Electrospun Core–Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
3. Characteristics
3.1. Surface Morphology and Internal Structure of Nanofibers
3.2. Physical Form and Chemical Compatibility
3.3. Water Contact Angle (WCA) and Swelling Performance
3.4. Drug Release
3.5. Antibacterial Activity
4. Results and Discussion
4.1. Modified Triaxial Electrospinning Process
4.2. Surface Morphology and Internal Structure of Nanofibers
4.3. XRD and ATR-FTIR Characteristics
4.4. Performance of Functional Fibers
4.4.1. Surface Wettability and Swelling Performance
4.4.2. Drug Release
4.4.3. Antibacterial Activity
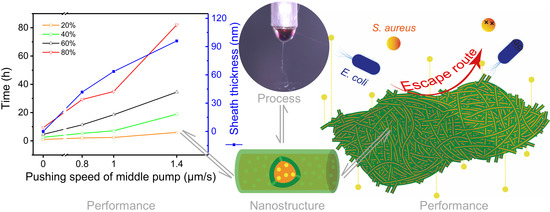
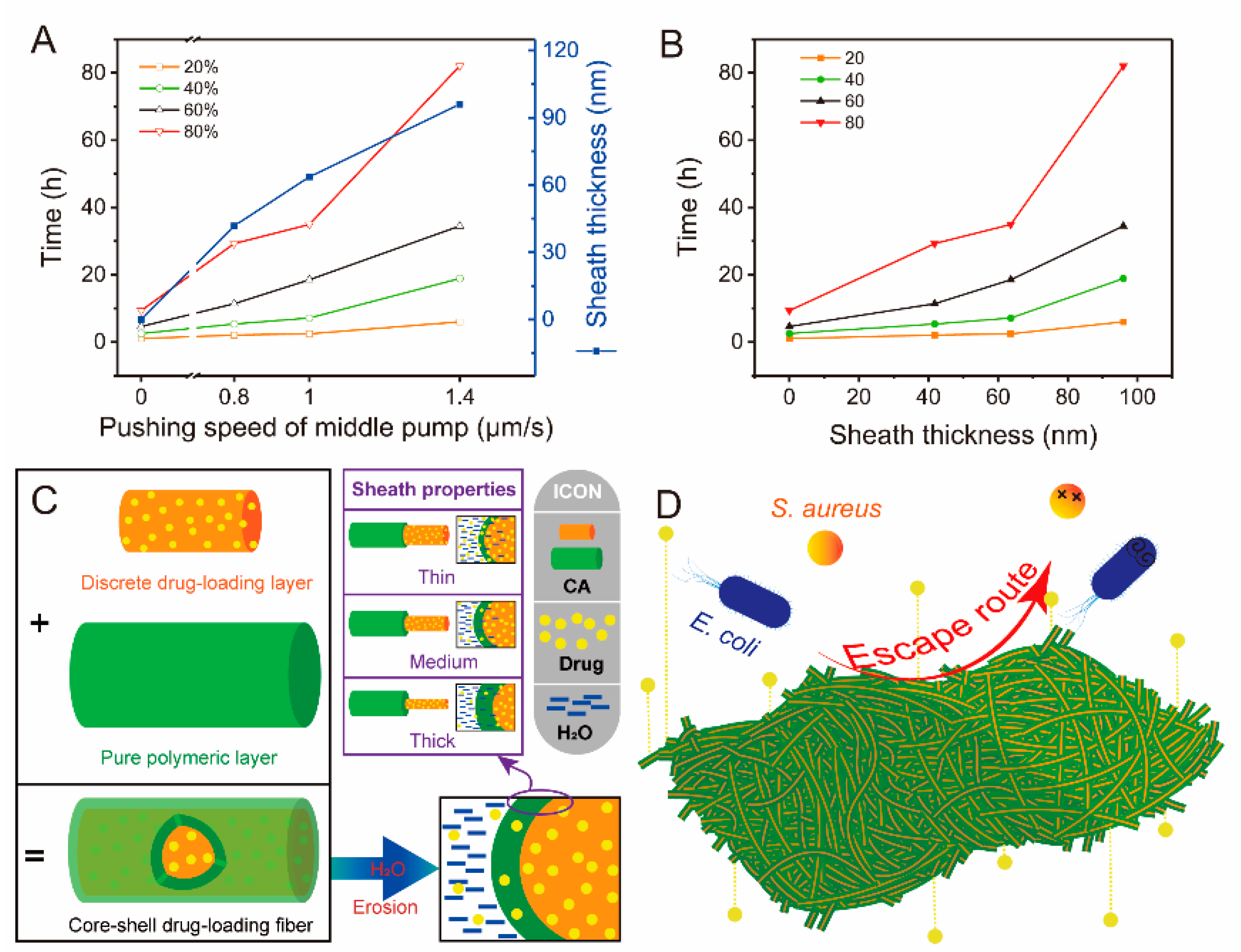
4.4.4. Performance and Mechanism Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Chen, X.; Radacsi, N. New tricks of old drugs: Repurposing non-chemo drugs and dietary phytochemicals as adjuvants in anti-tumor therapies. J. Controled Release 2021, 329, 96–120. [Google Scholar] [CrossRef] [PubMed]
- Agossa, K.; Delepierre, A.; Lizambard, M.; Delcourt-Debruyne, E.; Siepmann, J.; Siepmann, F.; Neut, C. In-Situ forming implants for dual controlled release of chlorhexidine and ibuprofen for periodontitis treatment: Microbiological and mechanical key properties. J. Drug Deliv. Sci. Technol. 2020, 60, 101956. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan oligosaccharide/alginate nanoparticles as an effective carrier for astaxanthin with improving stability, in vitro oral bioaccessibility, and bioavailability. Food Hydrocoll. 2022, 124, 107246. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising carrier of novel curcumin diethyl diglutarate. Int. J. Biol. Macromol. 2019, 131, 1125–1136. [Google Scholar] [CrossRef]
- Yu, D. Preface-bettering drug delivery knowledge from pharmaceutical techniques and excipients. Curr. Drug Deliv. 2021, 18, 2–3. [Google Scholar] [CrossRef]
- Basso, J.; Mendes, M.; Cova, T.; Sousa, J.; Pais, A.; Fortuna, A.; Vitorino, R.; Vitorino, C. A stepwise framework for the systematic development of lipid nanoparticles. Biomolecules 2022, 12, 233. [Google Scholar] [CrossRef]
- Ahmad, J. Lipid nanoparticles based cosmetics with potential application in alleviating skin disorders. Cosmetics 2021, 8, 84. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-delivery of imiquimod and curcumin by nanoemugel for improved topical delivery and reduced psoriasis-like skin lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Albarqi, H.A.; Alyami, H.S.; Alyami, M.H.; Alqahtani, A.A.; Alasiri, A.; Algahtani, T.S.; Mohammed, A.A.; et al. Preparation and characterization of curcumin nanoemulgel utilizing ultrasonication technique for wound healing: In vitro, ex vivo, and in vivo evaluation. Gels 2021, 7, 213. [Google Scholar] [CrossRef]
- Farkas, B.; Balogh, A.; Farkas, A.; Marosi, G.; Nagy, Z.K. Frequency and waveform dependence of alternating current electrospinning and their uses for drug dissolution enhancement. Int. J. Pharm. 2020, 586, 119593. [Google Scholar] [CrossRef]
- Tiboni, M.; Curzi, G.; Aluigi, A.; Casettari, L. An easy 3D printing approach to manufacture vertical diffusion cells for in vitro release and permeation studies. J. Drug Deliv. Sci. Technol. 2021, 65, 102661. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Torchio, A.; Cassino, C.; Lavella, M.; Gallina, A.; Stefani, A.; Boffito, M.; Ciardelli, G. Injectable supramolecular hydrogels based on custom-made poly(ether urethane)s and α-cyclodextrins as efficient delivery vehicles of curcumin. Mater. Sci. Eng. C 2021, 127, 112194. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Abrami, M.; Grassi, M.; Zoso, A.; Chiono, V.; Ciardelli, G. Dual stimuli-responsive polyurethane-based hydrogels as smart drug delivery carriers for the advanced treatment of chronic skin wounds. Bioact. Mater. 2021, 6, 3013–3024. [Google Scholar] [CrossRef]
- Farkas, B.; Balogh, A.; Cselkó, R.; Molnár, K.; Farkas, A.; Borbás, E.; Marosi, G.; Nagy, Z.K. Corona alternating current electrospinning: A combined approach for increasing the productivity of electrospinning. Int. J. Pharm. 2019, 561, 219–227. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.-G.; Liu, X.; Shen, H. Electrospun hierarchical structural films for effective wound healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Liu, P.; Chen, X.; Yu, D.-G. Electrospun multiple-chamber nanostructure and its potential self-healing applications. Polymers 2020, 12, 2413. [Google Scholar] [CrossRef]
- Ji, Y.; Song, W.; Xu, L.; Yu, D.-G.; Annie Bligh, S.W. A review on electrospun poly(amino acid) nanofibers and their applications of hemostasis and wound healing. Biomolecules 2022, 12, 794. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Lv, H.; Zhou, Y.; Yang, Y.; Yu, D.-G. Electrospun polyacrylonitrile-based lace nanostructures and their Cu (II) adsorption. Sep. Purif. Technol. 2022, 288, 120643. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Yang, Z.; Chen, X.; Yu, D.-G.; Shao, J. Hybrid films prepared from a combination of electrospinning and casting for offering a dual-phase drug release. Polymers 2022, 14, 2132. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Lu, X.; Murugadoss, V.; Huang, M.; Yang, H.; Wan, F.; Yu, D.-G.; Guo, Z. Electrospun structural nanohybrids combining three composites for fast helicide delivery. Adv. Compos. Hybrid Mater. 2022, 5, 1017–1029. [Google Scholar] [CrossRef]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.-G. Engineered spindles of little molecules around electrospun nanofibers for biphasic drug release. Adv. Fiber Mater. 2022, 4, 305–317. [Google Scholar] [CrossRef]
- Ning, T.; Zhou, Y.; Xu, H.; Guo, S.; Wang, K.; Yu, D.-G. Orodispersible membranes from a modified coaxial electrospinning for fast dissolution of diclofenac sodium. Membranes 2021, 11, 802. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Gao, Y.; Yu, D.-G.; Liu, P. Elaborate design of shell component for manipulating the sustained release behavior from core–shell nanofibres. J. Nanobiotechnol. 2022, 20, 244. [Google Scholar] [CrossRef]
- Irfan, A.; Feng, W.; Liu, K.; Habib, K.; Qu, Q.; Yang, L. TiO2-modified fibrous core-shell mesoporous material to selectively enrich endogenous phosphopeptides with proteins exclusion prior to CE-MS analysis. Talanta 2021, 235, 122737. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Bondi, E.; Posati, T.; Sotgiu, G.; Zamboni, R.; Torreggiani, A.; Corticelli, F.; Lotti, N.; Aluigi, A. Effects of the blending ratio on the design of keratin/poly(butylene succinate) nanofibers for drug delivery applications. Biomolecules 2021, 11, 1194. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yu, D.-G.; Liu, H.; Liu, Y.; Liu, P. Electrospun PVP-core/PHBV-shell fibers to eliminate tailing off for an improved sustained release of curcumin. Mol. Pharm. 2021, 18, 4170–4178. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Razak, S.I.A.; Bohari, S.P.M.; Shahir, S.; Salihu, R.; Kadir, M.R.A.; Nayan, N.H.M. Vitamin D3-loaded electrospun cellulose acetate/polycaprolactone nanofibers: Characterization, in-vitro drug release and cytotoxicity studies. Int. J. Biol. Macromol. 2021, 181, 82–98. [Google Scholar] [CrossRef]
- Peng, Y.; Ma, Y.; Bao, Y.; Liu, Z.; Chen, L.; Dai, F.; Li, Z. Electrospun PLGA/SF/artemisinin composite nanofibrous membranes for wound dressing. Int. J. Biol. Macromol. 2021, 183, 68–78. [Google Scholar] [CrossRef]
- Faralli, A.; Shekarforoush, E.; Ajalloueian, F.; Mendes, A.C.; Chronakis, I.S. In vitro permeability enhancement of curcumin across Caco-2 cells monolayers using electrospun xanthan-chitosan nanofibers. Carbohydr. Polym. 2019, 206, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Alba-Perez, A.; Jayawarna, V.; Childs, P.G.; Dalby, M.J.; Salmeron-Sanchez, M. Plasma polymerised nanoscale coatings of controlled thickness for efficient solid-phase presentation of growth factors. Mater. Sci. Eng. C 2020, 113, 110966. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Thaweesest, W.; Ratnatilaka Na Bhuket, P.; Jantaratana, P.; Rojsitthisak, P.; Rojsitthisak, P. Polyethylene glycol-chitosan oligosaccharide-coated superparamagnetic iron oxide nanoparticles: A novel drug delivery system for curcumin diglutaric acid. Biomolecules 2020, 10, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef] [PubMed]
- Luckanagul, J.A.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Wang, Q.; Rojsitthisak, P. Self-assembled thermoresponsive nanogel from grafted hyaluronic acid as a biocompatible delivery platform for curcumin with enhanced drug loading and biological activities. Polymers 2021, 13, 194. [Google Scholar] [CrossRef]
- Zheng, X.; Kang, S.; Wang, K.; Yang, Y.; Yu, D.-G.; Wan, F.; Williams, G.R.; Bligh, S.-W.A. Combination of structure-performance and shape-performance relationships for better biphasic release in electrospun Janus fibers. Int. J. Pharm. 2021, 596, 120203. [Google Scholar] [CrossRef]
- Xu, H.; Xu, X.; Li, S.; Song, W.-L.; Yu, D.-G.; Annie Bligh, S.W. The effect of drug heterogeneous distributions within core-sheath nanostructures on its sustained release profiles. Biomolecules 2021, 11, 1330. [Google Scholar] [CrossRef]
- Peppas, N. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun coaxial fibers to optimize the release of poorly water-soluble drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef]
- Parin, F.N.; Ullah, S.; Yildirim, K.; Hashmi, M.; Kim, I.S. Fabrication and characterization of electrospun folic acid/hybrid fibers: In vitro controlled release study and cytocompatibility assays. Polymers 2021, 13, 3594. [Google Scholar] [CrossRef]
- Rezk, A.I.; Bhattarai, D.P.; Park, J.; Park, C.H.; Kim, C.S. Polyaniline-coated titanium oxide nanoparticles and simvastatin-loaded poly(ε-caprolactone) composite nanofibers scaffold for bone tissue regeneration application. Colloids Surf. B 2020, 192. [Google Scholar] [CrossRef]
- Khan, A.u.R.; Huang, K.; Khalaji, M.S.; Yu, F.; Xie, X.; Zhu, T.; Morsi, Y.; Jinzhong, Z.; Mo, X. Multifunctional bioactive core-shell electrospun membrane capable to terminate inflammatory cycle and promote angiogenesis in diabetic wound. Bioact. Mater. 2021, 6, 2783–2800. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial mechanism of curcumin: A review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, N.D.; Rashkov, I.B. Electrospun curcumin-loaded cellulose acetate/polyvinylpyrrolidone fibrous materials with complex architecture and antibacterial activity. Mater. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef]
- Tsekova, P.; Spasova, M.; Manolova, N.; Rashkov, I.; Markova, N.; Georgieva, A.; Toshkova, R. Electrospun cellulose acetate membranes decorated with curcumin-PVP particles: Preparation, antibacterial and antitumor activities. J. Mater. Sci. Mater. Med. 2018, 29, 1–14. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun medical sutures for wound healing: A review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Jin, C.; Wu, Z.; Zhang, R.; Qian, X.; Xu, H.; Che, R. 1D electromagnetic-gradient hierarchical carbon microtube via coaxial electrospinning design for enhanced microwave absorption. ACS Appl. Mater. Interfaces 2021, 13, 15939–15949. [Google Scholar] [CrossRef]
- Zhang, X.; Chi, C.; Chen, J.; Zhang, X.; Gong, M.; Wang, X.; Yan, J.; Shi, R.; Zhang, L.; Xue, J. Electrospun quad-axial nanofibers for controlled and sustained drug delivery. Mater. Des. 2021, 206, 109732. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, Y.-S.; Shao, Y.-W.; Huang, T.; Zhang, N.; Yang, J.-H.; Qi, X.-D.; Wang, Y. Coaxial electrospun membranes with thermal energy storage and shape memory functions for simultaneous thermal/moisture management in personal cooling textiles. Eur. Polym. J. 2021, 145, 110245. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater. Sci. Eng. C 2021, 118, 111514. [Google Scholar] [CrossRef]
- Jaworska, J.; Smolarczyk, R.; Musiał-Kulik, M.; Cichoń, T.; Karpeta-Jarząbek, P.; Włodarczyk, J.; Stojko, M.; Janeczek, H.; Kordyka, A.; Kaczmarczyk, B.; et al. Electrospun paclitaxel delivery system based on PGCL/PLGA in local therapy combined with brachytherapy. Int. J. Pharm. 2021, 602, 120596. [Google Scholar] [CrossRef] [PubMed]
- Huo, P.; Han, X.; Zhang, W.; Zhang, J.; Kumar, P.; Liu, B. Electrospun nanofibers of polycaprolactone/collagen as a sustained-release drug delivery system for artemisinin. Pharmaceutics 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jiang, W.; Shen, L.; Zhang, G.; Gao, Y.; Yang, Y.; Yu, D.-G. Electrospun hybrid films for fast and convenient delivery of active herb extracts. Membranes 2022, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Czapka, T.; Winkler, A.; Maliszewska, I.; Kacprzyk, R. Fabrication of photoactive electrospun cellulose acetate nanofibers for antibacterial applications. Energies 2021, 14, 2598. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, C.; Zou, Y.; Li, Y.; Zhang, H. Immobilization of lysozyme on layer-by-layer self-assembled electrospun nanofibers treated by post-covalent crosslinking. Food Hydrocoll. 2021, 121, 106999. [Google Scholar] [CrossRef]
- Prakash, J.; Venkataprasanna, K.; Bharath, G.; Banat, F.; Niranjan, R.; Venkatasubbu, G.D. In-vitro evaluation of electrospun cellulose acetate nanofiber containing Graphene oxide/TiO2/Curcumin for wound healing application. Colloids Surf. A 2021, 627, 127166. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Majumder, S.; Gupta, K.C.; Kumar, A. Dextran based amphiphilic nano-hybrid hydrogel system incorporated with curcumin and cerium oxide nanoparticles for wound healing. Colloids Surf. B 2020, 195, 111263. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Maya Nandkumar, A.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA—hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef]
- Sharma, D.; Satapathy, B.K. Understanding release kinetics and collapse proof suture retention response of curcumin loaded electrospun mats based on aliphatic polyesters and their blends. J. Mech. Behav. Biomed. Mater. 2021, 120, 104556. [Google Scholar] [CrossRef]
- Ko, S.W.; Lee, J.Y.; Lee, J.; Son, B.C.; Jang, S.R.; Aguilar, L.E.; Oh, Y.M.; Park, C.H.; Kim, C.S. Analysis of drug release behavior utilizing the swelling characteristics of cellulosic nanofibers. Polymers 2019, 11, 1376. [Google Scholar] [CrossRef] [Green Version]
- Rostamitabar, M.; Subrahmanyam, R.; Gurikov, P.; Seide, G.; Jockenhoevel, S.; Ghazanfari, S. Cellulose aerogel micro fibers for drug delivery applications. Mater. Sci. Eng. C 2021, 127, 112196. [Google Scholar] [CrossRef]
- Moutaharrik, S.; Maroni, A.; Melocchi, A.; Zema, L.; Foppoli, A.; Cerea, M.; Palugan, L.; Neut, C.; Siepmann, F.; Siepmann, J.; et al. Oral colon delivery platform based on a novel combination approach: Design concept and preliminary evaluation. J. Drug Deliv. Sci. Technol. 2021, 66, 102919. [Google Scholar] [CrossRef]
- Guo, M.; Wei, Y.; Lee, H.; Maia, J.; Morrison, E. One-step extrusion of concentrated lidocaine lipid nanocarrier (LNC) dispersions. Int. J. Pharm. 2020, 589, 119817. [Google Scholar] [CrossRef]
- Bassand, C.; Verin, J.; Lamatsch, M.; Siepmann, F.; Siepmann, J. How agarose gels surrounding PLGA implants limit swelling and slow down drug release. J. Controled Release 2022, 343, 255–266. [Google Scholar] [CrossRef]
- Huang, G.; Yan, Y.; Xu, D.; Wu, J.; Xu, C.; Fu, L.; Lin, B. Curcumin-loaded nanoMOFs@CMFP: A biological preserving paste with antibacterial properties and long-acting, controllable release. Food Chem. 2021, 337, 127987. [Google Scholar] [CrossRef]
- Kour, P.; Afzal, S.; Gani, A.; Zargar, M.I.; Nabi Tak, U.; Rashid, S.; Dar, A.A. Effect of nanoemulsion-loaded hybrid biopolymeric hydrogel beads on the release kinetics, antioxidant potential and antibacterial activity of encapsulated curcumin. Food Chem. 2022, 376, 131925. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Devahastin, S.; Liu, K. Fabrication of curcumin encapsulated in casein-ethyl cellulose complexes and its antibacterial activity when applied in combination with blue LED irradiation. Food Control 2022, 134, 108702. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Gimeno-Alcañiz, J.V.; Ocio, M.J.; Lagaron, J.M. Controlled delivery of gentamicin antibiotic from bioactive electrospun polylactide-based ultrathin fibers. Adv. Eng. Mater. 2012, 14, B112–B122. [Google Scholar] [CrossRef]
- Jalvandi, J.; White, M.; Truong, Y.B.; Gao, Y.; Padhye, R.; Kyratzis, I.L. Release and antimicrobial activity of levofloxacin from composite mats of poly(ɛ-caprolactone) and mesoporous silica nanoparticles fabricated by core–shell electrospinning. J. Mater. Sci. 2015, 50, 7967–7974. [Google Scholar] [CrossRef]
- Domokos, A.; Balogh, A.; Dénes, D.; Nyerges, G.; Ződi, L.; Farkas, B.; Marosi, G.; Nagy, Z.K. Continuous manufacturing of orally dissolving webs containing a poorly soluble drug via electrospinning. Eur. J. Pharm. Sci. 2019, 130, 91–99. [Google Scholar] [CrossRef]
- Casian, T.; Borbás, E.; Ilyés, K.; Démuth, B.; Farkas, A.; Rapi, Z.; Bogdan, C.; Iurian, S.; Toma, V.; Știufiuc, R.; et al. Electrospun amorphous solid dispersions of meloxicam: Influence of polymer type and downstream processing to orodispersible dosage forms. Int. J. Pharm. 2019, 569, 118593. [Google Scholar] [CrossRef]
- Benzine, Y.; Siepmann, F.; Neut, C.; Danede, F.; Francois Willart, J.; Siepmann, J.; Karrout, Y. Injection-molded capsule bodies and caps based on polymer blends for controlled drug delivery. Eur. J. Pharm. Biopharm. 2021, 168, 1–14. [Google Scholar] [CrossRef]
- Salahuddin, N.; Gaber, M.; Elneanaey, S.; Abdelwahab, M.A. Norfloxacin-tenoxicam dual drug delivery system based on poly(lactic acid) microspheres and electrospun fibers: Release and in vivo study. Express Polym. Lett. 2022, 16, 368–387. [Google Scholar] [CrossRef]
- Yu, D.G.; Wang, M.; Ge, R. Strategies for sustained drug release from electrospun multi-layer nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 14, e1772. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Shi, X.; Li, J.; Yao, Q.; Zhang, P. Fabrication of epirubicin loaded core/shell electrospun fibers with effective transdermal sustained-release properties. Mater. Lett. 2021, 299, 130117. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Song, W.; Zhang, Y.; Yu, D.-G.; Liu, Y. Electrospun core (HPMC-acetaminophen)-shell (PVP-sucralose) nanohybrids for rapid drug delivery. Gels 2022, 8, 357. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Liu, Y.; Gao, Y.; Kim, H.Y.; Ouyang, Y.; Yu, D.-G. Progresses on electrospun metal–organic frameworks nanofibers and their wastewater treatment applications. Mater. Today Chem. 2022, 25, 100974. [Google Scholar] [CrossRef]

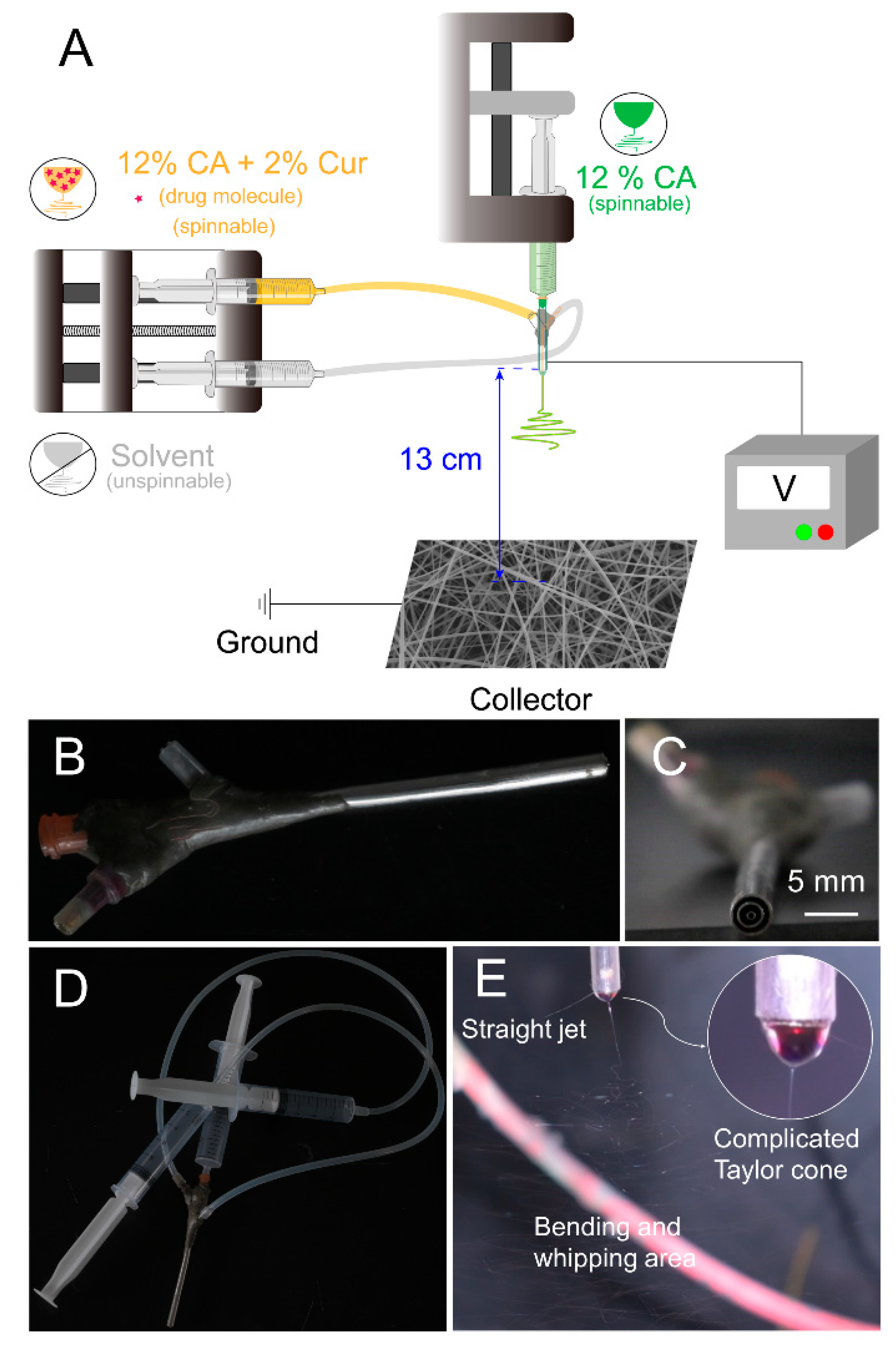
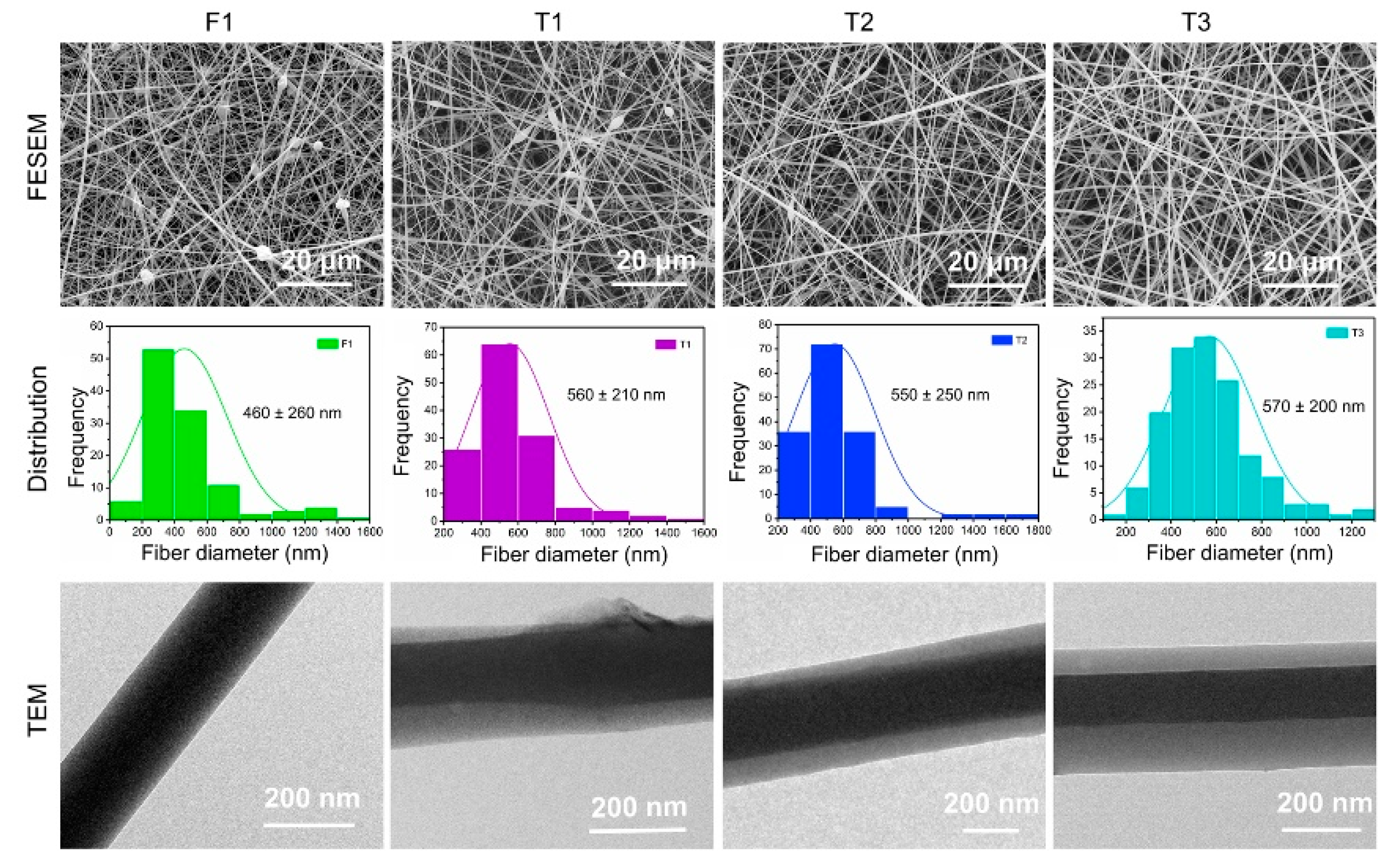


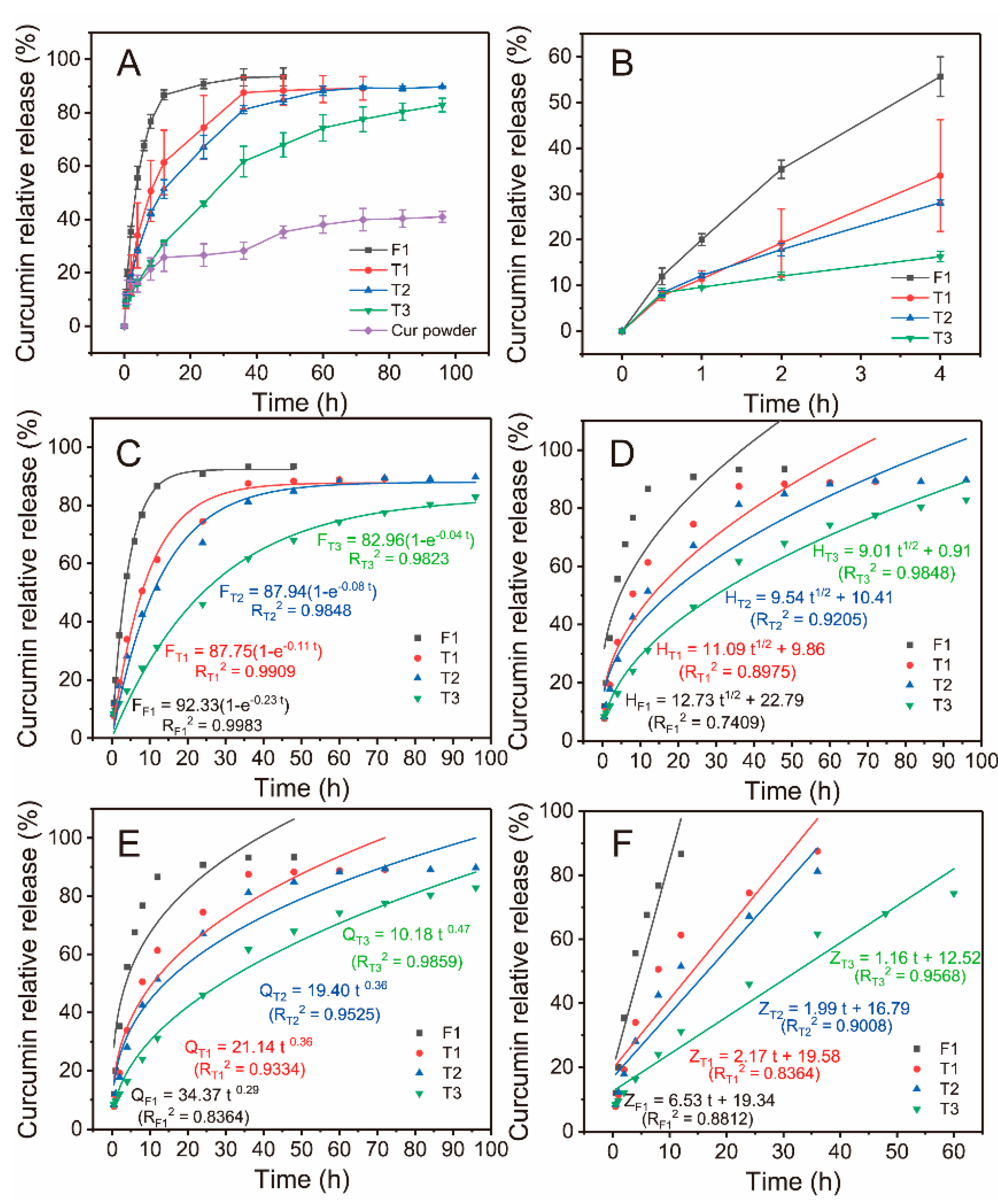

| No. | Working Process | Working Fluid a (w/v) | The Pushing Speed of Pump (μm/s) | Structure | |||
|---|---|---|---|---|---|---|---|
| Inner | Middle | Inner | Middle | Outer b | |||
| F1 | Single-fluid electrospinning | 12% CA + 2% Cur | - | 1.4 | - | - | Monolithic |
| T1 | Modified triaxial electrospinning | 12% CA + 2% Cur | 12% CA | 1.4 | 0. 8 | 1.4 | Core–sheath |
| T2 | 12% CA + 2% Cur | 12% CA | 1.4 | 1.0 | 1.4 | Core–sheath | |
| T3 | 12% CA + 2% Cur | 12% CA | 1.4 | 1.4 | 1.4 | Core–sheath | |
| No. | Sheath Layer Thickness | Core Layer Thickness | Thickness Ratio of Core and Sheath Layer | Upper Sheath Thickness | Thickness Of Lower Sheath |
|---|---|---|---|---|---|
| T1 | 225 ± 16 nm | 148 ± 9 nm | 0.66 | 26 ± 5 nm | 57 ± 23 nm |
| T2 | 382 ± 7 nm | 257 ± 3 nm | 0.67 | 48 ± 18 nm | 79 ± 10 nm |
| T3 | 319 ± 3 nm | 130 ± 0 nm | 0.41 | 61 ± 4 nm | 124 ± 2 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, X.; Gao, Y.; Liu, Y.; Yu, D.; Liu, P. Electrospun Core–Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance. Biomolecules 2022, 12, 1057. https://doi.org/10.3390/biom12081057
Liu Y, Chen X, Gao Y, Liu Y, Yu D, Liu P. Electrospun Core–Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance. Biomolecules. 2022; 12(8):1057. https://doi.org/10.3390/biom12081057
Chicago/Turabian StyleLiu, Yubo, Xiaohong Chen, Yuhang Gao, Yuyang Liu, Dengguang Yu, and Ping Liu. 2022. "Electrospun Core–Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance" Biomolecules 12, no. 8: 1057. https://doi.org/10.3390/biom12081057
APA StyleLiu, Y., Chen, X., Gao, Y., Liu, Y., Yu, D., & Liu, P. (2022). Electrospun Core–Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance. Biomolecules, 12(8), 1057. https://doi.org/10.3390/biom12081057