A New Serum Macrophage Checkpoint Biomarker for Innate Immunotherapy: Soluble Signal-Regulatory Protein Alpha (sSIRPα)
Abstract
:1. Introduction
2. Methods
2.1. Human Monocyte-Derived Macrophages
2.2. Flow Cytometry
2.3. Optimized ELISA
2.4. Western Blotting
2.5. Human LPS Exposure
2.6. Statistical Methods
3. Results
3.1. Expression of SIRPα and Shedding of sSIRPα by Macrophage Subtypes In Vitro
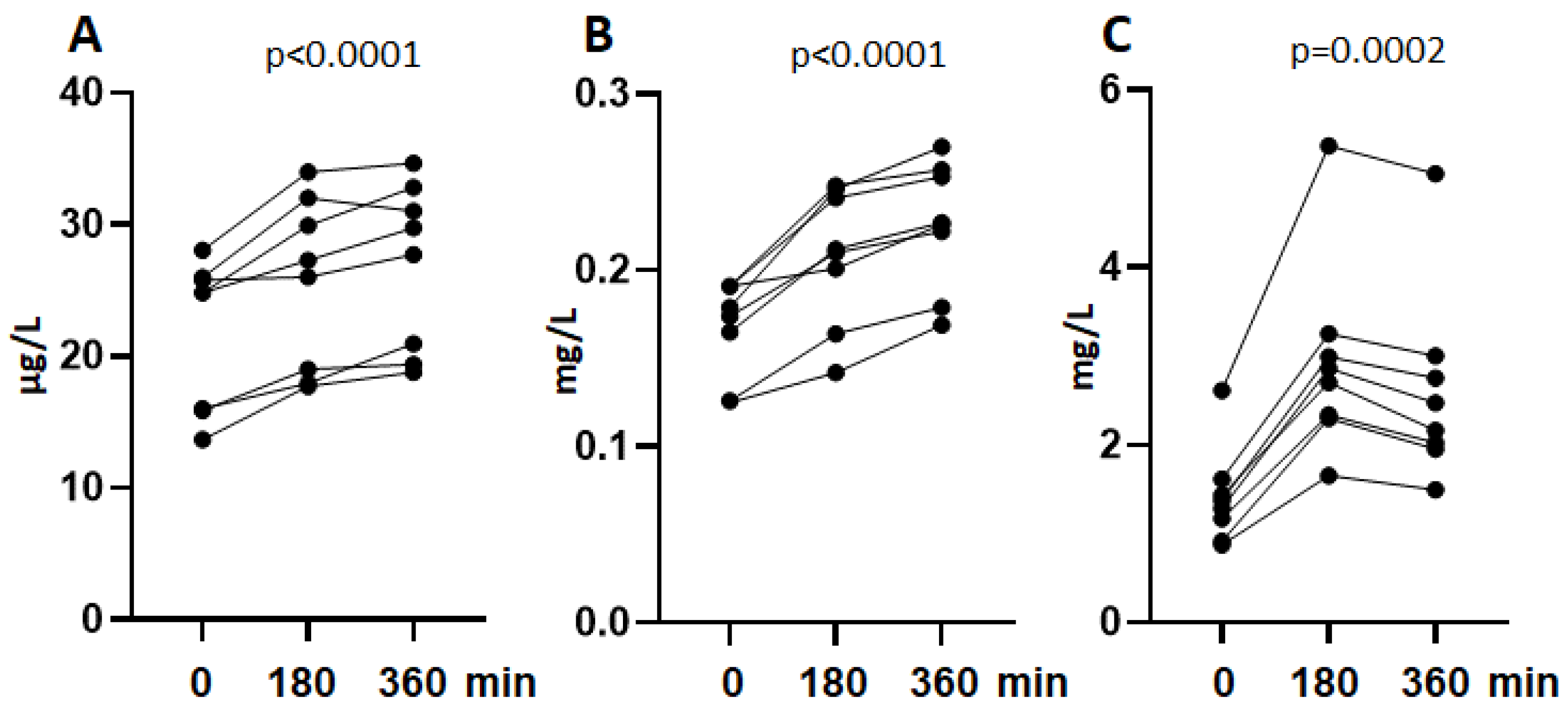
3.2. Shedding of sSIRPα In Vivo
3.3. Validation of an ELISA for sSIRPα
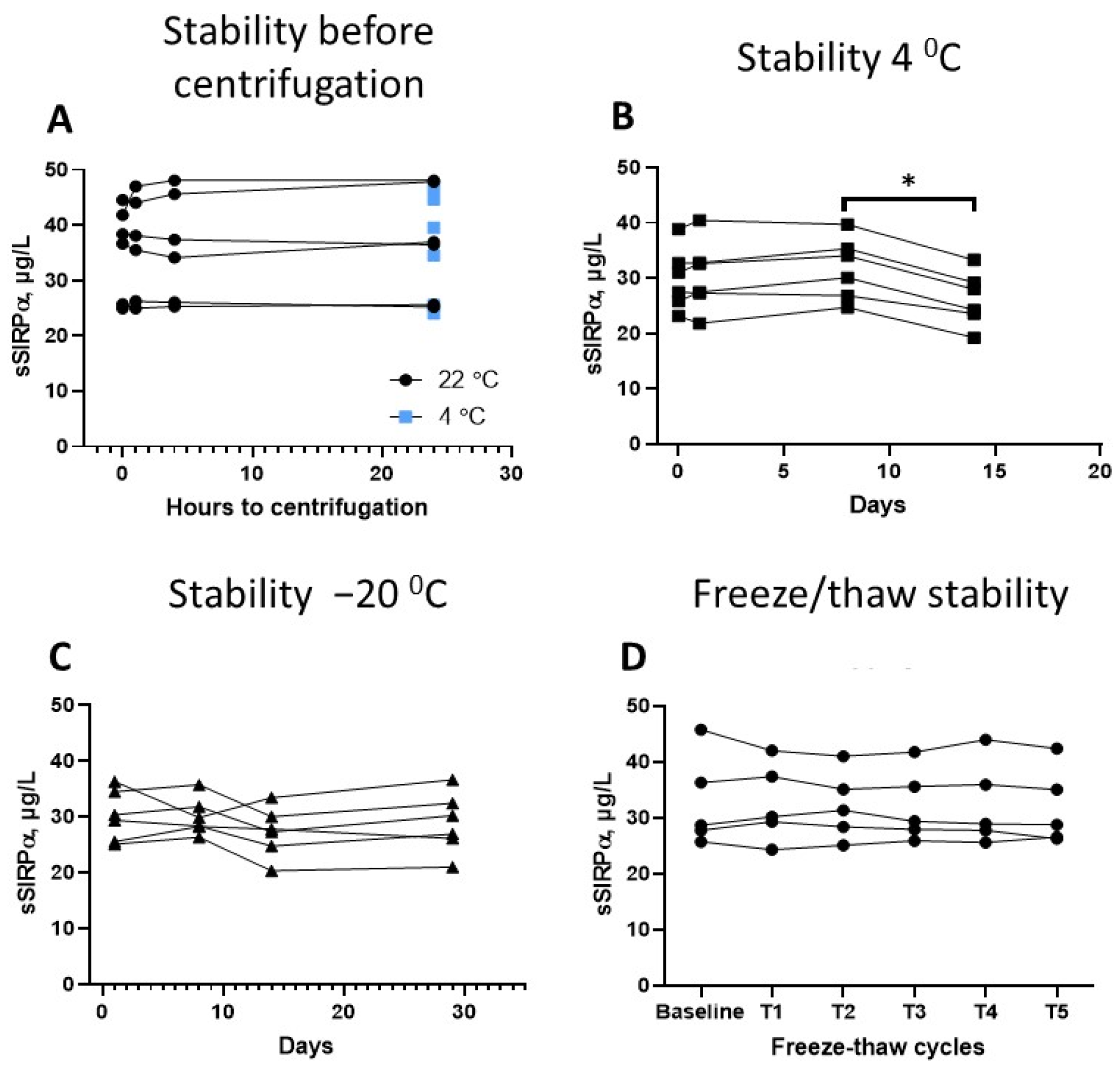
3.4. Preanalytical Factors
3.5. Serum Concentration of sSIRPα in Healthy Individuals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lentz, R.W.; Colton, M.D.; Mitra, S.S.; Messersmith, W.A. Innate Immune Checkpoint Inhibitors: The Next Breakthrough in Medical Oncology? Mol. Cancer Ther. 2021, 20, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Melo Garcia, L.; Barabé, F. Harnessing Macrophages through the Blockage of CD47: Implications for Acute Myeloid Leukemia. Cancers 2021, 13, 6258. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Matozaki, T.; Noguchi, T.; Iwamatsu, A.; Yamao, T.; Takahashi, N.; Tsuda, M.; Takada, T.; Kasuga, M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol. 1996, 16, 6887–6899. [Google Scholar] [CrossRef] [Green Version]
- Kharitonenkov, A.; Chen, Z.; Sures, I.; Wang, H.; Schilling, J.; Ullrich, A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 1997, 386, 181–186. [Google Scholar] [CrossRef]
- Takahashi, S. Molecular functions of SIRPα and its role in cancer. Biomed. Rep. 2018, 9, 3–7. [Google Scholar] [CrossRef] [PubMed]
- van Beek, E.M.; Cochrane, F.; Barclay, A.N.; van den Berg, T.K. Signal regulatory proteins in the immune system. J. Immunol. 2005, 175, 7781–7787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.; van der Laan, L.J.; Vernon-Wilson, E.; Renardel de Lavalette, C.; Döpp, E.A.; Dijkstra, C.D.; Simmons, D.L.; van den Berg, T.K. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J. Immunol. 1998, 161, 1853–1859. [Google Scholar]
- Barclay, A.N.; Brown, M.H. The SIRP family of receptors and immune regulation. Nat. Rev. Immunol. 2006, 6, 457–464. [Google Scholar] [CrossRef]
- Seiffert, M.; Brossart, P.; Cant, C.; Cella, M.; Colonna, M.; Brugger, W.; Kanz, L.; Ullrich, A.; Bühring, H.J. Signal-regulatory protein alpha (SIRPalpha) but not SIRPbeta is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34(+)CD38(−) hematopoietic cells. Blood 2001, 97, 2741–2749. [Google Scholar] [CrossRef] [Green Version]
- Veillette, A.; Thibaudeau, E.; Latour, S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem. 1998, 273, 22719–22728. [Google Scholar] [CrossRef] [Green Version]
- Koga, N.; Hu, Q.; Sakai, A.; Takada, K.; Nakanishi, R.; Hisamatsu, Y.; Ando, K.; Kimura, Y.; Oki, E.; Oda, Y.; et al. Clinical significance of signal regulatory protein alpha (SIRPα) expression in esophageal squamous cell carcinoma. Cancer Sci. 2021, 112, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Londino, J.D.; Gulick, D.; Isenberg, J.S.; Mallampalli, R.K. Cleavage of Signal Regulatory Protein α (SIRPα) Enhances Inflammatory Signaling. J. Biol. Chem. 2015, 290, 31113–31125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, H.; Kobayashi, H.; Okazawa, H.; Ohe, Y.; Tomizawa, K.; Sato, R.; Matozaki, T. Ectodomain shedding of SHPS-1 and its role in regulation of cell migration. J. Biol. Chem. 2004, 279, 27878–27887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemori, H.; Sanes, J.R. Signal regulatory proteins (SIRPS) are secreted presynaptic organizing molecules. J. Biol. Chem. 2008, 283, 34053–34061. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.C.; Andersen, M.N.; Møller, H.J. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology 2020, 159, 63–74. [Google Scholar] [CrossRef]
- Rittig, N.; Bach, E.; Thomsen, H.H.; Johannsen, M.; Jørgensen, J.O.; Richelsen, B.; Jessen, N.; Møller, N. Amino acid supplementation is anabolic during the acute phase of endotoxin-induced inflammation: A human randomized crossover trial. Clin. Nutr. 2016, 35, 322–330. [Google Scholar] [CrossRef]
- Møller, H.J.; Hald, K.; Moestrup, S.K. Characterization of an enzyme-linked immunosorbent assay for soluble CD163. Scand. J. Clin. Lab. Invest 2002, 62, 293–299. [Google Scholar] [CrossRef]
- Rødgaard-Hansen, S.; Rafique, A.; Christensen, P.A.; Maniecki, M.B.; Sandahl, T.D.; Nexø, E.; Møller, H.J. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clin. Chem. Lab. Med. 2014, 52, 453–461. [Google Scholar] [CrossRef]
- Khan, M.; Zhao, Z.; Arooj, S.; Fu, Y.; Liao, G. Soluble PD-1: Predictive, Prognostic, and Therapeutic Value for Cancer Immunotherapy. Front. Immunol. 2020, 11, 587460. [Google Scholar] [CrossRef]
- Etzerodt, A.; Maniecki, M.B.; Møller, K.; Møller, H.J.; Moestrup, S.K. Tumor necrosis factor α-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J. Leukoc. Biol. 2010, 88, 1201–1205. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Mitrakas, A.; Anestopoulos, I.; Kontosis, A.; Koukourakis, I.M.; Pappa, A.; Panayiotidis, M.I.; Koukourakis, M.I. Expression of CD47 and SIRPα Macrophage Immune-Checkpoint Pathway in Non-Small-Cell Lung Cancer. Cancers 2022, 14, 1801. [Google Scholar] [CrossRef] [PubMed]
- Toda, Y.; Kohashi, K.; Yamamoto, H.; Ishihara, S.; Ito, Y.; Susuki, Y.; Kawaguchi, K.; Kiyozawa, D.; Takamatsu, D.; Kinoshita, I.; et al. Tumor microenvironment in giant cell tumor of bone: Evaluation of PD-L1 expression and SIRPα infiltration after denosumab treatment. Sci. Rep. 2021, 11, 14821. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zeng, H.; Liu, Z.; Jin, K.; Chang, Y.; Wang, Y.; Liu, L.; Zhu, Y.; Xu, L.; Wang, Z.; et al. Poor clinical outcomes and immunoevasive contexture in SIRPα(+) tumor-associated macrophages enriched muscle-invasive bladder cancer patients. Urol. Oncol. 2022, 40, 109.e111–109.e120. [Google Scholar] [CrossRef]
- Yang, H.; Yan, M.; Li, W.; Xu, L. SIRPα and PD1 expression on tumor-associated macrophage predict prognosis of intrahepatic cholangiocarcinoma. J. Trans. l. Med. 2022, 20, 140. [Google Scholar] [CrossRef]
- Jalil, A.R.; Tobin, M.P.; Discher, D.E. Suppressing or Enhancing Macrophage Engulfment through the Use of CD47 and Related Peptides. Bioconjug. Chem. 2022. [Google Scholar] [CrossRef]
- Lin, Y.; Yan, X.Q.; Yang, F.; Yang, X.W.; Jiang, X.; Zhao, X.C.; Zhu, B.K.; Liu, L.; Qin, H.Y.; Liang, Y.M.; et al. Soluble extracellular domains of human SIRPα and CD47 expressed in Escherichia coli enhances the phagocytosis of leukemia cells by macrophages in vitro. Protein Expr. Purif. 2012, 85, 109–116. [Google Scholar] [CrossRef]

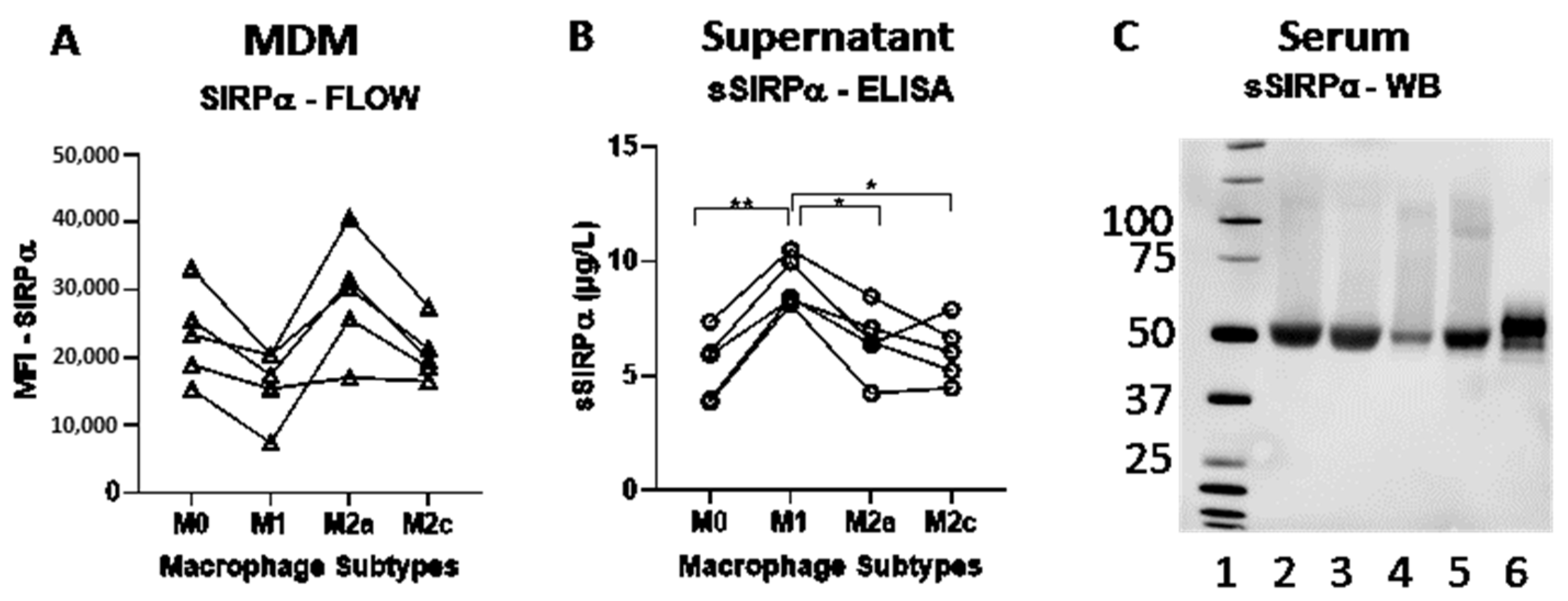
| MDM Subtype | Mean SIRPα (MFI) | MFI Range | Test (p-Value) | Relative Difference (%) |
|---|---|---|---|---|
| M0 | 22,982 | (15,835; 32,596) | 0.078 | 45.83 |
| M1 | 15,760 | (8,010; 20,130) | - | - |
| M2a | 28,522 | (16,921; 39,051) | 0.065 | 80.97 |
| M2c | 20,631 | (16,434; 26,940) | 0.243 | 30.90 |
| MDM Subtype | Mean sSIRPα (µg/L) | sSRIPα Range | Test (p-Value) | Relative Difference (%) |
|---|---|---|---|---|
| M0 | 5.43 | (3.87; 7.37) | 0.0024 ** | −40.09 |
| M1 | 9.07 | (8.13; 10.5) | - | - |
| M2a | 6.54 | (4.26; 8.47) | 0.0229 * | −27.92 |
| M2c | 6.07 | (4.48;7.90) | 0.0494 * | −33.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladimirova, Y.V.; Mølmer, M.K.; Antonsen, K.W.; Møller, N.; Rittig, N.; Nielsen, M.C.; Møller, H.J. A New Serum Macrophage Checkpoint Biomarker for Innate Immunotherapy: Soluble Signal-Regulatory Protein Alpha (sSIRPα). Biomolecules 2022, 12, 937. https://doi.org/10.3390/biom12070937
Vladimirova YV, Mølmer MK, Antonsen KW, Møller N, Rittig N, Nielsen MC, Møller HJ. A New Serum Macrophage Checkpoint Biomarker for Innate Immunotherapy: Soluble Signal-Regulatory Protein Alpha (sSIRPα). Biomolecules. 2022; 12(7):937. https://doi.org/10.3390/biom12070937
Chicago/Turabian StyleVladimirova, Yoanna V., Marie K. Mølmer, Kristian W. Antonsen, Niels Møller, Nikolaj Rittig, Marlene C. Nielsen, and Holger J. Møller. 2022. "A New Serum Macrophage Checkpoint Biomarker for Innate Immunotherapy: Soluble Signal-Regulatory Protein Alpha (sSIRPα)" Biomolecules 12, no. 7: 937. https://doi.org/10.3390/biom12070937
APA StyleVladimirova, Y. V., Mølmer, M. K., Antonsen, K. W., Møller, N., Rittig, N., Nielsen, M. C., & Møller, H. J. (2022). A New Serum Macrophage Checkpoint Biomarker for Innate Immunotherapy: Soluble Signal-Regulatory Protein Alpha (sSIRPα). Biomolecules, 12(7), 937. https://doi.org/10.3390/biom12070937






