Monoterpenes as Sirtuin-1 Activators: Therapeutic Potential in Aging and Related Diseases
Abstract
1. Introduction
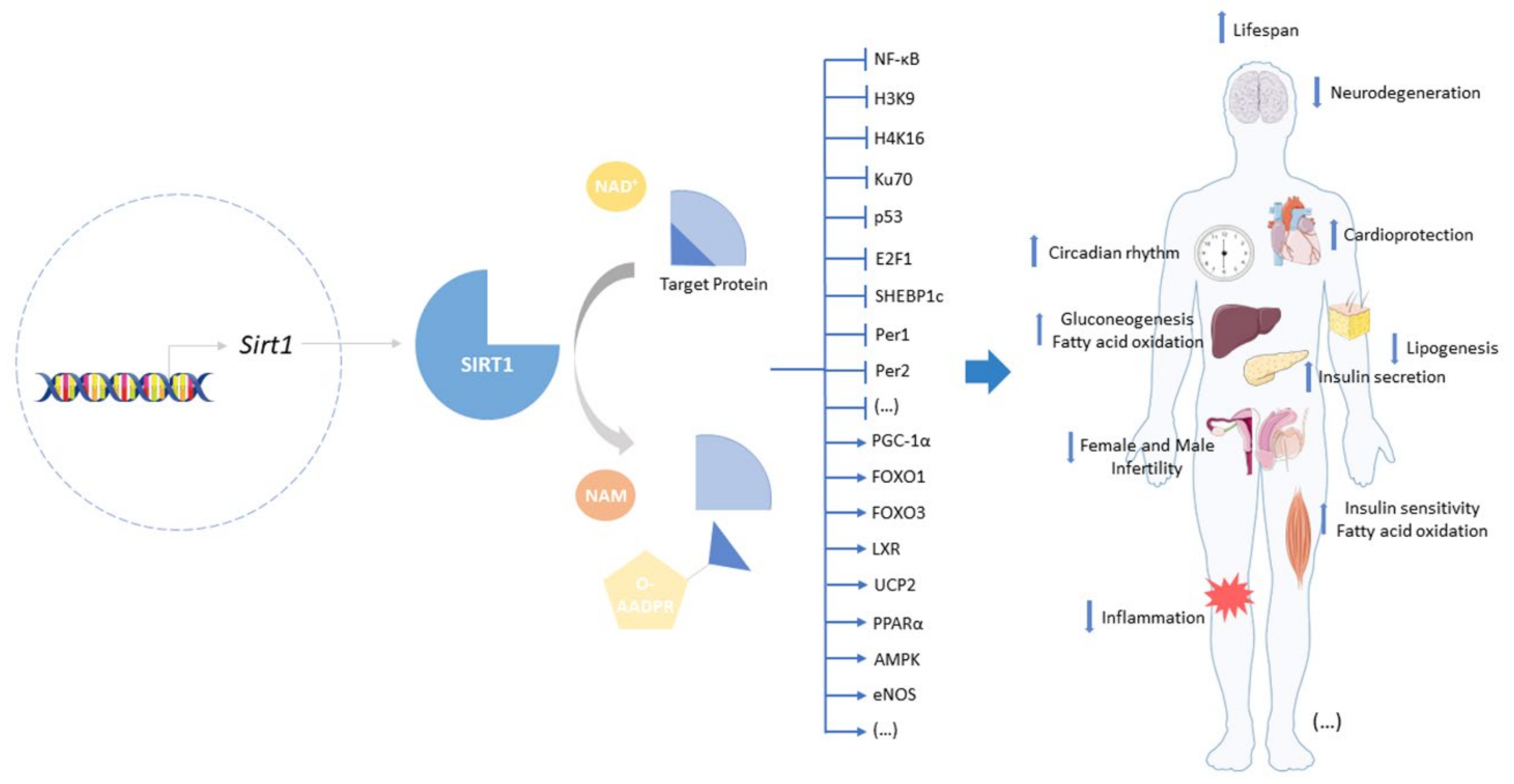
2. Sirtuin-1: A Target to Offset Aging and Age-Related Diseases
3. Monoterpenes and SIRTs
3.1. Monoterpenes
3.1.1. Bakuchiol
3.1.2. (S)-(+)-Carvone
3.1.3. Hinokitiol
3.1.4. Paeoniflorin
| Compound | Structure | Model and (Dose or Concentration of Test Compound) | SIRT1 | Outcome | Ref. | |
|---|---|---|---|---|---|---|
| Activity | Expression | |||||
| Bakuchiol | 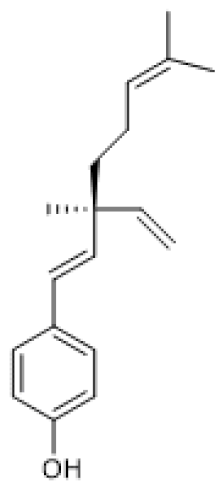 | Normal Rat Heart (0.25–1 µM) IR-Injured Rat Heart (0.25–1 µM) | NA | ↑ | Attenuation of myocardial IR injury | [73] |
| STZ-induced mouse (60 mg/Kg/d) HG-treated H9c2 cells (10 µM) | NA | ↑ | Alleviation of hyperglycemia-induced cardiomyopathy | [74] | ||
| (S)-(+)-carvone |  | LPS-stimulated Raw 264.7 cells | ↑ | = | Reduction of LPS-induced pro-inflammatory mediators | [21] |
| Hinokitiol | 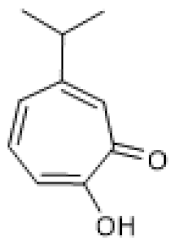 | LPS-stimulated NHEKs cells (2.5–20 µmol/L) | NA | ↑ | Attenuation of LPS-induced inflammation | [78] |
| Paeoniflorin | 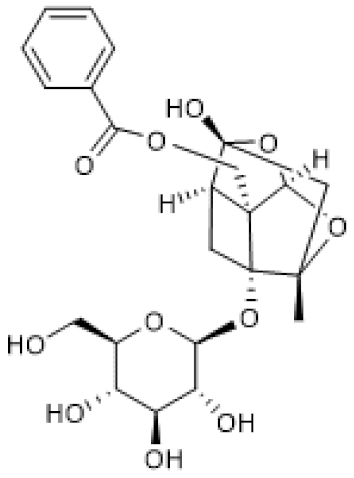 | chronic constriction injury of rat sciatic nerve (50–100 mg/kg) | NA | ↑ | Attenuation of neuropathic pain | [81] |
| Ox-LDL-treated HUVEC cells (100 µg/mL) | NA | ↑ | Attenuation of Ox-LDL-induced apoptosis and adhesion molecule expression | [83] | ||
3.2. Iridoids
3.2.1. Non-Glycoside Iridoids
3.2.2. Iridoid Glycosides
| Compound | Structure | Model and (Dose or Concentration of Test Compound) | SIRT1 Expression | Outcome | Ref. |
|---|---|---|---|---|---|
| Genipin | 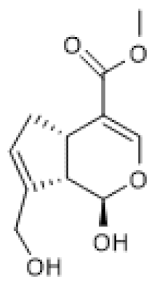 | IR-induced hepatic mice injury (100 mg/Kg) | ↑ | Protection against IR-induced hepatic injury | [86] |
| Catalpol |  | TNBS-induced rat colitis (20 mg/kg) Brefeldin A-treated Caco 2 cells (10–80 µM) | ↑ | Attenuation of ER stress in colitis | [88] |
| Adriamycin-induced mice nephrotoxicity (40–120 mg/Kg) | ↑ | Alleviation of adriamycin-induced nephrotoxicity | [89] | ||
| HFD/STZ-induced diabetic nephropathy mice (100–200 mg/Kg) HG-induced podocyte (10 µM) | ↑ | Inhibition of oxidative stress and inflammation | [90] | ||
| IMQ-induced psoriasis-like lesions in mice (2.5–10 mg/Kg) TNF-α-stimulated HaCat cells (7.5–30 µM) | ↑ | Amelioration of psoriasis-like phenotypes | [91] | ||
| Geniposide |  | ISO-induced cardiac fibrosis in mice (50 mg/Kg/d) TGF-β-treated cardiac fibroblasts (100 µM) | ↑ | Alleviation of ISO-induced cardiac fibrosis | [93] |
| HFD-fed mice (50 mg/Kg) Cardiomyocytes (50 µmol/L) | ↑ | Protection against obesity-related cardiac injury | [94] | ||
| HFD/STZ-induced diabetic nephropathy in mice (25–50 mg/Kg) HG-induced podocyte (200 µg/mL) | ↑ | Alleviation of diabetic nephropathy | [97] | ||
| DSS-induced acute colitis in mice (25–100 mg/Kg) LPS+ATP-stimulated Raw 264.7/BMDM cells (25–100 µM) | ↑ | Amelioration of inflammatory responses in colitis | [98] | ||
| Genipin- 1-β-D-gentiobioside | 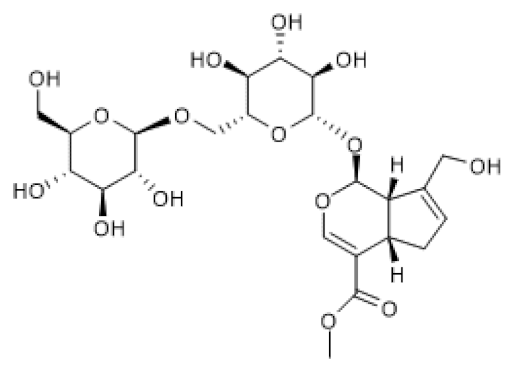 | HFD/STZ-induced diabetic nephropathy in mice (25–50 mg/Kg) HG-induced podocyte (20 µM) | ↑ | Protection against diabetic nephropathy | [99] |
| Loganin | 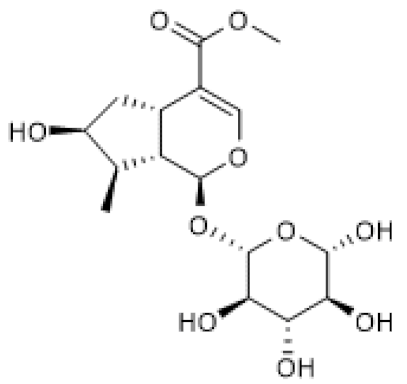 | DSS-induced ulcerative colitis in mice (50–100 mg/Kg/day) | ↑ | Attenuation of DSS-induced ulcerative colitis | [101] |
| Monotropein | 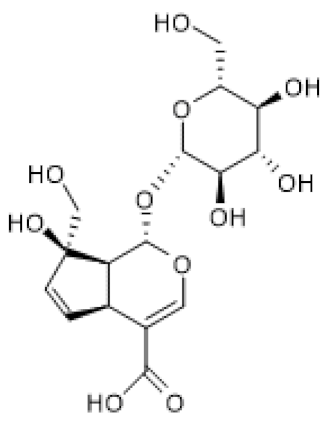 | H2O2-treated primary osteoblasts (1.5–10 µg/mL) | ↑ | Suppression of apoptosis and inflammation in H2O2-treated primary osteoblasts | [102] |
| Picroside II | 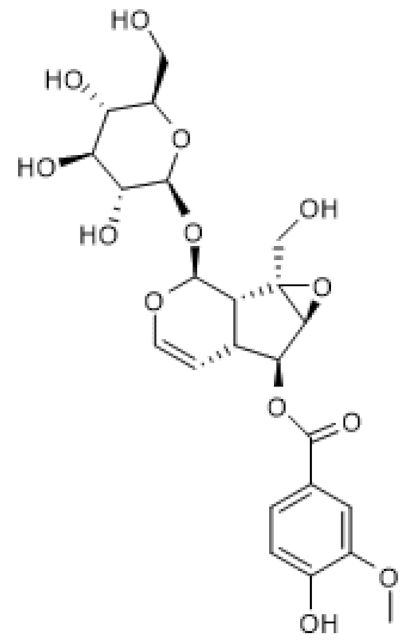 | Hyperhomo-cysteinemic mice (60 mg/Kg) Hcy-treated HUVECs (100–200 µg/mL) | ↑ | Attenuation of hyperhomo-cysteinemia-induced endothelial injury | [103] |
| Sweroside | 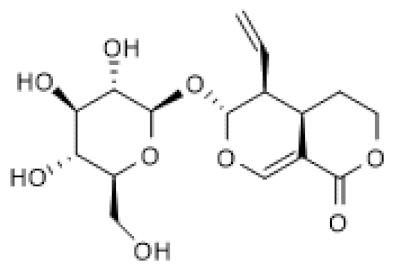 | LPS-stimulated Raw 264.7 cells (40–80 µM) | ↑ | Attenuation of LPS-induced inflammation | [104] |
3.2.3. Secoiridoids
| Compound | Structure | Model | SIRT1 Expression | Outcome | Ref. |
|---|---|---|---|---|---|
| Gentio-picroside | 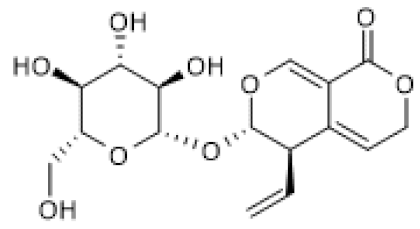 | OVA-induced allergic mouse asthma (20–80 mg/Kg) | ↑ | Amelioration of OVA-induced inflammation | [106] |
| Oleuropein | 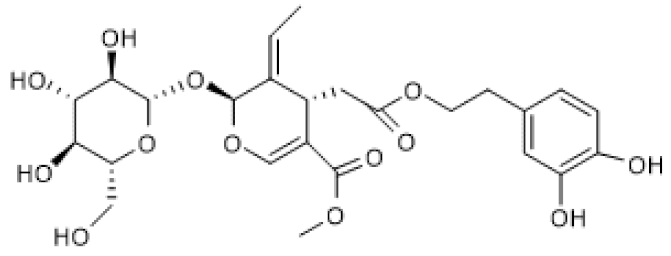 | Avian muscle cells (50 µM) | ↑ | Induction of mitochondrial biogenesis and decrease of ROS | [108] |
| Growing broiler chicken (0.1–2.5 ppm) | ↑ | Reduction of muscle oxidative damage | [109] | ||
| Oleuropein aglycone | 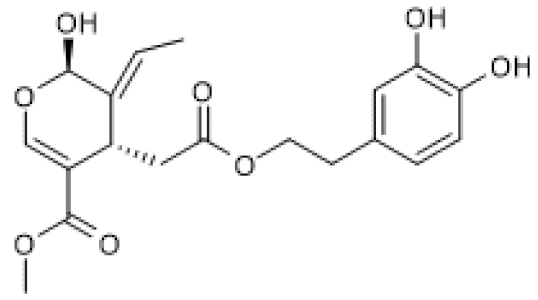 | TgCRND8 mice(50 mg/Kg) MNNG-treated N2a cells (100 µM) | ↑ | Counteraction of neuronal damage | [110] |
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kaplan, W.; Wirtz, V.; Mantel, A.; Béatrice, P.S.U. Priority medicines for Europe and the world update 2013 report. Methodology 2013, 2, 99–102. [Google Scholar]
- World Health Organization. Decade of Healthy Ageing: Baseline Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- De Magalhães, J.P. Longevity pharmacology comes of age. Drug Discov. Today 2021, 26, 1559–1562. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Pennypacker, J.K. Drugs that modulate aging: The promising yet difficult path ahead. Transl. Res. 2013, 163, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Prospective Treatment of Age-Related Diseases by Slowing Down Aging. Am. J. Pathol. 2012, 181, 1142–1146. [Google Scholar] [CrossRef]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef]
- Sauve, A.A. Sirtuin chemical mechanisms. Biochim. et Biophys. Acta (BBA)-Proteins Proteom. 2010, 1804, 1591–1603. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian Sirtuins: Biological Insights and Disease Relevance. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Nakagawa, T.; Guarente, L. SnapShot: Sirtuins, NAD, and Aging. Cell Metab. 2014, 20, 192. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Jęśko, H.; Wencel, P.; Strosznajder, R.; Strosznajder, J. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem. Res. 2016, 42, 876–890. [Google Scholar] [CrossRef]
- Bellet, M.M.; Nakahata, Y.; Boudjelal, M.; Watts, E.; Mossakowska, D.E.; Edwards, K.A.; Cervantes, M.; Astarita, G.; Loh, C.; Ellis, J.L.; et al. Pharmacological modulation of circadian rhythms by synthetic activators of the deacetylase SIRT1. Proc. Natl. Acad. Sci. USA 2013, 110, 3333–3338. [Google Scholar] [CrossRef]
- Kawahara, T.L.; Michishita, E.; Adler, A.S.; Damian, M.; Berber, E.; Lin, M.; McCord, R.A.; Ongaigui, K.C.; Boxer, L.D.; Chang, H.Y.; et al. SIRT6 Links Histone H3 Lysine 9 Deacetylation to NF-κB-Dependent Gene Expression and Organismal Life Span. Cell 2009, 136, 62–74. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Bonkowski, M.; Sinclair, M.S.B.D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol. Ther. 2018, 188, 140–154. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Liao, M.; Hu, M.; Li, W.; Ouyang, H.; Wang, X.; Ye, T.; Zhang, Y.; Ouyang, L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2018, 161, 48–77. [Google Scholar] [CrossRef]
- Gomes, P.; Leal, H.; Mendes, A.F.; Reis, F.; Cavadas, C. Dichotomous Sirtuins: Implications for Drug Discovery in Neurodegenerative and Cardiometabolic Diseases. Trends Pharmacol. Sci. 2019, 40, 1021–1039. [Google Scholar] [CrossRef]
- Du, N.; Wu, K.; Zhang, J.; Wang, L.; Pan, X.; Zhu, Y.; Wu, X.; Liu, J.; Chen, Y.; Ye, Y.; et al. Inonotsuoxide B regulates M1 to M2 macrophage polarization through sirtuin-1/endoplasmic reticulum stress axis. Int. Immunopharmacol. 2021, 96, 107603. [Google Scholar] [CrossRef]
- Pillai, V.B.; Kanwal, A.; Fang, Y.H.; Sharp, W.W.; Samant, S.; Arbiser, J.; Gupta, M.P. Honokiol, an activator of Sirtuin-3 (SIRT3) preserves mitochondria and protects the heart from doxorubicin-induced cardiomyopathy in mice. Oncotarget 2017, 8, 34082–34098. [Google Scholar] [CrossRef]
- Sousa, C.; Neves, B.; Leitão, A.; Mendes, A. Elucidation of the Mechanism Underlying the Anti-Inflammatory Properties of (S)-(+)-Carvone Identifies a Novel Class of Sirtuin-1 Activators in a Murine Macrophage Cell Line. Biomedicines 2021, 9, 777. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Borzì, R.; Flamigni, F. Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death. Osteoarthr. Cartil. 2017, 25, 600–610. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, Z.; Linghu, K.-G.; Liu, J.; Gan, L.; Lin, L. Small molecule-driven SIRT3-autophagy-mediated NLRP3 inflam-masome inhibition ameliorates inflammatory crosstalk between macrophages and adipocytes. Br. J. Pharmacol. 2020, 177, 4645–4665. [Google Scholar] [CrossRef]
- Treviño-Saldaña, N.; García-Rivas, G. Regulation of Sirtuin-Mediated Protein Deacetylation by Cardioprotective Phytochem-icals. Oxidative Med. Cell. Longev. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Agarwal, B.; Baur, J.A. Resveratrol and life extension. Ann. New York Acad. Sci. 2011, 1215, 138–143. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharma-col. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Landry, J.; Sutton, A.; Tafrov, S.T.; Heller, R.C.; Stebbins, J.; Pillus, L.; Sternglanz, R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl. Acad. Sci. USA 2000, 97, 5807–5811. [Google Scholar] [CrossRef]
- Imai, S.-I.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Lin, S.-J.; Defossez, P.-A.; Guarente, L. Requirement of NAD and SIR2 for Life-Span Extension by Calorie Restriction in Sac-charomyces cerevisiae. Science 2000, 289, 2126–2128. [Google Scholar] [CrossRef]
- Morselli, E.; Maiuri, M.C.; Markaki, M.; Megalou, E.; Pasparaki, A.; Palikaras, K.; Criollo, A.; Galluzzi, L.; Malik, S.A.; Vitale, I.; et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010, 1, e10. [Google Scholar] [CrossRef]
- Boily, G.; Seifert, E.L.; Bevilacqua, L.; He, X.H.; Sabourin, G.; Estey, C.; Moffat, C.; Crawford, S.; Saliba, S.; Jardine, K.; et al. SirT1 Regulates Energy Metabolism and Response to Caloric Restriction in Mice. PLoS ONE 2008, 3, e1759. [Google Scholar] [CrossRef]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Cardioprotective Effects of Sirtuin-1 and Its Downstream Effectors. Circ. Heart Fail. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, X.; Li, X.; Dong, X.; Li, P.; Zhao, L. Resveratrol attenuates intermittent hypoxia-induced insulin resistance in rats: Involvement of Sirtuin 1 and the phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT pathway. Mol. Med. Rep. 2014, 11, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; McBurney, M.W.; Fang, D. Sirtuin 1 in immune regulation and autoimmunity. Immunol. Cell Biol. 2011, 90, 6–13. [Google Scholar] [CrossRef]
- Serravallo, M.; Jagdeo, J.; Glick, S.A.; Siegel, D.M.; Brody, N.I. Sirtuins in dermatology: Applications for future research and therapeutics. Arch. Dermatol. Res. 2013, 305, 269–282. [Google Scholar] [CrossRef]
- Yao, H.; Sundar, I.K.; Huang, Y.; Gerloff, J.; Sellix, M.T.; Sime, P.J.; Rahman, I. Disruption of Sirtuin 1–Mediated Control of Circadian Molecular Clock and Inflammation in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2015, 53, 782–792. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Chen, H.; Wu, Y. Fisetin inhibits IL-1β-induced inflammatory re-sponse in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef]
- Kume, S.; Uzu, T.; Horiike, K.; Chin-Kanasaki, M.; Isshiki, K.; Araki, S.-I.; Sugimoto, T.; Haneda, M.; Kashiwagi, A.; Koya, D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Investig. 2010, 120, 1043–1055. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.W.; Lee, S.Y.; Hong, K.W.; Bae, S.S.; Kim, K.; Kim, C.D. SIRT1/Adenosine Monophosphate-Activated Protein Kinase α Signaling Enhances Macrophage Polarization to an Anti-inflammatory Phenotype in Rheumatoid Arthritis. Front. Immunol. 2017, 8, 1135. [Google Scholar] [CrossRef]
- Zhong, Y.; Lee, K.; He, J.C. SIRT1 Is a Potential Drug Target for Treatment of Diabetic Kidney Disease. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Hwang, J.-W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senes-cence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef]
- Imai, S.-I.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Osum, M.; Serakinci, N. Impact of circadian disruption on health; SIRT1 and Telomeres. DNA Repair 2020, 96, 102993. [Google Scholar] [CrossRef]
- Wang, R.-H.; Zhao, T.; Cui, K.; Hu, G.; Chen, Q.; Chen, W.; Wang, X.-W.; Soto-Gutierrez, A.; Zhao, K.; Deng, C.-X. Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging. Sci. Rep. 2016, 6, 28633. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-Dependent Deacetylase SIRT1 Modulates CLOCK-Mediated Chromatin Remodeling and Circadian Con-trol. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian Control of the NAD + Salvage Pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, D.; Liu, W.; Li, S.; Chen, J.; Shen, Y.; Wang, F.; Hu, L.-F.; Liu, C.-F. Disruption of the Circadian Clock Alters Antioxidative Defense via the SIRT1-BMAL1 Pathway in 6-OHDA-Induced Models of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Sato, S.; Solanas, G.; Peixoto, F.O.; Bee, L.; Symeonidi, A.; Schmidt, M.S.; Brenner, C.; Masri, S.; Benitah, S.A.; Sassone-Corsi, P. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell 2017, 170, 664–677.e11. [Google Scholar] [CrossRef]
- Yang, W.; Kang, X.; Liu, J.; Li, H.; Ma, Z.; Jin, X.; Qian, Z.; Xie, T.; Qin, N.; Feng, D.; et al. Clock Gene Bmal1 Modulates Human Cartilage Gene Expression by Crosstalk With Sirt1. Endocrinology 2016, 157, 3096–3107. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Clauson, C.L.; Niedernhofer, L.J.; Robbins, P.D. NF-ΚB in Aging and Disease. Aging Dis. 2011, 2, 449–465. [Google Scholar]
- Butt, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The Senescence-Associated Secretory Phenotype (SASP) in the Challenging Future of Cancer Therapy and Age-Related Diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef]
- Salminen, A.; Huskonen, J.; Ojala, J.; Kauppinen, A.; Carnirantha, K.; Suuronen, T. Activation of the innate immune system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Aging Res. Rev. Fr. 2008, 7, 83–105. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Carnirantha, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- Caito, S.; Rajendrasozhan, S.; Cook, S.; Chung, S.; Yao, H.; Friedman, A.E.; Brookes, P.S.; Rahman, I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. 2010, 24, 3145–3159. [Google Scholar] [CrossRef]
- Dvir-Ginzberg, M.; Gagarina, V.; Lee, E.J.; Booth, R.; Gabay, O.; Hall, D.J. Tumor necrosis factor α-mediated cleavage and in-activation of SirT1 in human osteoarthritic chondrocytes. Arthritis Care Res. 2011, 63, 2363–2373. [Google Scholar] [CrossRef]
- Guo, B.; Yang, N.; Borysiewicz, E.; Dudek, M.; Williams, J.; Li, J.; Maywood, E.; Adamson, A.; Hastings, M.; Bateman, J.; et al. Catabolic cytokines disrupt the circadian clock and the expression of clock-controlled genes in cartilage via an NFκB-dependent pathway. Osteoarthr. Cartel. 2015, 23, 1981–1988. [Google Scholar] [CrossRef]
- Dudek, M.; Yang, N.; Ruckshanthi, J.P.; Williams, J.; Borysiewicz, E.; Wang, P.; Adamson, A.; Li, J.; Bateman, J.F.; White, M.R.; et al. The intervertebral disc contains intrinsic circadian clocks that are regulated by age and cytokines and linked to degeneration. Ann. Rheum. This. 2016, 76, 576–584. [Google Scholar] [CrossRef]
- Hong, H.-K.; Maury, E.; Ramsey, K.M.; Perelis, M.; Marcheva, B.; Mura, C.; Kobayashi, Y.; Guttridge, D.C.; Barish, G.D.; Bass, J. Requirement for NF-κB in maintenance of molecular and behavioral circadian rhythms in mice. Genes Dev. 2018, 32, 1367–1379. [Google Scholar] [CrossRef]
- Spengler, M.L.; Kuropatwinski, K.K.; Comas, M.; Gasparian, A.V.; Fedtsova, N.; Gleiberman, A.S.; Gitlin, I.I.; Artemicheva, N.M.; Deluca, K.A.; Gudkov, A.V.; et al. The core circadian protein CLOCK is a positive regulator of NF- B-mediated transcription. Proc. Natl. Acad. Sci. USA 2012, 109, E2457–E2465. [Google Scholar] [CrossRef] [PubMed]
- Delerive, P.; Monte, D.; Dubois, G.; Trottein, F.; Fruchart-Najib, J.; Mariani, J.; Fruchart, J.-C.; Staels, B. The orphan nuclear receptor RORα is a negative regulator of the inflammatory response. EMBO Rep. 2001, 2, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Kim, D.; Kim, K.; Boo, K.; You, Y.S.; Kim, I.S.; Jeon, Y.; Im, S.-K.; Lee, S.-H.; Lee, J.M.; et al. RORα is crucial for at-tenuated inflammatory response to maintain intestinal homeostasis. Proc. Natl. Acad. Sci. USA 2019, 116, 21140–21149. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M. Terpenoids. In Pharmacognosy; Academic Press: Cambridge, MA, USA, 2017; pp. 233–266. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants: From Farm to Pharmacy; Nirmal, J., Sadanand, A.D., Parajuli, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar] [CrossRef]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. In Terpenes and Terpenoids; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Sałat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An Overview of the Pharma-cological Properties and Potential Applications of Natural Monoterpenes. Mini-Rev. Med. Chem. 2015, 14, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Shim, S.H.; Ahn, H.R.; Jung, S.H. Protective effects of the compounds isolated from the seed of Psoralea corylifolia on oxidative stress-induced retinal damage. Toxicol. Appl. Pharmacol. 2013, 269, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.K.; Bojanowski, K. Bakuchiol: A retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects. Int. J. Cosmet. Sci. 2014, 36, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sawhney, G.; Kumar Nagar, R.; Chauhan, N.; Gupta, N.; Kaul, A.; Ahmed, Z.; Sangwan, P.L.; Satheesh Kumar, P.; Yadav, G. Evaluation of the immunomodulatory and anti-inflammatory activity of Bakuchiol using RAW 264.7 macrophage cell lines and in animal models stimulated by lipopolysaccharide (LPS). Int. Immunopharmacol. 2021, 91, 107264. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Zhang, X.; Wang, J.; Gu, D.; Wang, Y. In vitro evidence for bakuchiol’s influence towards drug metabolism through inhibition of UDP-glucuronosyltransferase (UGT) 2B7. Afr. Health Sci. 2014, 14, 564–569. [Google Scholar] [CrossRef]
- Feng, J.; Yang, Y.; Zhou, Y.; Wang, B.; Xiong, H.; Fan, C.; Jiang, S.; Liu, J.; Ma, Z.; Hu, W.; et al. Bakuchiol attenuates myocardial ischemia reperfusion injury by maintaining mitochondrial function: The role of silent information regulator 1. Apoptosis 2016, 21, 532–545. [Google Scholar] [CrossRef]
- Ma, W.; Guo, W.; Shang, F.; Li, Y.; Li, W.; Liu, J.; Ma, C.; Teng, J. Bakuchiol Alleviates Hyperglycemia-Induced Diabetic Car-diomyopathy by Reducing Myocardial Oxidative Stress via Activating the SIRT1/Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Pina, L.T.; Serafini, M.R.; Oliveira, M.A.; Sampaio, L.A.; Guimarães, J.O.; Guimarães, A.G. Carvone and its pharmacological activities: A systematic review. Phytochemistry 2022, 196, 113080. [Google Scholar] [CrossRef]
- Sousa, C.; Leitão, A.J.; Neves, B.M.; Judas, F.; Cavaleiro, C.; Mendes, A.F. Standardised comparison of limonene-derived monoterpenes identifies structural determinants of anti-inflammatory activity. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Lakhdar, F.; Khouchlaa, A.; Bakrim, S.; El Omari, N.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Orlando, G.; et al. Health Benefits and Pharmacological Properties of Hinokitiol. Processes 2021, 9, 1680. [Google Scholar] [CrossRef]
- Lee, J.-H.; Moon, J.-H.; Lee, Y.-J.; Park, S.-Y. SIRT1, a Class III Histone Deacetylase, Regulates LPS-Induced Inflammation in Human Keratinocytes and Mediates the Anti-Inflammatory Effects of Hinokitiol. J. Investig. Dermatol. 2017, 137, 1257–1266. [Google Scholar] [CrossRef]
- Kaneda, M.; Iitaka, Y.; Shibata, S. Chemical studies on the oriental plant drugs—XXXIII. Tetrahedron 1972, 28, 4309–4317. [Google Scholar] [CrossRef]
- Jiao, F.; Varghese, K.; Wang, S.; Liu, Y.; Yu, H.; Booz, G.W.; Roman, R.J.; Liu, R.; Fan, F. Recent Insights into the Protective Mechanisms of Paeoniflorin in Neurological, Cardiovascular, and Renal Diseases. J. Cardiovasc. Pharmacol. 2021, 77, 728–734. [Google Scholar] [CrossRef]
- Tao, T.; Zheng, H.; Yang, L.; Guo, X.; Zhang, J.; Lv, C.; Shi, D.S. Paeoniflorin attenuates neuropathic pain through the regulation of Sirt1 in rats. Int. J. Clin. Exp. Med. 2017, 10, 4678–4686. [Google Scholar]
- Cappoli, N.; Tabolacci, E.; Aceto, P.; Russo, C.D. The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J. Neuroimmunol. 2020, 349, 577406. [Google Scholar] [CrossRef]
- Wang, Y.; Che, J.; Zhao, H.; Tang, J.; Shi, G. Paeoniflorin attenuates oxidized low-density lipoprotein-induced apoptosis and adhesion molecule expression by autophagy enhancement in human umbilical vein endothelial cells. J. Cell. Biochem. 2018, 120, 9291–9299. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phyto-chemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef]
- Bryś, M.; Urbańska, K.; Olas, B. Novel Findings regarding the Bioactivity of the Natural Blue Pigment Genipin in Human Diseases. Int. J. Mol. Sci. 2022, 23, 902. [Google Scholar] [CrossRef]
- Shin, J.-K.; Lee, S.-M. Genipin protects the liver from ischemia/reperfusion injury by modulating mitochondrial quality control. Toxicol. Appl. Pharmacol. 2017, 328, 25–33. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Yap, K.H.; Rao, V.; Choudhury, H. Multiple Biological Effects of an Iridoid Glucoside, Catalpol, and Its Underlying Molecular Mechanisms. Biomolecules 2019, 10, 32. [Google Scholar] [CrossRef]
- Xiong, Y.; Shi, L.; Wang, L.; Zhou, Z.; Wang, C.; Lin, Y.; Luo, D.; Qiu, J.; Chen, D. Activation of sirtuin 1 by catalpol-induced down-regulation of microRNA-132 attenuates endoplasmic reticulum stress in colitis. Pharmacol. Res. 2017, 123, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bi, R.; Meng, Q.; Wang, C.; Huo, X.; Liu, Z.; Wang, C.; Sun, P.; Sun, H.; Ma, X.; et al. Catalpol alleviates adriamycin-induced nephropathy by activating the SIRT1 signalling pathway in vivo and in vitro. J. Cereb. Blood Flow Metab. 2019, 176, 4558–4573. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Y.; Lv, Z.; Shu, A.; Du, Q.; Wang, W.; Chen, Y.; Xu, H. Study on the inhibitive effect of Catalpol on diabetic nephropathy. Life Sci. 2020, 257, 118120. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhang, B.; Zhao, W.; Tu, Y.; Wang, Q.; Li, J. Catalpol ameliorates psoriasis-like phenotypes via SIRT1 mediated sup-pression of NF-κB and MAPKs signaling pathways. Bioengineered 2020, 12, 183–195. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Zhang, R.-Q.; Rahman, K.; Cao, Z.-X.; Zhang, H.; Peng, C. Diverse Pharmacological Activities and Potential Me-dicinal Benefits of Geniposide. Evidence-Based Complement. Altern. Med. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Ma, Z.-G.; Zhu, J.-X.; Liu, C.; Song, P.; Kong, C.-Y.; Wu, H.-M.; Deng, W.; Tang, Q.-Z. Geniposide Alleviates Isoproterenol-Induced Cardiac Fibrosis Partially via SIRT1 Activation in vivo and in vitro. Front. Pharmacol. 2018, 9, 854. [Google Scholar] [CrossRef]
- Ma, Z.-G.; Kong, C.-Y.; Song, P.; Zhang, X.; Yuan, Y.-P.; Tang, Q.-Z. Geniposide Protects against Obesity-Related Cardiac Injury through AMPKα- and Sirt1-Dependent Mechanisms. Oxidative Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Gong, N.; Fan, H.; Ma, A.-N.; Xiao, Q.; Wang, Y.-X. Geniposide and its iridoid analogs exhibit antinociception by acting at the spinal GLP-1 receptors. Neuropharmacology 2014, 84, 31–45. [Google Scholar] [CrossRef]
- Hu, X.; Jin, G.; Shi, Z.; Zhang, X.; Sun, W.; Chen, F. Geniposide reduces development of streptozotocin-induced diabetic nephropathy via regulating NF-κB signaling pathways. Fundam. Clin. Pharmacol. 2016, 31, 54–63. [Google Scholar] [CrossRef]
- Li, F.; Chen, Y.; Li, Y.; Huang, M.; Zhao, W. Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-κB pathway. Eur. J. Pharmacol. 2020, 886, 173449. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, Y.; Li, C.; Xu, M.; Xie, H.; Zhao, J. Using Network Pharmacology for Systematic Understanding of Geniposide in Ameliorating Inflammatory Responses in Colitis through Suppression of NLRP3 Inflammasome in Macrophage by AMPK/Sirt1 Dependent Signaling. Am. J. Chin. Med. 2020, 48, 1693–1713. [Google Scholar] [CrossRef]
- Li, F.; Song, L.; Chen, J.; Chen, Y.; Li, Y.; Huang, M.; Zhao, W. Effect of genipin-1-β-d-gentiobioside on diabetic nephropathy in mice by activating AMP-activated protein kinase/silencing information regulator-related enzyme 1/nuclear factor-κB path-way. J. Pharm. Pharmacol. 2021, 73, 1201–1211. [Google Scholar] [CrossRef]
- Yuan, J.; Cheng, W.; Zhang, G.; Ma, Q.; Li, X.; Zhang, B.; Hu, T.; Song, G. Protective effects of iridoid glycosides on acute colitis via inhibition of the inflammatory response mediated by the STAT3/NF-κB pathway. Int. Immunopharmacol. 2020, 81, 106240. [Google Scholar] [CrossRef]
- Liu, S.; Shen, H.; Li, J.; Gong, Y.; Bao, H.; Zhang, J.; Hu, L.; Wang, Z.; Gong, J. Loganin inhibits macrophage M1 polarization and modulates sirt1/NF-κB signaling pathway to attenuate ulcerative colitis. Bioengineered 2020, 11, 628–639. [Google Scholar] [CrossRef]
- Zhu, F.-B.; Wang, J.-Y.; Zhang, Y.-L.; Hu, Y.-G.; Yue, Z.-S.; Zeng, L.-R.; Zheng, W.-J.; Hou, Q.; Yan, S.-G.; Quan, R.-F. Mecha-nisms underlying the antiapoptotic and anti-inflammatory effects of monotropein in hydrogen peroxide-treated osteoblasts. Mol. Med. Rep. 2016, 14, 5377–5384. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, Y.; Zhang, C.; Shen, Y.; Pan, Y.S.; Chen, R.Z.; Zhang, Q.; Chen, Y.H. Picroside II attenuates hyperhomocys-teinemia-induced endothelial injury by reducing inflammation, oxidative stress and cell apoptosis. J. Cell. Mol. Med. 2018, 23, 464–475. [Google Scholar] [CrossRef]
- Wang, R.; Dong, Z.; Lan, X.; Liao, Z.; Chen, M. Sweroside Alleviated LPS-Induced Inflammation via SIRT1 Mediating NF-κB and FOXO1 Signaling Pathways in RAW264. 7 Cells. Molecules 2019, 24, 872. [Google Scholar] [CrossRef]
- Wu, S.; Ning, Y.; Zhao, Y.-Y.; Sun, W.; Thorimbert, S.; Dechoux, L.; Sollogoub, M.; Zhang, Y. Research Progress of Natural Product Gentiopicroside-a Secoiridoid Compound. Mini-Rev. Med. Chem. 2016, 17, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Fu, Y.; Cao, C.; Pan, D.; Wang, W.; Kong, L. Gentiopicroside ameliorates ovalbumin-induced airway inflammation in a mouse model of allergic asthma via regulating SIRT1/NF-κB signaling pathway. Pulm. Pharmacol. Ther. 2021, 68, 102034. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L. As a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M.; Muroi, H.; Uwabe, Y.; Furukawa, K.; Toyomizu, M. Oleuropein induces mitochondrial biogenesis and decreases reactive oxygen species generation in cultured avian muscle cells, possibly via an up-regulation of peroxisome prolifera-tor-activated receptor γ coactivator-1α. Anim. Sci. J. 2016, 87, 1371–1378. [Google Scholar] [CrossRef]
- Shimao, R.; Muroi, H.; Furukawa, K.; Toyomizu, M.; Kikusato, M. Effects of low-dose oleuropein diet supplementation on the oxidative status of skeletal muscles and plasma hormonal concentration of growing broiler chickens. Br. Poult. Sci. 2019, 60, 784–789. [Google Scholar] [CrossRef]
- Luccarini, I.; Pantano, D.; Nardiello, P.; Cavone, L.; Lapucci, A.; Miceli, C.; Nediani, C.; Berti, A.; Stefani, M.; Casamenti, F. The Polyphenol Oleuropein Aglycone Modulates the PARP1-SIRT1 Interplay: An In Vitro and In Vivo Study. J. Alzheimer’s Dis. 2016, 54, 737–750. [Google Scholar] [CrossRef]
- Park, H.; Kam, T.-I.; Dawson, T.M.; Dawson, V.L. Poly (ADP-ribose) (PAR)-dependent cell death in neurodegenerative dis-eases. Int. Rev. Cell Mol. Biol. 2020, 353, 1–29. [Google Scholar] [CrossRef]
- Bai, P.; Cantó, C.; Oudart, H.; Brunyánszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.H.; et al. PARP-1 Inhibition Increases Mitochondrial Metabolism through SIRT1 Activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef]
- Brahmbhatt, H.; Gupta, R.; Gupta, A.; Rastogi, S.; Misri, R.; Mobeen, A.; Ghosh, A.; Kothari, P.; Sitaniya, S.; Scaria, V.; et al. The long noncoding RNA MALAT1 suppresses miR-211 to confer protection from ultraviolet-mediated DNA damage in vitiligo epidermis by upregulating sirtuin 1*. Br. J. Dermatol. 2020, 184, 1132–1142. [Google Scholar] [CrossRef]
- Kong, P.; Yu, Y.; Wang, L.; Dou, Y.-Q.; Zhang, X.-H.; Cui, Y.; Wang, H.-Y.; Yong, Y.-T.; Liu, Y.; Hu, H.-J.; et al. circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019, 47, 3580–3593. [Google Scholar] [CrossRef]
- Song, L.; Chen, T.; Zhao, X.; Xu, Q.; Jiao, R.; Li, J.; Kong, L. Pterostilbene prevents hepatocyte epithelial-mesenchymal transition in fructose-induced liver fibrosis through suppressing miR-34a/Sirt1/p53 and TGF-β1/Smads signalling. J. Cereb. Blood Flow Metab. 2019, 176, 1619–1634. [Google Scholar] [CrossRef]
- Nikseresht, S.; Khodagholi, F.; Ahmadiani, A. Protective effects of ex-527 on cerebral ischemia–reperfusion injury through necroptosis signaling pathway attenuation. J. Cell. Physiol. 2018, 234, 1816–1826. [Google Scholar] [CrossRef]
- Huang, J.; Tian, R.; Yang, Y.; Jiang, R.; Dai, J.; Tang, L.; Zhang, L. The SIRT1 inhibitor EX-527 suppresses mTOR activation and alleviates acute lung injury in mice with endotoxiemia. Innate Immun. 2017, 23, 678–686. [Google Scholar] [CrossRef]
- Smith, M.R.; Syed, A.; Lukacsovich, T.; Purcell, J.; Barbaro, B.A.; Worthge, S.A.; Wei, S.R.; Pollio, G.; Magnoni, L.; Scali, C.; et al. A potent and selective Sirtuin 1 inhibitor alleviates pathology in multiple animal and cell models of Huntington’s disease. Hum. Mol. Genet. 2014, 23, 2995–3007. [Google Scholar] [CrossRef]
- Brandl, L.; Kirstein, N.; Neumann, J.; Sendelhofert, A.; Vieth, M.; Kirchner, T.; Menssen, A. The c-MYC/NAMPT/SIRT1 feedback loop is activated in early classical and serrated route colorectal cancer and represents a therapeutic target. Med. Oncol. 2018, 36, 5. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Sellitto, C.; Corbi, G.; Stefanelli, B.; Manzo, V.; Trucillo, M.; Charlier, B.; Mensitieri, F.; Izzo, V.; Lucariello, A.; Perna, A.; et al. Antioxidant Supplementation Hinders the Role of Exercise Training as a Natural Activator of SIRT1. Nutrients 2022, 14, 2092. [Google Scholar] [CrossRef]
- Guarente, L. Calorie restriction and sirtuins revisited. Genes Dev. 2013, 27, 2072–2085. [Google Scholar] [CrossRef]
- Ferrara, N.; Rinaldi, B.; Corbi, G.; Conti, V.; Stiuso, P.; Boccuti, S.; Rengo, G.; Rossi, F.; Filippelli, A. Exercise Training Promotes SIRT1 Activity in Aged Rats. Rejuvenation Res. 2008, 11, 139–150. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chandrasekaran, B.; Namasivayam, N. Geraniol attenuates oxidative stress by Nrf2 activation in di-et-induced experimental atherosclerosis. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 335–346. [Google Scholar] [CrossRef]
- Yang, S.-M.; Hua, K.-F.; Lin, Y.-C.; Chen, A.; Chang, J.-M.; Chao, L.K.; Ho, C.-L.; Ka, S.-M. Citral Is Renoprotective for Focal Segmental Glomerulosclerosis by Inhibiting Oxidative Stress and Apoptosis and Activating Nrf2 Pathway in Mice. PLoS ONE 2013, 8, e74871. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.L.; Ramasamy, T.S. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res. Rev. 2018, 43, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Niu, H.; Sha, G.; Zhang, Y.; Liu, P.; Li, Y. Serum SIRT1 is Associated with Frailty and Adipokines in Older Adults. J. Nutr. Health Aging 2018, 23, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.; Benson, V.; McMahon, A.C.; Blyth, F.; Handelsman, D.J.; Seibel, M.; Kennerson, M.; Naganathan, V.; Cumming, R.; de Cabo, R. Determinants of Serum-Induced SIRT1 Expression in Older Men: The CHAMP Study. J. Gerontol. Ser. A 2010, 66, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, Frailty and Cardiovascular Disease. Frailty Cardiovasc. Diseases 2020, 1216, 55–64. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, C.; Mendes, A.F. Monoterpenes as Sirtuin-1 Activators: Therapeutic Potential in Aging and Related Diseases. Biomolecules 2022, 12, 921. https://doi.org/10.3390/biom12070921
Sousa C, Mendes AF. Monoterpenes as Sirtuin-1 Activators: Therapeutic Potential in Aging and Related Diseases. Biomolecules. 2022; 12(7):921. https://doi.org/10.3390/biom12070921
Chicago/Turabian StyleSousa, Cátia, and Alexandrina Ferreira Mendes. 2022. "Monoterpenes as Sirtuin-1 Activators: Therapeutic Potential in Aging and Related Diseases" Biomolecules 12, no. 7: 921. https://doi.org/10.3390/biom12070921
APA StyleSousa, C., & Mendes, A. F. (2022). Monoterpenes as Sirtuin-1 Activators: Therapeutic Potential in Aging and Related Diseases. Biomolecules, 12(7), 921. https://doi.org/10.3390/biom12070921







