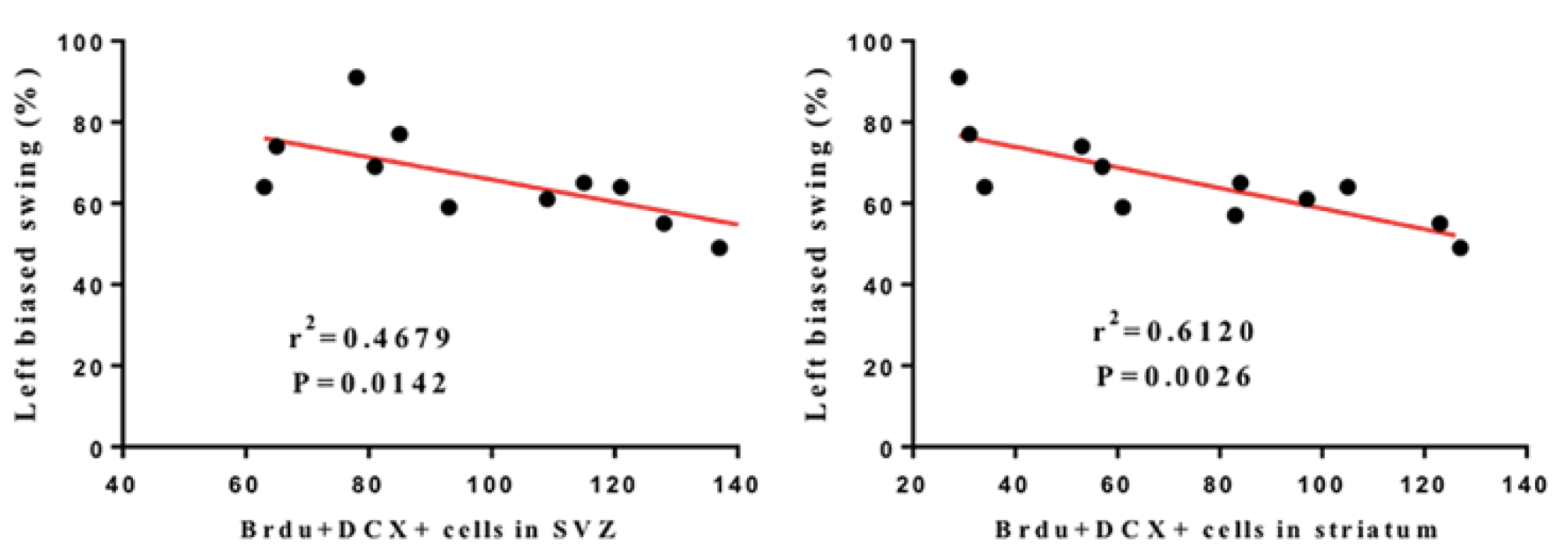
3.2. LRIC Promote the Proliferation and Migration of SVZ Neural Stem Cells
3.3. LRIC Promoted Activated Notch1 (NICD) Protein Expression in SVZ
3.4. Noth1 shRNA Abolished the Beneficial Effects of LRIC on Proliferation of Neuronal Stem Cells in MCAO Mice
3.5. LRIC Down-Regulated the Expression of miR-449b in SVZ
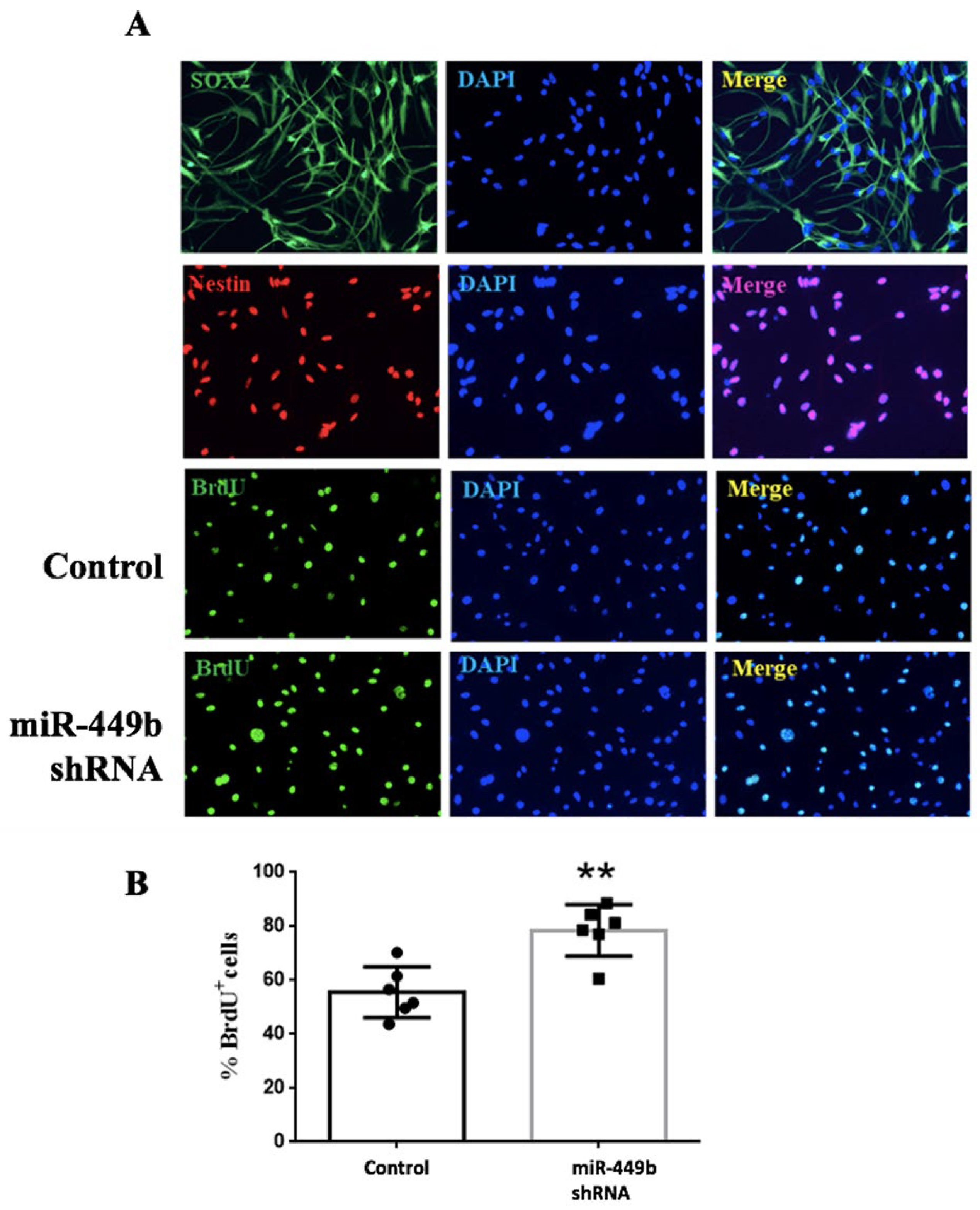
3.6. miR-449b Was Involved in the Proliferation of Neural Stem Cells
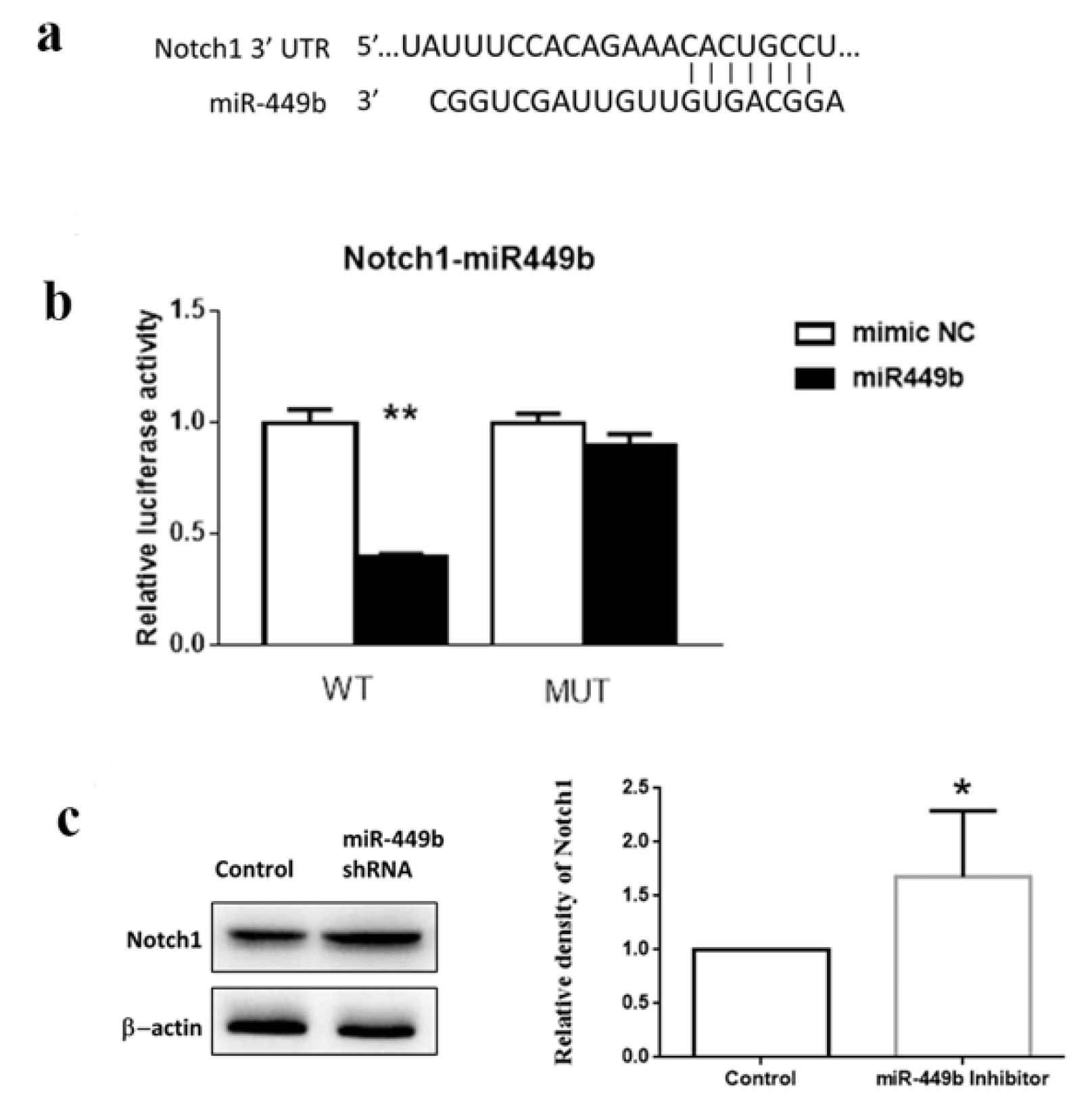
3.7. miR-449b Targeted Notch1 and Modulated the Notch1 Level in Cells
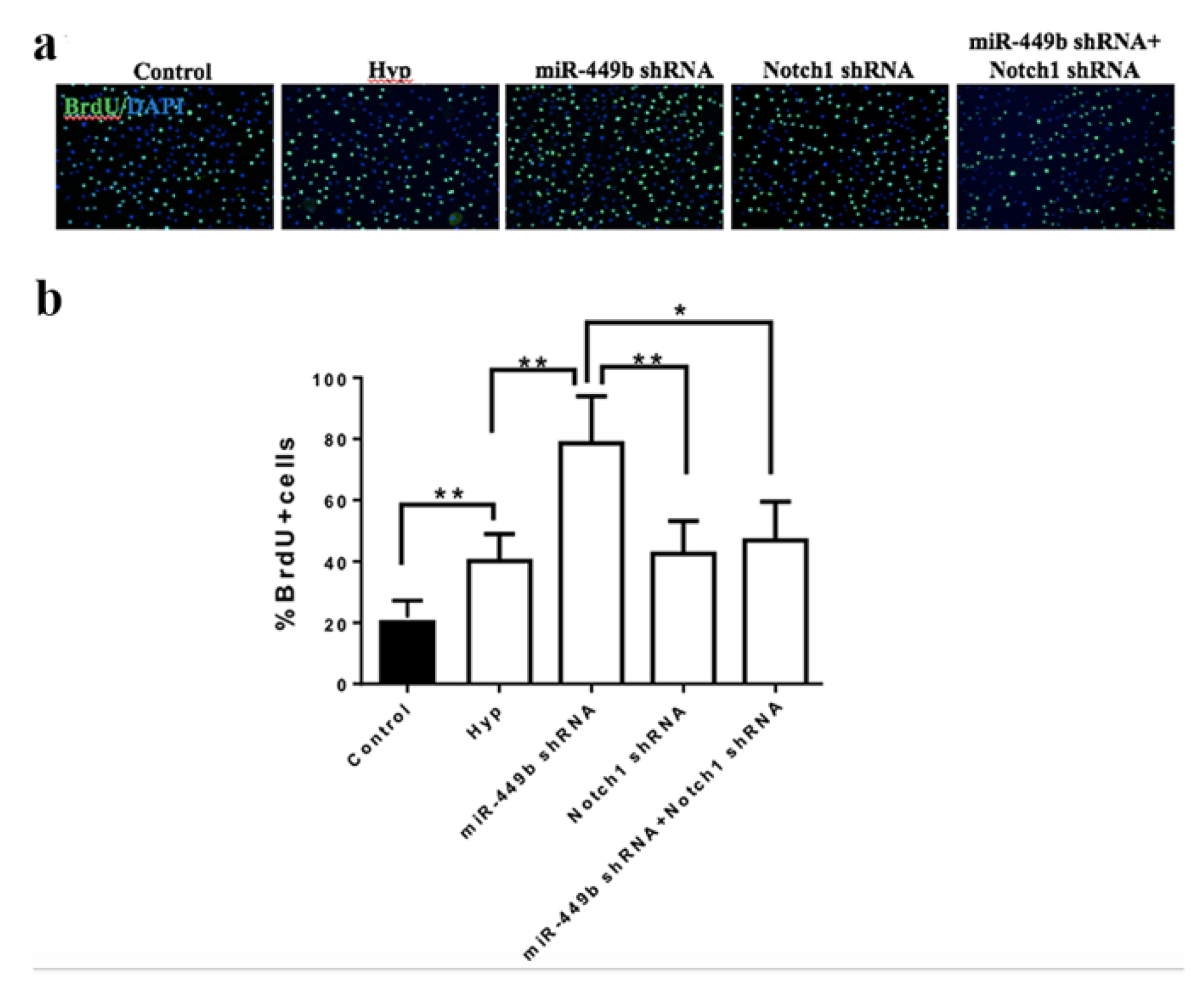
3.8. miR-449b/Notch1 Signaling Pathway Modulated NE-4C Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Hamidi, S. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Kolb, B.; Saber, H.; Fadel, H.; Rajah, G. The endocannabinoid system and stroke: A focused review. Brain Circ. 2019, 5, 1–7. [Google Scholar]
- Leng, T.; Xiong, Z.G. Treatment for ischemic stroke: From thrombolysis to thrombectomy and remaining challenges. Brain Circ. 2019, 5, 8–11. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, C.; Dornbos, D., 3rd; Li, S.; Song, H.; Wang, Y.; Ding, Y.; Ji, X. Multiphase adjuvant neuroprotection: A novel paradigm for improving acute ischemic stroke outcomes. Brain Circ. 2020, 6, 11–18. [Google Scholar]
- Donega, V.; van Velthoven, C.T.; Nijboer, C.H.; Kavelaars, A.; Heijnen, C.J. The endogenous regenerative capacity of the damaged newborn brain: Boosting neurogenesis with mesenchymal stem cell treatment. J. Cereb. Blood Flow Metab. 2013, 33, 625–634. [Google Scholar] [CrossRef]
- Lagace, D.C. Does the endogenous neurogenic response alter behavioral recovery following stroke? Behav. Brain Res. 2012, 227, 426–432. [Google Scholar] [CrossRef]
- Sun, F.; Wang, X.; Mao, X.; Xie, L.; Jin, K. Ablation of neurogenesis attenuates recovery of motor function after focal cerebral ischemia in middle-aged mice. PLoS ONE 2012, 7, e46326. [Google Scholar] [CrossRef]
- Ruan, L.; Lau, B.W.; Wang, J.; Huang, L.; Zhuge, Q.; Wang, B.; Jin, K.; So, K.F. Neurogenesis in neurological and psychiatric diseases and brain injury: From bench to bedside. Prog. Neurobiol. 2014, 115, 116–137. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Wang, Y.; Zhang, R.; Chopp, M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 2004, 35, 1732–1737. [Google Scholar] [CrossRef]
- Hess, D.C.; Blauenfeldt, R.A.; Andersen, G.; Hougaard, K.D.; Hoda, M.N.; Ding, Y.; Ji, X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015, 11, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Gidday, J. Cerebrovascular ischemic protection by pre- and post-conditioning. Brain Circ. 2015, 1, 97–103. [Google Scholar] [CrossRef]
- Ren, C.; Liu, Y.; Stone, C.; Li, N.; Li, S.; Li, H.; Cheng, Z.; Hu, J.; Li, W.; Jin, K.; et al. Limb Remote Ischemic Conditioning Ameliorates Cognitive Impairment in Rats with Chronic Cerebral Hypoperfusion by Regulating Glucose Transport. Aging Dis. 2021, 12, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sapolsky, R.M.; Steinberg, G.K. Interrupting reperfusion as a stroke therapy: Ischemic postconditioning reduces infarct size after focal ischemia in rats. J. Cereb. Blood Flow Metab. 2006, 26, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Maida, C.; Arnao, V.; Della Corte, V.; Simonetta, I.; Corpora, F.; Di Bona, D.; Maugeri, R.; et al. Early High-dosage Atorvastatin Treatment Improved Serum Immune-inflammatory Markers and Functional Outcome in Acute Ischemic Strokes Classified as Large Artery Atherosclerotic Stroke: A Randomized Trial. Medicine 2016, 95, e3186. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, A.; Shimazaki, T.; Gregg, C.; Weinmaster, G.; Weiss, S. Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J. Neurosci. 2003, 23, 1730–1741. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef]
- Givogri, M.I.; de Planell, M.; Galbiati, F.; Superchi, D.; Gritti, A.; Vescovi, A.; de Vellis, J.; Bongarzone, E.R. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev. Neurosci. 2006, 28, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, X.; Xie, L.; Greenberg, D.A.; Jin, K. Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J. Cereb. Blood Flow Metab. 2009, 29, 1644–1654. [Google Scholar] [CrossRef]
- Kim, V.N. Small RNAs: Classification; biogenesis; and function. Mol. Cells 2005, 19, 1–15. [Google Scholar]
- Liu, D.Z.; Tian, Y.; Ander, B.P.; Xu, H.; Stamova, B.S.; Zhan, X.; Turner, R.J.; Jickling, G.; Sharp, F.R. Brain and blood microRNA expression profiling of ischemic stroke; intracerebral hemorrhage; and kainate seizures. J. Cereb. Blood Flow Metab. 2010, 30, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Morris-Blanco, K.C.; Lopez, M.S.; Yang, T.; Zhao, H.; Vemuganti, R.; Luo, Y. Impact of microRNAs on ischemic stroke: From pre- to post-disease. Prog. Neurobiol. 2018, 163, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Zhang, Y.; Liu, L.; Yang, L.; Cai, Y.; Zhang, J.; Xu, S. Neuroinflammation in Ischemic Stroke: Focus on MicroRNA-mediated Polarization of Microglia. Front. Mol. Neurosci. 2020, 13, 612439. [Google Scholar] [CrossRef] [PubMed]
- Fededa, J.P.; Esk, C.; Mierzwa, B.; Stanyte, R.; Yuan, S.; Zheng, H.; Ebnet, K.; Yan, W.; Knoblich, J.A.; Gerlich, D.W. MicroRNA-34/449 controls mitotic spindle orientation during mammalian cortex development. EMBO J. 2016, 35, 2386–2398. [Google Scholar] [CrossRef]
- Marcet, B.; Chevalier, B.; Luxardi, G.; Coraux, C.; Zaragosi, L.E.; Cibois, M.; Robbe-Sermesant, K.; Jolly, T.; Cardinaud, B.; Moreilhon, C.; et al. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat. Cell Biol. 2011, 13, 693–699. [Google Scholar] [CrossRef]
- Ren, C.; Li, S.; Wang, B.; Han, R.; Li, N.; Gao, J.; Li, X.; Jin, K.; Ji, X. Limb remote ischemic conditioning increases Notch signaling activity and promotes arteriogenesis in the ischemic rat brain. Behav. Brain Res. 2018, 340, 87–93. [Google Scholar] [CrossRef]
- Ren, C.; Wang, B.; Li, N.; Jin, K.; Ji, X. Herbal Formula Danggui-Shaoyao-San Promotes Neurogenesis and Angiogenesis in Rat Following Middle Cerebral Artery Occlusion. Aging Dis. 2015, 6, 245–253. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Sanberg, P.R. Elevated body swing test: A new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J. Neurosci. 1995, 15, 5372–5378. [Google Scholar] [CrossRef]
- Belayev, L.; Alonso, O.F.; Busto, R.; Zhao, W.; Ginsberg, M.D. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 1996, 27, 1616–1622. [Google Scholar] [CrossRef]
- Ren, C.; Yao, Y.; Han, R.; Huang, Q.; Li, H.; Wang, B.; Li, S.; Li, M.; Mao, Y.; Mao, X.; et al. Cerebral ischemia induces angiogenesis in the peri-infarct regions via Notch1 signaling activation. Exp. Neurol. 2018, 304, 30–40. [Google Scholar] [CrossRef]
- Jiang, L.; Cao, Y.; Liu, Z.; Ni, S.; Liu, J.; Ha, Y.; Luo, Z.; Li, C.; Liu, S.; Li, J.; et al. SRμCT Reveals 3D Microstructural Alterations of the Vascular and Neuronal Network in a Rat Model of Chronic Compressive Thoracic Spinal Cord Injury. Aging Dis. 2020, 11, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Wang, P.; Wang, B.; Li, N.; Li, W.; Zhang, C.; Jin, K.; Ji, X. Limb remote ischemic per-conditioning in combination with post-conditioning reduces brain damage and promotes neuroglobin expression in the rat brain after ischemic stroke. Restor. Neurol. Neurosci. 2015, 33, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Onose, G.; Anghelescu, A.; Blendea, D.; Ciobanu, V.; Daia, C.; Firan, F.C.; Oprea, M.; Spinu, A.; Popescu, C.; Ionescu, A.; et al. Cellular and Molecular Targets for Non-Invasive; Non-Pharmacological Therapeutic/Rehabilitative Interventions in Acute Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Wang, B.; ZhuGe, Q.; Jin, K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015, 1623, 166–173. [Google Scholar] [CrossRef]
- Esposito, E.; Hayakawa, K.; Maki, T.; Arai, K.; Lo, E.H. Effects of Postconditioning on Neurogenesis and Angiogenesis During the Recovery Phase After Focal Cerebral Ischemia. Stroke 2015, 46, 2691–2694. [Google Scholar] [CrossRef]
- Veighey, K.; Macallister, R.J. Clinical applications of remote ischemic preconditioning. Cardiol. Res. Pract. 2012, 2012, 620681. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Ren, C.; Gao, C.; Li, N.; Wang, C.; Wang, L.; Zhao, W.; Ji, X.; Jin, K. Hypoxic postconditioning promotes neurogenesis by modulating the metabolism of neural stem cells after cerebral ischemia. Exp. Neurol. 2022, 347, 113871. [Google Scholar] [CrossRef]
- Felling, R.J.; Song, H. Epigenetic mechanisms of neuroplasticity and the implications for stroke recovery. Exp. Neurol. 2015, 268, 37–45. [Google Scholar] [CrossRef]
- Wu, J.; Bao, J.; Kim, M.; Yuan, S.; Tang, C.; Zheng, H.; Mastick, G.S.; Xu, C.; Yan, W. Two miRNA clusters; miR-34b/c and miR-449; are essential for normal brain development; motile ciliogenesis; and spermatogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, E2851–E2857. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Chopp, M. Function of neural stem cells in ischemic brain repair processes. J. Cereb. Blood Flow Metab. 2016, 36, 2034–2043. [Google Scholar] [CrossRef]
- Tobin, M.K.; Bonds, J.A.; Minshall, R.D.; Pelligrino, D.A.; Testai, F.D.; Lazarov, O. Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. J. Cereb. Blood Flow Metab. 2014, 34, 1573–1584. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yang, Y.; Li, N.; Li, H.; Xu, J.; Zhao, W.; Wang, X.; Ma, L.; Gao, C.; Ding, Y.; et al. Limb Remote Ischemic Conditioning Promotes Neurogenesis after Cerebral Ischemia by Modulating miR-449b/Notch1 Pathway in Mice. Biomolecules 2022, 12, 1137. https://doi.org/10.3390/biom12081137
Li S, Yang Y, Li N, Li H, Xu J, Zhao W, Wang X, Ma L, Gao C, Ding Y, et al. Limb Remote Ischemic Conditioning Promotes Neurogenesis after Cerebral Ischemia by Modulating miR-449b/Notch1 Pathway in Mice. Biomolecules. 2022; 12(8):1137. https://doi.org/10.3390/biom12081137
Chicago/Turabian StyleLi, Sijie, Yong Yang, Ning Li, Haiyan Li, Jiali Xu, Wenbo Zhao, Xiaojie Wang, Linqing Ma, Chen Gao, Yuchuan Ding, and et al. 2022. "Limb Remote Ischemic Conditioning Promotes Neurogenesis after Cerebral Ischemia by Modulating miR-449b/Notch1 Pathway in Mice" Biomolecules 12, no. 8: 1137. https://doi.org/10.3390/biom12081137
APA StyleLi, S., Yang, Y., Li, N., Li, H., Xu, J., Zhao, W., Wang, X., Ma, L., Gao, C., Ding, Y., Ji, X., & Ren, C. (2022). Limb Remote Ischemic Conditioning Promotes Neurogenesis after Cerebral Ischemia by Modulating miR-449b/Notch1 Pathway in Mice. Biomolecules, 12(8), 1137. https://doi.org/10.3390/biom12081137







