YTHDF3 Is Involved in the Diapause Process of Bivoltine Bombyx mori Strains by Regulating the Expression of Cyp307a1 and Cyp18a1 Genes in the Ecdysone Synthesis Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents
2.3. Cell Culture
2.4. Construction of the Baculovirus Expression System
2.5. dsRNA Synthesis
2.6. In Vivo Injection
2.7. RIP: RNA Immunoprecipitation
2.8. RNA Isolation and Quantitative RT-PCR Analysis
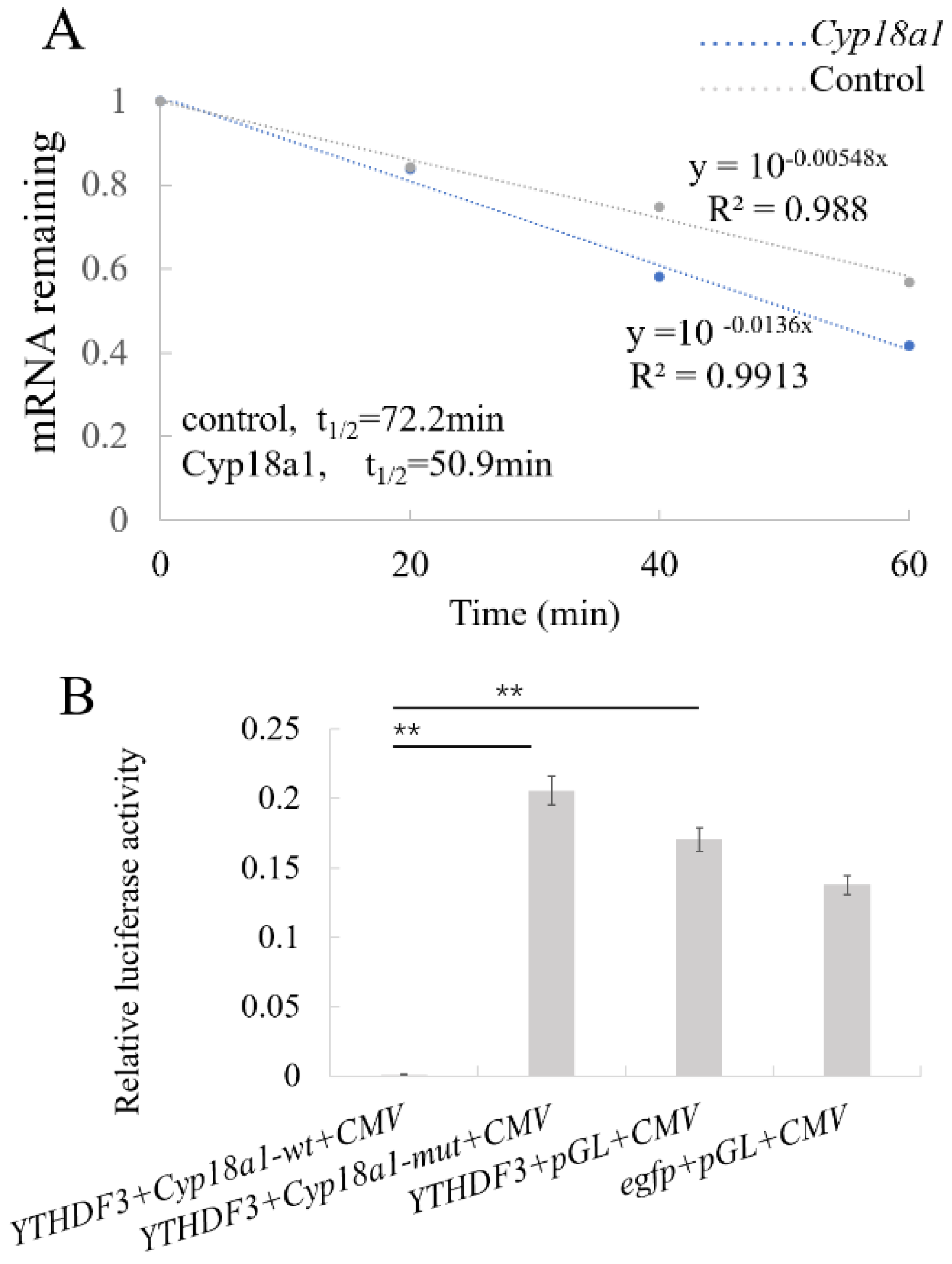
2.9. mRNA Stability Assay
2.10. m6A Site Validation
2.11. Western Blotting
2.12. Dual-Luciferase Reporter Assay
2.13. Statistical Analysis
3. Results
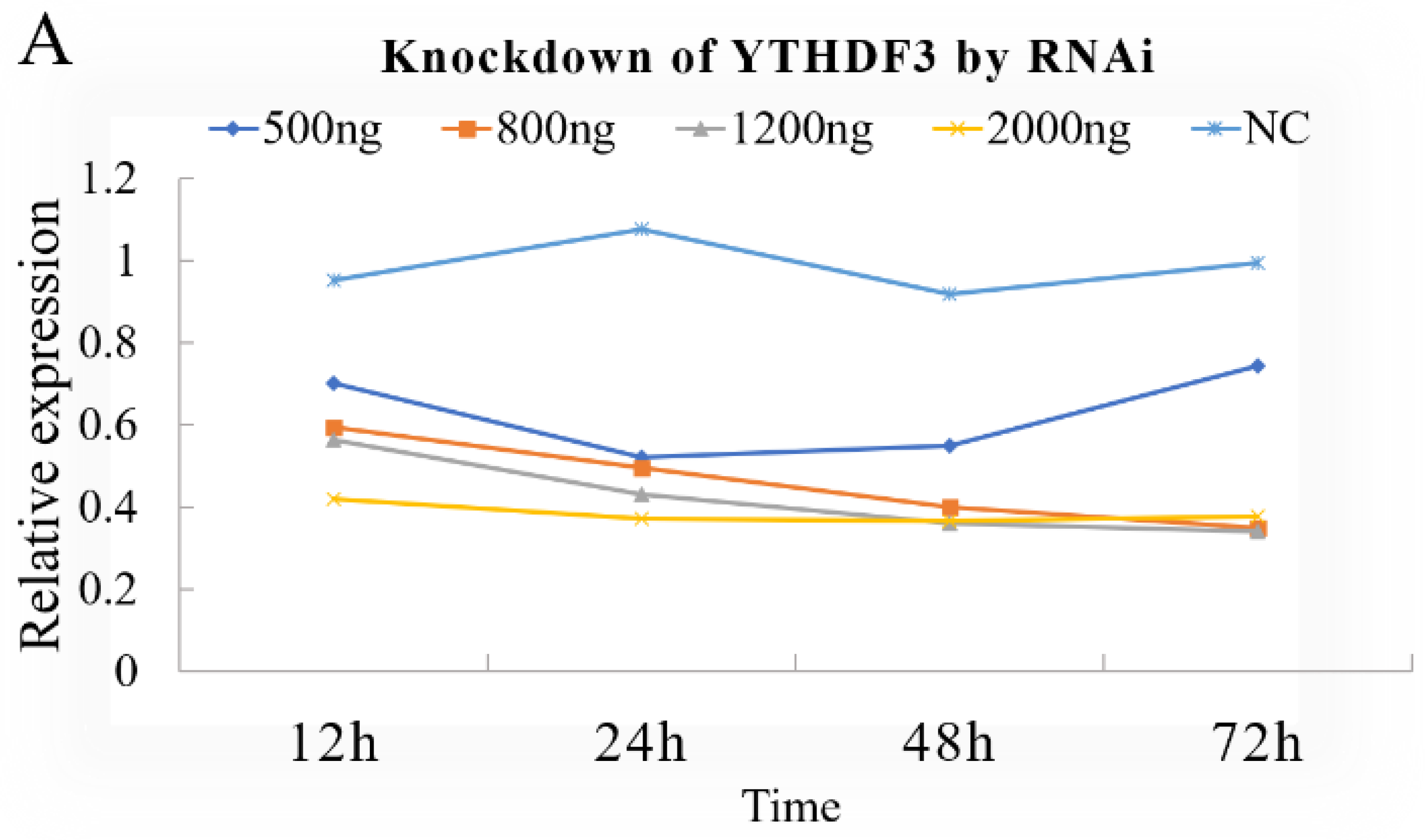
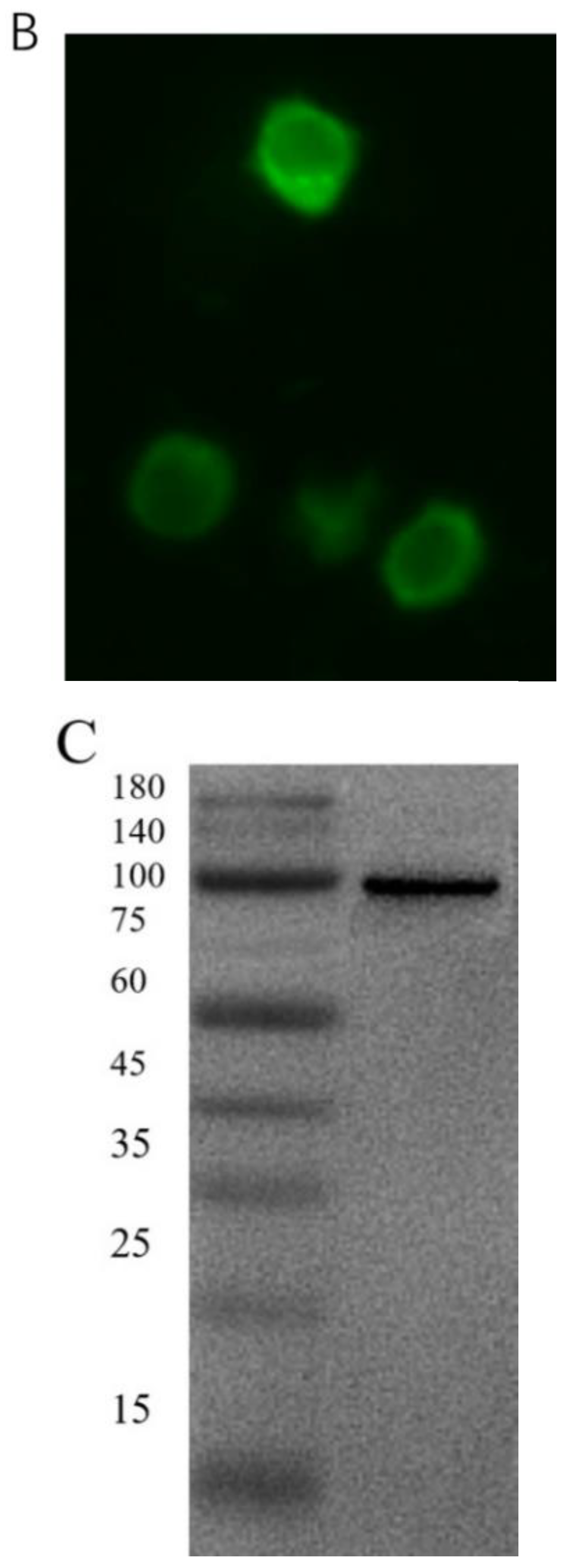
3.1. Knockdown or Overexpression of YTHDF3 in BmN Cells
3.2. YTHDF3 Regulates B. mori Diapause
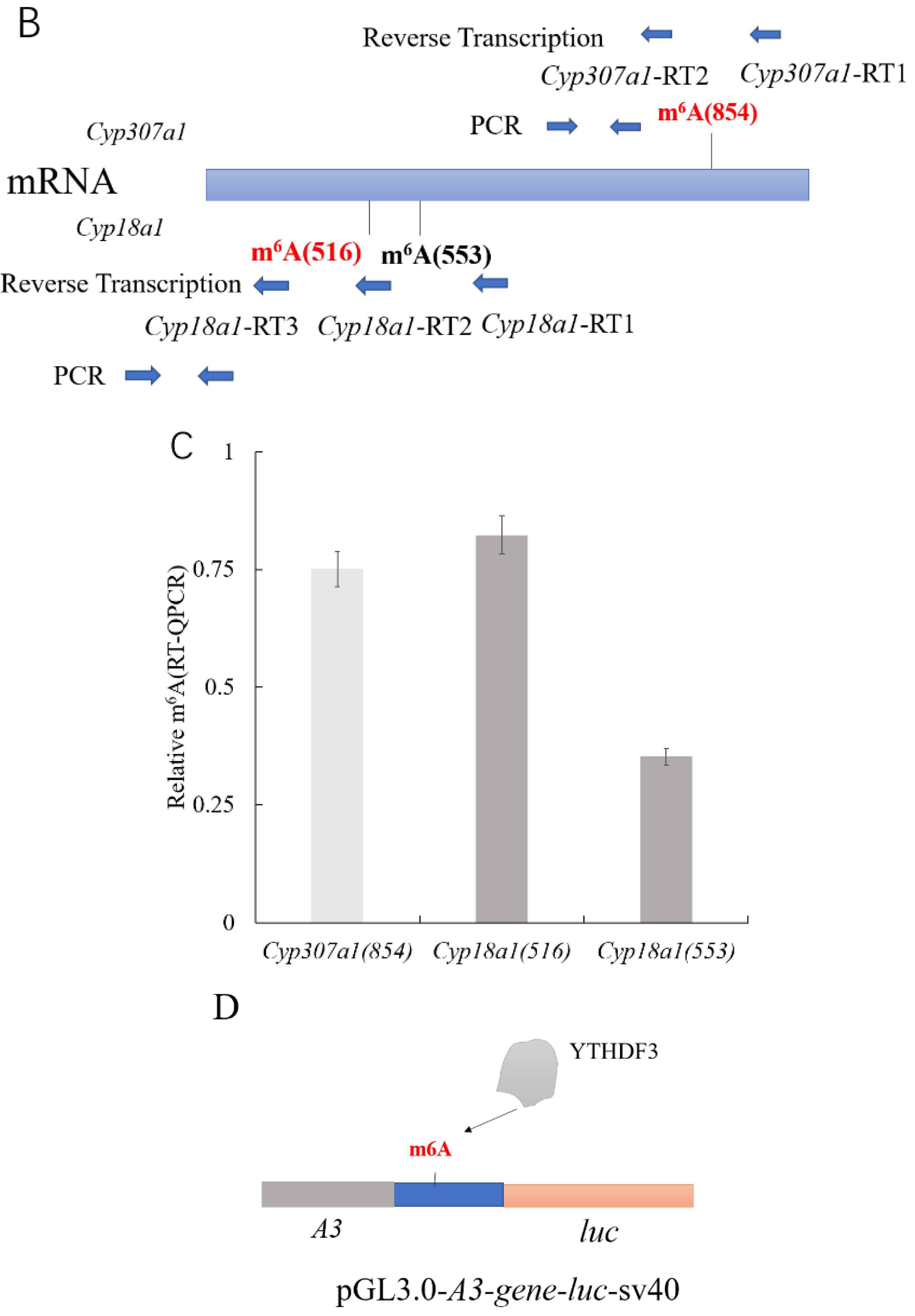
3.3. Identification of Target Genes in the Ecdysone Synthesis Pathway Regulated by YTHDF3
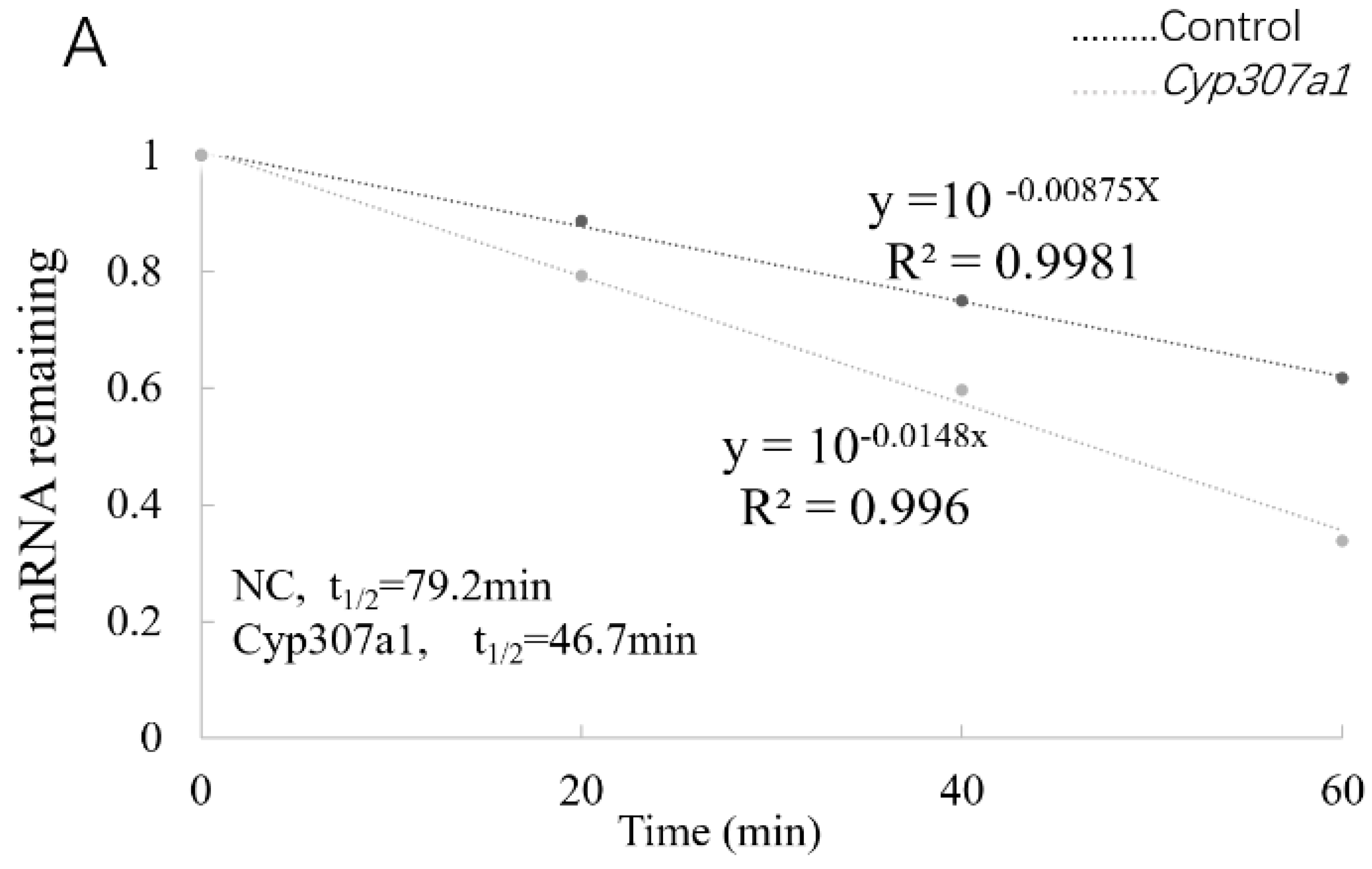
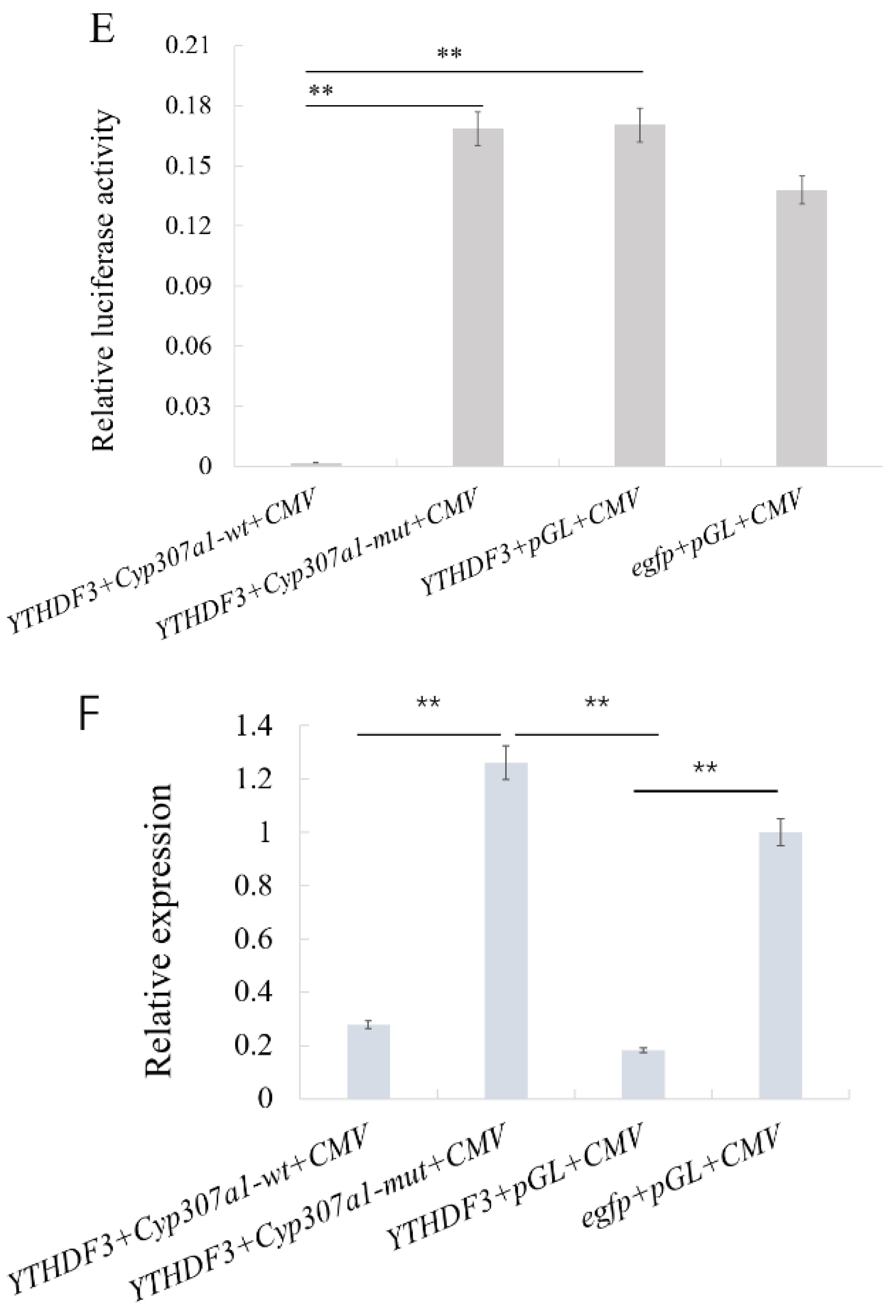
3.4. YTHDF3 Mediates Cyp307a1 mRNA Degradation in the Ecdysone Pathway
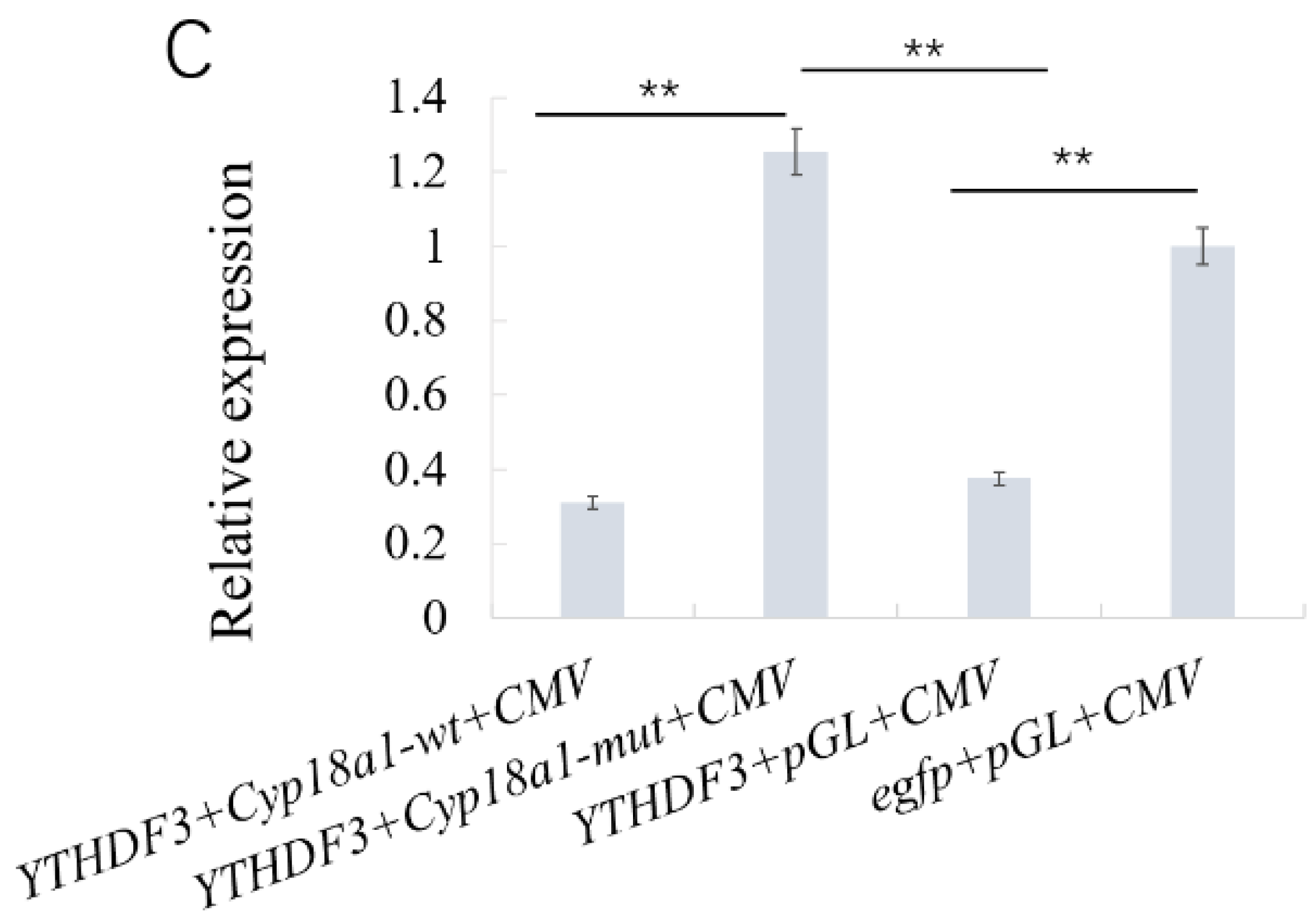
3.5. YTHDF3 Mediates Cyp18a1 mRNA Degradation in the Ecdysone Synthesis Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.-B.; Tong, J.; Zhu, S.; Batista, P.J.; Duffy, E.E.; Zhao, J.; Bailis, W.; Cao, G.; Kroehling, L.; Chen, Y.; et al. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 2017, 548, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Taketo, K.; Konno, M.; Asai, A.; Koseki, J.; Toratani, M.; Satoh, T.; Doki, Y.; Mori, M.; Ishii, H.; Ogawa, K. The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int. J. Oncol. 2018, 52, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Zhao, X.; Li, L.; Yang, Q.; Liu, X.; Caihu, H.; Runhui, L.; Chen, R.; Wang, Y.; Jiang, B.; et al. SUMOylation of YTHDF2 promotes mRNA degradation and cancer progression by increasing its binding affinity with m6A-modified mRNAs. Nucleic Acids Res. 2021, 49, 2859–2877. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovit, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mousem6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef]
- Slobodin, B.; Han, R.; Calderone, V.; Vrielink, J.A.F.O.; Loayza-Puch, F.; Elkon, R.; Agami, R. Transcription Impacts the Efficiency of mRNA Translation via Co-transcriptional N6-adenosine Methylation. Cell 2017, 169, 326–337.e12. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N6-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; He, J.; Zhang, Y.; Xi, H.; Liu, M.; Ma, J.; Wu, L. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nat. Commun. 2016, 7, 12626. [Google Scholar] [CrossRef]
- Alarcón, C.R.; Goodarzi, H.; Lee, H.; Liu, X.; Tavazoie, S.; Tavazoie, S.F. HNRNPA2B1 Is a Mediator of m6A-Dependent Nuclear RNA Processing Events. Cell 2015, 162, 1299–1308. [Google Scholar] [CrossRef]
- Liu, N.; Dai, Q.; Zheng, G.; He, C.; Parisien, M.; Pan, T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 2015, 518, 560–564. [Google Scholar] [CrossRef]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef]
- Lacerda, R.; Menezes, J.; Romão, L. More than just scanning: The importance of cap-independent mRNA translation initiation for cellular stress response and cancer. Cell Mol. Life Sci. 2017, 74, 1659–1680. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, X.; Liu, K.; Roundtree, I.A.; Tempel, W.; Li, Y.; Lu, Z.; He, C.; Min, J. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat. Chem. Biol. 2014, 10, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Lu, Z.; Zhao, B.S.; Ma, H.; Hsu, P.J.; Liu, C.; He, C. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017, 27, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Lasman, L.; Krupalnik, V.; Viukov, S.; Mor, N.; Aguilera Castrejon, A.; Schneir, D.; Bayerl, J.; Mizrahi, O.; Peles, S.; Tawil, S.; et al. Context-dependent functional compensation between Ythdf m6A reader proteins. Genes Dev. 2020, 34, 1373–1391. [Google Scholar] [CrossRef]
- Lennon, J.T.; Jones, S.E. Microbial seed banks: The ecological and evolutionary implications of dormancy. Nat. Rev. Microbiol. 2011, 9, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.; Li, R.; Qi, R.; Dick, T. Proteins of Mycobacterium bovis BCG induced in the Wayne dormancy model. J. Bacteriol. 2001, 183, 2672–2676. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D. Insect Clocks. In Comparative Biochemistry and Physiology Part A: Physiology; Elsevier: Amsterdam, The Netherlands, 1977; Volume 56, pp. 1–5. [Google Scholar] [CrossRef]
- Bonasio, R.; Tu, S.; Reinberg, D. Molecular signals of epigenetic states. Science 2010, 330, 612–616. [Google Scholar] [CrossRef]
- Pegoraro, M.; Bafna, A.; Davies, N.J.; Shuker, D.M.; Tauber, E. DNA methylation changes induced by long and short photoperiods in Nasonia. Genome Res. 2016, 26, 203–210. [Google Scholar] [CrossRef]
- Delaney, C.E.; Chen, A.T.; Graniel, J.V.; Dumas, K.J.; Hu, P.J. A histone H4 lysine 20 methyltransferase couples environmental cues to sensory neuron control of developmental plasticity. Development 2017, 144, 1273–1282. [Google Scholar] [CrossRef]
- George, S.; Palli, S.R. Histone deacetylase 3 is required for development and metamorphosis in the red flour beetle, Tribolium castaneum. BMC Genom. 2020, 21, 420. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Xu, G.; Chen, P.; Song, Q.; Feng, Q.; Yi, Y.; Zheng, S. 20-Hydroxyecdysone receptor-activated Bombyx mori CCAAT/enhancer-binding protein gamma regulates the expression of BmCBP and subsequent histone H3 lysine 27 acetylation in Bo. mori. Insect Mol. Biol. 2020, 29, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Hůla, P.; Kostál, V. Potential role for histone H3K4 methylation in diapause induction in the larva of Chymomyza costata. In Proceedings of the 7th International Symposium of the Environmental Physiology of Ectotherms and Plants, Tartu, Estonia, 31 July–4 August 2017. [Google Scholar]
- Lekha, G.; Gupta, T.; Awasthi, A.K.; Murthy, G.N.; Trivedy, K.; Ponnuvel, K.M. Genome wide microarray based expression profiles associated with BmNPV resistance and susceptibility in Indian silkworm races of Bombyx mori. Genomics 2015, 106, 393–403. [Google Scholar] [CrossRef]
- Li, B.; Hu, P.; Zhang, S.Z.; Toufeeq, S.; Wang, J.; Zhao, K.; Xu, X.; Xu, J.P.; Huang, S.J. DNA methyltransferase BmDnmt1 and BmDnmt2 in silkworm (Bombyx mori) and the regulation of silkworm embryonic development. Arch. Insect Biochem. Physiol. 2019, 100, e21529. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Li, J.; Qian, P.; Xue, P.; Xu, J.; Chen, Y.; Zhu, J.; Shunming, T.; Zhao, Q.; Qian, H.; et al. The role of N6-methyladenosine modification on diapause in silkworm (Bombyx mori) strains that exhibit different voltinism. Mol. Reprod. Dev. 2019, 86, 1981–1992. [Google Scholar] [CrossRef]
- Castellanos-Rubio, A.; Santin, I.; Olazagoitia-Garmendia, A.; Romero-Garmendia, I.; Jauregi-Miguel, A.; Legarda, M.; Bilbao, J.R. A novel RT-QPCR-based assay for the relative quantification of residue specific m6A RNA methylation. Sci. Rep. 2019, 9, 4220. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.A. Chapter Five—Epigenetic Influences on Diapause. In Advances in Insect Physiology; Verlinden, H., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 53, pp. 115–144. [Google Scholar]
- Santos, P.K.F.; de Souza Araujo, N.; Françoso, E.; Zuntini, A.R.; Arias, M.C. Diapause in a tropical oil-collecting bee: Molecular basis unveiled by RNA-Seq. BMC Genom. 2018, 19, 305. [Google Scholar] [CrossRef]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.; Hao, Y.J.; Sun, B.F.; Sun, H.Y.; Li, A.; Ping, X.; Lai, W.; et al. Nuclear m6A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef]
- Li, A.; Chen, Y.-S.; Ping, X.-L.; Yang, X.; Xiao, W.; Yang, Y.; Sun, H.-Y.; Zhu, Q.; Baidya, P.; Wang, X.; et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017, 27, 444–447. [Google Scholar] [CrossRef]
- Guittard, E.; Blais, C.; Maria, A.; Parvy, J.-P.; Pasricha, S.; Lumb, C.; Lafont, R.; Daborn, P.J.; Dauphin-Villemant, C. CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev. Biol. 2011, 349, 35–45. [Google Scholar] [CrossRef]
- Namiki, T.; Niwa, R.; Sakudoh, T.; Shirai, K.; Takeuchi, H.; Kataoka, H. Cytochrome P450 CYP307A1/Spook: A regulator for ecdysone synthesis in insects. Biochem. Biophys. Res. Commun. 2005, 337, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Kadono-Okuda, K.; Amornsak, W.; Yamashita, O. Controlled Ecdysteroid Accumulation in Eggs of the Silkworm, Bombyx mori, by an lmidazole Compound (KK-42), and Embryogenesis in These Eggs. Arch. Insect Biochem. Physiol. 1994, 25, 121–135. [Google Scholar] [CrossRef]
- Haruyuki, S.; Hideki, T.; Toshihiro, M.; Hibiki, T.; Noriyuki, H.; Yoshinori, F. Comparative Studies of Ecdysteroid Metabolism between Diapause Eggs and Non-diapause Eggs of the Silkworm, Bombyx mori. Zool. Sci. 1999, 16, 935–943. [Google Scholar] [CrossRef]
- Seino, A.; Ogura, T.; Tsubota, T.; Shimomura, M.; Nakakura, T.; Tan, A.; Mita, K.; Shinoda, T.; Nakagawa, Y.; Shiotsuki, T. Characterization of Juvenile Hormone Epoxide Hydrolase and Related Genes in the Larval Development of the Silkworm Bombyx mori. Biosci. Biotechnol. Biochem. 2010, 74, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Jiang, T.; Zhu, J.; Xie, Y.C.; Tan, Z.C.; Chen, Y.H.; Tang, S.M.; Hao, B.F.; Wang, S.P.; Huang, J.S.; et al. Transcriptome sequencing reveals potential mechanisms of diapause preparation in bivoltine silkworm Bombyx mori (Lepidoptera: Bombycidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 24, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zaccara, S.; Jaffrey, S.R. A Unified Model for the Function of YTHDF Proteins in Regulating m6A-Modified mRNA. Cell 2020, 181, 1582–1595.e18. [Google Scholar] [CrossRef]
- Patil, D.P.; Pickering, B.F.; Jaffrey, S.R. Reading m6A in the Transcriptome: m6A-Binding Proteins. Trends Cell Biol. 2018, 28, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Dai, K.; Liang, Z.; Zhu, M.; Pan, J.; Zhang, M.; Yan, B.; Zhu, H.; Zhang, Z.; et al. N (6)-Methyladenosine Level in Silkworm Midgut/Ovary Cell Line Is Associated with Bombyx mori Nucleopolyhedrovirus Infection. Front. Microbiol. 2019, 10, 2988. [Google Scholar] [CrossRef]
- Xue, P.; Jiang, T.; Zhu, J.; Wang, M.; Zhao, Q.; Huang, J.; Tang, S.; Shen, X. Low METTL3 level in midgut of the Bombyx mori inhibit the proliferation of nucleopolyhedrovirus. J. Asia-Pac. Entomol. 2021, 24, 42–49. [Google Scholar] [CrossRef]

| Primer Name | Sequence | Primer Purpose |
|---|---|---|
| egfp-F | ATGGTGAGCAAGGGC | pFastBac Dual |
| egfp-R | CTTGTACAGCTCGTCC | pFastBac Dual |
| YTHDF3-F | ATGTCAGCAGGCGTGTCAG | pFastBac Dual |
| YTHDF3-R | TTAATTGCGGGGACGTCCTCG | pFastBac Dual |
| YTHDF3T7-F | GGATCCTAATACGACTCACTATAGGTCGCAACTATCGTGAGCATC | dsRNA |
| YTHDF3-R | TGCATTTTCTGATTCCCCTC | dsRNA |
| YTHDF3-F | TCGCAACTATCGTGAGCATC | dsRNA |
| YTHDF3T7-R | GGATCCTAATACGACTCACTATAGGTGCATTTTCTGATTCCCCTC | dsRNA |
| YTHDF3-F | ATCAGCGGATGAAAGGGCAA | Real-time PCR |
| YTHDF3-R | AGGCGCATAAGAAGGTTGCT | Real-time PCR |
| Cyp307a1-RT1 | CGTGTAGCACCCTGAGGAGGCC | Reverse Transcription |
| Cyp307a1-RT2 | CCGGAGCTTCCGTGTCTAAGC | Reverse Transcription |
| Cyp307a1-F | TGGGAGATCAACCAGGGCTA | Real-time PCR |
| Cyp307a1-R | GAAGGATCGGATGTCCTGGG | Real-time PCR |
| Cyp18a1-RT1 | CCTCGTCCTTCCATGATTTCG | Reverse Transcription |
| Cyp18a1-RT2 | GGTCTCGCGATAGAATCCGAT | Reverse Transcription |
| Cyp18a1-RT3 | TCGGCGAATTGAGACAGACGA | Reverse Transcription |
| Cyp18a1-F | TGTGGACGAAACTCGACACG | Real-time PCR |
| Cyp18a1-R | AAACGGTACACCCCATGGTC | Real-time PCR |
| Cyp307a1-wtF | AGAAGGACTTTCTGGACGGC | pGL-A3-luc |
| Cyp307a1-mutF | AGAAGGTCTTTCTGGACGGC | pGL-A3-luc |
| Cyp307a1-R | TACGGGTCTTTTGCCTCTGG | pGL-A3-luc |
| Cyp18a1-wtF | CGCCGAAACTTGTGAAGGAA | pGL-A3-luc |
| Cyp18a1-mutF | CGCCGAAACTTGTGAAGGAT | pGL-A3-luc |
| Cyp18a1-R | CGGCCAAACCTTGACTCCAA | pGL-A3-luc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Fan, B.; Yasen, A.; Zhu, J.; Wang, M.; Shen, X. YTHDF3 Is Involved in the Diapause Process of Bivoltine Bombyx mori Strains by Regulating the Expression of Cyp307a1 and Cyp18a1 Genes in the Ecdysone Synthesis Pathway. Biomolecules 2022, 12, 1127. https://doi.org/10.3390/biom12081127
Chen Y, Fan B, Yasen A, Zhu J, Wang M, Shen X. YTHDF3 Is Involved in the Diapause Process of Bivoltine Bombyx mori Strains by Regulating the Expression of Cyp307a1 and Cyp18a1 Genes in the Ecdysone Synthesis Pathway. Biomolecules. 2022; 12(8):1127. https://doi.org/10.3390/biom12081127
Chicago/Turabian StyleChen, Yanhua, Bingyan Fan, Ayinuer Yasen, Juan Zhu, Meixian Wang, and Xingjia Shen. 2022. "YTHDF3 Is Involved in the Diapause Process of Bivoltine Bombyx mori Strains by Regulating the Expression of Cyp307a1 and Cyp18a1 Genes in the Ecdysone Synthesis Pathway" Biomolecules 12, no. 8: 1127. https://doi.org/10.3390/biom12081127
APA StyleChen, Y., Fan, B., Yasen, A., Zhu, J., Wang, M., & Shen, X. (2022). YTHDF3 Is Involved in the Diapause Process of Bivoltine Bombyx mori Strains by Regulating the Expression of Cyp307a1 and Cyp18a1 Genes in the Ecdysone Synthesis Pathway. Biomolecules, 12(8), 1127. https://doi.org/10.3390/biom12081127







