Extracellular Heat Shock Protein-90 (eHsp90): Everything You Need to Know
Abstract
1. Introduction
2. History of eHsp90 Discovery
3. eHsp90α vs. eHsp90β: Who Calls the Shots and Why?
4. Is eHsp90 Secreted by Living Cells on Purpose or Leaked by Dead Cells by Accident?
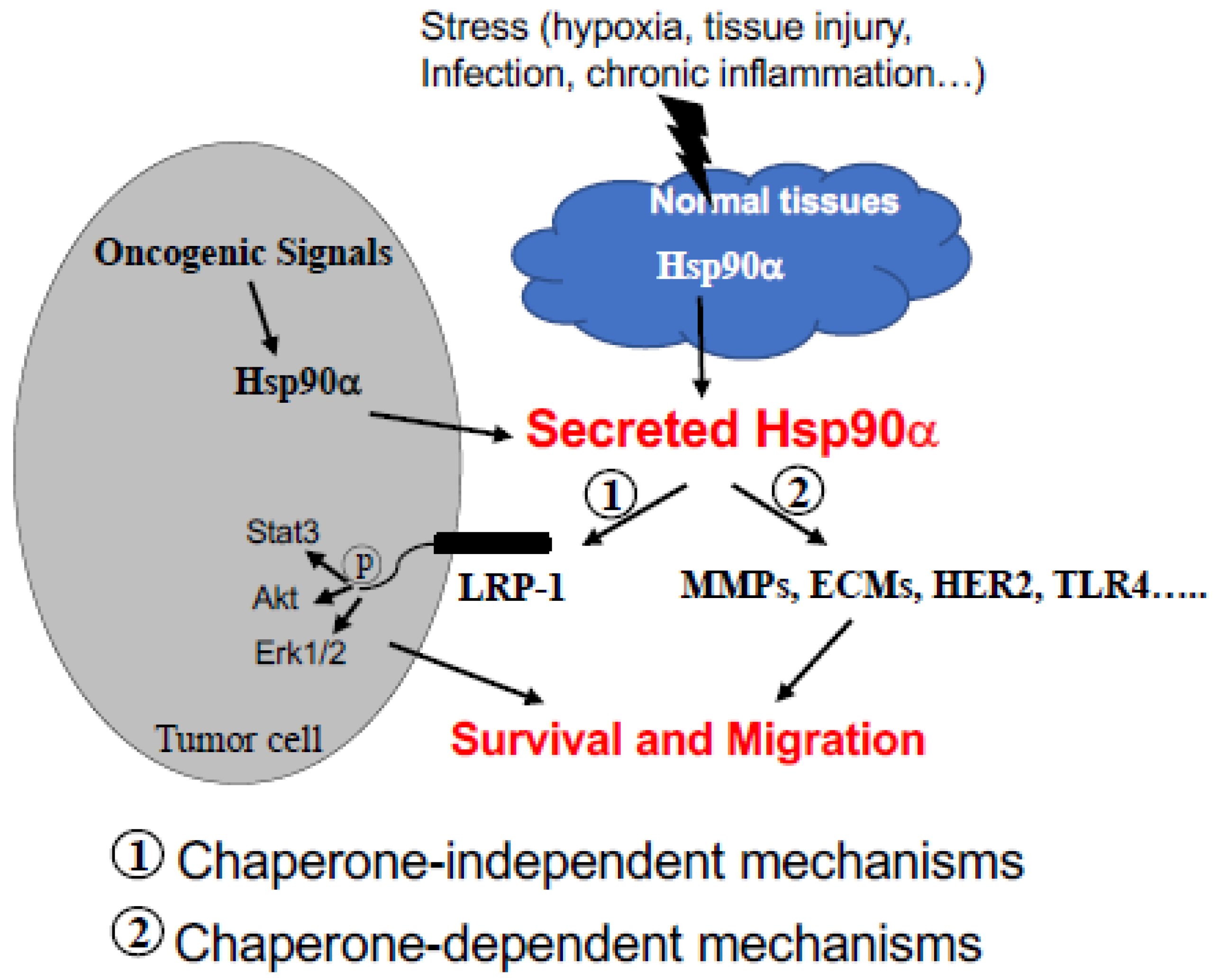
5. Two Main Biological Functions of eHsp90α
5.1. Promoting Cell Survival under Ischemic Stress
5.2. Promoting Cell Motility (Not Growth) during Tissue Repair and Tumour Invasion
6. Mechanisms of Action by eHsp90α
7. Preclinical Studies of eHsp90α
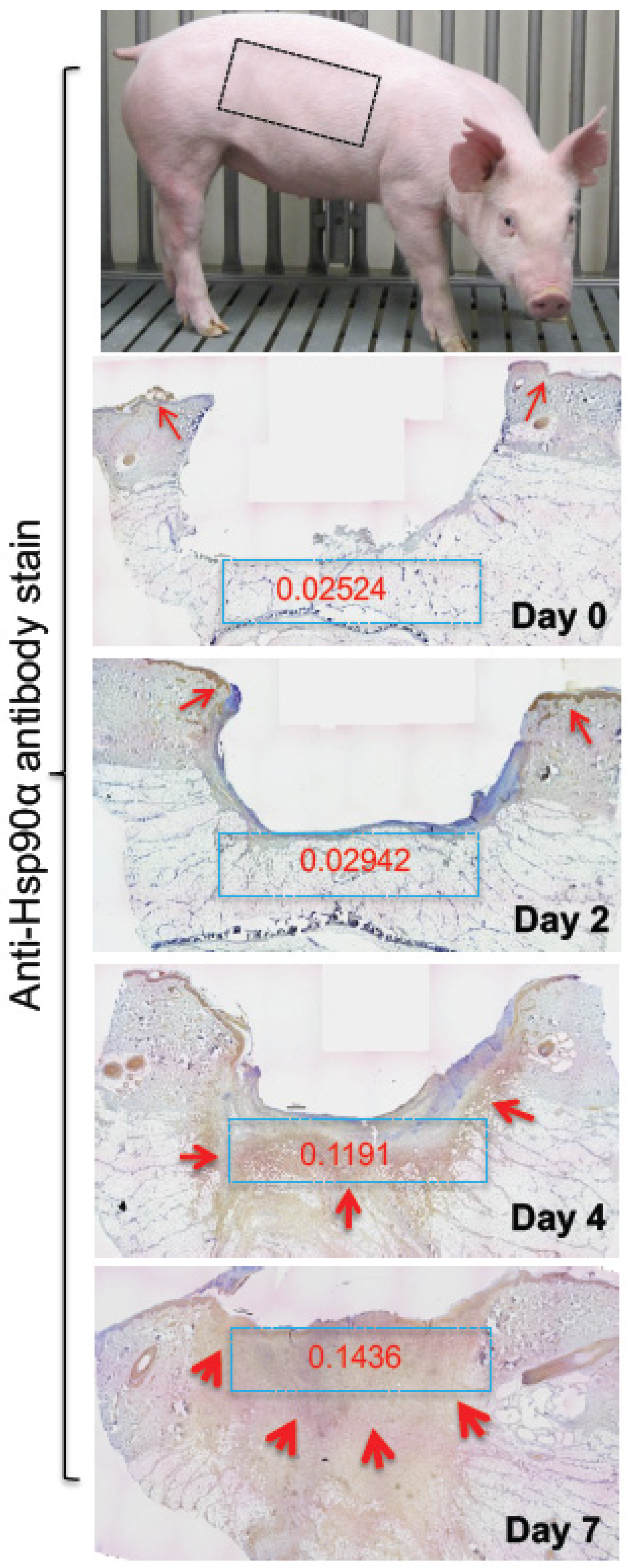
7.1. Wound Healing
7.2. Tissue Fibrosis
7.3. Wasting Syndrome
7.4. Tumorigenesis
8. Clinical Studies of eHsp90α in Patients with Cancer and Inflammatory Disorders
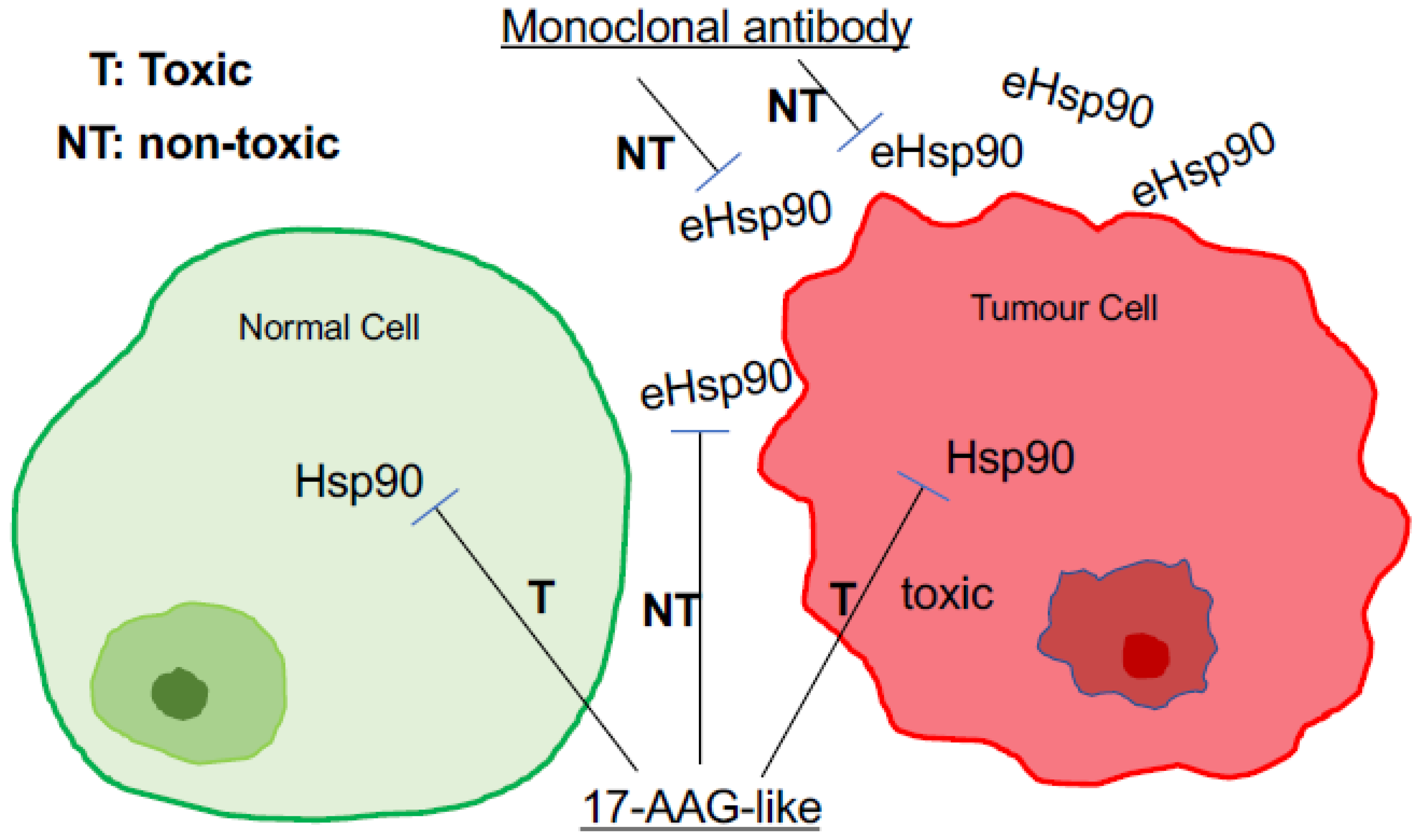
9. Is eHsp90α a More Effective and Safer Drug Target than Intracellular Hsp90?
10. Why Is eHsp90α Co-Opted for Extracellular Duties?
11. Conclusions and Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Young, J.C.; Moarefi, I.; Hartl, F.U. Hsp90: A specialized but essential protein-folding tool. J. Cell Biol. 2001, 154, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.; Taldone, T.; Modi, S.; Chiosis, G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim. Biophys. Acta Mol. 2012, 1823, 742–755. [Google Scholar] [CrossRef]
- Neckers, L.; Blagg, B.; Haystead, T.; Trepel, J.B.; Whitesell, L.; Picard, D. Methods to validate Hsp90 inhibitor specificity, to identify off-target effects, and to rethink approaches for further clinical development. Cell Stress Chaperones 2018, 23, 467–482. [Google Scholar] [CrossRef]
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S. Old and new approaches to target the Hsp90 chaperone. Curr. Cancer Drug Targets 2020, 20, 253–270. [Google Scholar] [CrossRef]
- Csermely, P.; Tamás, S.; Csaba, S.; Zoltán, P.; Gábor, N. The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998, 79, 129–168. [Google Scholar] [CrossRef]
- Stone, K.R.; Ralph, E.S.; Wolfgang, K.J. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology 1974, 58, 86–100. [Google Scholar] [CrossRef]
- Shiu, R.P.; Pouyssegur, J.; Pastan, I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc. Natl. Acad. Sci. USA 1977, 74, 3840–3844. [Google Scholar] [CrossRef]
- Pouysségur, J.; Shiu, R.P.; Pastan, I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell 1977, 11, 941–947. [Google Scholar] [CrossRef]
- Pouysségur, J.; Yamada, K.M. Isolation and immunological characterization of a glucose-regulated fibroblast cell surface glycoprotein and its nonglycosylated precursor. Cell 1978, 13, 139–140. [Google Scholar] [CrossRef]
- McCormick, P.J.; Keys, B.J.; Pucci, C.; Millis, A.J. Human fibroblast-conditioned medium contains a 100K dalton glucose-regulated cell surface protein. Cell 1979, 18, 173–182. [Google Scholar] [CrossRef]
- Hughes, E.N.; Colombatti, A.; August, J.T. Murine cell surface glycoproteins. Purification of the polymorphic Pgp-1 antigen and analysis of its expression on macrophages and other myeloid cells. J. Biol. Chem. 1983, 258, 1014–1021. [Google Scholar] [CrossRef]
- Srivastava, P.K.; DeLeo, A.B.; Old, L.J. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc. Natl. Acad. Sci. USA 1986, 83, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.J.; Robinson, E.A.; Law, L.W.; Willingham, M.; Appella, E. A mouse tumor-specific transplantation antigen is a heat shock-related protein. Proc. Natl. Acad. Sci. USA 1986, 83, 3121–3125. [Google Scholar] [CrossRef]
- La Thangue, N.B.; Latchman, D.S. A cellular protein related to heat-shock protein 90 accumulates during herpes simplex virus infection and is overexpressed in transformed cells. Exp. Cell Res. 1988, 178, 169–179. [Google Scholar] [CrossRef]
- Erkeller-Yüksel, F.M.; Isenberg, D.A.; Dhillon, V.B.; Latchman, D.S.; Lydyard, P.M. Surface expression of heat shock protein 90 by blood mononuclear cells from patients with systemic lupus erythematosus. J. Autoimmun. 1992, 5, 803–814. [Google Scholar] [CrossRef]
- Thomaidou, D.; Dori, I.; Patsavoudi, E. Developmental expression and functional characterization of the 4C5 antigen in the postnatal cerebellar cortex. J. Neurochem. 1995, 64, 1937–1944. [Google Scholar] [CrossRef]
- Sidera, K.; Samiotaki, M.; Yfanti, E.; Panayotou, G.; Patsavoudi, E. Involvement of cell surface HSP90 in cell migration reveals a novel role in the developing nervous system. J. Biol. Chem. 2004, 279, 45379–45388. [Google Scholar] [CrossRef]
- Eustace, B.K.T.; Sakurai, J.K.; Stewart, D.; Yimlamai, C.; Unger, C.; Zehetmeier, B.; Lain, C.; Torella, S.W.; Henning, G.; Beste, B.T.; et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat. Cell Biol. 2004, 6, 507–514. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Guan, S.; Fan, J.; Cheng, C.F.; Bright, A.M.; Chinn, C.; Chen, M.; Woodley, D.T. Extracellular heat shock protein-90alpha: Linking hypoxia to skin cell motility and wound healing. EMBO J. 2007, 26, 1221–1233. [Google Scholar] [CrossRef]
- Cheng, C.F.; Fan, J.; Fedesco, M.; Guan, S.; Li, Y.; Bandyopadhyay, B.; Bright, A.M.; Yerushalmi, D.; Liang, M.; Chen, M.; et al. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: Using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol. Cell. Biol. 2008, 28, 3344–3358. [Google Scholar] [CrossRef]
- Gopal, U.; Bohonowych, J.E.; Lema-Tome, C.; Liu, A.; Garrett-Mayer, E.; Wang, B.; Isaacs, J.S. A novel extracellular Hsp90 mediated co-receptor function for LRP1 regulates EphA2 dependent glioblastoma cell invasion. PLoS ONE 2011, 6, e17649. [Google Scholar] [CrossRef]
- Eustace, B.K.; Jay, D.G. Extracellular roles for the molecular chaperone, hsp90. Cell Cycle 2004, 3, 1098–1100. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Neckers, L. Extracellular heat shock protein 90: A role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007, 98, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Sidera, K.; Patsavoudi, E. Extracellular HSP90: Conquering the cell surface. Cell Cycle 2008, 7, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- McCready, J.; Sims, J.D.; Chan, D.D.; Jay, D.G. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: A role for plasminogen activation. BMC Cancer 2010, 10, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sahu, D.; Tsen, F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim. Biophys. Acta 2012, 1823, 730–741. [Google Scholar] [CrossRef]
- Li, W.; Tsen, F.; Sahu, D.; Bhatia, A.; Chen, M.; Multhoff, G.; Woodley, D.T. Extracellular Hsp90 (eHsp90) as the actual target in clinical trials: Intentionally or unintentionally. Int. Rev. Cell Mol. Biol. 2013, 303, 203–235. [Google Scholar]
- Hance, M.W.; Nolan, K.D.; Isaacs, J.S. The double-edged sword: Conserved functions of extracellular hsp90 in wound healing and cancer. Cancers 2014, 6, 1065–1097. [Google Scholar] [CrossRef]
- Sidera, K.; Patsavoudi, E. HSP90 inhibitors: Current development and potential in cancer therapy. Recent Pat. Anticancer Drug Discov. 2014, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.S.; Jay, D.G. Emerging roles of extracellular Hsp90 in cancer. Adv. Cancer Res. 2016, 129, 141–163. [Google Scholar] [PubMed]
- Kim, H.; Seo, E.H.; Lee, S.H.; Kim, B.J. The telomerase-derived anticancer peptide vaccine GV1001 as an extracellular heat shock protein-mediated cell-penetrating peptide. Int. J. Mol. Sci. 2016, 17, 2054. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Alves, P. Recent patents on heat shock proteins targeting antibodies. Recent Pat. Anticancer Drug Discov. 2017, 12, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K. Heat shock proteins and cancer: Intracellular chaperones or extracellularsignalling ligands? Philos. Trans. R Soc. Lond. B Biol. Sci. 2018, 373, 20160524. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of extracellular HSPs as biomarkers in immune surveillance and immune evasion. Int. J. Mol. Sci. 2019, 20, 4588. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Borges, T.J.; Eguchi, T.; Lang, B.J.; Murshid, A.; Okusha, Y.; Prince, T.L. Extracellular Hsp90 and protection of neuronal cells through Nrf2. Biochem. Soc. Trans. 2021, 49, 2299–2306. [Google Scholar] [CrossRef]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of HSP90 in cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef]
- Chakraborty, A.; Edkins, A.L. HSP90 as a regulator of extracellular matrix dynamics. Biochem. Soc. Trans. 2021, 49, 2611–2625. [Google Scholar] [CrossRef]
- Poggio, P.; Sorge, M.; Seclì, L.; Brancaccio, M. Extracellular HSP90 machineries build tumor microenvironment and boost cancer progression. Front. Cell Dev. Biol. 2021, 9, 735529. [Google Scholar] [CrossRef]
- Cheng, C.F.; Fan, J.; Zhao, Z.; Woodley, D.T.; Li, W. Secreted heat shock protein-90alpha: A more effective and safer target for anti-cancer drugs? Curr. Signal Transduct. Ther. 2010, 5, 121–127. [Google Scholar] [CrossRef]
- Yu, X.; Harris, S.L.; Levine, A.J. The regulation of exosome secretion: A novel function of the p53 protein. Cancer Res. 2006, 66, 4795–4801. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kulkarni, A.B. Extracellular heat shock protein HSP90beta secreted by MG63 osteosarcoma cells inhibits activation of latent TGF-beta1. Biochem. Biophys. Res. Commun. 2010, 398, 525–5331. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, P.; Dong, H.; Zou, M.; Bhatia, A.; O’Brien, K.; Chen, M.; Woodley, D.T.; Li, W. Hsp90α and Hsp90β together operate a hypoxia and nutrient paucity stress-response mechanism during wound healing. J. Cell Sci. 2015, 128, 1475–1480. [Google Scholar] [PubMed]
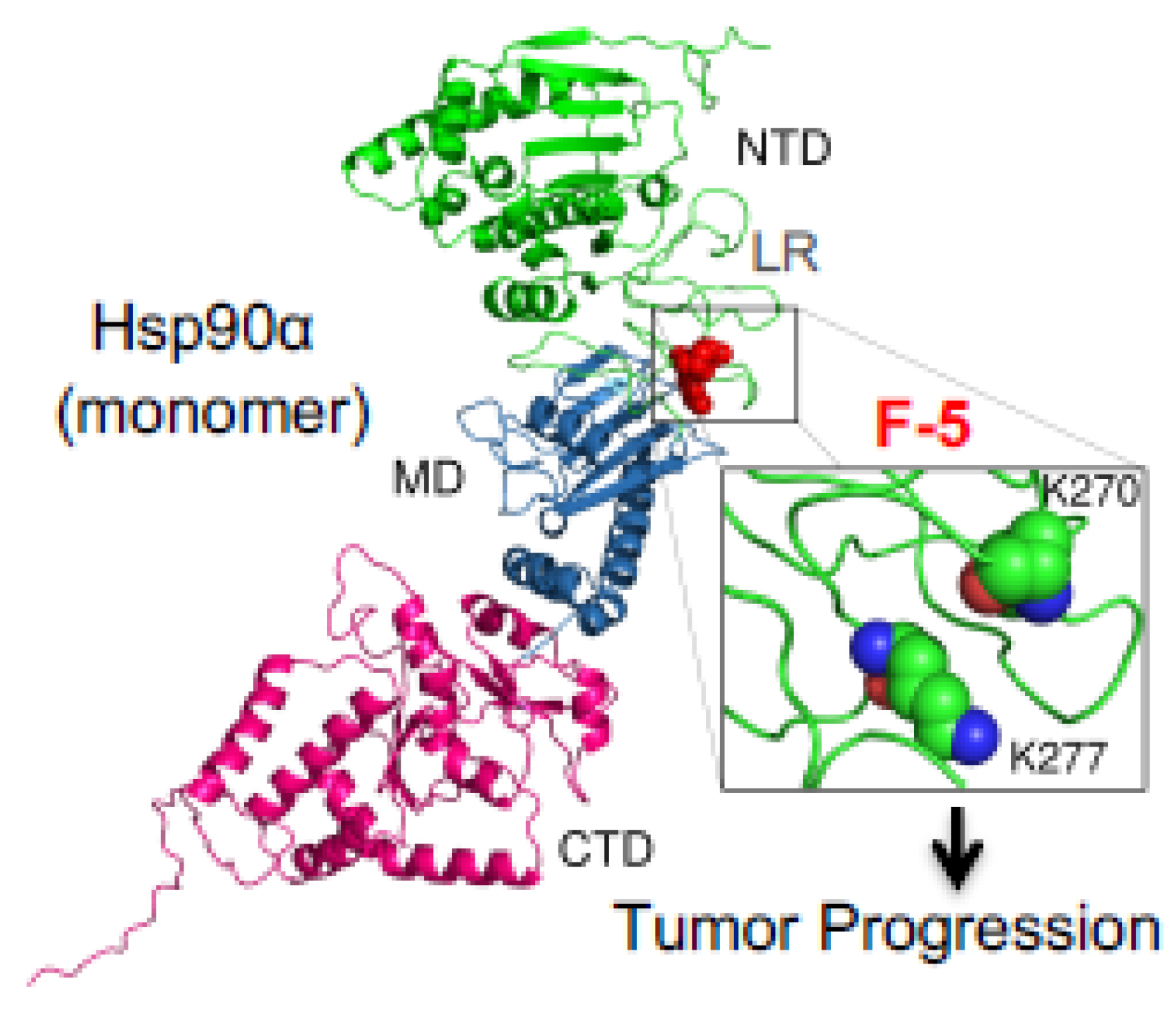
- Zou, M.; Bhatia, A.; Dong, H.; Jayaprakash, P.; Guo, J.; Sahu, D.; Hou, Y.; Tsen, F.; Tong, C.; O’Brien, K. Evolutionarily conserved dual lysine motif determines the non-chaperone function of secreted Hsp90alpha in tumour progression. Oncogene 2017, 36, 2160–2171. [Google Scholar] [CrossRef]
- Cheng, C.F.; Sahu, D.; Tsen, F.; Zhao, Z.; Fan, J.; Kim, R.; Wang, X.; O’Brien, K.; Li, Y.; Kuang, Y.; et al. A fragment of secreted Hsp90α carries properties that enable it to accelerate effectively both acute and diabetic wound healing in mice. J. Clin. Investig. 2011, 121, 4348–4361. [Google Scholar] [CrossRef]
- Tsen, F.; Bhatia, A.; O’Brien, K.; Cheng, C.F.; Chen, M.; Hay, N.; Stiles, B.; Woodley, D.T.; Li, W. Extracellular heat shock protein 90 signals through subdomain II and the NPVY motif of LRP-1 receptor to Akt1 and Akt2: A circuit essential for promoting skin cell migration in vitro and wound healing in vivo. Mol. Cell. Biol. 2013, 33, 4947–4959. [Google Scholar] [CrossRef]
- Voss, A.K.; Thomas, T.; Gruss, P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development 2000, 127, 1. [Google Scholar] [CrossRef]
- Grad, I.; Cederroth, C.R.; Walicki, J.; Grey, C.; Barluenga, S.; Winssinger, N.; De Massy, B.; Nef, S.; Picard, D. The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS ONE 2010, 5, e15770. [Google Scholar] [CrossRef]
- Imai, T.; Kato, Y.; Kajiwara, C.; Mizukami, S.; Ishige, I.; Ichiyanagi, T.; Hikida, M.; Wang, J.-Y.; Udono, H. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16363–16368. [Google Scholar] [CrossRef]
- Tang, X.; Chang CHao, M.; Chen, M.; Woodley, D.T.; Schönthal, A.H.; Li, W. Heat shock protein-90alpha (Hsp90α) stabilizes hypoxia-inducible factor-1α (HIF-1α) in support of spermatogenesis and tumorigenesis. Cancer Gene Ther. 2021, 28, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.F.; Jin, Z.G.; Baas, A.S.; Daum, G.; Gygi, S.P.; Aebersold, R.; Berk, B.C. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J. Biol. Chem. 2000, 275, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kuroita, T.; Tachibana, H.; Ohashi, H.; Shirahata, S.; Murakami, H. Growth stimulating activity of heat shock protein 90 alpha to lymphoid cell lines in serum-free medium. Cytotechnology 1992, 8, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, C.; Ma, B.; Xu, M.; Xu, S.; Liu, J.; Tian, Y.; Fu, Y.; Luo, Y. Mutant p53 drives cancer metastasis via RCP-mediated Hsp90α secretion. Cell Rep. 2020, 32, 107879. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; O’Brien, K.; Guo, J.; Lincoln, V.; Kajiwara, C.; Chen, M.; Woodley, D.T.; Udono, H.; Li, W. Extracellular and non-chaperone function of heat shock protein−90α is required for skin wound healing. J. Investig. Dermatol. 2018, 138, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, X.; Zhuo, W.; Fu, Y.; Shi, H.; Liang, Y.; Tong, M.; Chang, G.; Luo, Y. The regulatory mechanism of Hsp90α secretion and its function in tumor malignancy. Proc. Natl. Acad. Sci. USA 2009, 106, 21288–21293. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Mollapour, M.; Graf, C.; Lee, C.T.; Scroggins, B.T.; Xu, W.; Haslerova, L.; Hessling, M.; Konstantinova, A.A.; Trepel, J.B.; et al. Neckers Hsp90 charged-linker truncation reverses the functional consequences of weakened hydrophobic contacts in the N domain. Nat. Struct. Mol. Biol. 2009, 16, 1141–1147. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Febbraio, M.A. Exosome-dependent trafficking of HSP70: A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005, 280, 23349–23355. [Google Scholar] [CrossRef]
- Savina, A.; Furlan, M.; Vidal, M.; Colombo, M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 2003, 278, 20083–20090. [Google Scholar] [CrossRef]
- Mignot, G.; Roux, S.; Thery, C.; Segura, E.; Zitvogel, L. Prospects for exosomes in immunotherapy of cancer. J. Cell. Mol. Med. 2006, 10, 376–388. [Google Scholar] [CrossRef]
- Hegmans, J.P.; Bard, M.P.; Hemmes, A.; Luider, T.M.; Kleijmeer, M.J.; Prins, J.B.; Zitvogel, L.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am. J. Pathol. 2004, 164, 1807–1815. [Google Scholar] [CrossRef]
- Guo, J.; Jayaprakash, P.; Dan, J.; Wise, P.; Jang, G.B.; Liang, C.; Chen, M.; Woodley, D.T.; Fabbri, M.; Li, W. PRAS40 connects microenvironmental stress signaling to exosome-mediated secretion. Mol. Cell. Biol. 2017, 37, e00171-17. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chang, C.; Guo, J.; Lincoln, V.; Liang, C.; Chen, M.; Li, W. Tumor-secreted Hsp90α on external surface of exosomes mediates tumor—Stromal cell communication via autocrine and paracrine mechanisms. Sci. Rep. 2019, 9, 15108. [Google Scholar] [CrossRef] [PubMed]
- Bertout, J.; Patel, S.; Simon, M. The impact of O2 availability on human cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef]
- Bhatia, A.; O’brien, K.; Chen, M.; Wong, A.; Garner, W.; Woodley, D.T.; Li, W. Dual therapeutic functions of F-5 fragment in burn wounds: Preventing wound progression and promoting wound healing in pigs. Mol. Ther. Methods Clin. Dev. 2016, 3, 16041. [Google Scholar] [CrossRef]
- Dong, H.; Zou, M.; Bhatia, A.; Jayaprakash, P.; Hofman, F.; Ying, Q.; Chen, M.; Woodley, D.T.; Li, W. Breast cancer MDA-MB-231 cells use secreted heat shock protein-90alpha (Hsp90α) to survive a hostile hypoxic environment. Sci. Rep. 2016, 6, 20605. [Google Scholar] [CrossRef]
- Gao, F.; Hu, X.Y.; Xie, X.J.; Xu, Q.Y.; Wang, Y.P.; Liu, X.B.; Xiang, M.X.; Sun, Y.; Wang, J.A. Heat shock protein 90 protects rat mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways. J. Zhejiang Univ. Sci. B 2010, 11, 608–617. [Google Scholar] [CrossRef]
- Thomaidou, D.; Yfanti, E.; Patsavoudi, E.J. Expression of the 4C5 antigen during development and after injury of the rat sciatic nerve. Neurosci. Res. 1996, 46, 24–33. [Google Scholar] [CrossRef]
- Yfanti, E.; Nagata, I.; Patsavoudi, E.J. Migration behavior of rodent granule neurons in the presence of antibody to the 4C5 antigen. J. Neurochem. 1998, 71, 1381–1389. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Scroggins, B.; Koga, F.; Lee, M.J.; Trepel, J.; Felts, S.; Carreras, C.; Neckers, L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene 2008, 27, 2478–2487. [Google Scholar] [CrossRef]
- Sims, J.D.; McCready, J.; Jay, D.G. Extracellular heat shock protein (Hsp)70 and Hsp90a assist in matrix metalloproteinase-2 activation and breast cancer cell migration and invasion. PLoS ONE 2011, 6, e18848. [Google Scholar] [CrossRef] [PubMed]
- Baker-Williams, A.J.; Hashmi, F.; Budzyński, M.A.; Woodford, M.R.; Gleicher, S.; Himanen, S.V.; Makedon, A.M.; Friedman, D.; Cortes, S.; Namek, S.; et al. Co-chaperones TIMP2 and AHA1 competitively regulate extracellular HSP90: Client MMP2 activity and matrix proteolysis. Cell Rep. 2019, 28, 1894–1906. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, X.; Zhuo, W.; Shi, H.; Feng, D.; Sun, Y.; Liang, Y.; Fu, Y.; Zhou, D.; Luo, Y. The regulatory mechanism of extracellular Hsp90{alpha} on matrix metalloproteinase-2 processing and tumor angiogenesis. J. Biol. Chem. 2010, 285, 40039–40049. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, C.; Chen, S.; Liu, J.; Fu, Y.; Luo, Y. Extracellular Hsp90α and clusterin synergistically promote breast cancer epithelial-to-mesenchymal transition and metastasis via LRP1. J. Cell Sci. 2019, 132, jcs228213. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Merino, D.; Gómez, J.M.; Nistal, J.F.; Hurlé, M.A.; Cortajarena, A.L.; Villar, A.V. Extracellular heat shock protein 90 binding to TGFβ receptor I participates in TGFβ-mediated collagen production in myocardial fibroblasts. Cell. Signal. 2016, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Luo, Y. The regulatory mechanism of Hsp90alpha secretion from endothelial cells and its role in angiogenesis during wound healing. Biochem. Biophys. Res. Commun. 2010, 398, 111–117. [Google Scholar] [CrossRef]
- O’Brien, K.; Bhatia, A.; Tsen, F.; Chen, M.; Wong, A.K.; Woodley, D.T.; Li, W. Identification of the critical therapeutic entity in secreted Hsp90α that promotes wound healing in newly re-standardized healthy and diabetic pig models. PLoS ONE 2014, 9, e113956. [Google Scholar] [CrossRef]
- Bellaye, P.S.; Burgy, O.; Causse, S.; Garrido, C.; Bonniaud, P. Heat shock proteins in fibrosis and wound healing: Good or evil? Pharmacol. Ther. 2014, 143, 119–132. [Google Scholar] [CrossRef]
- Dong, H.; Luo, L.; Zou, M.; Huang, C.; Wan, X.; Hu, Y.; Le, Y.; Zhao, H.; Li, W.; Zou, F.; et al. Blockade of extracellular heat shock protein 90a by 1G6-D7 attenuates pulmonary fibrosis through inhibiting ERK signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, 1006–1015. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, W.; Liu, Y.; Chen, W.; Lu, Y.; Zeng, Z.; Qiao, Y.; Huang, H.; Wan, X.; Li, W.; et al. Extracellular HSP90α interacts with ER stress to promote fibroblasts activation through PI3K/AKT pathway in pulmonary fibrosis. Front. Pharmacol. 2021, 12, 708462. [Google Scholar] [CrossRef]
- Bellaye, P.S.; Shimbori, C.; Yanagihara, T.; Carlson, D.A.; Hughes, P.; Upagupta, C.; Sato, S.; Wheildon, N.; Haystead, T.; Ask, K.; et al. Synergistic role of HSP90α and HSP90β to promote myofibroblast persistence in lung fibrosis. Eur. Respir. J. 2018, 51, 1700386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Z.; Ding, H.; Zhou, Y.; Doan, H.A.; Sin, K.W.T.; Zhu, Z.J.; Flores, R.; Wen, Y.; Gong, X.; et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 2017, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Stellas, D.; Karameris, A.; Patsavoudi, E. Monoclonal antibody 4C5 immunostains human melanomas and inhibits melanoma cell invasion and metastasis. Clin. Cancer Res. 2007, 13, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Stellas, D.; El Hamidieh, A.; Patsavoudi, E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol. 2010, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Chen, S.; An, Q.; Li, B.; Fu, Y.; Luo, Y. Extracellular Hsp90α promotes tumor lymphangiogenesis and lymph node metastasis in breast cancer. Int. J. Mol. Sci. 2021, 22, 7747. [Google Scholar] [CrossRef] [PubMed]
- Stivarou, T.; Stellas, D.; Vartzi, G.; Thomaidou, D.; Patsavoudi, E. Targeting highly expressed extracellular HSP90 in breast cancer stem cells inhibits tumor growth in vitro and in vivo. Cancer Biol. Ther. 2016, 17, 799–812. [Google Scholar] [CrossRef]
- Seclì, L.; Avalle, L.; Poggio, P.; Fragale, G.; Cannata, C.; Conti, L.; Iannucci, A.; Carrà, G.; Rubinetto, C.; Miniscalco, B.; et al. Targeting the extracellular HSP90 co-chaperone morgana inhibits cancer cell migration and promotes anticancer immunity. Cancer Res. 2021, 81, 4794–4807. [Google Scholar] [CrossRef]
- Milani, M.; Laranjeira, A.B.; de Vasconcellos, J.F.; Brandalise, S.R.; Nowill, A.E.; Yunes, J.A. Plasma Hsp90 level as a marker of early acute lymphoblastic Leukemia engraftment and progression in mice. PLoS ONE 2015, 10, e0129298. [Google Scholar]
- Wang, C.; Zhang, S.; Liu, J.; Tian, Y.; Ma, B.; Xu, S.; Fu, Y.; Luo, Y. Secreted pyruvate kinase M2 promotes lung cancer metastasis through activating the integrin Beta1/FAK signaling pathway. Cell Rep. 2020, 30, 1780–1797. [Google Scholar] [CrossRef]
- Xu, Q.; Tu, J.; Dou, C.; Zhang, J.; Yang, L.; Liu, X.; Lei, K.; Liu, Z.; Wang, Y.; Li, L.; et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol. Cancer 2017, 16, 178. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, P.; Hou, Q.; Feng, S.; Liu, L.; Cui, D.; Shi, H.; Fu, Y.; Luo, Y. A novel pan-cancer biomarker plasma heat shock protein 90alpha and its diagnosis determinants in clinic. Cancer Sci. 2019, 110, 2941–2959. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhou, J.H.; Chen, L.P.; Liu, H.Z.; Zhang, F.Y.; Li, J.L.; Ning, S.F.; Li, S.R.; Wang, C.; Huang, Y.; et al. Plasma Levels of Heat Shock Protein 90 Alpha Associated With Colorectal Cancer Development. Front. Mol. Biosci. 2021, 8, 684836. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, X.; Huang, D.; Cui, D.; Liu, L.; Liu, J.; He, Z.; Liu, J.; Zheng, S.; Luo, Y. Plasma heat shock protein 90alpha as a biomarker for the diagnosis of liver cancer: An official, large-scale, and multicenter clinical trial. EBioMedicine 2017, 24, 56–63. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Lou, J.; Han, X.; Zhang, L.; Wang, Q.; Li, B.; Dong, M.; Zhang, Y. Plasma levels of heat shock protein 90 alpha associated with lung cancer development and treatment responses. Clin. Cancer Res. 2014, 20, 6016–6022. [Google Scholar] [CrossRef] [PubMed]
- Kasanga, M.; Liu, L.; Xue, L.; Song, X. Plasma heat shock protein 90-alpha have an advantage in diagnosis of colorectal cancer at early stage. Biomark. Med. 2018, 12, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Bilgin, E.; Erturk, K.; Duranyildiz, D. Clinical significance of circulating serum cellular heat shock protein 90 (HSP90) level in patients with cutaneous malignant melanoma. Asian Pac. J. Cancer Prev. 2017, 18, 599–601. [Google Scholar]
- Fredly, H.; Reikvam, H.; Gjertsen, B.T.; Bruserud, O. Disease-stabilizing treatment with all-trans retinoic acid and valproic acid in acute myeloid Leukemia: Serum hsp70 and hsp90 levels and serum cytokine profiles are determined by the disease, patient age, and anti-leukemic treatment. Am. J. Hematol. 2012, 87, 368–376. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, L.L.; Li, C.P.; Hsu, Y.T.; Jiang, S.S.; Fan, C.S.; Chua, K.V.; Huang, S.X.; Shyr, Y.M.; Chen, L.T.; et al. Myeloid-derived macrophages and secreted HSP90α induce pancreatic ductal adenocarcinoma development. Oncoimmunology 2018, 7, e1424612. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, X.; Zang, N.; Li, H.; Li, G.; Li, C.; He, M. Transcriptomic and proteomic investigation of HSP90A as a potential biomarker for HCC. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 4039. [Google Scholar] [CrossRef][Green Version]
- Wei, W.; Liu, M.; Ning, S.; Wei, J.; Zhong, J.; Li, J.; Cai, Z.; Zhang, L. Diagnostic value of plasma HSP90α levels for detection of hepatocellular carcinoma. BMC Cancer 2020, 20, 6. [Google Scholar] [CrossRef]
- Wang, X.T.; An, D.Z.; Wang, X.L.; Liu, X.M.; Li, B.S. Extracellular Hsp90α clinically correlates with tumor malignancy and promotes migration and invasion in esophageal squamous cell carcinoma. OncoTargets Ther. 2019, 12, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Miao, C.; Lu, Q.; Wu, W.; He, Y.; Wu, S.; Liu, H.; Lian, C. Clinical significance of monitoring circulating free DNA and plasma heat shock protein 90alpha in patients with esophageal squamous cell carcinoma. Cancer Manag. Res. 2021, 13, 2223. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Cheng, Z.; Zhao, X.; Huang, Y. Diagnostic value of heat shock protein 90α and squamous cell carcinoma antigen in detection of cervical cancer. J. Int. Med. Res. 2019, 47, 5518–5525. [Google Scholar] [CrossRef] [PubMed]
- Burgess, E.F.; Ham, A.J.L.; Tabb, D.L.; Billheimer, D.; Roth, B.J.; Chang, S.S.; Cookson, M.S.; Hinton, T.J.; Cheek, K.L.; Hill, S.; et al. Prostate cancer serum biomarker discovery through proteomic analysis of alpha-2 macroglobulin protein complexes. Proteom. Clin. Appl. 2008, 2, 1223–1233. [Google Scholar] [CrossRef]
- Pawlik-Gwozdecka, D.; Górska-Ponikowska, M.; Adamkiewicz-Drożyńska, E.; Niedźwiecki, M. Serum heat shock protein 90 as a future predictive biomarker in childhood acute lymphoblastic leukemia. Central Eur. J. Immunol. 2021, 46, 63. [Google Scholar] [CrossRef]
- Liang, X.Q.; Li, K.Z.; Li, Z.; Xie, M.Z.; Tang, Y.P.; Du, J.B.; Huang, Y.; Li, J.L.; Hu, B.L. Diagnostic and prognostic value of plasma heat shock protein 90alpha in gastric cancer. Int. Immunopharmacol. 2021, 90, 107145. [Google Scholar] [CrossRef]
- Yuan, Z.; Hong, S.; Li, L.; He, L.; Xiao, P.; Tang, T.; Shi, C.; Mu, Y.; Sun, C.; Shang, Y.; et al. Diagnostic value of HSP90α and related markers in lung cancer. J. Clin. Lab. Anal. 2019, 36, e24462. [Google Scholar] [CrossRef]
- Fu, M.; Du, F.; Wei, Z.; Xu, C.; Wang, X.; Zhao, X. Hsp90α Is Suitable for Therapy Monitoring in Multiple Cancers. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Zhong, B.; Shen, J.; Zhang, C.; Zhou, G.; Yu, Y.; Qin, E.; Tang, J.; Wu, D.; Liang, X. Plasma heat shock protein 90 alpha: A valuable predictor of early chemotherapy effectiveness in advanced non-small-cell lung cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e924778-1. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Zhang, Y.; Wang, Q.; Wanga, H.; Gua, K. Diagnostic and prognostic value of heat shock protein 90α in malignant melanoma. Melanoma Res. 2021, 31, 152–161. [Google Scholar] [CrossRef]
- Mao, M.; Wang, X.; Sheng, H.; Li, H.; Liu, W.; Han, R.; Wen, W.; Liu, W. Heat shock protein 90α provides an effective and novel diagnosis strategy for nasopharyngeal carcinoma. Adv. Ther. 2021, 38, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, T.; Kvivik, I.; Kvaløy, J.T.; Aabakken, L.; Omdal, R. Heat-shock protein 90 α in plasma reflects severity of fatigue in patients with Crohn’s disease. Innate Immune 2020, 26, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Damasiewicz-Bodzek, A.; Szumska, M.; Tyrpień-Golder, K. Antibodies to heat shock proteins 90α and 90β in psoriasis. Arch. Immunol. Ther. Exp. 2020, 68, 9. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.; Vinogradov, A.; Gindis, A.; Tao, E.; Moiseev, S. Heat shock protein 90 and NFkB levels in serum and urine in patients with chronic glomerulonephritis. Cell Stress Chaperones 2020, 25, 495–501. [Google Scholar] [CrossRef]
- Miyazaki, D.; Nakamura, A.; Hineno, A.; Kobayashi, C.; Kinoshita, T.; Yoshida, K.; Ikeda, S.I. Elevation of serum heat-shock protein levels in amyotrophic lateral sclerosis. Neurol. Sci. 2016, 37, 1277–1281. [Google Scholar] [CrossRef]
- Bălănescu, A.; Stan, I.; Codreanu, I.; Comănici, V.; Bălănescu, E.; Bălănescu, P. Circulating Hsp90 isoform levels in overweight and obese children and the relation to nonalcoholic fatty liver disease: Results from a cross-sectional study. Dis. Markers 2019, 2019, 9560247. [Google Scholar] [CrossRef]
- Štorkánová, H.; Oreská, S.; Špiritović, M.; Heřmánková, B.; Bubová, K.; Komarc, M.; Pavelka, K.; Vencovský, J.; Distler, J.H.; Šenolt, L.; et al. Plasma Hsp90 levels in patients with systemic sclerosis and relation to lung and skin involvement: A cross-sectional and longitudinal study. Sci. Rep. 2021, 11, 1. [Google Scholar] [CrossRef]
- Ding, X.; Meng, C.; Dong, H.; Zhang, S.; Zhou, H.; Tan, W.; Huang, L.; He, A.; Li, J.; Huang, J.; et al. Extracellular Hsp90α, which participates in vascular inflammation, is a novel serum predictor of atherosclerosis in type 2 diabetes. BMJ Open Diabetes Res. Care 2022, 10, e002579. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Sethi, N.; Kang, Y. Unravelling the complexity of metastasis—Molecular understanding and targeted therapies. Nat. Rev. Cancer 2011, 11, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Turajlic, S.; Swanton, C. Metastasis as an evolutionary process. Science 2016, 352, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Center Watch. Available online: www.centerwatch.com/directories/1067-fda-approved-drugs/topic/103-oncology (accessed on 17 August 2020).
- Yuno, A.; Lee, M.J.; Lee, S.; Tomita, Y.; Rekhtman, D.; Moore, B.; Trepel, J.B. Clinical evaluation and biomarker profiling of Hsp90 inhibitors. Chaperones 2018, 1709, 423–441. [Google Scholar]
- Tang, X.; Chang, C.; Mosallaei, D.; Woodley, D.T.; Schönthal, A.H.; Chen, M.; Li, W. Heterogeneous responses and isoform compensation dim the therapeutic window of Hsp90 ATP-binding inhibitors in cancer. Mol. Cell. Biol. 2022, 42, e0045921. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Zhao, Z.; Tsen, F.; Cheng, C.F.; Park, R.; Situ, A.J.; Dai, J.; Eginli, A.; Shams, S.; Chen, M.; et al. A potentially common peptide target in secreted heat shock protein-90α for hypoxia-inducible factor-1α–positive tumors. Mol. Biol. Cell. 2012, 23, 602–613. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]


| Cancer Type | # of Patients | Plasma eHsp90α | # of Healthy Humans | Plasma eHsp90α | Refs. |
|---|---|---|---|---|---|
| Mix of liver, lung, breast, colorectal, stomach, pancreatic, esophagus cancer, and lymphoma. | 300 | IQR 87.01–235.5 Median 157.80 (ng/mL) | 132 | IQR 22.87–44.46 Median 31.19 (ng/mL) | [91] |
| Colon (CRC) | 635 | 51.4 (33.8, 80.3) ng/mL | 295 | 43.7 (34.3, 54.8) ng/mL | [92] |
| Mix of Breast & Other cancers | 85 | >50 (ng/mL) | 16 | 50.00 (ng/mL) | [56] |
| Liver | 782 | IQR 96.7–246.8 Median 159.9 (ng/mL) | 572 | IQR 21.1–42.2 Median 30 (ng/mL) | [93] |
| Lung | 1046 | Ave. 220.46 (ng/mL) | 592 | Ave. 48.0 (ng/mL) | [94] |
| Colon (CRC) | 77 | 135 ± 101.94 (ng/mL) | 76 | 44 ± 15.35 (ng/mL) | [95] |
| Melanoma | 98 | Median. 49.76 (ng/mL) | 43 | Median 25.7(ng/mL) | [96] |
| AML | 82 | Ave. 295 (ng/mL) | 20 | Ave. 12.1 (ng/mL) | [97] |
| Pancreas | 20 | 0.57 ± 0.23 (mg/mL) | 10 | 0.18 ± 0.05 (mg/mL) | [98] |
| Pancreatic ductal adenocarcinoma | 114 | 1 ± 0.86 (mg/mL) | 10 | 0.18 ± 0.05 (mg/mL) | [98] |
| Hepatocellular carcinoma | 76 | 274 ± 20.3 (μg/mL) | 14 | 186 ± 18.3 (μg/mL) | [99] |
| Hepatocellular carcinoma | 659 | 144 ± 4.98 (ng/mL) | 230 | 46 ± 1.11 (ng/mL) | [100] |
| Esophageal squamous cell carcinoma | 193 | ≥82.06 (ng/mL) | [101] | ||
| Esophageal squamous cell carcinoma | 93 | Ave. 85 (ng/mL) | 0 | 0 | [102] |
| Cervical cancer | 220 | 80.6–212.8 (ng/mL) | 75 | 48.6–89.6 (ng/mL) | [103] |
| Prostate cancer | 18 | Median 50.7 (25.5–378.1) (ng/mL) | 13 | Median 27.6 (13.9–46.5) (ng/mL) | [104] |
| Childhood acute lymphoblastic leukemia | 21 | 1.22–23.85 (ng/mL) | No exact number | 3.16–33.58 (ng/mL) | [105] |
| Gastric cancer | 976 | Median 64.3 (ng/mL) | 100 | 45.16 (ng/mL) | [106] |
| Lung cancer | 560 | 97.64 ± 103.36 (ng/mL) | 78 | 38.44 ± 15.4 (ng/mL) | [107] |
| Mix of Breast, Liver, Lung, Colon, Esophageal, Gastric and Colorectal | 370 | 57.97–294.63 (ng/mL) | Reference range | 0~82.06 (ng/mL) | [108] |
| Non-small-cell lung cancer | 60 Pre-chemotherapy | 0.29–0.93 (ng/mL) | 60 After 4-cycles of chemotherapy | 0.12–0.24 (ng/mL) | [109] |
| Malignant melanoma | 60 | 70.8–140.77 (ng/mL) | 60 | 42.56–61.42 (ng/mL) | [110] |
| Nasopharyngeal carcinoma | 196 | 212 ± 144.32 (ng/mL) | 106 | 35 ± 17.47 (ng/mL) | [111] |
| Non-cancer diseases | |||||
| Crohn’s disease | 53 | 6.4~55.1 | [112] | ||
| Psoriasis | 80 | 100 ± 193.66 (AU/mL) | 80 | 63 ± 49.71 (AU/mL) | [113] |
| Chronic glomerulonephritis | 32 | 33.31–77.25 (ng/mL) | 10 | 22.32 | [114] |
| Amyotrophic lateral sclerosis | 58 | 17.02 ± 10.55 | 85 | 12.7 ± 9.23 | [115] |
| Overweight and obese children with Nonalcoholic fatty liver disease | 26 | 3.59–119.85 (ng/mL) | Overweight & obese children without Nonalcoholic fatty liver disease | 0–105.4 (ng/mL) | [116] |
| Chronic glomerulonephritis with nephrotic syndrome | 21 | 33.31–77.25 (ng/mL) | 10 | Approx. 25–30 (ng/mL) | [114] |
| Systemic sclerosis | 92 | 9.6–17.9 (ng/mL) | 92 | 7.7–12.4 (ng/mL) | [117] |
| Diabetic lower extremity arterial disease (DLEAD) | 46 | Ave. 263.88 (pg/mL) | [11] | ||
| Idiopathic pulmonary fibrosis (IPF) | 31 | Ave. 60 (ng/mL) | 9 | Ave. 35 (ng/mL) | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jay, D.; Luo, Y.; Li, W. Extracellular Heat Shock Protein-90 (eHsp90): Everything You Need to Know. Biomolecules 2022, 12, 911. https://doi.org/10.3390/biom12070911
Jay D, Luo Y, Li W. Extracellular Heat Shock Protein-90 (eHsp90): Everything You Need to Know. Biomolecules. 2022; 12(7):911. https://doi.org/10.3390/biom12070911
Chicago/Turabian StyleJay, Daniel, Yongzhang Luo, and Wei Li. 2022. "Extracellular Heat Shock Protein-90 (eHsp90): Everything You Need to Know" Biomolecules 12, no. 7: 911. https://doi.org/10.3390/biom12070911
APA StyleJay, D., Luo, Y., & Li, W. (2022). Extracellular Heat Shock Protein-90 (eHsp90): Everything You Need to Know. Biomolecules, 12(7), 911. https://doi.org/10.3390/biom12070911







