Minor Phytocannabinoids: A Misleading Name but a Promising Opportunity for Biomedical Research
Abstract
1. Introduction
2. Discovery Studies
2.1. Biochemical Assays
2.1.1. Type-1 Cannabinoid Receptor (CB1)
2.1.2. Type 2-Cannabinoid Receptor (CB2)
2.1.3. Non-CB GPCRs (GPR6, GPR18, GPR55 and GPR119)
2.1.4. TRP Channels
2.1.5. Serotonin (5HT) Receptors
2.1.6. GABA Receptors
2.1.7. Anti-Inflammatory Targets
2.1.8. PPARs
2.1.9. Estrogen Receptors
2.2. Phenotypic Assays
2.2.1. Anti-Cancer Activity and Pro-Carcinogenic Activity
2.2.2. Anti-Bacterial Activity
2.3. Acne
3. Preclinical In Vivo Studies
3.1. Epilepsy
3.2. Autism Spectrum Disorders (ASD)
3.3. Rett Syndrome (RTTS)
3.4. Ulcerative Colitis (UC)
3.5. Duchenne Muscular Dystrophy (DMD)
3.6. Diabetes and Metabolic Syndrome
3.7. Nausea
3.8. Analgesic and Anti-Inflammatory Activity
3.9. Cachexia
3.10. Neuroprotective Activity
3.11. Glaucoma
3.12. Sleep Induction
3.13. Tetrad Test
3.14. Pharmacokinetic Studies
4. Clinical Studies
4.1. Autism Spectrum Disorders (ASD)
4.2. Epileptic Conditions
4.3. HIV-Associated Neuropathic Pain
4.4. Obesity
4.5. Diabetes
4.6. Addiction
4.7. Sleep Induction
5. Strategy to Increase the Availability of Minor Phytocannabinoids
5.1. Selective Breeding and Metabolic Engineering of Cannabis
5.2. Synthetic Biology
5.3. Semi-Synthesis
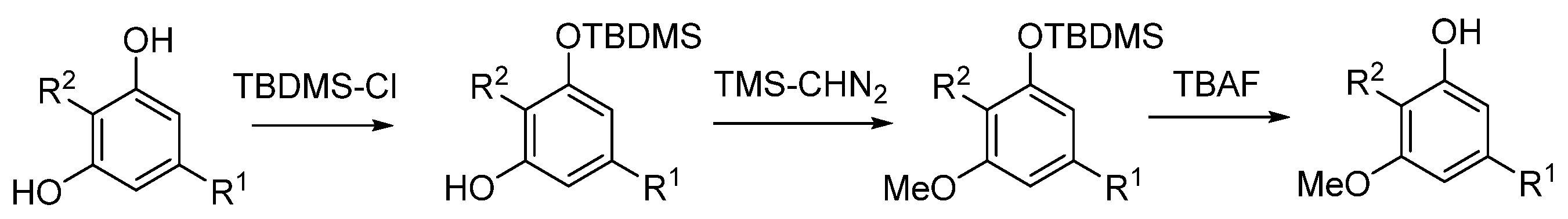
5.3.1. O-Methylphytocannabinoids
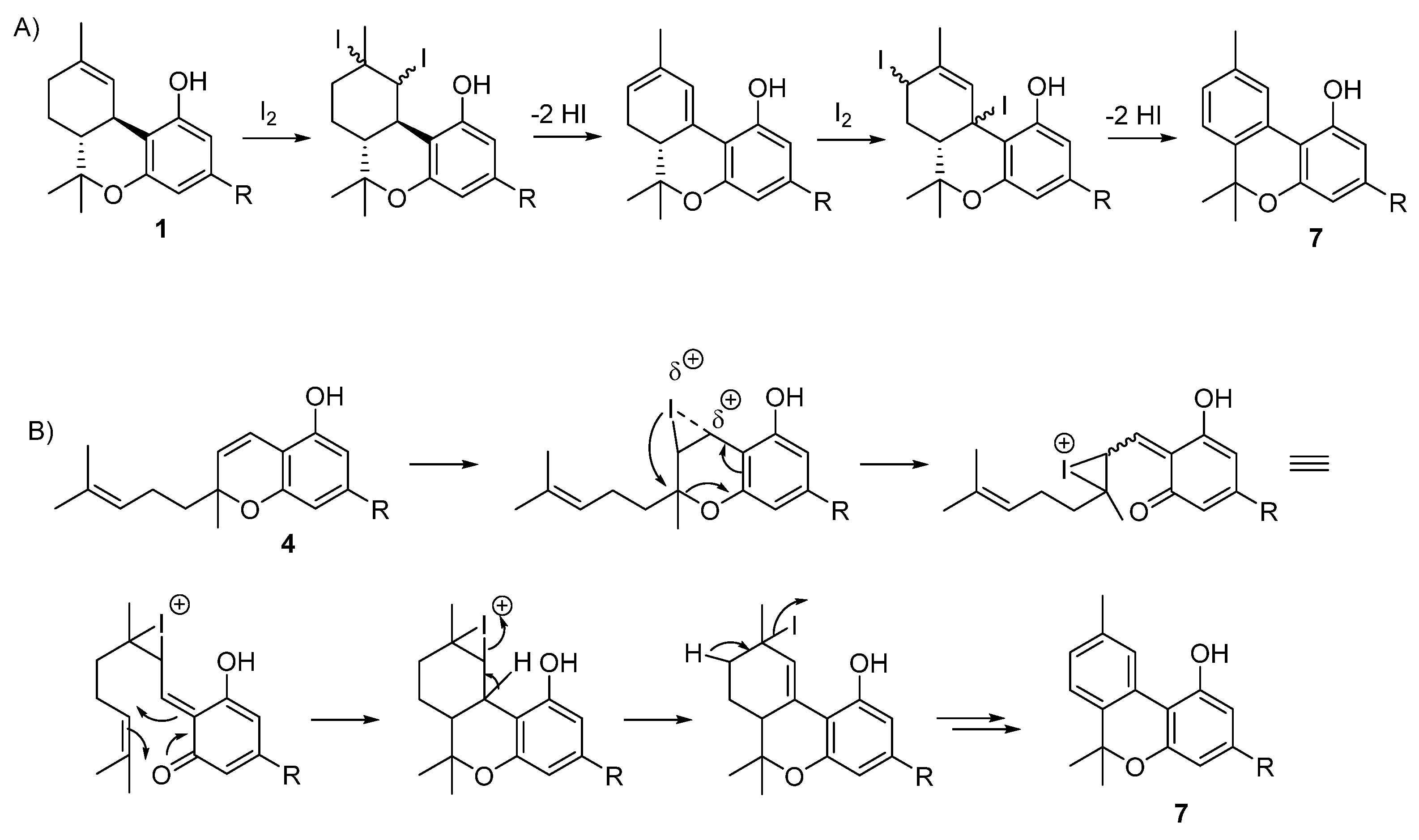
5.3.2. Cannabinol (CBN, 7)
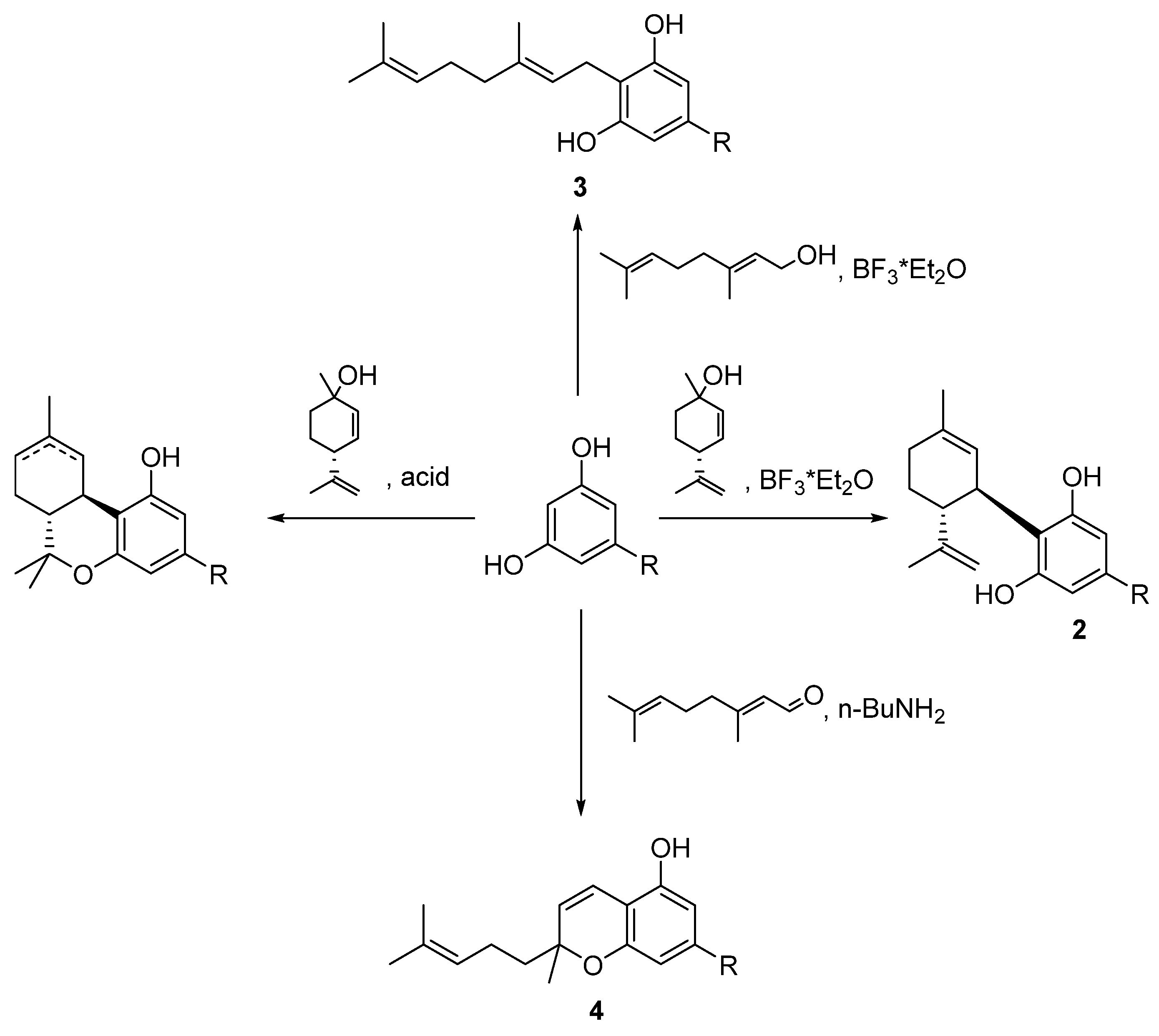
5.3.3. Cannabicyclol
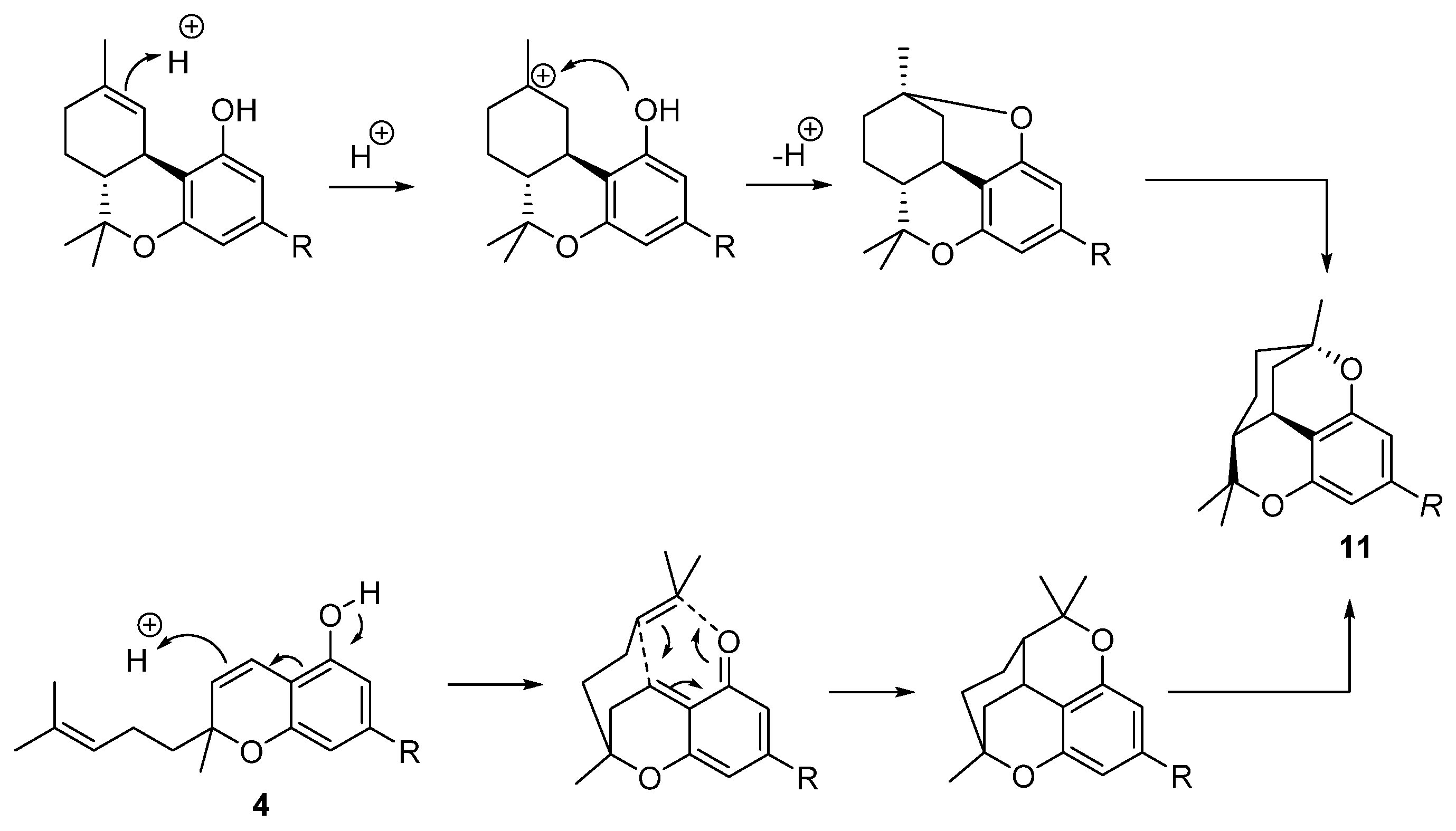
5.3.4. Cannabicitran (CBT, 11)
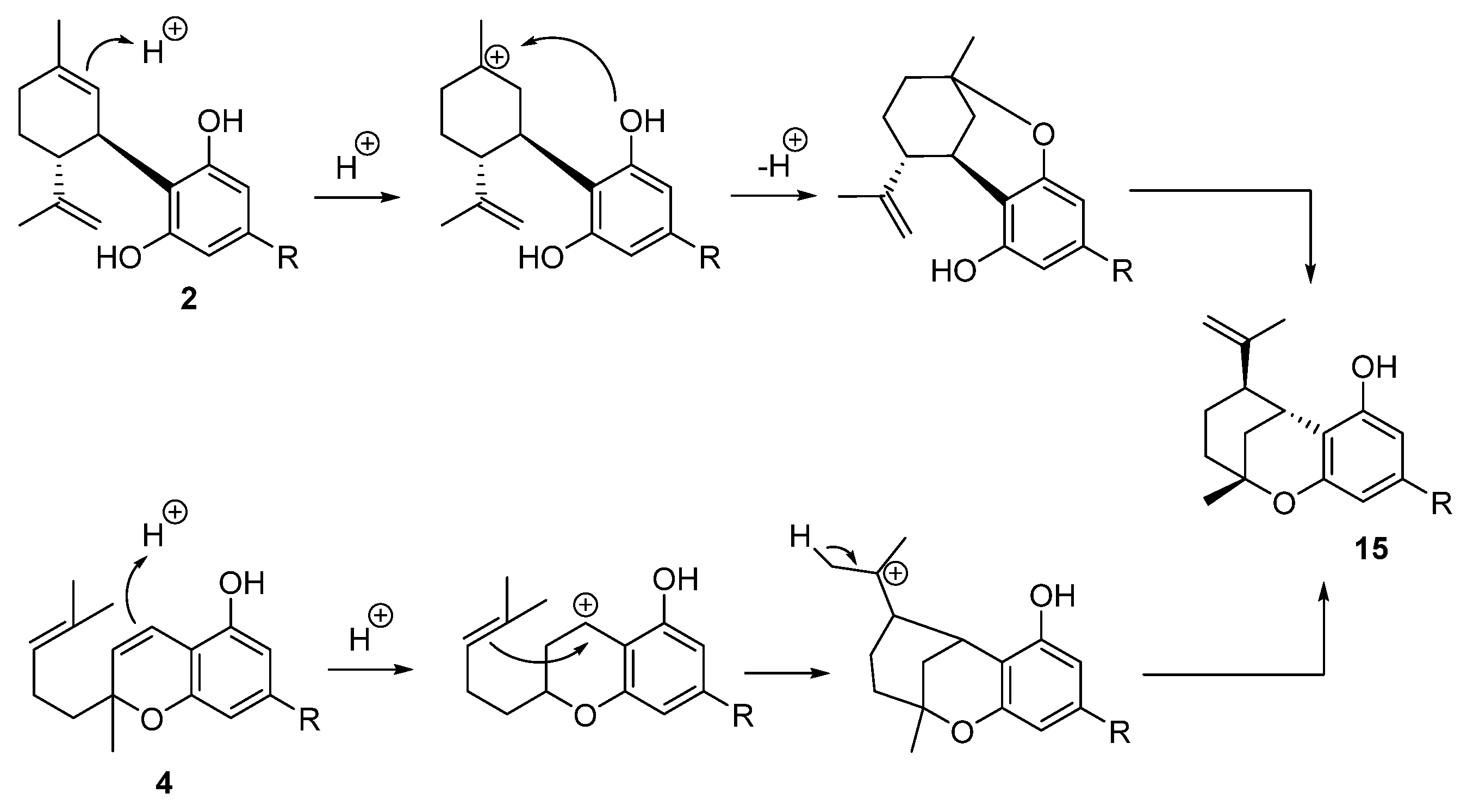
5.3.5. Iso-THC (15)
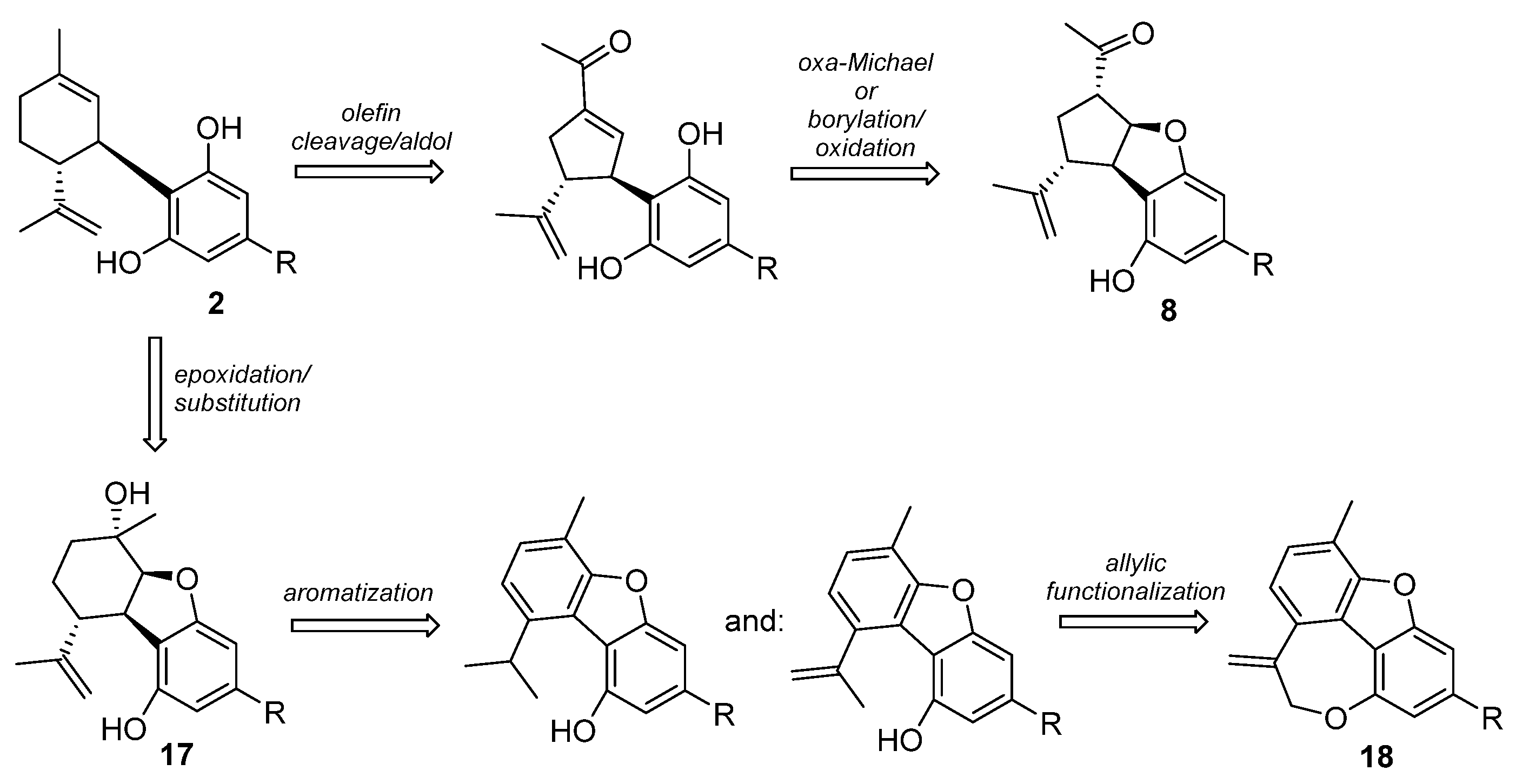
5.3.6. Furanoid Phytocannabinoids and Cannabimovone (16)
5.4. Total Synthesis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appendino, G. The Early History of Cannabinoid Research. Rend. Lincei. Sci. Fis. Nat. 2020, 31, 919–929. [Google Scholar] [CrossRef]
- Di Marzo, V. New Approaches and Challenges to Targeting the Endocannabinoid System. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Pollastro, F.; Caprioglio, D.; Del Prete, D.; Rogati, F.; Minassi, A.; Taglialatela-Scafati, O.; Muñoz, E.; Appendino, G. Cannabichromene. Nat. Prod. Commun. 2018, 13, 1189–1194. [Google Scholar] [CrossRef]
- de Meijer, E.P.M.; Hammond, K.M.; Micheler, M. The Inheritance of Chemical Phenotype in Cannabis sativa L. (III): Variation in Cannabichromene Proportion. Euphytica 2009, 165, 293–311. [Google Scholar] [CrossRef]
- Singh, A.; Bilichak, A.; Kovalchuk, I. The Genetics of Cannabis—Genomic Variations of Key Synthases and their Effect on Cannabinoid Content. Genome 2021, 64, 490–501. [Google Scholar] [CrossRef]
- Sampson, P.B. Phytocannabinoid Pharmacology: Medicinal Properties of Cannabis sativa Constituents Aside from the “Big Two”. J. Nat. Prod. 2021, 84, 142–160. [Google Scholar] [CrossRef]
- Nguyen, G.N.; Jordan, E.N.; Kayser, O. Synthetic Strategies for Rare Cannabinoids Derived from Cannabis sativa. J. Nat. Prod. 2022, 85, 1555–1568. [Google Scholar] [CrossRef]
- Cerrato, A.; Citti, C.; Cannazza, G.; Capriotti, A.L.; Cavaliere, C.; Grassi, G.; Marini, F.; Montone, C.M.; Paris, R.; Piovesana, S.; et al. Phytocannabinomics: Untargeted Metabolomics as a Tool for Cannabis Chemovar Differentiation. Talanta 2021, 230, 122313. [Google Scholar] [CrossRef] [PubMed]
- Carmen del Río, C.; Carmen Navarrete, C.; Juan, A.; Collado, J.A.; Bellido, M.L.; Gómez-Cañas, M.; Pazos, M.R.; Fernández-Ruiz, J.; Pollastro, F.; Appendino, G.; et al. The Cannabinoid Quinol VCE-004.8 Alleviates Bleomycin-induced Scleroderma and Exerts Potent Antifibrotic Effects through Peroxisome Proliferator-Activated Receptor-γ and CB2 Pathways. Sci. Rep. 2016, 6, 21703. [Google Scholar] [CrossRef]
- Linciano, P.; Citti, C.; Luongo, L.; Belardo, C.; Maione, S.; Vandelli, M.A.; Forni, F.; Gigli, G.; Laganà, A.; Montone, C.M.; et al. Isolation of a High-Affinity Cannabinoid for the Human CB1 Receptor from a Medicinal Cannabis sativa Variety: Δ 9-Tetrahydrocannabutol, the Butyl Homologue of Δ 9-Tetrahydrocannabinol. J. Nat. Prod. 2020, 83, 88–98. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Vandelli, M.A.; Gigli, G.; Cannazza, G. A Novel Phytocannabinoid Isolated from Cannabis Sativa, L. With an In Vivo Cannabimimetic Activity Higher Than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef] [PubMed]
- Raïch, I.; Rivas-Santisteban, R.; Lillo, A.; Lillo, J.; Reyes-Resina, I.; Nadal, X.; Ferreiro-Vera, C.; Sánchez de Medina, V.; Majellaro, M.; Sotelo, E.; et al. Similarities and Differences upon Binding of Naturally Occurring Δ9-Tetrahydrocannabinol-Derivatives to Cannabinoid CB1 and CB2 Receptors. Pharm. Res. 2021, 174, 105970. [Google Scholar] [CrossRef] [PubMed]
- Schafroth, M.A.; Mazzoccanti, G.; Reynoso-Moreno, I.; Erni, R.; Pollastro, F.; Caprioglio, D.; Botta, B.; Allegrone, G.; Grassi, G.; Chicca, A.; et al. Δ9- cis-Tetrahydrocannabinol: Natural Occurrence, Chirality, and Pharmacology. J. Nat. Prod. 2021, 84, 2502–2510. [Google Scholar] [CrossRef]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular Pharmacology of Phytocannabinoids. Prog. Chem. Org. Nat. Prod. 2017, 103, 61–101. [Google Scholar]
- Taglialatela-Scafati, O.; Pagani, A.; Scala, F.; De Petrocellis, L.; Di Marzo, V.; Grassi, G.; Appendino, G. Cannabimovone, a Cannabinoid with a Rearranged Terpenoid Skeleton from Hemp. Eur. J. Org. Chem. 2010, 2010, 2067–2072. [Google Scholar] [CrossRef]
- Sylantyev, S.; Jensen, T.P.; Ross, R.A.; Rusakov, D.A. Cannabinoid- and Lysophosphatidylinositol-sensitive Receptor GPR55 Boosts Neurotransmitter Release at Central Synapses. Proc. Natl. Acad. Sci. USA 2013, 110, 5193–5198. [Google Scholar] [CrossRef]
- Hofmann, N.A.; Yang, J.; Trauger, S.A.; Nakayama, H.; Huang, L.; Strunk, D.; Moses, M.A.; Klagsbrun, M.; Bischoff, J.; Graier, W.F. The GPR 55 Agonist, L-α-Lysophosphatidylinositol, Mediates Ovarian Carcinoma Cell-induced Angiogenesis. Br. J. Pharmacol. 2015, 172, 4107–4118. [Google Scholar] [CrossRef]
- Caldwell, M.D.; Shu-Jung Hu, S.; Viswanathan, S.; Bradshaw, H.; Kelly, M.E.M.; Straiker, A. A GPR18-Based Signalling System Regulates IOP in Murine Eye. Br. J. Pharmacol. 2013, 169, 834–843. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Schiano Moriello, A.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Göthert, M.; Bönisch, H.; Malinowska, B.; Schlicker, E. Serotonin Discovery and Stepwise Disclosure of 5-HT Receptor Complexity over Four Decades. Part II. Some Contributions of Manfred Göthert. Pharmacol. Rep. 2020, 72, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, D.; Rock, E.M.; Cluny, N.L.; Cascio, M.G.; Limebeer, C.L.; Duncan, M.; Stott, C.G.; Javid, F.A.; Parker, L.A.; Pertwee, R.G. Cannabidiolic Acid Prevents Vomiting in Suncus Murinus and Nausea-Induced Behaviour in Rats by Enhancing 5-HT1A Receptor Activation. Br. J. Pharmacol. 2013, 168, 1456–1470. [Google Scholar] [CrossRef] [PubMed]
- Salaga, A.B.; Tichkule, R.B.; Thakur, G.A.; Makriyannis, A.; Storr, M.; Fichna, J. The Novel Peripherally Active Cannabinoid Type 1 and Serotonin Type 3 Receptor Agonist AM9405 Inhibits Gastrointestinal Motility and Reduces Abdominal Pain in Mouse Models Mimicking Irritable Bowel Syndrome. Eur. J. Pharmacol. 2018, 836, 34–43. [Google Scholar] [CrossRef]
- Cifelli, P.; Ruffolo, G.; De Felice, E.; Alfano, V.; van Vliet, E.A.; Aronica, E.; Palma, E. Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission? Int. J. Mol. Sci. 2020, 21, 723. [Google Scholar] [CrossRef]
- Morano, A.; Cifelli, P.; Nencini, P.; Antonilli, L.; Fattouch, J.; Ruffolo, G.; Roseti, C.; Aronica, E.; Limatola, C.; Di Bonaventura, C.; et al. Cannabis in Epilepsy: From Clinical Practice to Basic Research Focusing on the Possible Role of Cannabidivarin. Epilepsia 2016, 1, 145–151. [Google Scholar] [CrossRef]
- Nallathambi, R.; Mazuz, M.; Ion, A.; Selvaraj, G.; Weininger, S.; Fridlender, M.; Nasser, A.; Sagee, O.; Kumari, P.; Diana Nemichenizer, D.; et al. Anti-Inflammatory Activity in Colon Models Is Derived from Δ9-Tetrahydrocannabinolic Acid that Interacts with Additional Compounds in Cannabis Extracts. Cannabis Cannabinoid Res. 2017, 2, 167–182. [Google Scholar] [CrossRef]
- Takeda, S.; Misawa, K.; Yamamoto, I.; Watanabe, K. Cannabidiolic Acid as a Selective Cyclooxygenase-2 Inhibitory Component in Cannabis. Drug Metab. Dispos. 2008, 36, 1917–1921. [Google Scholar] [CrossRef]
- Cuadari, A.; Pollastro, F.; Unciti-Broceta, J.D.; Caprioglio, D.; Minassi, A.; Lopatriello, A.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. The Dimerization of Δ9-Tetrahydrocannabinolic Acid A (THCA-A). Acta Pharm. Sin. B 2019, 9, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hanh, J.; Váradi, L.; Cairns, R.; Sjöström, H.; Liao, V.W.; Peta Wood, P.; Balaban, S.; Ong, J.A.; Lin, H.-Y.J.; et al. Identification of Dual PPARα/γ Agonists and Their Effects on Lipid Metabolism. Bioorg. Med. Chem. 2015, 23, 7676–7684. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, E.; Fellous, T.; Iannotti, F.A.; Gentile, A.; Allarà, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; Di Marzo, V. Identification and Characterization of Phytocannabinoids as Novel PPARα/γ Dual Agonists by a Computational and In Vitro Experimental Approach. Biochim. Biophys. Acta 2019, 1863, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Palomares, B.; Ruiz-Pino, F.; Garrido-Rodriguez, M.; Eugenia Prados, M.; Sánchez- Garrido, M.A.; Velasco, I.; Vazquez, M.J.; Nadal, X.; Ferreiro-Vera, C.; Morrugares, R.; et al. Tetrahydrocannabinolic Acid A (THCA-A) Reduces Adiposity and Prevents Metabolic Disease Caused by Diet-Induced Obesity. Biochem. Pharmacol. 2020, 171, 113693. [Google Scholar] [CrossRef]
- Iannotti, F.A.; De Maio, F.; Panza, E.; Appendino, G.; Taglialatela-Scafati, O.; De Petrocellis, L.; Amodeo, P.; Vitale, R.M. Identification and Characterization of Cannabimovone, a Cannabinoid from Cannabis sativa, as a Novel PPARγ Agonist via a Combined Computational and Functional Study. Molecules 2020, 25, 1119. [Google Scholar] [CrossRef]
- Kikiowo, B.; Ogunleye, A.J.; Iwaloye, O.; Ijatuyi, T.; Adelakun, N.S.; Alashe, W.O. Induced Fit Docking and Automated QSAR Studies Reveal the ER-α Inhibitory Activity of Cannabis sativa in Breast Cancer. Recent Pat. Anticancer Drug Discov. 2021, 16, 273–284. [Google Scholar] [CrossRef]
- Takeda, S.; Okajima, S.; Miyoshi, H.; Yoshida, K.; Okamoto, Y.; Okada, T.; Amamoto, T.; Watanabe, K.; Omiecinski, C.J.; Aramaki, H. Cannabidiolic acid, a major cannabinoid in fiber-type cannabis, is an inhibito of MDA-MB-231 breast cancer cell migration. Toxicol. Lett. 2012, 214, 314–319. [Google Scholar] [CrossRef]
- Hirao-Suzuki, M.; Takeda, S.; Koga, T.; Takiguchi, M.; Toda, A. Cannabidiolic Acid dampens the expression of cyclooxygenase-2 in MDA-MB-231 breast cancer cells: Possible implication of the Peroxisome Proliferator-Activated Receptor abrogation. J. Toxicol. Sci. 2020, 45, 227–236. [Google Scholar] [CrossRef]
- Suzuki, M.; Takeda, S.; Okazaki, H.; Watanabe, K.; Takiguchi, M.; Aramaki, H. Cannabidiolic Acid-mediated Interference with AP-1 Transcriptional Activity in MDA-MB-231 Breast Cancer Cells. Nat. Prod. Commun. 2017, 12, 759–761. [Google Scholar] [CrossRef]
- Scott, K.A.; Shah, S.; Dalgleish, A.G.; Liu, W.M. Enhancing the Activity of Cannabidiol and Other Cannabinoids In Vitro throu Modifications to Drug Combinations and Treatment Schedules. Anticancer Res. 2013, 33, 4373–4380. [Google Scholar]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. Anticancer Effects of Phytocannabinoids Used with Chemotherapy in Leukaemia Cells Can Be Improved by Altering the Sequence of Their Administration. Int. J. Oncol. 2017, 51, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Ferk, F.; Mišík, M.; Ropek, N.; Nersesyan, A.; Mejri, D.; Holzmann, K.; Lavorgna, M.; Isidori, M.; Knasmüller, S. Low Doses of Widely Consumed Cannabinoids (Cannabidiol and Cannabidivarin) Cause DNA Damage and Chromosomal Aberrations in Human-derived Cells. Arch. Toxicol. 2019, 93, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis sativa: A Structure-activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef]
- Russo, C.; Lavorgna, M.; Nugnes, R.; Orlo, E.; Isidori, M. Comparative Assessment of Antimicrobial, Antiradical and Cytotoxic Activities of Cannabidiol and its Propyl Analogue Cannabidivarin. Sci. Rep. 2021, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Pecoraro, M.T.; Formato, M.; Piccolella, S.; Ragucci, S.; Mallardo, M.; Russo, R.; Di Maro, A.; Daniele, A.; Pacifico, S. Cannabidiolic acid in Hemp Seed Oil Table Spoon and Beyond. Molecules 2022, 27, 2566. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The Antimicrobial Potential of Cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Wassmann, C.S.; Højrup, P.; Klitgaard, J.K. Cannabidiol is an Effective Helper Compound in Combination with Bacitracin to kill Gram-positive Bacteria. Sci. Rep. 2020, 10, 4112. [Google Scholar] [CrossRef]
- Oláh, A.; Tóth, B.J.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol Exerts Sebostatic and Antiinflammatory Effects on Human Sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef]
- Di Meo, C.; Tortolani, D.; Standoli, S.; Angelucci, C.B.; Fanti, F.; Leuti, A.; Sergi, M.; Kadhim, S.; Hsu, E.; Rapino, C.; et al. Effects of Rare Phytocannabinoids on the Endocannabinoid System of Human Keratinocytes. Int. J. Mol. Sci. 2022, 23, 5430. [Google Scholar] [CrossRef]
- Río, C.D.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The Endocannabinoid System of the Skin. A Potential Approach for the Treatment of Skin Disorders. Biochem. Pharmacol. 2018, 157, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Low, I.K.; Banister, S.D.; McGregor, I.S.; Arnold, J.C. Pharmacokinetics of Phytocannabinoid Acids and Anticonvulsant effect of Cannabidiolic acid in a Mouse Model of Dravet Syndrome. J. Nat. Prod. 2019, 82, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Goerl, B.; Watkins, S.; Metcalf, C.; Smith, M.; Beenhakker, M. Cannabidiolic Acid Exhibits Entourage-like Improvements of Anticonvulsant Activity in an Acute Rat Model of Seizures. Epilepsy Res. 2011, 169, 106525. [Google Scholar] [CrossRef]
- Hill, A.J.; Weston, S.E.; Jones, N.A.; Smith, I.; Bevan, S.A.; Williamson, E.M.; Stephens, G.J.; Williams, C.M.; Whalley, B.J. Δ⁹-Tetrahydrocannabivarin Suppresses in vitro Epileptiform and in vivo Seizure Activity in Adult Rats. Epilepsia 2010, 51, 1522–1532. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Woolley-Roberts, M.; Bingham, S.; Rubino, T.; Parolaro, D. Cannabidivarin Treatment Ameliorates Autism-Like Behaviors and Restores Hippocampal Endocannabinoid System and Glia Alterations Induced by Prenatal Valproic Acid Exposure in Rats. Front. Cell Neurosci. 2019, 13, 367. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Piscitelli, F.; Brodie, J.S.; Woolley-Roberts, M.; Barbiero, I.; Tramarin, M.; Binelli, G.; Landsberger, N.; Kilstrup-Nielsen, C.; et al. Cannabidivarin Completely Rescues Cognitive Deficits and Delays Neurological and Motor Defects in Male Mecp2 Mutant Mice. J. Psychopharmacol. 2019, 33, 894–907. [Google Scholar] [CrossRef]
- Pagano, E.; Romano, B.; Iannotti, F.A.; Parisi, O.A.; D’Armiento, M.; Pignatiello, S.; Coretti, L.; Lucafò, M.; Venneri, T.; Stocco, G.; et al. The Non-euphoric Phytocannabinoid Cannabidivarin Counteracts Intestinal Inflammation in Mice and Cytokine Expression in Biopsies from UC Pediatric Patients. Pharmacol. Res. 2019, 149, 104464. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Pagano, E.; Schiano Moriello, A.; Alvino, F.A.; Sorrentino, N.A.; D’Orsi, L.; Gazzerro, E.; Capasso, R.; De Leonibus, E.; De Petrocellis, L.; et al. Effects of Non-euphoric Plant Cannabinoids on Muscle Quality and Performance of Dystrophic mdx Mice. Br. J. Pharmacol. 2019, 176, 1568–1584. [Google Scholar] [CrossRef]
- Le Foll, B.; Trigo, J.M.; Sharkey, K.A.; Le Strat, Y. Cannabis and Δ9-Tetrahydrocannabinol (THC) for Weight Loss? Med. Hypotheses 2013, 80, 564–567. [Google Scholar] [CrossRef]
- Wargent, E.T.; Zaibi, M.S.; Silvestri, C.; Hislop, D.C.; Stocker, C.J.; Stott, C.G.; Guy, G.W.; Duncan, M.; Di Marzo, V.; Cawthorne, M.A. The Cannabinoid Δ9-Tetrahydrocannabivarin (THCV) Ameliorates Insulin Sensitivity in Two Mouse Models of Obesity. Nutr. Diabetes 2013, 3, e68. [Google Scholar] [CrossRef]
- Carmona-Hidalgo, B.; González-Mariscal, I.; García-Martín, A.; Prados, M.E.; Ruiz-Pino, F.; Appendino, G.; Tena-Sempere, M.; Muñoz, E. Δ9-Tetrahydrocannabinolic Acid Markedly Alleviates Liver Fibrosis and Inflammation in Mice. Phytomedicine 2021, 81, 153426. [Google Scholar] [CrossRef]
- Rock, E.M.; Bolognini, D.; Limebeer, C.L.; Cascio, M.G.; Anavi-Goer, S.; Fletcher, P.J.; Mechoulam, R.; Pertwee, R.G.; Parker, L.A. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomitingand nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsalraphe nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Parker, L.A. Suppression of Lithium Chloride-Induced Conditioned Gaping (A Model of Nausea-Induced Behaviour) in Rats (Using the Taste Reactivity Test) with Metoclopramide is Enhanced by Cannabidiolic Acid. Pharmacol. Biochem. Behav. 2013, 111, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Cairns, B.E. Cannabidiol, Cannabinol and Their Combinations Act as Peripheral Analgesics in a Rat Model of Myofascial Pain. Arch. Oral Biol. 2019, 104, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jan, T.R.; Farraj, A.K.; Harkema, J.R.; Kaminski, N.E. Attenuation of the Ovalbumin-Induced Allergic Airway Response by Cannabinoid Treatment in A/J Mice. Toxicol. Appl. Pharmacol. 2003, 188, 24–35. [Google Scholar] [CrossRef]
- Bolognini, D.; Costa, B.; Maione, S.; Comelli, F.; Marini, P.; Di Marzo, V.; Parolaro, D.; Ross, R.A.; Gauson, L.A.; Cascio, M.G.; et al. The Plant Cannabinoid Δ9-Tetrahydrocannabivarin can Decrease Signs of Inflammation and Inflammatory Pain in Mice. Br. J. Pharmacol. 2010, 160, 677–687. [Google Scholar] [CrossRef]
- Rock, E.M.; Limebeer, C.L.; Parker, L.A. Effect of cannabidiolic acid and D9-tetrahydrocannabinol on carrageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain. Psychopharmacology 2018, 235, 3259–3271. [Google Scholar] [CrossRef]
- Farrimond, J.A.; Whalley, B.J.; Williams, C.M. Non-Δ⁹-Tetrahydrocannabinol Phytocannabinoids Stimulate Feeding in Rats. Behav. Pharmacol. 2012, 23, 113–117. [Google Scholar] [CrossRef]
- Nadal, X.; Del Río, C.; Casano, S.; Palomares, B.; Ferreiro-Vera, C.; Navarrete, C.; Sánchez-Carnerero, C.; Cantarero, I.; Bellido, M.L.; Meyer, S.; et al. Tetrahydrocannabinolic Acid is a Potent PPARγ Agonist with Neuroprotective Activity. Br. J. Pharmacol. 2017, 174, 4263–4276. [Google Scholar] [CrossRef]
- García, C.; Palomo-Garo, C.; García-Arencibia, M.; Ramos, J.A.; Pertwee, R.G.; Fernández-Ruiz, J. Symptom-relieving and Neuroprotective Effects of the Phytocannabinoid delta9-THCV in Animal Models of Parkinson’s Disease. Br. J. Pharmacol. 2011, 163, 1495–1506. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Harland, E.C.; Benigni, D.A.; Waller, C.W. Cannabinoids in glaucoma II: The effect of different cannabinoids on intraocular pressure of the rabbit. Curr. Eye Res. 1984, 3, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Corroon, J. Cannabinol and Sleep: Separating Fact from Fiction. Cannabis Cannabinoid Res. 2021, 6, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Usami, N.; Ohishi, Y.; Watanabe, K.; Yamamoto, I.; Yoshimura, H. Synthesis and Pharmacological Effects in Mice of Halogenated Cannabinol Derivatives. Chem. Pharm. Bull. 1995, 43, 335–337. [Google Scholar] [CrossRef]
- Chesner, G.B.; Jackson, D.M.; Stamer, G.A. Interaction of Cannabisand General Anesthetic Agents in Mice. Br. J. Pharmacol. 1974, 50, 593–599. [Google Scholar] [CrossRef]
- Takahashi, R.N.; Karniol, I.G. Pharmacologic Interaction Between Cannabinol and Delta9-tetrahydrocannabinol. Psychopharmacologia 1975, 41, 277–284. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are Cannabidiol and Δ9-Tetrahydrocannabivarin Negative Modulators of the Endocannabinoid System? A Systematic Review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef]
- Stott, C.; Jones, N.; Williams, R.; Whalley, B. Use of Cannabinoids in the Treatment of Epilepsy. Patent WO2017025712A1, 10 August 2015. [Google Scholar]
- Huff, H.C.; Vasan, A.; Roy, P.; Kaul, A.; Tajkhorshid, E.; Das, A. Differential Interactions of Selected Phytocannabinoids with Human CYP2D6 Polymorphisms. Biochemistry 2021, 60, 2749–2760. [Google Scholar] [CrossRef]
- Pretzsch, C.M.; Voinescu, B.; Lythgoe, D.; Horder, J.; Mendez, M.A.; Wichers, R.; Ajram, L.; Ivin, G.; Heasman, M.; Edden, R.A.E.; et al. Effects of Cannabidivarin (CBDV) on Brain Excitation and Inhibition Systems in Adults with and without Autism Spectrum Disorder (ASD): A Single Dose Trial During Magnetic Resonance Spectroscopy. Transl. Psychiatry 2019, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, C.M.; Floris, D.L.; Voinescu, B.; Elsahib, M.; Mendez, M.A.; Wichers, R.; Ajram, L.; Ivin, G.; Heasman, M.; Pretzsch, E.; et al. Modulation of Striatal Functional Connectivity Differences in Adults with and without Autism Spectrum Disorder in a Single-dose Randomized Trial of Cannabidivarin. Mol. Autism. 2021, 12, 49. [Google Scholar] [CrossRef]
- Zamberletti, E.; Rubino, T.; Parolaro, D. Therapeutic Potential of Cannabidivarin for Epilepsy and Autism Spectrum Disorder. Pharmacol. Ther. 2021, 226, 107878. [Google Scholar] [CrossRef]
- Brodie, M.J.; Czapinski, P.; Pazdera, L.; Sander, J.W.; Toledo, M.; Napoles, M.; Sahebkar, F.; Schreiber, A. GWEP1330 Study Group. A Phase 2 Randomized Controlled Trial of the Efficacy and Safety of Cannabidivarin as Add-on Therapy in Participants with Inadequately Controlled Focal Seizures. Cannabis Cannabinoid Res. 2021, 6, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.N.; Ellaway, C.J.; Johnson, A.M.; Truong, L.; Gordon, R.; Galettis, P.; Martin, J.H.; Lawson, J.A. Efficacy and Safety of Cannabidivarin Treatment of Epilepsy in Girls with Rett Syndrome: A Phase 1 Clinical Trial. Epilepsia 2022, 63, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Eibach, L.; Scheffel, S.; Cardebring, M.; Lettau, M.; Özgür, C.; Morguet, A.; Roehle, R.; Stein, C. Cannabidivarin for HIV-Associated Neuropathic Pain: A Randomized, Blinded, Controlled Clinical Trial. Clin. Pharmacol. Ther. 2021, 109, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, E.K.F.F.; Costa, A.B.; Salla, D.H.; Silva, M.R.D.; Mendes, T.F.; Silva, L.E.D.; Turatti, C.D.R.; Bitencourt, R.M.; Rezin, G.T. Cannabis sativa as a Treatment for Obesity: From Anti-Inflammatory Indirect Support to a Promising Metabolic Re-Establishment Target. Cannabis Cannabinoid Res. 2022, 7, 135–151. [Google Scholar] [CrossRef]
- Tudge, L.; Williams, C.; Cowen, P.J.; McCabe, C. Neural Eeffects of Cannabinoid CB1 Neutral Antagonist Tetrahydrocannabivarin on Food Reward and Aversion in Healthy Volunteers. Int. J. Neuropsychopharmacol. 2014, 18, pyu094. [Google Scholar]
- Rzepa, E.; Tudge, L.; McCabe, C. The CB1 Neutral Antagonist Tetrahydrocannabivarin Reduces Default Mode Network and Increases Executive Control Network Resting State Functional Connectivity in Healthy Volunteers. Int. J. Neuropsychopharmacol. 2015, 19, pyv092. [Google Scholar] [CrossRef][Green Version]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 2016, 9, 1777–1786. [Google Scholar] [CrossRef]
- Englund, A.; Atakan, Z.; Kralj, A.; Tunstall, N.; Murray, R.; Morrison, P. The Effect of Five Day Dosing with THCV on THC-Induced Cognitive, Psychological and Physiological Effects in Healthy Male Human Volunteers: A Placebo-Controlled, Double-blind, Crossover Pilot Trial. J. Psychopharmacol. 2016, 30, 140–151. [Google Scholar] [CrossRef]
- Karniol, I.G.; Shirakawa, I.; Takahashi, R.N.; Knobel, E.; Musty, R.E. Effects of Delta9-tetrahydrocannabinol and Cannabinol in Man. Pharmacology 1975, 13, 502–512. [Google Scholar] [CrossRef]
- Deguchi, M.; Kane, S.; Potlakayala, S.; George, H.; Proano, R.; Sheri, V.; Curtis, W.R.; Rudrabhatla, S. Metabolic Engineering Strategies of Industrial Hemp (Cannabis sativa L.): A Brief Review of the Advances and Challenges. Plant Sci. 2020, 11, 580621. [Google Scholar] [CrossRef]
- Meijer, E.P.M.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. The Inheritance of Chemical Phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Complete Biosynthesis of Cannabinoids and Their Unnatural Analogues in Yeast. Nature 2019, 567, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Tchilibon, S.; Mechoulam, R. Synthesis of a Primary Metabolite of Cannabidiol. Org. Lett. 2000, 2, 3301–3303. [Google Scholar] [CrossRef] [PubMed]
- Bloemendal, V.R.L.J.; van Hest, J.C.M.; Rutjes, F.P.J.T. Synthetic Pathways to Tetrahydrocannabinol (THC): An Overview. Org. Biomol. Chem. 2020, 18, 3203–3215. [Google Scholar] [CrossRef]
- Caprioglio, D.; Allegrone, G.; Pollastro, F.; Valera, S.; Lopatriello, A.; Collado, J.A.; Muñoz, E.; Appendino, G.; Taglialatela-Scafati, O. O-Methyl Phytocannabinoids: Semi-synthesis, Analysis in Cannabis Flowerheads, and Biological Activity. Planta Med. 2019, 85, 981–986. [Google Scholar] [CrossRef]
- Pollastro, F.; Caprioglio, D.; Marotta, P.; Moriello, A.S.; De Petrocellis, L.; Taglialatela-Scafati, O.; Appendino, G. Iodine-Promoted Aromatization of p-Menthane-Type Phytocannabinoids. J. Nat. Prod. 2018, 81, 630–633. [Google Scholar] [CrossRef]
- Caprioglio, D.; Mattoteia, D.; Minassi, A.; Pollastro, F.; Lopatriello, A.; Muňoz, E.; Taglialatela-Scafati, O.; Appendino, G. One-Pot Total Synthesis of Cannabinol via Iodine-Mediated Deconstructive Annulation. Org. Lett. 2019, 21, 6122–6125. [Google Scholar] [CrossRef]
- Bercht, C.L.; Lousberg, R.J.; Küppers, F.J.; Salemink, C.A. Cannabicitran: A New Naturally Occurring Tetracyclic Diether from Lebanese Cannabis sativa. Phytochemistry 1974, 13, 619–621. [Google Scholar] [CrossRef]
- Mazzoccanti, G.; Ismail, O.H.; D’Acquarica, I.; Villani, C.; Manzo, C.; Wilcox, M.; Cavazzini, A.; Gasparrini, F. Cannabis Through the Looking Glass: Chemo- and Enantioselective Separation of Phytocannabinoids by Enantioselective Ultra High Performance Supercritical Fluid Chromatography. Chem. Commun. 2017, 53, 12262–12265. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. The Iso-Tetrahydrocannabinols. Isr. J. Chem. 1968, 6, 679–690. [Google Scholar] [CrossRef]
- Yamamoto, I.; Gohda, H.; Narimatsu, S.; Watanabe, K.; Yoshimura, H. Cannabielsoin as a New Metabolite of Cannabidiol in Mammals. Pharmacol. Biochem. Behav. 1991, 40, 541–546. [Google Scholar] [CrossRef]
- Chianese, G.; Sirignano, C.; Benetti, E.; Marzaroli, V.; Collado, J.A.; de la Vega, L.; Appendino, G.; Muñoz, E.; Taglialatela-Scafati, O. A Nrf-2 Stimulatory Hydroxylated Cannabidiol Derivative from Hemp (Cannabis sativa). J. Nat. Prod. 2022, 85, 1089–1097. [Google Scholar] [CrossRef]
- Pagani, A.; Scala, F.; Chianese, G.; Grassi, G.; Appendino, G.; Taglialatela-Scafati, O. Cannabioxepane, a Novel Tetracyclic Cannabinoid from Hemp, Cannabis sativa L. Tetrahedron 2011, 67, 3369–3373. [Google Scholar] [CrossRef]
- Dennis, D.G.; Anand, S.D.; Lopez, A.J.; Petrovčič, J.; Das, A.; Sarlah, D. Synthesis of the Cannabimovone and Cannabifuran Class of Minor Phytocannabinoids and Their Anti-inflammatory Activity. J. Org. Chem. 2022, 87, 6075–6086. [Google Scholar] [CrossRef]
- Razdan, R.K. The Total Synthesis of Cannabinoids. In The Total Synthesis of Natural Products; ApSimon, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1981; Volume 4, pp. 185–262. [Google Scholar]
- Madhusoodanan, J. Hungering for Obesity Treatments. ACS Cent. Sci. 2017, 3, 150–152. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caprioglio, D.; Amin, H.I.M.; Taglialatela-Scafati, O.; Muñoz, E.; Appendino, G. Minor Phytocannabinoids: A Misleading Name but a Promising Opportunity for Biomedical Research. Biomolecules 2022, 12, 1084. https://doi.org/10.3390/biom12081084
Caprioglio D, Amin HIM, Taglialatela-Scafati O, Muñoz E, Appendino G. Minor Phytocannabinoids: A Misleading Name but a Promising Opportunity for Biomedical Research. Biomolecules. 2022; 12(8):1084. https://doi.org/10.3390/biom12081084
Chicago/Turabian StyleCaprioglio, Diego, Hawraz Ibrahim M. Amin, Orazio Taglialatela-Scafati, Eduardo Muñoz, and Giovanni Appendino. 2022. "Minor Phytocannabinoids: A Misleading Name but a Promising Opportunity for Biomedical Research" Biomolecules 12, no. 8: 1084. https://doi.org/10.3390/biom12081084
APA StyleCaprioglio, D., Amin, H. I. M., Taglialatela-Scafati, O., Muñoz, E., & Appendino, G. (2022). Minor Phytocannabinoids: A Misleading Name but a Promising Opportunity for Biomedical Research. Biomolecules, 12(8), 1084. https://doi.org/10.3390/biom12081084








