Degradation of Exogenous Fatty Acids in Escherichia coli
Abstract
1. Introduction
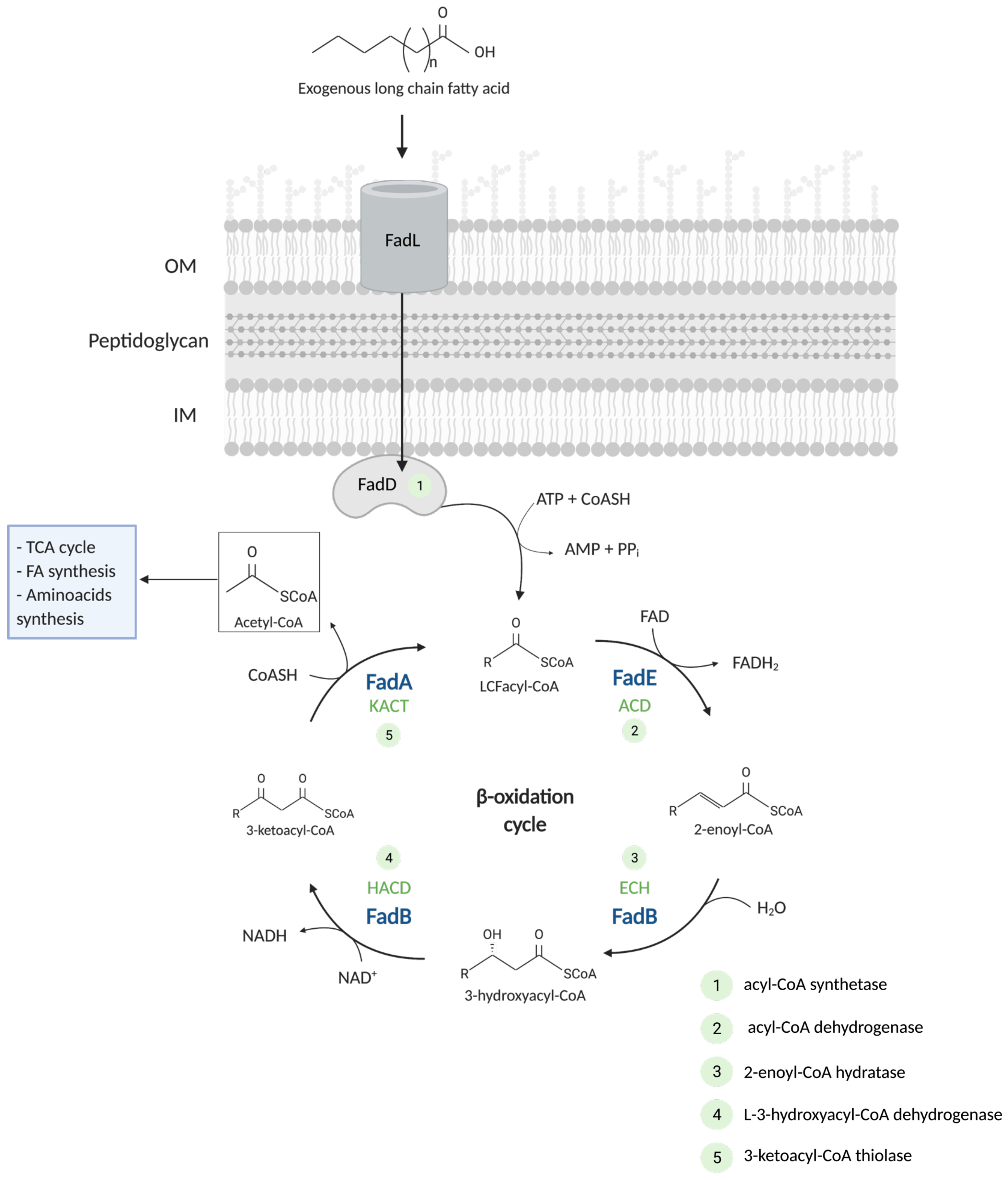
2. Pathways of Fatty Acid Degradation
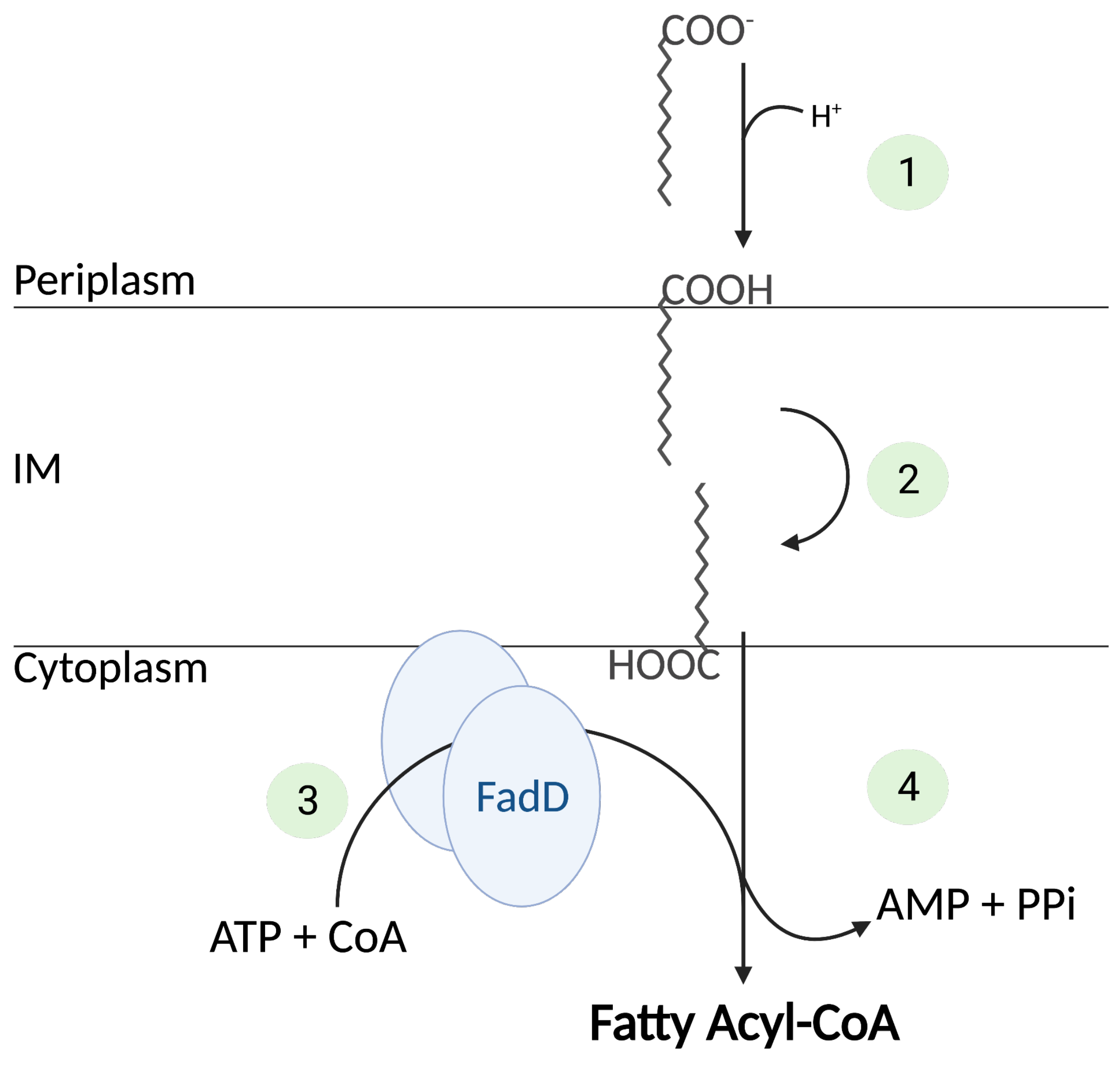
2.1. Translocation of Fatty Acid in the Cell
2.2. The β-Oxidation Cycle
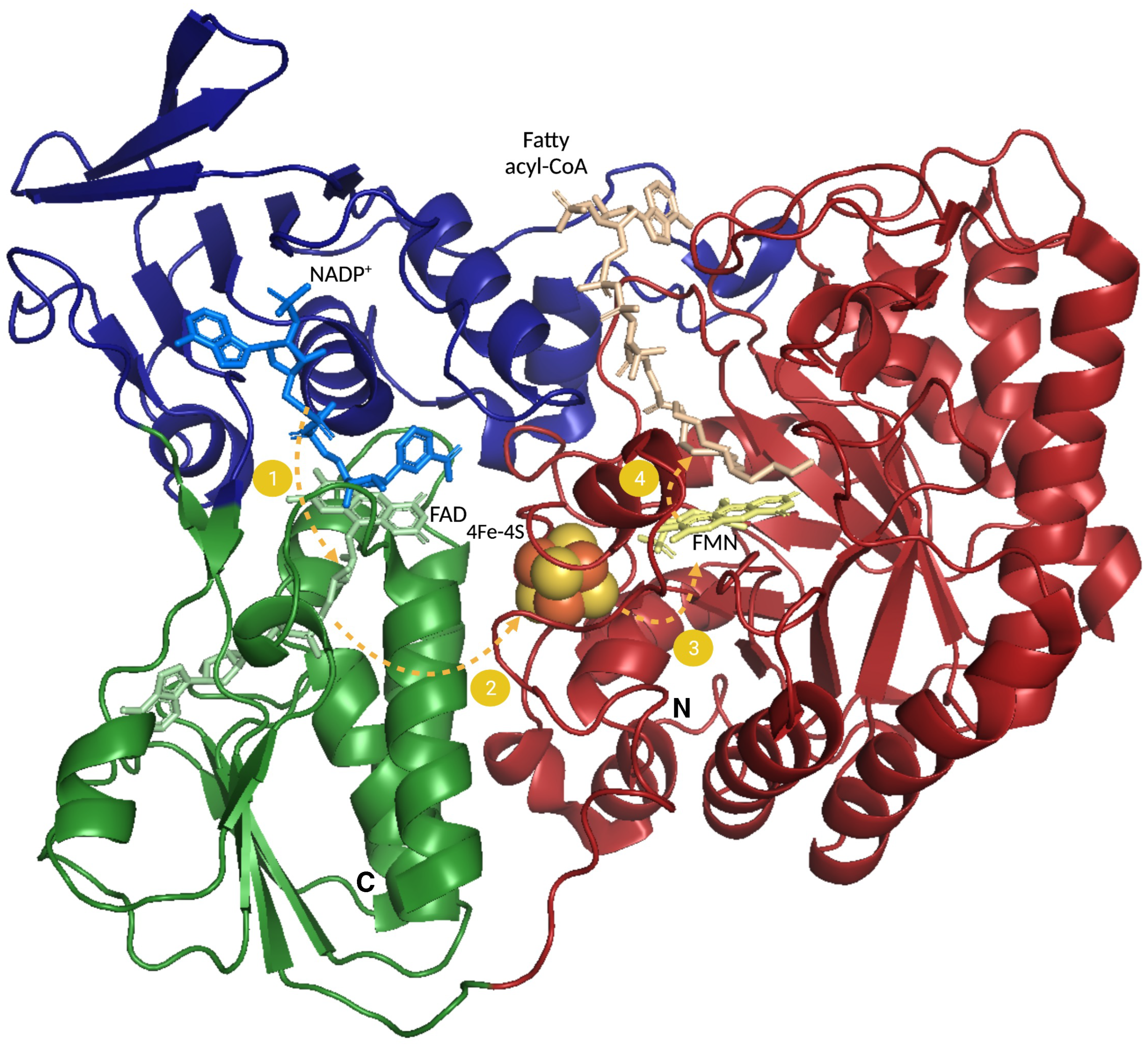
2.2.1. Degradation of Saturated Fatty Acids
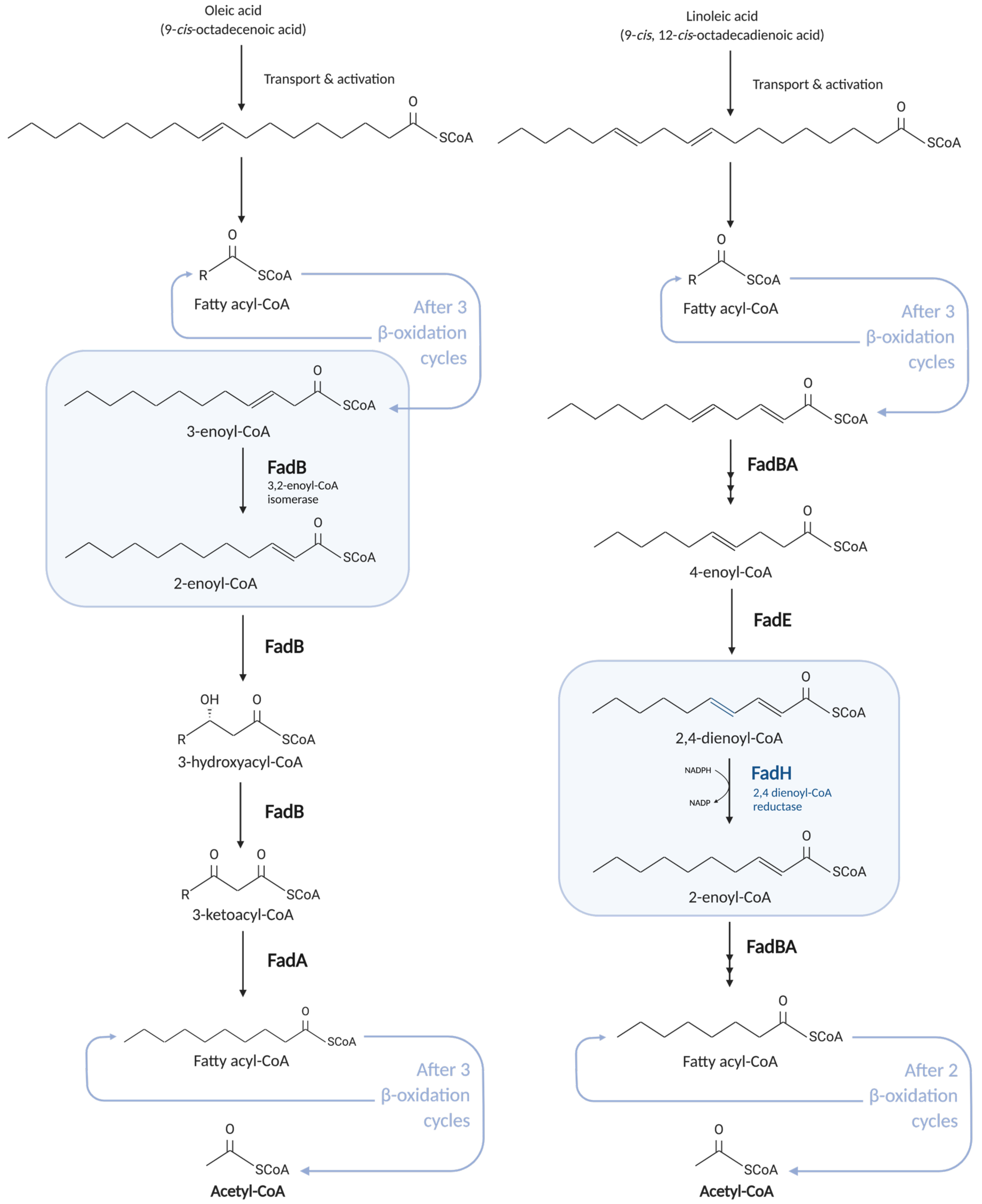
2.2.2. Degradation of Unsaturated Fatty Acids
2.2.3. S/MCFA Degradation
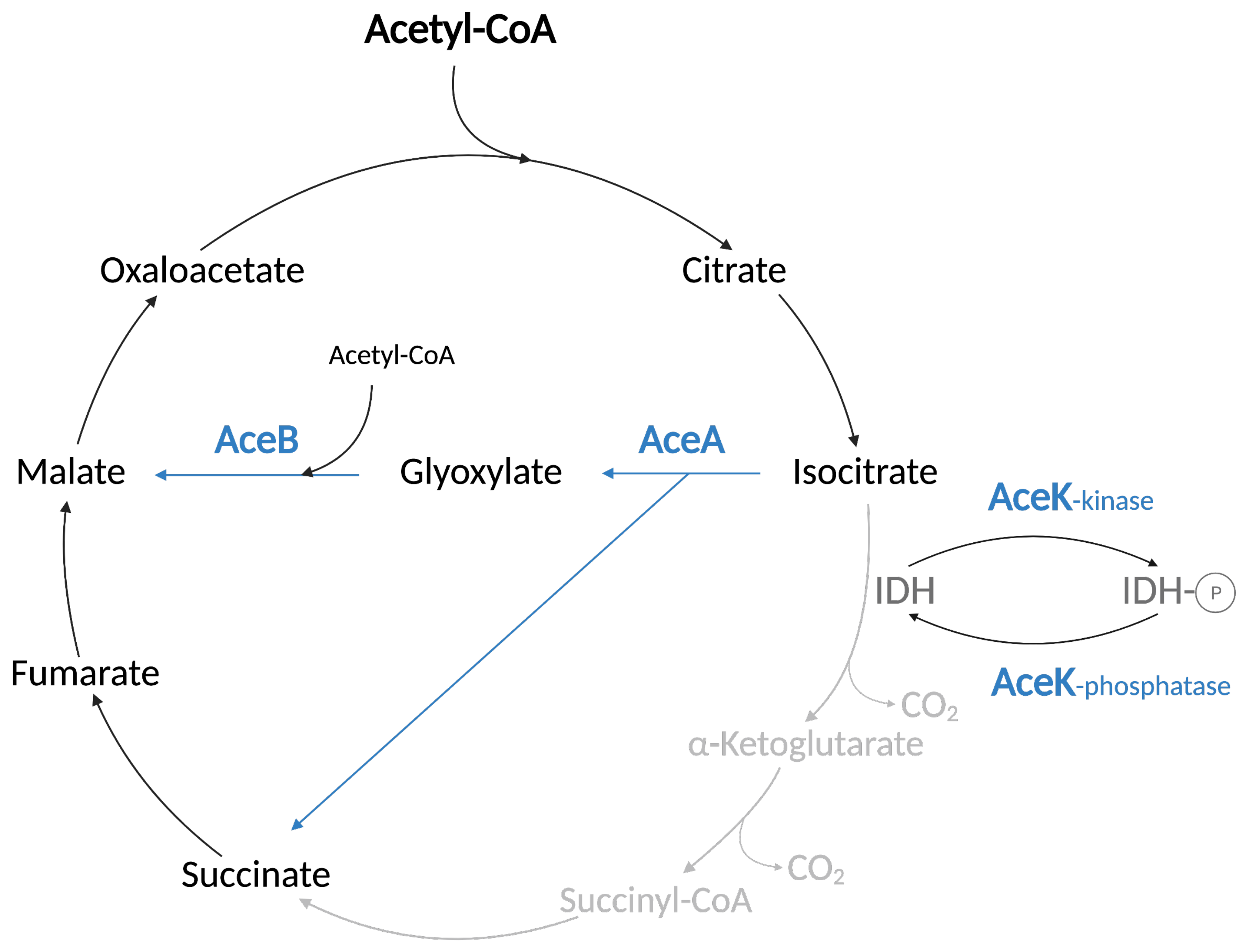
2.2.4. The TCA Cycle and Glyoxylate Shunt
2.2.5. Fatty Acid β-Oxidation and the Respiratory Chain
2.3. Diversity of the Fad Enzymes
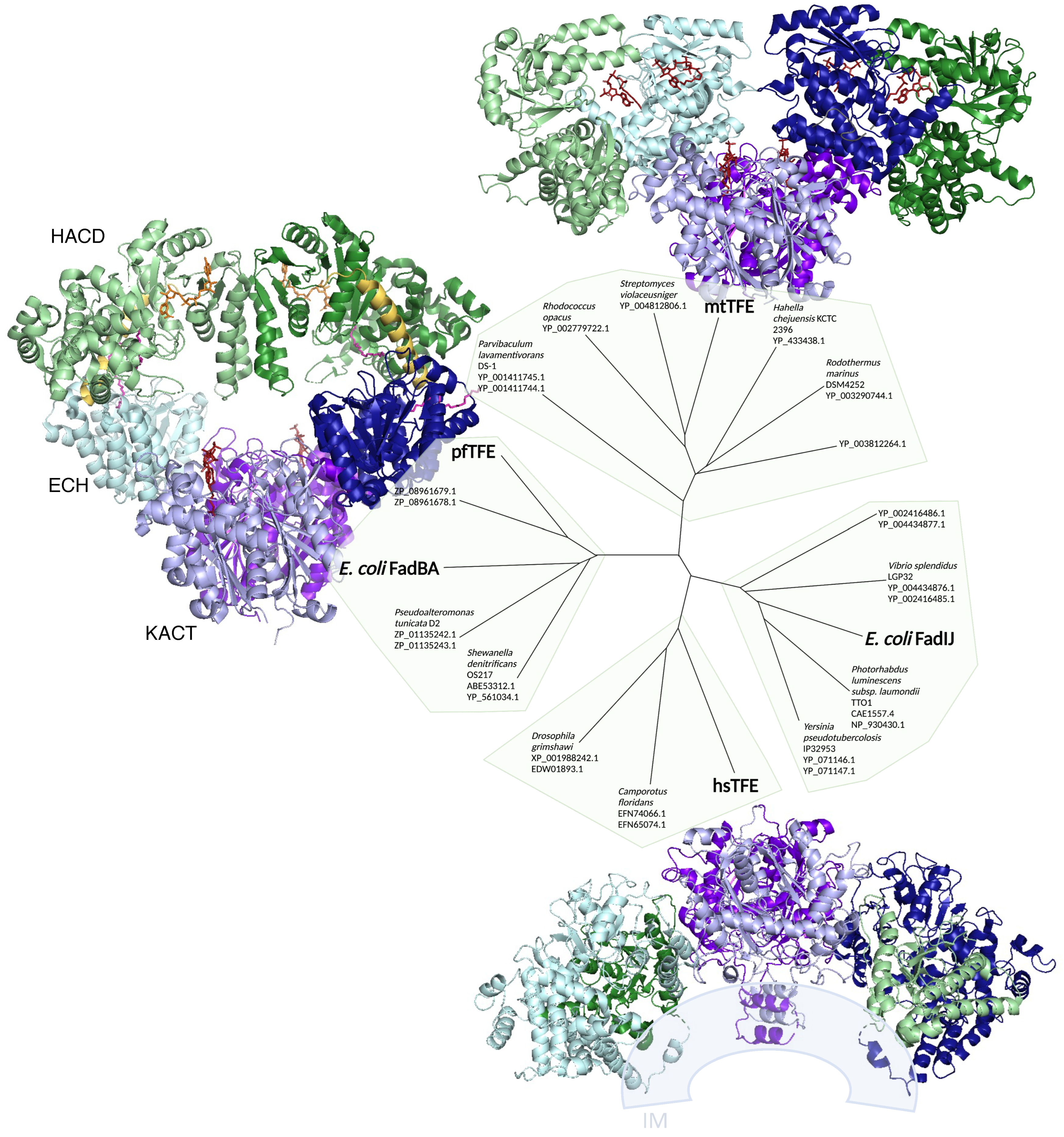
2.3.1. TFE Diversity in Bacteria
2.3.2. A Second TFE in E. coli: The FadIJ Proteins
2.3.3. Acyl-CoA Synthetase Diversity in Bacteria
2.3.4. The Acyl-CoA Synthetase FadK
3. Genetic Regulation of Fatty Acid Degradation in E. coli
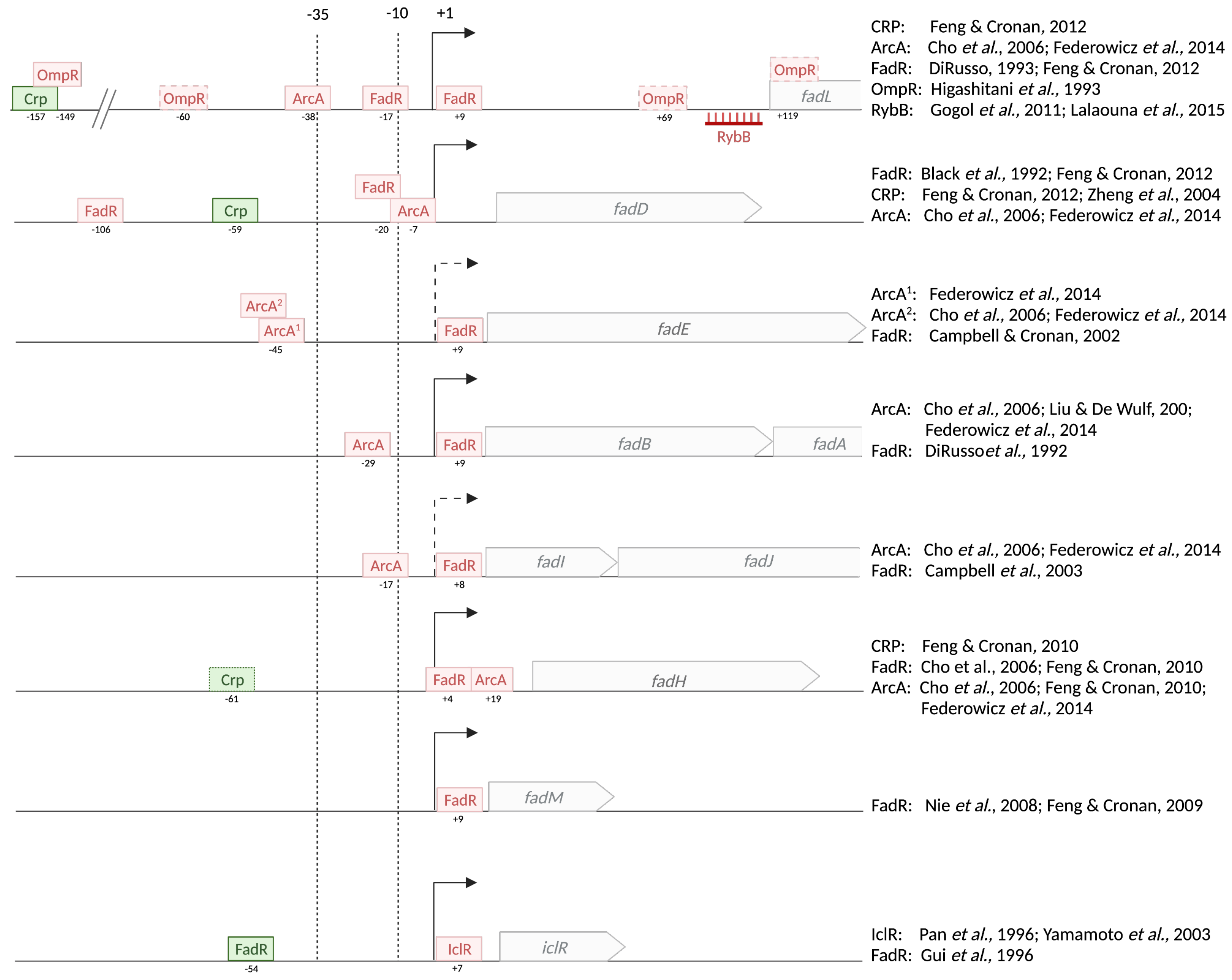
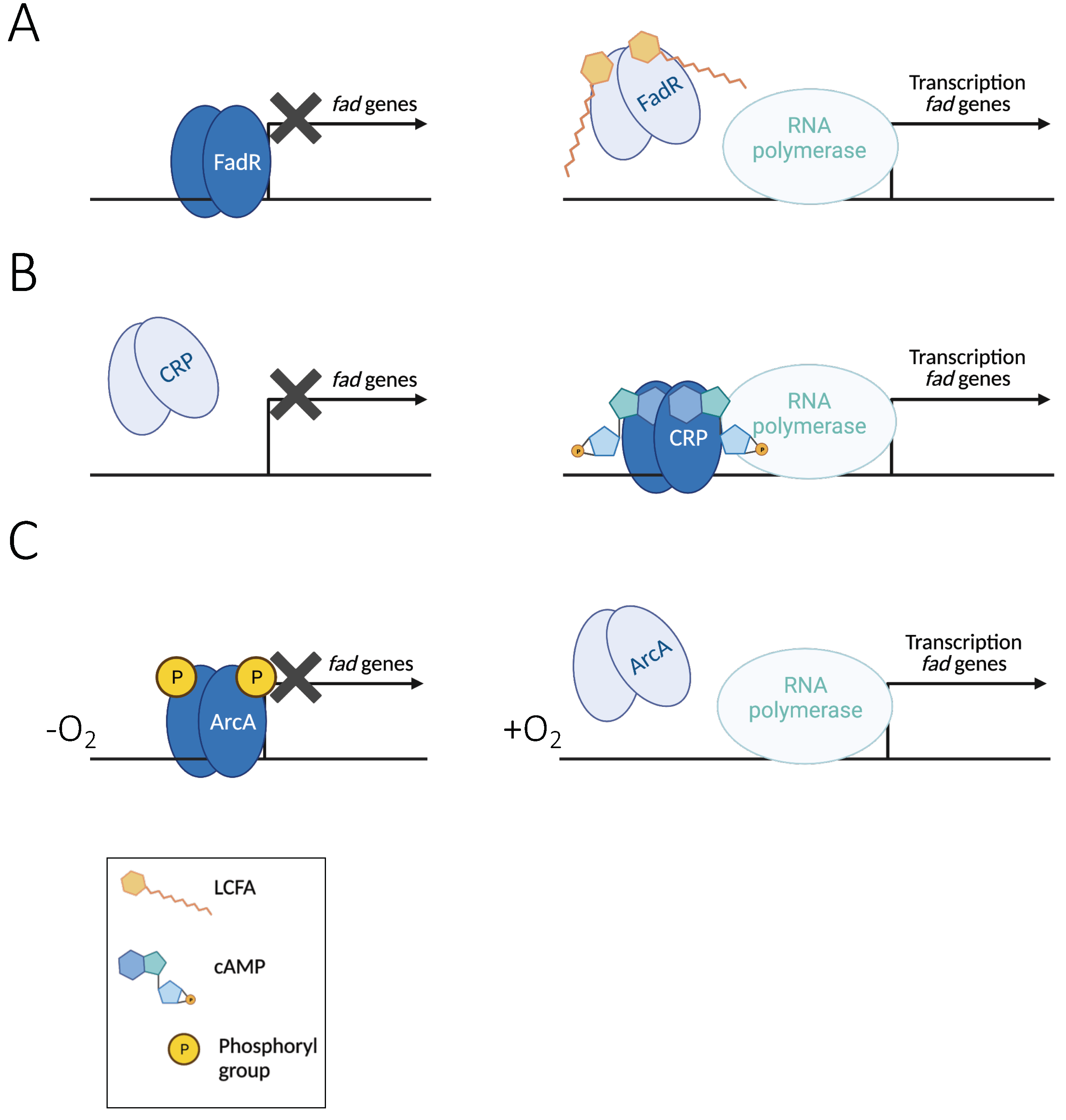
3.1. Regulation by Substrate Availability: The Transcriptional Repressor FadR
3.1.1. FadR DNA Binding Features
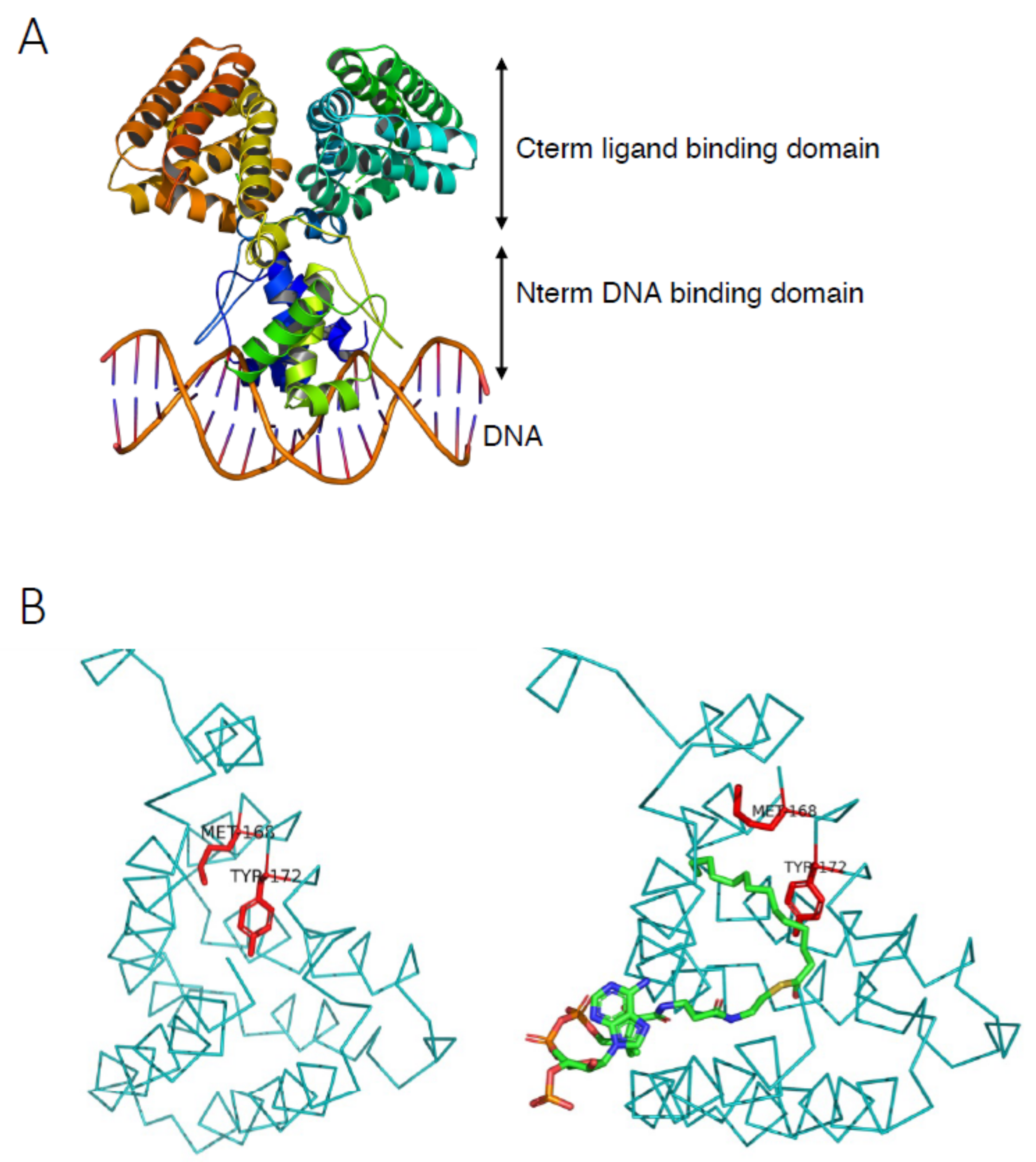
3.1.2. FadR Ligand Binding Properties
3.1.3. The FadR Regulon
3.2. Regulation by Carbon Source Availability: The CRP/cAMP Activator

3.3. Repression in Anaerobiosis by the ArcAB Two-Component System
3.4. Regulation of fadL by Stress Response
4. Open Questions and Research Needs
Author Contributions
Funding
Conflicts of Interest
References
- Pifer, R.; Russell, R.M.; Kumar, A.; Curtis, M.M.; Sperandio, V. Redox, amino acid, and fatty acid metabolism intersect with bacterial virulence in the gut. Proc. Natl. Acad. Sci. USA 2018, 115, E10712–E10719. [Google Scholar] [CrossRef] [PubMed]
- May, K.L.; Silhavy, T.J. The Escherichia coli Phospholipase PldA Regulates Outer Membrane Homeostasis via Lipid Signaling. mBio 2018, 9, e00379-18. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, M.; Jimenez, A.G.; Pifer, R.; Ruiz, N.; Sperandio, V. The Canonical Long-Chain Fatty Acid Sensing Machinery Processes Arachidonic Acid to Inhibit Virulence in Enterohemorrhagic Escherichia coli. mBio 2021, 12, e03247-20. [Google Scholar] [CrossRef] [PubMed]
- Beites, T.; Jansen, R.S.; Wang, R.; Jinich, A.; Rhee, K.Y.; Schnappinger, D.; Ehrt, S. Multiple acyl-CoA dehydrogenase deficiency kills Mycobacterium tuberculosis in vitro and during infection. Nat. Commun. 2021, 12, 6593. [Google Scholar] [CrossRef]
- DiRusso, C.C.; Black, P.N.; Weimar, J.D. Molecular inroads into the regulation and metabolism of fatty acids, lessons from bacteria. Prog. Lipid Res. 1999, 38, 129–197. [Google Scholar] [CrossRef]
- Pech-Canul, Á.; Nogales, J.; Miranda-Molina, A.; Alvarez, L.; Geiger, O.; Soto, M.J.; López-Lara, I.M. FadD is required for utilization of endogenous fatty acids released from membrane lipids. J. Bacteriol. 2011, 193, 6295–6304. [Google Scholar] [CrossRef]
- Pech-Canul, Á.C.; Rivera-Hernández, G.; Nogales, J.; Geiger, O.; Soto, M.J.; López-Lara, I.M. Role of Sinorhizobium meliloti and Escherichia coli Long-Chain Acyl-CoA Synthetase FadD in Long-Term Survival. Microorganisms 2020, 8, 470. [Google Scholar] [CrossRef]
- Black, P.N.; DiRusso, C.C. Transmembrane movement of exogenous long-chain fatty acids: Proteins, enzymes, and vectorial esterification. Microbiol. Mol. Biol. Rev. 2003, 67, 454–472. [Google Scholar] [CrossRef]
- Van den Berg, B.; Black, P.N.; Clemons, W.M.; Rapoport, T.A. Crystal structure of the long-chain fatty acid transporter FadL. Science 2004, 304, 1506–1509. [Google Scholar] [CrossRef]
- Hearn, E.M.; Patel, D.R.; Lepore, B.W.; Indic, M.; van den Berg, B. Transmembrane passage of hydrophobic compounds through a protein channel wall. Nature 2009, 458, 367–370. [Google Scholar] [CrossRef]
- Lepore, B.W.; Indic, M.; Pham, H.; Hearn, E.M.; Patel, D.R.; van den Berg, B. Ligand-gated diffusion across the bacterial outer membrane. Proc. Natl. Acad. Sci. USA 2011, 108, 10121–10126. [Google Scholar] [CrossRef] [PubMed]
- Overath, P.; Pauli, G.; Schairer, H.U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur. J. Biochem. 1969, 7, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Steinberg, R.; Fiethen, B.; Overath, P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur. J. Biochem. 1971, 19, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Weimar, J.D.; DiRusso, C.C.; Delio, R.; Black, P.N. Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous long-chain fatty acids. Amino acid residues within the ATP/AMP signature motif of Escherichia coli FadD are required for enzyme activity and fatty acid transport. J. Biol. Chem. 2002, 277, 29369–29376. [Google Scholar]
- Nunn, W.D.; Simons, R.W. Transport of long-chain fatty acids by Escherichia coli: Mapping and characterization of mutants in the fadL gene. Proc. Natl. Acad. Sci. USA 1978, 75, 3377–3381. [Google Scholar] [CrossRef]
- Black, P.N.; Kianian, S.F.; DiRusso, C.C.; Nunn, W.D. Long-chain fatty acid transport in Escherichia coli. Cloning, mapping, and expression of the fadL gene. J. Biol. Chem. 1985, 260, 1780–1789. [Google Scholar] [CrossRef]
- Black, P.N. Characterization of FadL-specific fatty acid binding in Escherichia coli. Biochim. Biophys Acta. 1990, 1046, 97–105. [Google Scholar] [CrossRef]
- Rock, C.O.; Jackowski, S. Pathways for the incorporation of exogenous fatty acids into phosphatidylethanolamine in Escherichia coli. J. Biol. Chem. 1985, 260, 12720–12724. [Google Scholar] [CrossRef]
- Campbell, J.W.; Cronan, J.E. The enigmatic Escherichia coli fadE gene is yafH. J. Bacteriol. 2002, 184, 3759–3764. [Google Scholar] [CrossRef]
- Pawar, S.; Schulz, H. The structure of the multienzyme complex of fatty acid oxidation from Escherichia coli. J. Biol. Chem. 1981, 256, 3894–3899. [Google Scholar] [CrossRef]
- Yang, S.Y.; Li, J.M.; He, X.Y.; Cosloy, S.D.; Schulz, H. Evidence that the fadB gene of the fadAB operon of Escherichia coli encodes 3-hydroxyacyl-coenzyme A (CoA) epimerase, delta 3-cis-delta 2-trans-enoyl-CoA isomerase, and enoyl-CoA hydratase in addition to 3-hydroxyacyl-CoA dehydrogenase. J. Bacteriol. 1988, 170, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Smeland, T.E.; Cuebas, D.; Schulz, H. Epimerization of 3-hydroxy-4-trans-decenoyl coenzyme A by a dehydration/hydration mechanism catalyzed by the multienzyme complex of fatty acid oxidation from Escherichia coli. J. Biol. Chem. 1991, 266, 23904–23908. [Google Scholar] [CrossRef]
- Ishikawa, M.; Tsuchiya, D.; Oyama, T.; Tsunaka, Y.; Morikawa, K. Structural basis for channelling mechanism of a fatty acid beta-oxidation multienzyme complex. EMBO J. 2004, 23, 2745–2754. [Google Scholar] [CrossRef]
- Venkatesan, R.; Wierenga, R.K. Structure of mycobacterial β-oxidation trifunctional enzyme reveals its altered assembly and putative substrate channeling pathway. ACS Chem. Biol. 2013, 8, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Fu, Z.; Battaile, K.P.; Kim, J.P. Crystal structure of human mitochondrial trifunctional protein, a fatty acid β-oxidation metabolon. Proc. Natl. Acad. Sci. USA 2019, 116, 6069–6074. [Google Scholar] [CrossRef]
- Sah-Teli, S.K.; Hynönen, M.J.; Sulu, R.; Dalwani, S.; Schmitz, W.; Wierenga, R.K.; Venkatesan, R. Insights into the stability and substrate specificity of the E. coli aerobic β-oxidation trifunctional enzyme complex. J. Struct Biol. 2020, 210, 107494. [Google Scholar] [CrossRef]
- Kunau, W.H.; Dommes, V.; Schulz, H. beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: A century of continued progress. Prog Lipid Res. 1995, 34, 267–342. [Google Scholar] [CrossRef]
- Liang, K.; Li, N.; Wang, X.; Dai, J.; Liu, P.; Wang, C.; Chen, X.-W.; Gao, N.; Xiao, J. Cryo-EM structure of human mitochondrial trifunctional protein. Proc. Natl. Acad. Sci. USA 2018, 115, 7039–7044. [Google Scholar] [CrossRef]
- Pramanik, A.; Pawar, S.; Antonian, E.; Schulz, H. Five different enzymatic activities are associated with the multienzyme complex of fatty acid oxidation from Escherichia coli. J. Bacteriol. 1979, 137, 469–473. [Google Scholar] [CrossRef]
- Clark, D.P.; Cronan, J.E. Two-Carbon Compounds and Fatty Acids as Carbon Sources. EcoSal Plus 2005, 1. [Google Scholar] [CrossRef]
- Caldeira, J.; Feicht, R.; White, H.; Teixeira, M.; Moura, J.J.G.; Simon, H.; Moura, I. EPR and Mössbauer spectroscopic studies on enoate reductase. J. Biol. Chem. 1996, 271, 18743–18748. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Thorpe, C.; Schulz, H. 2,4-Dienoyl-CoA reductase from Escherichia coli is a novel iron-sulfur flavoprotein that functions in fatty acid beta-oxidation. Arch. Biochem. Biophys. 2000, 380, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Hubbard, P.A.; Kim, J.J.; Schulz, H. Two distinct proton donors at the active site of Escherichia coli 2,4-dienoyl-CoA reductase are responsible for the formation of different products. Biochemistry 2008, 47, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- You, S.Y.; Cosloy, S.; Schulz, H. Evidence for the essential function of 2,4-dienoyl-coenzyme A reductase in the beta-oxidation of unsaturated fatty acids in vivo. Isolation and characterization of an Escherichia coli mutant with a defective 2,4-dienoyl-coenzyme A reductase. J. Biol. Chem. 1989, 264, 16489–16495. [Google Scholar] [CrossRef]
- Hubbard, P.A.; Liang, X.; Schulz, H.; Kim, J.J. The crystal structure and reaction mechanism of Escherichia coli 2,4-dienoyl-CoA reductase. J. Biol. Chem. 2003, 278, 37553–37560. [Google Scholar] [CrossRef]
- Ren, Y.; Aguirre, J.; Ntamack, A.G.; Chu, C.; Schulz, H. An alternative pathway of oleate beta-oxidation in Escherichia coli involving the hydrolysis of a dead end intermediate by a thioesterase. J. Biol. Chem. 2004, 279, 11042–11050. [Google Scholar] [CrossRef]
- Nie, L.; Ren, Y.; Schulz, H. Identification and characterization of Escherichia coli thioesterase III that functions in fatty acid beta-oxidation. Biochemistry 2008, 47, 7744–7751. [Google Scholar] [CrossRef]
- Nie, L.; Ren, Y.; Janakiraman, A.; Smith, S.; Schulz, H. A novel paradigm of fatty acid beta-oxidation exemplified by the thioesterase-dependent partial degradation of conjugated linoleic acid that fully supports growth of Escherichia coli. Biochemistry 2008, 47, 9618–9626. [Google Scholar] [CrossRef]
- Feng, Y.; Cronan, J.E. A new member of the Escherichia coli fad regulon: Transcriptional regulation of fadM (ybaW). J. Bacteriol. 2009, 191, 6320–6328. [Google Scholar] [CrossRef]
- Iram, S.H.; Cronan, J.E. The beta-oxidation systems of Escherichia coli and Salmonella enterica are not functionally equivalent. J. Bacteriol. 2006, 188, 599–608. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Qi, Q. Molecular effect of FadD on the regulation and metabolism of fatty acid in Escherichia coli. FEMS Microbiol. Lett. 2006, 259, 249–253. [Google Scholar] [CrossRef]
- Pauli, G.; Overath, P. ato Operon: A highly inducible system for acetoacetate and butyrate degradation in Escherichia coli. Eur. J. Biochem. 1972, 29, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Lolkema, J.S. Domain structure and pore loops in the 2-hydroxycarboxylate transporter family. J. Mol. Microbiol. Biotechnol. 2006, 11, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Nunn, W.D. A molecular view of fatty acid catabolism in Escherichia coli. Microbiol. Rev. 1986, 50, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, L.S.; Nunn, W.D. Genetic and molecular characterization of the genes involved in short-chain fatty acid degradation in Escherichia coli: The ato system. J. Bacteriol. 1987, 169, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.E.; Laporte, D.C. Tricarboxylic Acid Cycle and Glyoxylate Bypass. EcoSal Plus 2005, 1, 206–216. [Google Scholar] [CrossRef]
- Dolan, S.K.; Welch, M. The Glyoxylate Shunt, 60 Years On. Annu. Rev. Microbiol. 2018, 72, 309–330. [Google Scholar] [CrossRef]
- Agrawal, S.; Jaswal, K.; Shiver, A.L.; Balecha, H.; Patra, T.; Chaba, R. A genome-wide screen in Escherichia coli reveals that ubiquinone is a key antioxidant for metabolism of long-chain fatty acids. J. Biol. Chem. 2017, 292, 20086–20099. [Google Scholar] [CrossRef]
- Henriques, B.J.; Jentoft Olsen, R.K.; Gomes, C.M.; Bross, P. Electron transfer flavoprotein and its role in mitochondrial energy metabolism in health and disease. Gene 2021, 776, 145407. [Google Scholar] [CrossRef]
- Walt, A.; Kahn, M.L. The fixA and fixB genes are necessary for anaerobic carnitine reduction in Escherichia coli. J. Bacteriol. 2002, 184, 4044–4047. [Google Scholar] [CrossRef][Green Version]
- Campbell, J.W.; Morgan-Kiss, R.M.; Cronan, J.E. A new Escherichia coli metabolic competency: Growth on fatty acids by a novel anaerobic beta-oxidation pathway. Mol. Microbiol. 2003, 47, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Spector, M.P.; DiRusso, C.C.; Pallen, M.J.; Del Portillo, F.G.; Dougan, G.; Finlay, B.B. The medium-/long-chain fatty acyl-CoA dehydrogenase (fadF) gene of Salmonella typhimurium is a phase 1 starvation-stress response (SSR) locus. Microbiology 1999, 145, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Olivera, E.R.; Carnicero, D.; García, B.; Minambres, B.; Moreno, M.A.; Canedo, L.; DiRusso, C.C.; Naharro, G.; Luengo, J.M. Two different pathways are involved in the beta-oxidation of n-alkanoic and n-phenylalkanoic acids in Pseudomonas putida U: Genetic studies and biotechnological applications. Mol. Microbiol. 2001, 39, 863–874. [Google Scholar] [CrossRef]
- Son, M.S.; Matthews, W.J.; Kang, Y.; Nguyen, D.T.; Hoang, T.T. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun. 2007, 75, 5313–5324. [Google Scholar] [CrossRef]
- Kang, Y.; Nguyen, D.T.; Son, M.S.; Hoang, T.T. The Pseudomonas aeruginosa PsrA responds to long-chain fatty acid signals to regulate the fadBA5 beta-oxidation operon. Microbiology 2008, 154, 1584–1598. [Google Scholar] [CrossRef]
- Menendez-Bravo, S.; Paganini, J.; Avignone-Rossa, C.; Gramajo, H.; Arabolaza, A. Identification of FadAB Complexes Involved in Fatty Acid β-Oxidation in Streptomyces coelicolor and Construction of a Triacylglycerol Overproducing strain. Front Microbiol. 2017, 8, 1428. [Google Scholar] [CrossRef] [PubMed]
- Snell, K.D.; Feng, F.; Zhong, L.; Martin, D.; Madison, L.L. YfcX enables medium-chain-length poly(3-hydroxyalkanoate) formation from fatty acids in recombinant Escherichia coli fadB strains. J. Bacteriol. 2002, 184, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Marrakchi, H.; Rock, C.O. The FabR (YijC) transcription factor regulates unsaturated fatty acid biosynthesis in Escherichia coli. J. Biol. Chem. 2002, 277, 15558–15565. [Google Scholar] [CrossRef] [PubMed]
- Sah-Teli, S.K.; Hynönen, M.J.; Schmitz, W.; Geraets, J.A.; Seitsonen, J.; Pedersen, J.S.; Butcher, S.J.; Wierenga, R.K.; Venkatesan, R. Complementary substrate specificity and distinct quaternary assembly of the Escherichia coli aerobic and anaerobic β-oxidation trifunctional enzyme complexes. Biochem. J. 2019, 476, 1975–1994. [Google Scholar] [CrossRef]
- Kang, Y.; Zarzycki-Siek, J.; Walton, C.B.; Norris, M.H.; Hoang, T.T. Multiple FadD acyl-CoA synthetases contribute to differential fatty acid degradation and virulence in Pseudomonas aeruginosa. PLoS ONE. 2010, 5, e13557. [Google Scholar] [CrossRef]
- Zarzycki-Siek, J.; Norris, M.H.; Kang, Y.; Sun, Z.; Bluhm, A.P.; McMillan, I.A.; Hoang, T.T. Elucidating the Pseudomonas aeruginosa fatty acid degradation pathway: Identification of additional fatty acyl-CoA synthetase homologues. PLoS ONE. 2013, 8, e64554. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.R.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., III; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Schnappinger, D.; Ehrt, S.; Voskuil, M.I.; Liu, Y.; Mangan, J.A.; Monahan, I.M.; Dolganov, G.; Efron, B.; Butcher, P.D.; Nathan, C.; et al. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J. Exp. Med. 2003, 198, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Kiss, R.M.; Cronan, J.E. The Escherichia coli fadK (ydiD) gene encodes an anerobically regulated short chain acyl-CoA synthetase. J. Biol. Chem. 2004, 279, 37324–37333. [Google Scholar] [CrossRef]
- Thomason, M.K.; Bischler, T.; Eisenbart, S.K.; Förstner, K.U.; Zhang, A.; Herbig, A.; Nieselt, K.; Sharma, C.M.; Storz, G. Global transcriptional start site mapping using differential RNA sequencing reveals novel antisense RNAs in Escherichia coli. J. Bacteriol. 2015, 197, 18–28. [Google Scholar] [CrossRef]
- DiRusso, C.C.; Metzger, A.K.; Heimert, T.L. Regulation of transcription of genes required for fatty acid transport and unsaturated fatty acid biosynthesis in Escherichia coli by FadR. Mol. Microbiol. 1993, 7, 311–322. [Google Scholar] [CrossRef]
- Feng, Y.; Cronan, J.E. Crosstalk of Escherichia coli FadR with global regulators in expression of fatty acid transport genes. PLoS ONE. 2012, 7, e46275. [Google Scholar] [CrossRef]
- Cho, B.K.; Knight, E.M.; Palsson, B.Ø. Transcriptional regulation of the fad regulon genes of Escherichia coli by ArcA. Microbiology 2006, 152, 2207–2219. [Google Scholar] [CrossRef][Green Version]
- Federowicz, S.; Kim, D.; Ebrahim, A.; Lerman, J.; Nagarajan, H.; Cho, B.-K.; Zengler, K.; Palsson, B. Determining the control circuitry of redox metabolism at the genome-scale. PLoS Genet. 2014, 10, e1004264. [Google Scholar] [CrossRef]
- Higashitani, A.; Nishimura, Y.; Hara, H.; Aiba, H.; Mizuno, T.; Horiuchi, K. Osmoregulation of the fatty acid receptor gene fadL in Escherichia coli. Mol. Gen Genet. 1993, 240, 339–347. [Google Scholar] [CrossRef]
- Gogol, E.B.; Rhodius, V.A.; Papenfort, K.; Vogel, J.; Gross, C.A. Small RNAs endow a transcriptional activator with essential repressor functions for single-tier control of a global stress regulon. Proc. Natl. Acad. Sci. USA 2011, 108, 12875–12880. [Google Scholar] [CrossRef] [PubMed]
- Lalaouna, D.; Carrier, M.C.; Semsey, S.; Brouard, J.-S.; Wang, J.; Wade, J.T.; Massé, E. A 3’ external transcribed spacer in a tRNA transcript acts as a sponge for small RNAs to prevent transcriptional noise. Mol. Cell. 2015, 58, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; DiRusso, C.C.; Metzger, A.K.; Heimert, T.L. Cloning, sequencing, and expression of the fadD gene of Escherichia coli encoding acyl coenzyme A synthetase. J. Biol. Chem. 1992, 267, 25513–25520. [Google Scholar] [CrossRef]
- Zheng, D.; Constantinidou, C.; Hobman, J.L.; Minchin, S.D. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 2004, 32, 5874–5893. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.S.; Yan, H.; Ong, I.M.; Chung, D.; Liang, K.; Tran, F.; Keleş, S.; Landick, R.; Kiley, P.J. Genome-scale analysis of Escherichia coli FNR reveals complex features of transcription factor binding. PLoS Genet. 2013, 9, e1003565. [Google Scholar] [CrossRef]
- DiRusso, C.C.; Heimert, T.L.; Metzger, A.K. Characterization of FadR, a global transcriptional regulator of fatty acid metabolism in Escherichia coli. Interaction with the fadB promoter is prevented by long chain fatty acyl coenzyme A. J. Biol. Chem. 1992, 267, 8685–8691. [Google Scholar] [CrossRef]
- Liu, X.; De Wulf, P. Probing the ArcA-P modulon of Escherichia coli by whole genome transcriptional analysis and sequence recognition profiling. J. Biol. Chem. 2004, 279, 12588–12597. [Google Scholar] [CrossRef]
- Li, Z.; Pan, Q.; Xiao, Y.; Fang, X.; Shi, R.; Fu, C.; Danchin, A.; You, C. Deciphering global gene expression and regulation strategy in Escherichia coli during carbon limitation. Microb. Biotechnol. 2019, 12, 360–376. [Google Scholar] [CrossRef]
- Feng, Y.; Cronan, J.E. Overlapping repressor binding sites result in additive regulation of Escherichia coli FadH by FadR and ArcA. J. Bacteriol. 2010, 192, 4289–4299. [Google Scholar] [CrossRef]
- Gui, L.; Sunnarborg, A.; LaPorte, D.C. Regulated expression of a repressor protein: FadR activates iclR. J. Bacteriol. 1996, 178, 4704–4709. [Google Scholar] [CrossRef]
- Pan, B.; Unnikrishnan, I.; LaPorte, D.C. The binding site of the IclR repressor protein overlaps the promoter of aceBAK. J. Bacteriol. 1996, 178, 3982–3984. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ishihama, A. Two different modes of transcription repression of the Escherichia coli acetate operon by IclR. Mol. Microbiol. 2003, 47, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Heath, R.J.; Li, Z.; Rock, C.O.; White, S.W. The FadR.DNA complex. Transcriptional control of fatty acid metabolism in Escherichia coli. J. Biol. Chem. 2001, 276, 17373–17379. [Google Scholar] [CrossRef]
- Raman, N.; Black, P.N.; DiRusso, C.C. Characterization of the fatty acid-responsive transcription factor FadR. Biochemical and genetic analyses of the native conformation and functional domains. J. Biol. Chem. 1997, 272, 30645–30650. [Google Scholar] [CrossRef]
- Van Aalten, D.M.; DiRusso, C.C.; Knudsen, J.; Wierenga, R.K. Crystal structure of FadR, a fatty acid-responsive transcription factor with a novel acyl coenzyme A-binding fold. EMBO J. 2000, 19, 5167–5177. [Google Scholar] [CrossRef]
- Van Aalten, D.M.; DiRusso, C.C.; Knudsen, J. The structural basis of acyl coenzyme A-dependent regulation of the transcription factor FadR. EMBO J. 2001, 20, 2041–2050. [Google Scholar] [CrossRef]
- Farewell, A.; Diez, A.A.; DiRusso, C.C.; Nyström, T. Role of the Escherichia coli FadR regulator in stasis survival and growth phase-dependent expression of the uspA, fad, and fab genes. J. Bacteriol. 1996, 178, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, A.E.; Rodionov, D.A.; Alm, E.; Arkin, A.P.; Dubchak, I.; Gelfand, M.S. Comparative genomics of regulation of fatty acid and branched-chain amino acid utilization in proteobacteria. J. Bacteriol. 2009, 191, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; DiRusso, C.C. Analysis of acyl coenzyme A binding to the transcription factor FadR and identification of amino acid residues in the carboxyl terminus required for ligand binding. J. Biol. Chem. 1995, 270, 1092–1097. [Google Scholar] [CrossRef]
- Bacik, J.P.; Yeager, C.M.; Twary, S.N.; Martí-Arbona, R. Modulation of FadR binding capacity for acyl-CoA fatty acids through structure-guided mutagenesis. Protein J. 2015, 34, 359–366. [Google Scholar] [CrossRef]
- Iram, S.H.; Cronan, J.E. Unexpected functional diversity among FadR fatty acid transcriptional regulatory proteins. J. Biol. Chem. 2005, 280, 32148–32156. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Kovacikova, G.; Lin, W.; Taylor, R.K.; Skorupski, K.; Kull, F.J. The 40-residue insertion in Vibrio cholerae FadR facilitates binding of an additional fatty acyl-CoA ligand. Nat. Commun. 2015, 6, 6032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ouellet, M.; Batth, T.S.; Adams, P.; Petzold, C.; Mukhopadhyay, A.; Keasling, J.D. Enhancing fatty acid production by the expression of the regulatory transcription factor FadR. Metab. Eng. 2012, 14, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Dabirian, Y.; Gonçalves Teixeira, P.; Nielsen, J.; Siewers, V.; David, F. FadR-Based Biosensor-Assisted Screening for Genes Enhancing Fatty Acyl-CoA Pools in Saccharomyces cerevisiae. ACS Synth Biol. 2019, 8, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, Q.; Zhang, J.; Zhang, M.; Wang, C.; Qu, R.; Wang, Y.; Yang, G.; Wang, J. A Ratiometric Fluorescent Biosensor Reveals Dynamic Regulation of Long-Chain Fatty Acyl-CoA Esters Metabolism. Angew. Chem. Int. Ed. Engl. 2021, 60, 13996–14004. [Google Scholar] [CrossRef]
- Henry, M.F.; Cronan, J.E. Escherichia coli transcription factor that both activates fatty acid synthesis and represses fatty acid degradation. J. Mol. Biol. 1991, 222, 843–849. [Google Scholar] [CrossRef]
- Henry, M.F.; Cronan, J.E. A new mechanism of transcriptional regulation: Release of an activator triggered by small molecule binding. Cell 1992, 70, 671–679. [Google Scholar] [CrossRef]
- Campbell, J.W.; Cronan, J.E. Escherichia coli FadR positively regulates transcription of the fabB fatty acid biosynthetic gene. J. Bacteriol. 2001, 183, 5982–5990. [Google Scholar] [CrossRef]
- My, L.; Rekoske, B.; Lemke, J.J.; Viala, J.P.; Gourse, R.L.; Bouveret, E. Transcription of the Escherichia coli fatty acid synthesis operon fabHDG is directly activated by FadR and inhibited by ppGpp. J. Bacteriol. 2013, 195, 3784–3795. [Google Scholar] [CrossRef]
- My, L.; Ghandour Achkar, N.; Viala, J.P.; Bouveret, E. Reassessment of the Genetic Regulation of Fatty Acid Synthesis in Escherichia coli: Global Positive Control by the Dual Functional Regulator FadR. J. Bacteriol. 2015, 197, 1862–1872. [Google Scholar] [CrossRef]
- Cronan, J.E. Bacterial membrane lipids: Where do we stand. Annu. Rev. Microbiol. 2003, 57, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Weeks, G.; Shapiro, M.; Burns, R.O.; Wakil, S.J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J. Bacteriol. 1969, 97, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Fic, E.; Bonarek, P.; Gorecki, A.; Kedracka-Krok, S.; Mikolajczak, J.; Polit, A.; Tworzydlo, M.; Dziedzicka-Wasylewska, M.; Wasylewski, Z. cAMP receptor protein from Escherichia coli as a model of signal transduction in proteins--a review. J. Mol. Microbiol. Biotechnol. 2009, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kvint, K.; Hosbond, C.; Farewell, A.; Nybroe, O.; Nyström, T. Emergency derepression: Stringency allows RNA polymerase to override negative control by an active repressor. Mol. Microbiol. 2000, 35, 435–443. [Google Scholar] [CrossRef]
- Kralj, T.; Nuske, M.; Hofferek, V.; Sani, M.-A.; Lee, T.-H.; Separovic, F.; Aguilar, M.-I.; Reid, G.E. Multi-Omic Analysis to Characterize Metabolic Adaptation of the E. coli Lipidome in Response to Environmental Stress. Metabolites 2022, 12, 171. [Google Scholar] [CrossRef]
- Dong, T.; Schellhorn, H.E. Global effect of RpoS on gene expression in pathogenic Escherichia coli O157:H7 strain EDL933. BMC Genom. 2009, 10, 349. [Google Scholar] [CrossRef]
- Keseler, I.M.; Gama-Castro, S.; Mackie, A.; Billington, R.; Bonavides-Martínez, C.; Caspi, R.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Muñiz-Rascado, L.; et al. The EcoCyc Database in 2021. Front Microbiol. 2021, 12, 711077. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Brown, A.N.; Anderson, M.T.; Bachman, M.A.; Mobley, H.L.T. The ArcAB Two-Component System: Function in Metabolism, Redox Control, and Infection. Microbiol. Mol. Biol. Rev. 2022, 86, e0011021. [Google Scholar] [CrossRef]
- Groszewska, A.; Wroblewska, Z.; Olejniczak, M. The structure of fadL mRNA and its interactions with RybB sRNA. Acta Biochim. Pol. 2016, 63, 835–840. [Google Scholar] [CrossRef]
- Dellomonaco, C.; Clomburg, J.M.; Miller, E.N.; Gonzalez, R. Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature 2011, 476, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Pfleger, B.F. Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals. Metab. Eng. 2020, 58, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Cordell, W.T.; Jindra, M.A.; Courtney, D.K.; Kuckuk, M.K.; Chen, X.; Pfleger, B.F. Metabolic engineering strategies to produce medium-chain oleochemicals via acyl-ACP:CoA transacylase activity. Nat. Commun. 2022, 13, 1619. [Google Scholar] [CrossRef]
- Mangroo, D.; Gerber, G.E. Fatty acid uptake in Escherichia coli: Regulation by recruitment of fatty acyl-CoA synthetase to the plasma membrane. Biochem. Cell Biol. 1993, 71, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Fröhlich, K.S.; Radmer, J.; Papenfort, K. Switching fatty acid metabolism by an RNA-controlled feed forward loop. Proc. Natl. Acad. Sci. USA 2020, 117, 8044–8054. [Google Scholar] [CrossRef]
- Madikonda, A.K.; Shaikh, A.; Khanra, S.; Yakkala, H.; Yellaboina, S.; Lin-Chao, S.; Siddavattam, D. Metabolic remodeling in Escherichia coli MG1655. A prophage e14-encoded small RNA, co293, post-transcriptionally regulates transcription factors HcaR and FadR. FEBS J. 2020, 287, 4767–4782. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavoncello, V.; Barras, F.; Bouveret, E. Degradation of Exogenous Fatty Acids in Escherichia coli. Biomolecules 2022, 12, 1019. https://doi.org/10.3390/biom12081019
Pavoncello V, Barras F, Bouveret E. Degradation of Exogenous Fatty Acids in Escherichia coli. Biomolecules. 2022; 12(8):1019. https://doi.org/10.3390/biom12081019
Chicago/Turabian StylePavoncello, Viola, Frédéric Barras, and Emmanuelle Bouveret. 2022. "Degradation of Exogenous Fatty Acids in Escherichia coli" Biomolecules 12, no. 8: 1019. https://doi.org/10.3390/biom12081019
APA StylePavoncello, V., Barras, F., & Bouveret, E. (2022). Degradation of Exogenous Fatty Acids in Escherichia coli. Biomolecules, 12(8), 1019. https://doi.org/10.3390/biom12081019





