Abstract
HIV can traverse the BBB using a Trojan horse-like mechanism. Hidden within infected immune cells, HIV can infiltrate the highly safeguarded CNS and propagate disease. Once integrated within the host genome, HIV becomes a stable provirus, which can remain dormant, evade detection by the immune system or antiretroviral therapy (ART), and result in rebound viraemia. As ART targets actively replicating HIV, has low BBB penetrance, and exposes patients to long-term toxicity, further investigation into novel therapeutic approaches is required. Viral proteins can be produced by latent HIV, which may play a synergistic role alongside ART in promoting neuroinflammatory pathophysiology. It is believed that the ability to specifically target these proviral reservoirs would be a vital driving force towards a cure for HIV infection. A novel drug design platform, using the in-tandem administration of several therapeutic approaches, can be used to precisely target the various components of HIV infection, ultimately leading to the eradication of active and latent HIV and a functional cure for HIV. The aim of this review is to explore the pitfalls of ART and potential novel therapeutic alternatives.
1. Introduction
Infection with human immunodeficiency virus (HIV) was once considered to be near-certainly fatal. Approximately 40 million people are currently infected worldwide by HIV, including approximately 2 million children under the age of 15 [1]. Antiretroviral therapy (ART) has changed the landscape of the morbidity and mortality of HIV infection, providing HIV-infected (HIV+) individuals with a means to achieve long-term viral suppression within peripheral circulation while also quelling viral activity within the CNS. Most HIV+ patients undergo lifelong treatment with ART due to the aggressive nature of HIV infection and the potential for rebound viraemia [2]. Evidence suggests that the antiretroviral drugs (ARVds) commonly used to treat HIV infection can be toxic within the CNS and can result in the development of various pathophysiologies [3,4,5,6,7,8,9,10,11,12,13,14]. While ART can control and inhibit actively replicating HIV, the virus can persist undetected within the host genome in the form of a latent, replication-competent provirus, which can later become reactivated [2,15]. Thus, the current review focuses on pitfalls of ART, including the inability to specifically target the latent HIV provirus, long-term toxic exposure, and limited BBB penetrance, along with novel therapeutic approaches aimed at mitigating these concerns (Figure 1).
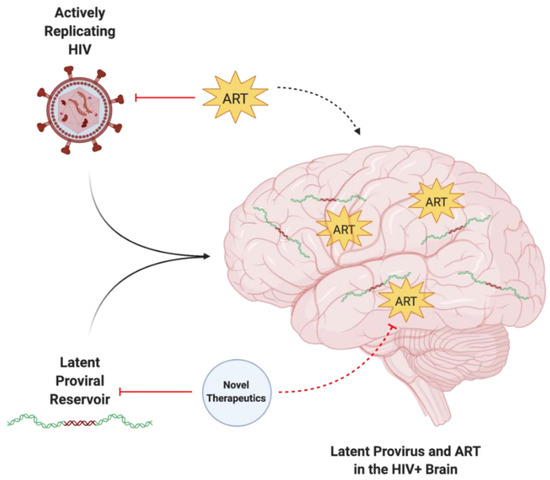
Figure 1.
The enigma of persisting latent HIV provirus despite ART. Actively replicating and latent forms of HIV can infiltrate the CNS, resulting in neuroinflammatory pathophysiology. While ART can target active HIV, it is unable to target latent proviral reservoirs. The in-tandem use of ART and novel therapeutic approaches is required to target and eliminate both active and latent HIV within the CNS. Created with BioRender.com. Abbreviations: ART = antiretroviral therapy; HIV = human immunodeficiency virus.
2. HIV Infection within the CNS
2.1. The Blood-Brain Barrier
The blood-brain barrier (BBB) is the crucial anatomic and biochemical interface responsible for regulating the microenvironments between peripheral circulation and the central nervous system (CNS) [16,17,18]. These highly regulated microenvironments are required for neural signaling and the maintenance of homeostasis within the CNS. Additional barriers including the blood–cerebrospinal fluid (CSF) barrier and the arachnoid barrier provide additional supportive functions in maintaining CNS homeostasis, but are not as crucial nor do they occupy as large of a surface area as the BBB. As such, the BBB is at the front line of defense in protecting the highly safeguarded CNS from the entrance of toxins and pathogens, including HIV and medications such as ART, adding an element of challenge to drug discovery and design.
At the BBB, a monolayer of cerebral microvascular endothelial cells (CMECs) forms the framework of capillary walls, which are interlocked by tight junctions (TJs) made up of proteins including claudin-5, occludin, and submembranous zona occludnes-1 (ZO-1). These TJs facilitate the regulation of BBB and CNS homeostasis by linking together CMECs, preventing the passage of many paracellular molecules into the brain parenchyma, while also providing a cytoskeletal matrix of intercellular protein filaments arranged as a series of membranous and submembranous barricades that enable the structural and functional maintenance of barrier integrity. CMECs are peripherally surrounded by a basement membrane (basal lamina), pericytes, astrocytic end-feet, and neurons, which together comprise the neurovascular unit and serve to strengthen barrier function and integrity at the BBB [18]. Pericytes and astrocytes have important roles in maintaining structural integrity at the BBB as they can modulate levels of TJ protein expression and vesicle trafficking in CMECs [19] and contribute to various aspects of CMEC phenotype, including development, proliferation, migration, and survival [17,19]. Interestingly, pericytes are also able to regulate the expression of BBB-specific genes in CMECs, influencing overall BBB integrity [20].
There are several pathways that restrict the entry of drugs into the CNS. Molecules that can diffuse or be transported through the endothelium, including ART, can be actively removed via efflux pumps including P-glycoprotein, multidrug resistance proteins, and organic anion transporters [3]. This provides a challenge for ART in reaching therapeutic concentrations within the CNS, allowing for the possibility of rebound viraemia. It is, however, essential to note that several factors can modulate the expression of these transport proteins, such as inflammatory, genetic, and drug-induced interactions [3], which can result in increased transport of ART across the brain endothelium, leading to increased toxic exposure. Of particular interest is the role of P-glycoprotein in limiting entry into the CNS for ARVds. Substantial effort has been made to dissect the regulatory mechanisms modulating the expression and/or activity of this protein. Exposure of capillaries to low levels of proinflammatory factors, such as lipopolysaccharide (LPS), tumor necrosis factor (TNF)-α, or endothelin-1 (ET-1), was demonstrated to cause a rapid loss of P-glycoprotein transport function with no change in protein expression. On the other hand, a prolonged exposure to proinflammatory factors, including TNF-α, had an opposing effect, i.e., upregulating P-glycoprotein expression via complex mechanisms that shared common signaling elements, such as TNF receptor 1, endothelin receptors, protein kinase C, and nuclear factor-κB (NF-κB) [21]. The role of inflammatory factors in the modulation of P-glycoprotein activity has been confirmed in several literature reports (reviewed in [22]). Interestingly, P-glycoprotein is also involved in the immune inflammatory response in the CNS by regulating microglia activation and mediating immune cell migration [23]. We demonstrated that exposure to HIV-1 Tat protein resulted in overexpression of P-glycoprotein both at mRNA and protein levels in brain endothelial cells and brain microvessels via mechanisms involving NF-κB, intact lipid rafts, and activation of Rho signaling [24,25]. Similar upregulation of P-glycoprotein was demonstrated upon exposure to HIV [26]. In addition, induction of P-glycoprotein in human brain microvessel endothelial cells was demonstrated upon treatment with ARVds via the mechanisms involving human pregnane X receptor (hPXR) and/or human constitutive androstane receptor (hCAR) [27].
Hydrophobicity and low molecular weight are positively correlated with BBB penetration [3,28]; however, efflux pumps may still actively remove these substances from the brain parenchyma. The inability of certain antiretroviral drugs (ARVds) to reach therapeutic concentrations within the CNS may play a role in the potential for rebound viraemia. Thus, future methods of drug delivery must be investigated and optimized to bypass the classical diffusion and transport mechanisms of ART across the BBB.
2.2. A Trojan Horse Mechanism for HIV Infection of the CNS
HIV attacks the immune system by infecting and eliminating cells that express the CD4 receptor (CD4+ cells) and coreceptors, including the C-C motif receptor 5 (CCR5) and C-X-C motif receptor 4 (CXCR4). The HIV genome can integrate into the host genome of many cell types; however, there are two major cellular reservoirs: CD4+ T lymphocytes and macrophages. CD4+ T cells are crucial for combating infection and maintaining immune responses, homeostasis, and memory, and as such, are associated with several inflammatory and autoimmune diseases [29]. Macrophages are derived from monocytes and myeloid cells of hematopoietic origin [23]. In the CNS, microglia and partially pericytes are cells of myeloid origin. These cells, along with astrocytes, can all be directly infected by HIV [30,31,32,33,34]. Indeed, a 12 h incubation period with two strains of HIV resulted in the cellular entry of HIV and low-level replication of HIV in human brain pericytes, astrocytes, and CMECs [35].
HIV can infiltrate the CNS early in the course of infection. HIV evades detection by the immune system primarily by using HIV+ CD4+ T cells and cells of the monocytic lineage in a Trojan horse approach to traverse the BBB [35] (Figure 2). The free virus is also able to cross the BBB through TJ openings that can result from HIV-induced dysfunction of CMECs [16]. In addition, HIV+ pericytes were shown to stimulate dysregulation of BBB integrity via decreased TJ protein expression [34]. This HIV-induced increase in BBB permeability can lead to the activation of microglial cells and uncontrolled migration of immune cells into the CNS, which are capable of causing neuroinflammation, loss of neural tissue, and infection due to the influx of pathogens [36]. In addition, studies have shown that CMECs can undergo apoptosis during HIV infection [16,34], which increases BBB permeability and can promote the infiltration of HIV+ cells and virions into the CNS. Intriguingly, HIV-specific proteins, such as transactivator of transcription (Tat), are capable of inducing CMEC dysfunction and subsequent BBB dysregulation [37,38,39], enhancing the infiltration of HIV across the BBB. The entry of HIV into the CNS can result in neuropathological dysfunctions ranging from sub-clinical and minor cognitive impairments or motor deficits to HIV-associated neurocognitive disorders (HAND), including dementia [40]. ART administration is negatively correlated to TJ protein expression and BBB permeability [19,36,41], illustrating the need for drug design to maximize the efficiency of BBB crossing and to overcome toxicities associated with the administration of ART.
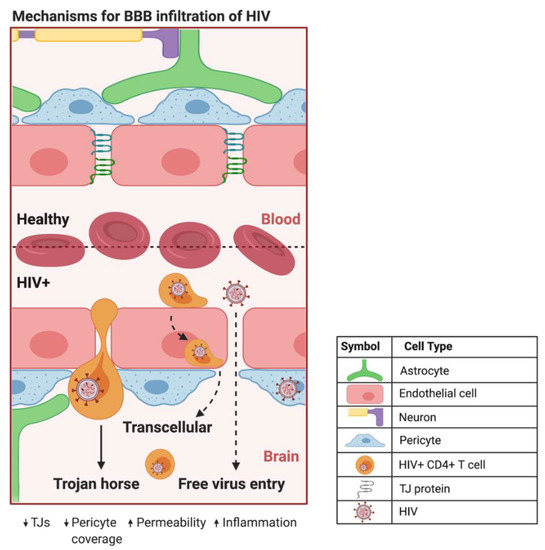
Figure 2.
Proposed mechanisms of BBB infiltration of HIV. At the HIV+ BBB, infected CD4+ T cells and monocytes can cross by several proposed mechanisms. The predominate method centers around HIV using infected CD4+ T cells and monocytes as a Trojan horse to paracellularly infiltrate brain parenchyma. HIV+ monocytes can also transcellularly pass through CMECs at the BBB. As HIV infection progresses in the CNS, increased BBB permeability and decreased expression of TJ proteins can provide a pathway for HIV to paracellularly invade the brain parenchyma. Created with BioRender.com. Abbreviations: HIV+ = human immunodeficiency virus-infected; TJs = tight junctions.
2.3. Elusive Latent Proviral Reservoirs within the CNS
The integration of reverse-transcribed viral DNA into the host genome is a crucial step in propagating both the active and dormant forms of HIV (Figure 3). Once integrated, the proviral DNA serves as the transcriptionally competent viral unit and the central source of viral protein production. The gene expression of HIV is controlled by promoter and enhancer sequences where transcription factors, including NF-κB, can bind, promoting RNA polymerase II activity, ultimately resulting in increased virus-specific protein levels [15]. Transcriptional inactivity of the HIV proviral DNA results in the latent proviral stage of HIV, where the virus can remain dormant in the host genome as a transcriptionally incompetent reservoir for later reactivation.
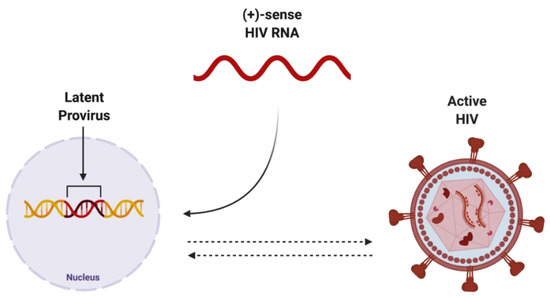
Figure 3.
Potential endpoints of positive-sense HIV RNA after integration into host genome. Once integrated into the host genome, (+)-sense HIV RNA can persist as either latent provirus, which is capable of being reactivated, or actively replicating HIV, which can be deactivated. Created with BioRender.com. Abbreviations: (+)-sense = positive-sense; HIV = human immunodeficiency virus; RNA = ribonucleic acid.
The HIV provirus can exist in three forms: latent, which is transcriptionally silent; intact, producing active virions; or defective, producing viral proteins but not able to successfully replicate [42] (Figure 4). Intact and latent HIV proviral reservoirs have the potential to cause rebound viraemia, whereas defective provirus does not. It is important to note that while defective HIV provirus is not replication-competent, these malfunctioning viral DNA sequences can produce viral HIV proteins, which can propagate pathogenesis. Furthermore, cells latently infected with HIV can release exosomes containing viral mRNA and protein, hijacking intercellular communication networks as a means to reactivate latent reservoirs, transmit infection, and further disease development [43], presenting another target required to fully eradicate infection with HIV.
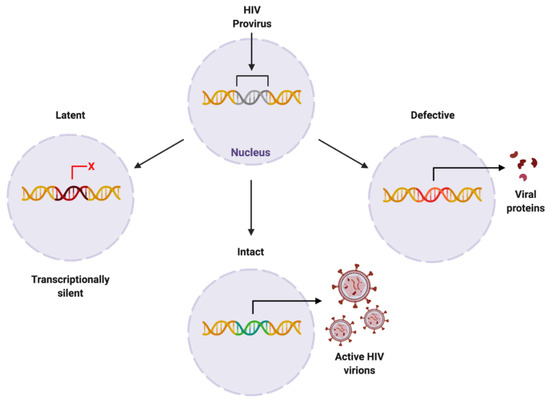
Figure 4.
Proposed forms of HIV proviral reservoir. HIV provirus can persist in three forms: latent, being transcriptionally silent; intact, producing active HIV virions; or defective, containing genetic mutations resulting in viral protein synthesis. Created with BioRender.com. Abbreviations: HIV = human immunodeficiency virus.
HIV can exist as a latent proviral reservoir in several cell types, namely CD4+ T cells and cells of monocytic lineage. Cells that are latently infected with HIV provirus can evade detection by the immune system and may be replicated via the homeostatic proliferation of their host cell [42]. Although microglial cells are the primary reservoir cell type within the CNS [44], there is novel evidence depicting astrocytes and pericytes as constituents of these dormant HIV cellular reservoirs [31,32]. For example, integrated viral DNA has been discovered in microglia and pericytes within the CNS tissue of post-mortem HIV+ patients [30,42], illustrating the likelihood of myeloid-derived reservoir sites within the brain. Intriguingly, novel research indicates a key role for neurons, as opposed to non-neuronal cell lines, in the stimulation of HIV latency in microglia [45]. In addition, neurons can prevent the emergence of active HIV from latency and neuronal damage can induce replication and activation of HIV [45]. As cells of the myeloid lineage are long-lasting and recent investigation has illustrated both inductive and preventative roles for neurons in HIV latency, it is crucial to further investigate the functional properties of HIV latency.
Activating the transcriptionally silent, latent HIV proviral reservoir can be achieved with the use of histone deacetylase inhibitors. Histone deacetylase inhibitors promote the acetylation of histones and consequential chromatin relaxation, facilitating the accessibility of transcription factors to DNA and enabling transcription of the viral genome via RNA polymerase II recruitment. For example, pericytes in the latent stage of HIV infection that were treated with histone deacetylase inhibitors and tumor necrosis factor (TNF) exhibited a significant increase in HIV-1 RNA and HIV p24 protein production, illustrating that pericytes can alternate between the latent and active viral stages [30]. The mechanisms underlying HIV proviral transcriptional silencing and reactivation are not yet fully understood. Recent investigation has revealed a method of measuring and discerning the intact versus defective proviral HIV genome [46], which is a crucial step toward curing HIV infection. Specific targeting of the latent proviral reservoir remains the central obstacle in achieving complete viral eradication from HIV+ individuals.
Perivascular spaces in the CNS also contain populations of cells capable of harboring HIV. In a macaque model, perivascular macrophages and microglia were shown to harbor SIV genomes, which could be reactivated, even after observed antiretroviral therapy suppression [47]. While there is still debate regarding the role that macrophages play in active viral reservoirs of HIV, findings in mice confirm the possible importance of this cell type. Indeed, studies indicated that HIV persists in humanized myeloid-only mice independent of other possible reservoir-capable cell types, such as T-cells, supporting the role of macrophages in HIV replication and formation of viral reservoirs. This mouse model is generated by transplanting CD34+ hematopoietic stem cells into immunodeficient nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice, which are characterized by an absence of functional T and B cells [48,49]. Furthermore, there is mounting evidence that macrophages play an important role in their susceptibility to HIV even after ART initiation (reviewed in [28]).
Evidence indicates that viral entry can occur through the choroid plexus [50,51]. It is well-known that resident macrophages, i.e., the cells that frequently become infected with HIV in the CNS, can line the epithelium of the choroid plexus [52]. As a separate dynamic reservoir for HIV accumulation, the choroid plexus provides a possible path for neuro-invasion events and a conduit for future ART drug delivery. It should also be noted that HIV trafficking via the choroid plexus barrier is coordinated by the high amount of multidrug resistance proteins and P-glycoprotein expressed on the surface [53]. Paradoxically, the P-glycoprotein pump is oriented in a way that opposes the action of P-glycoprotein efflux transporter located in the BBB, whereby it prevents substrates and other molecules from escaping the CSF. This complex relationship further accentuates CNS and BBB homeostasis in trafficking therapeutics to the CNS (reviewed in [28]).
3. Pitfalls of ART: Focus on the CNS
There are many shortcomings associated with ART, the current standard therapeutic approach in treating HIV infection. While ART has provided HIV+ patients with a means to control the actively replicating virus and decrease patient mortality rates, there are still several key issues that must be overcome when administering ARVds, e.g., limited BBB penetrance, toxic exposure, and the inability to target latent proviral HIV reservoirs, particularly in the CNS. A drug’s ability to traverse the BBB is dependent on an array of factors, including molecular size, polarity, protein–protein intractability, and physicochemical properties [54]. Moreover, drug-induced modulation of transport protein expression may increase the penetrability of brain endothelium to ART and, in turn, expose the CNS to a higher level of toxicity [54]. Taken together, alternative routes of delivery and mechanisms of CNS entry need to be further explored to achieve concentrations sufficient for optimizing the therapeutic effects of ART while minimizing any toxic side effects.
3.1. ART Does Not Protect against Latent HIV Infections
Latent proviral HIV reservoirs are established early in the course of infection and cannot be targeted by traditional therapeutic approaches, specifically ART, making them a primary obstacle in attaining a cure for HIV infection [54]. ART does, however, have the capability to target several stages within the viral replication cycle of HIV (Table 1) (Figure 5). Though this is sufficient to suppress levels of actively replicating HIV and maintain viraemia, latent HIV provirus does not actively replicate and therefore is not a therapeutic target of ARVds. Additionally, the limited BBB penetrance of traditional HIV therapeutics presents a significant obstacle in not only delivering effective doses, which are required for maintaining suppression of viraemia within the CNS in particular, but also in designing novel therapeutics to target latent HIV proviral reservoirs within the CNS.

Table 1.
Antiretroviral drug classes, function, examples, and dosage reference.
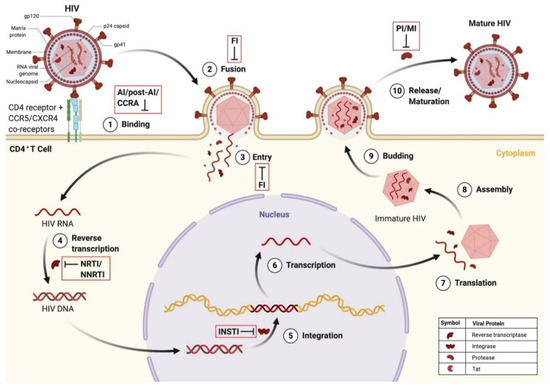
Figure 5.
HIV infection of CD4+ T cell and points of ARVd intervention in the HIV replication cycle. (1) HIV binds to the CD4 receptor and CCR5/CXCR4 co-receptors. This can be blocked by attachment inhibitors, post-attachment inhibitors, and CCR antagonists. (2) Fusion of the HIV and host cellular membrane occurs. This can be blocked by fusion inhibitors. (3) Entry of viral proteins into the host cell. This can be blocked by fusion inhibitors. (4) Reverse transcription of HIV RNA into proviral HIV DNA. This can be blocked by nucleoside/non-nucleoside reverse transcriptase inhibitors. (5) Integration of HIV DNA into the host genome. This can be blocked by integrase strand transfer inhibitors. (6) Transcription of HIV RNA. (7) Translation of HIV RNA into viral proteins. (8) Assembly of immature HIV. (9) Budding of immature HIV into the host cell membrane. (10) Release and maturation of HIV. This can be blocked by protease inhibitors and maturation inhibitors. Created with BioRender.com. Abbreviations: ARVd = antiretroviral drug; CD4 = cluster of differentiation 4; CCR5 = C-C motif receptor 5; CXCR4 = C-X-C motif receptor 4; DNA = deoxyribonucleic acid; FI = fusion inhibitor; HIV = human immunodeficiency virus; INSTI = integrase strand transfer inhibitor; Tat = transactivator of transcription.
Despite several years of ART treatment, HIV provirus can persist within the chromosomal DNA of latently infected CD4+ T cells [28]. These potent proviral reservoirs are established within hours of infection and are extremely stable, having an average half-life of approximately 44 months [55,56]. Currently, virologic suppression of HIV infection has been defined as having a viral load of fewer than 20 copies per milliliter [57], which can be achieved through lifelong adherence to an ART regimen. Interestingly, many HIV+ patients who have successfully gained control of the infection via ART experience intermittent episodes of detectable viraemia (blips) [58], which have been shown to be correlated to HIV reservoir size [59]. Specifically, HIV+ patients undergoing treatment regimens with protease inhibitor-based combination ART (cART) have exhibited a higher degree of residual viraemia [57], indicating the inability of ART to target the HIV provirus. In one study, a majority of the simian immunodeficiency virus (SIV)-infected (SIV+), ART-suppressed macaques contained latently infected brain macrophages [60]. Remarkably, latent HIV provirus can persist even when viral RNA is not detectable [60]. Taken together, further investigation is required to elucidate and understand the mechanisms underlying latent HIV proviral persistence despite ART.
3.2. ART Has Limited BBB Penetrance
The ability of ARVds to infiltrate the CNS has been hierarchically categorized by CNS penetration efficiency (CPE), which ranges from low (i.e., CPE of 1) to high (i.e., CPE of 4) [36] (see Table 1). When jointly administered, ART can have a cumulative CPE score varying from low (i.e., CPE < 8) to high (i.e., CPE > 8) [29]. Several chemical and physiological properties determine the ability of ARVds to cross the BBB, including pharmacodynamics, pharmacokinetics, and structural characteristics of the drugs (e.g., lipophilic or ligand-receptor interactions), which can alter their half-life and biodistribution. There is a debate centered around what combination of ARVds is ideal for overcoming the challenge of BBB penetrance and optimizing the therapeutic benefits while minimizing potential toxicities associated with ART. ARVds with a higher CPE score (i.e., better CNS penetration) may be advantageous for regulating HIV infection within the brain; however, these drugs may also result in a higher level of toxic exposure as they are able to reach higher levels in the brain [61]. Additionally, the limited ability of ARVds to penetrate the BBB may permit low levels of active HIV replication, potentially increasing latent reservoir size and the chance of rebound viraemia.
3.3. ART-Induced CNS Toxicity
Long-term administration of ARVds places patients at an increased risk of toxic exposure, which can result in neurodegeneration, inflammation, and co-/multi-morbidities such as cardiovascular, metabolic, and neurological diseases [40,54,61,62,63,64,65,66,67,68,69,70,71]. The comorbidities and long-term toxic exposure associated with ART can lead HIV+ patients to switch or discontinue their medication regimens, ultimately resulting in rebound viraemia. For example, the use of ART has been shown to impact various aspects of cellular function within the CNS (see Table 2), in particular, inducing neurotoxic effects [71] and reducing the viability of endothelial cells exposed to ART even at relatively low concentrations [2]. ART-induced vascular toxicity can result in decreased TJ protein expression and BBB dysregulation [3]. Primary CMECs that were treated with Efavirenz exhibited decreased claudin-5 expression and localization to the cellular membrane, which was attenuated via the inhibition of endoplasmic reticulum (ER) stress prior to ART exposure [36]. Notably, it was found that the use of ART induced oxidative damage and mitochondrial dysfunction in endothelial cells [3,11,12,41,72] and neurons [4,71,73] (see Table 2). These ART-mediated effects stimulate the induction of inflammasomes and lead to an increase in inflammatory cytokines [10,74,75], which can result in a loss of BBB integrity and increased permeability via decreased TJ protein expression [76].

Table 2.
Known effects of ART on cells of the CNS.
Table 2.
Known effects of ART on cells of the CNS.
| Cell Type | Impact from ART | References |
|---|---|---|
| Astrocyte | ↓ Mitochondria function and metabolism ↓ MMPs ↑ Senescence ↑ ER stress | [3,13,77] |
| Endothelial Cell | ↓ Viability ↓ Mitochondria function ↓ Autophagosome formation ↓ TJPs ↑ ROS production ↑ ER stress ↑ Inflammatory cytokine production | [3,12,36,75,78,79] |
| Microglial Cell | ↓ Lysosomal function ↓ Autophagosomal function ↑ ROS production ↑ Expression of pro-inflammatory cytokines | [10] |
| Neuron | ↓ Axonal length ↓ Neurogenesis ↑ Neuronal death ↑ Oxidative stress ↑ ROS accumulation | [4,73,80,81] |
| Neural Progenitor Cell | ↓ Cell proliferation ↓ Mitochondrial function ↑ Senescence ↑ ROS production ↑ MMP production | [82,83,84] |
| Oligodendrocyte | ↓ Maturation ↑ ROS production ↑ Oxidative stress ↑ Lysosomal stress | [85,86] |
| Pericyte | ↓ Coverage | [87] |
Abbreviations: ER = endoplasmic reticulum; MMP = matrix metalloproteinase; ROS = reactive oxygen species; TJPs = tight junction proteins.
The use of ART has been linked to mitochondrial toxicity [3,6,8,9,11,88,89] and oxidative stress [3,4,8,9,11,72]. ART-induced mitochondrial dysfunction may be explained, at least in part, by genetic or epigenetic modulations to mtDNA [74], which impair mitochondrial function and can increase oxidative stress. In particular, protease inhibitors and nucleoside reverse transcriptase inhibitors (NRTIs) are known to induce mitochondrial damage [3,4,70,90,91,92,93]. Exposure to NRTIs has been shown to reduce levels of mtDNA and dysregulate the mitochondrial proteome via the inhibition of polymerase-γ [3,90,92,93]. ART-treated mice exhibited modulated expression of mitochondrial transcription factor A (TFAM) [6]. TFAM occupies roles in the regulation of mitochondrial biogenesis and well as protecting mtDNA [6,94]. A decrease in TFAM observed in neurons of ARV-treated mice supports the notion that mitochondrial biogenesis may be dysregulated by ART. Additionally, NRTIs have been shown to inhibit telomerase, a crucial reverse transcriptase in charge of the de novo production of repeats in telomeric DNA, at therapeutic concentration [92,93]. The inhibition of telomerase can result in downstream interferences in mtDNA replication [93], which can lead to increased mitochondrial aging [91,92] and, ultimately, BBB dysfunction.
Mitochondria are the major source of reactive oxygen species (ROS) production in most mammalian cells, which can occur via the dysregulation of complexes I, II, and III [95,96,97]. Complex I is responsible for the oxidation of nicotinamide adenine dinucleotide (NADH), a crucial step in the electron transport chain, which has been shown to be inhibited in a dose-dependent manner by ARVds [3]. The inhibition and concomitant dysregulation of complex I by ART illustrate a possible mechanism for ART-mediated increase in ROS levels. Protease inhibitors are known to increase oxidative stress through the impairment of mitochondrial function, resulting in mitochondrial damage [4,89,91]. ROS provides a common point of activation for many downstream signaling pathways, which can directly mediate BBB function [98,99], TJ modification [98,99,100,101], and matrix metalloproteinase activation [102]. Therefore, ROS are potentially key mediators in the breakdown and dysfunction of the BBB in ART-treated HIV+ patients. Exposure to ART was shown to increase the production of nitric oxide, which resulted in subsequent inflammation [3]. This may be particularly damaging to BBB integrity as nitric oxide synthase activation results in BBB breakdown [103].
Interestingly, a novel study published by our group hints at the metabolic function of certain tight junction proteins, including occludin [104,105], which may result in crosstalk between metabolic function and BBB permeability. Specifically, occludin has NADH oxidase enzymatic activity, which regulates the expression and stimulation of the histone deacetylase sirtuin 1 (SIRT-1). As histone deacetylase inhibitors activate the latent HIV provirus, histone deacetylase, therefore, inhibits latent proviral activation, resulting in a negative correlation between occludin and HIV transcription. In fact, the silencing of occludin in various HIV+ cell types resulted in a significant increase in viral transcription via SIRT-1 activation [104]. Thus, further investigation is required to elucidate the signaling pathways tethering metabolic, transcriptional regulation, and BBB permeability functionality of TJ proteins, specifically in the context of ART.
The degradation, clearance, and removal of proteins from cells is a crucial process that converges on three primary organelles: the endoplasmic reticulum (ER), autophagosome, and the lysosome. The exposure of CMECs to ARVds was shown to induce ER stress and autophagy disruption [78], which has been associated with cellular dysfunction and increased permeability in several disease models [36]. For example, ART-treated CMECs exhibited a significant decrease in the expression of the secreted form of alkaline phosphate, which is an indication of ER stress. The elevated expression of stress-indicating ER proteins, including inositol-requiring enzyme 1a and pancreatic ER eukaryotic initiation factor 2a (eIF2a) kinase (PERK), further confirmed ER stress in ART-treated CMECs [78]. PERK was also shown to be upregulated in ART-treated astrocytes [76]. Downstream mediators of these stress-indicating ER proteins were also altered by ART exposure in CMECs [78]. Specifically, increased phosphorylation of eIF2a, which is carried out by PERK, was shown in ART-treated CMECs [78,106] and astrocytes [77]. This effect can result in the decline of a cell’s ability to withstand stressful insults by leading to a reduction in de novo protein synthesis [107].
Autolysosomes are formed from the fusion of autophagosomes and lysosomes and ultimately ensure the completion of autophagy and clearance of misfolded or aggregated proteins [108]. Autophagy is known to be influenced by ER stress and has been shown to be impacted by ART exposure [36]. Light chain 3B (LC3B) is a commonly utilized marker of autophagic function, which is processed from LC3BI (16kDa) to LC3BII (14kDa) upon autophagic activation [78]. Exposure of CMECs to ART was shown to result in decreased processing from LC3BI to LC3BII [78], indicating an ART-induced decrease in autophagic activity. ART was shown to interrupt the maturation stage of autophagy by impairing lysosomal function, ultimately inducing defects in autophagosome-lysosome fusion. Specifically, ART-treated microglia were shown to exhibit impaired lysosomal functioning, which resulted in the accumulation of autophagosomes [10]. In addition, ART was shown to stimulate lysosomal membrane permeabilization, decreased levels of lysosomal-associated membrane protein 2 [10], lysosomal deacidification [10,85], and decreased expression of cathepsin D [10], all of which contribute to lysosomal-mediated autophagy dysfunction and the activation of microglial cells. Interestingly, heat shock protein family A was shown to attenuate ART-mediated lysosomal impairments [10], illustrating promise in moderating the toxic effects associated with ARVds.
It is important to note that the ART-induced products of mitochondrial, ER, and autolysosomal stress can result in the induction of inflammasomes, including the nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome [109,110], which produces inflammatory cytokines such as interleukin-1b (IL-1b) and IL-18, whose discharge by caspase-1 mediates apoptosis [111,112], suggesting a possible means for ART-induced neuroinflammation. The NLRP3 inflammatory pathway responds to the presence of damage-associated molecular patterns (DAMPs), which are produced as a result of ART-induced organelle stress [113]. Remarkably, ART-mediated apoptosis was shown in endothelial cells, which was measured by upregulation in the expression of the pro-apoptotic cytokine pro-caspase-3 [73], which serves an important role in cell death. Furthermore, time-dependent ART-mediated upregulation in the mRNA of pro-inflammatory cytokines IL-1β, IL-6, and TNF was recently discovered in microglial cells [10]. Exposure to ART was also reported to increase in Aβ peptides [114,115] and Aβ deposition [116], as shown in the CSF of HIV+ individuals compared to those not utilizing the therapy [115]. These results correspond to elevated levels of Aβ in HIV+ individuals [114,115,116] and warrant further investigations on the impact of ART on cognitive functions and/or accentuated aging frequently observed in HIV+ patients [117].
4. Novel Strategies in Developing HIV Therapeutics
It is largely agreed that the eradication of HIV is dependent on the ability to target and eliminate both the latent viral reservoir and persistent low-level replication of active virions or inhibit infection with HIV altogether. This demands novel approaches, such as an effective vaccine, allogenic hematopoietic stem cell transplantation (allo-HSCT), gene therapy, and nanotherapeutics, or even a combination of these approaches. The tandem deployment of these novel therapeutic approaches can also serve to mitigate the long-term toxicity observed in ART-treated HIV+ patients.
4.1. CNS Targeting of Latent HIV Provirus
Targeting the latent HIV proviral reservoir has been attempted by using a “shock and kill” paradigm, which works by inducing the activation of HIV from latency (shock) and then eliminating HIV viral reservoir cells (kill) [118,119,120]. The kill phase of this paradigm primarily centers around the immunologic elimination of viral reservoir cell sites. As transcription factors such as NF-kB are known to induce HIV replication [15,121], activators of this pathway have been used to shock latent HIV reservoirs into a reactivated state [47,122]. Another approach utilized an activator of the cytokine IL-15 [123] as a shock mechanism, which has been shown to activate the transcription of HIV [124]. This strategy can be combined with the suppression of immune components that have an apparent, albeit unknown, role in the stabilization of HIV latency [124]. It is important to note that while NF-kB activation is a promising shock strategy to reactivate latent HIV reservoirs, inducers of this pathway have low efficacy in acting as latency-reversal agents, often leading to toxic exposure that restricts the clinical application of this potential therapeutic approach [125]. In addition, the reactivation of latent HIV alone is not sufficient to decrease the size of the HIV reservoir, and the kill phase of this paradigm primarily centers around the immunologic elimination of viral reservoir cell sites, which requires optimization [126].
A more recent “block-and-lock” paradigm has provided an alternative promise into a possible method for eradication of the latent HIV proviral reservoir. The “shock-and-kill” paradigm aims to entirely eradicate latent proviral reservoir sites, whereas the “block-and-lock” method is deployed to permanently silence all HIV proviruses, even after the termination of ART [126]. Permanent silencing of the HIV genome can be accomplished by targeting different components of the transcriptional machinery, including the use of a Tat inhibitor to silence the transcription and reactivation of HIV [126]. Indeed, it was shown that treatment with the Tat inhibitor didehydro-cortistatin A (dCA) was able to delay and reduce rebound viraemia in mice [127]. Other elements of viral transcription that can be targeted and inhibited in the “block-and-lock” paradigm include heat shock protein 90 (HSP90) [118], Janus kinus-signal transducer and activator of transcription (Jak-STAT) [119,120], mammalian target of Rapamycin (mTOR) [128], p21-activated kinase (PAK) [129], rapidly accelerated fibrosarcoma kinase (Raf) [129], and bromodomain-containing protein 4 (BRD4) [130], among several other proposed targets of HIV transcription.
Additional approaches for targeting latent HIV proviral reservoirs predominately revolve around gene-therapy methods, including clustered regularly interspaced short palindromic repeats-associated protein nuclease-9 (CRISPR/Cas-9) [15,131,132,133], broadly neutralizing antibodies (bNAbs) [134], and transcription activator-like effector nucleases (TALENs) [135]. CRISPR/Cas9-based systems have been used to precisely target latent HIV proviral DNA [15,136]. Similarly, TALENs have been utilized to accurately target latent HIV DNA, being more effective and having less off-target editing than CRISPR/Cas9-based systems [135]. Interestingly, the use of bNAbs in mice was shown to obstruct the development of the latent HIV proviral reservoir [134]. In fact, consistent with data from human and macaque studies, mice treated with bNAbs 4 days post-HIV infection exhibited viremia approximately 34% less often, which took longer to establish than in mice treated with ART [118]. Major advantages of CRISPR, TALEN, and bNAb-based platforms, besides their ability to specifically target latent HIV proviral DNA, are their pliability in design for individual target sites, high editing capabilities, and minimal toxic exposure [135].
4.2. Obstructing Infection by HIV
Along with precisely targeting the latent HIV provirus, the inhibition of infection by HIV has proven possible with the use of novel techniques such as allo-HSCT [137,138]. Allo-HSCT is focused predominantly on a homozygous deletion (CCR5D32/D32) within the C-C motif receptor 5 (CCR5) coreceptor, which is a crucial mediator in the cellular entry and subsequent infection of HIV. Indeed, CD4+ T cells lacking this receptor exhibit impaired binding to HIV, ultimately inhibiting downstream viral infection and providing resistance to HIV [139]. There have been reported cases of a functional, ART-free possible HIV cure by using allo-HSCT [137,138,140], including the Berlin patient. In a similar case of allo-HSCT, the Essen patient, rebound viraemia quickly occurred after the interruption of ART [130]. It was determined that the Essen patient had been co-infected with an alternative, pre-existing HIV variant, albeit at low levels, able to transmit the infection through the C-X-C motif receptor 4 (CXCR4) coreceptor [141]. This type of CXCR4 tropic switch may occur later in the course of HIV infection [142,143] and presents a significant challenge that needs to be considered in therapeutic design strategies, specifically regarding the use of gene therapy to cure HIV infection. In addition, risks including graft-versus-host interactions may occur [142].
5. Concluding Comments
While ART has provided HIV+ patients with a means to control the infection and live relatively normal lives, there are still several pitfalls and shortcomings that need to be further improved and investigated. For example, ART cannot target or eradicate the latent HIV provirus, ultimately failing to cure infection with HIV. A long-term regimen of ART places HIV+ patients at the risk of developing toxicity and the subsequent domino effect of pathogenesis, specifically in the CNS. In addition, ARVds have limited BBB penetrance, hindering delivery to the CNS, and failing to target another crucial component within the landscape of HIV infection. On the plus side, ART has the capability of targeting various elements within the HIV replication cycle of active virions, which is necessary to suppress viraemia.
A multimodal approach utilizing the in-tandem administration of several novel anti-HIV therapies may be an effective strategy in designing a cure for HIV infection (Figure 6). The use of these novel approaches in concert may prove to be less toxic and/or more effective than the use of ART alone [135,144]. A recent study demonstrated the use of magneto-electric nanoparticles bound with a CRISPR/Cas-9-based system to cross the BBB and inhibit latent HIV infection in microglial cells [131]. Additional studies have investigated the use of nano-formulations developed for the delivery of ART [145,146,147] and combining ART with mitochondria-targeted antioxidant therapy [148]. Specifically, nano-based drug-delivery systems have been developed for the delivery of tenofovir [146], and graphene quantum dot-based systems have been used to inhibit HIV replication similarly to NNRTIs [147]. As the investigation continues to deepen our understanding of the crucial elements that may provide systemic eradication of HIV infection, it is clear that new avenues of therapeutic innovation are required. Taken together, the use of these modern approaches illustrates the promise of novel therapeutic strategies in augmenting traditional ART, providing a higher degree of targeting efficiency and ultimately curing HIV infection.
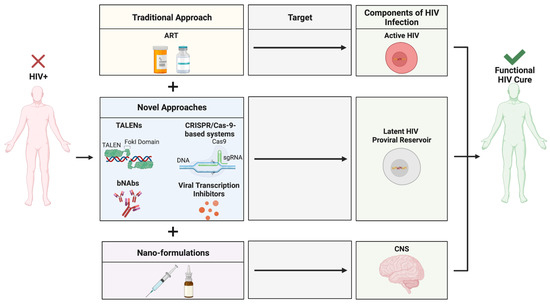
Figure 6.
Novel approaches for the eradication of HIV within the CNS. A novel, multimodal drug delivery platform for anti-HIV therapeutics can result in the discovery of a functional HIV cure, allowing for the eradication of actively replicating HIV, latent proviral reservoirs, and specific targeting of the highly safeguarded CNS. Created with BioRender.com. Abbreviations: ART = antiretroviral therapy; bNAbs = broadly neutralizing antibodies; CNS = central nervous system; CRISPR/Cas-9 = clustered regularly interspaced short palindromic repeats-associated protein nuclease-9; HIV = human immunodeficiency virus; TALENs = transcription activator-like effector nucleases.
Author Contributions
H.R. and M.T. made substantial contributions to the conception and design of the review. H.R. reviewed the literature, created all figures, and wrote the manuscript; M.T. reviewed and corrected the manuscript and provided funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health (NIH) grants MH128022, MH122235, MH072567, HL126559, DA044579, DA039576, DA040537, DA050528, and DA047157.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Centers for Disease Control and Prevention. HIV Suveillance Report. 2018. Available online: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed on 19 September 2019).
- Churchill, M.J.; Deeks, S.G.; Margolis, D.M.; Siliciano, R.F.; Swanstrom, R. HIV reservoirs: What, where and how to target them. Nat. Rev. Microbiol. 2015, 14, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, L.; Velichkovska, M.; Toborek, M. Cerebral vascular toxicity of antiretroviral therapy. J. Neuroimmune Pharmacol. 2019, 16, 74–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akay, C.; Cooper, M.; Odeleye, A.; Jensen, B.K.; White, M.G.; Vassoler, F.; Gannon, P.J.; Mankowski, J.; Dorsey, J.L.; Buch, A.M.; et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the Central Nervous System. J. NeuroVirology 2014, 20, 39–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, A.; Wang, C.; Patton, C.; Murray, M.; Punekar, Y.; de Ruiter, A.; Steinhart, C. A review of long-term toxicity of antiretroviral treatment regimens and implications for an aging population. Infect. Dis. Ther. 2018, 7, 183–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, J.A.; Swinton, M.K.; Carson, A.; Soontornniyomkij, B.; Lindsay, C.; Han, M.M.; Frizzi, K.; Sambhwani, S.; Murphy, A.; Achim, C.L.; et al. Tenofovir disoproxil fumarate induces peripheral neuropathy and alters inflammation and mitochondrial biogenesis in the Brains of Mice. Sci. Rep. 2019, 9, 17158. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.; Landay, A.; Miyahara, S.; Vecchio, A.; Masters, M.C.; Brown, T.T.; Taiwo, B.O. Limited correlation between systemic biomarkers and neurocognitive performance before and during HIV treatment. J. NeuroVirology 2019, 26, 107–113. [Google Scholar] [CrossRef]
- Williams, D.W.; Li, Y.; Dastgheyb, R.; Fitzgerald, K.C.; Maki, P.M.; Spence, A.B.; Gustafson, D.R.; Milam, J.; Sharma, A.; Adimora, A.A.; et al. Associations between antiretroviral drugs on depressive symptomatology in homogenous subgroups of women with HIV. J. Neuroimmune Pharmacol. 2020, 16, 181–194. [Google Scholar] [CrossRef]
- Velichkovska, M.; Surnar, B.; Nair, M.; Dhar, S.; Toborek, M. Targeted mitochondrial COQ10 delivery attenuates antiretroviral-drug-induced senescence of neural progenitor cells. Mol. Pharm. 2018, 16, 724–736. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.; Thangaraj, A.; Chivero, E.T.; Periyasamy, P.; Callen, S.; Burkovetskaya, M.E.; Guo, M.-L.; Buch, S. Antiretroviral-mediated microglial activation involves dysregulated autophagy and lysosomal dysfunction. Cells 2019, 8, 1168. [Google Scholar] [CrossRef] [Green Version]
- Schank, M.; Zhao, J.; Moorman, J.P.; Yao, Z.Q. The impact of HIV- and art-induced mitochondrial dysfunction in cellular senescence and aging. Cells 2021, 10, 174. [Google Scholar] [CrossRef]
- Smith, R.L.; Tan, J.M.; Jonker, M.J.; Jongejan, A.; Buissink, T.; Veldhuijzen, S.; van Kampen, A.H.; Brul, S.; van der Spek, H. Beyond the polymerase-γ theory: Production of ROS as a mode of NRTI-induced mitochondrial toxicity. PLoS ONE 2017, 12, e0187424. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; D’Agostino, L.; Wilson, J.; Tuzer, F.; Torres, C. Astrocyte senescence and metabolic changes in response to HIV antiretroviral therapy drugs. Front. Aging Neurosci. 2017, 9, 281. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zhang, J.; Geng, M.; Tang, S.-J.; Zhang, W.; Shu, J. Nucleoside reverse transcriptase inhibitors (nrtis) induce proinflammatory cytokines in the CNS via WNT5A signaling. Sci. Rep. 2017, 7, 4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebina, H.; Misawa, N.; Kanemura, Y.; Koyanagi, Y. Harnessing the CRISPR/cas9 system to disrupt latent HIV-1 provirus. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Toborek, M.; Lee, Y.W.; Flora, G.; Pu, H.; András, I.E.; Wylegala, E.; Hennig, B.; Nath, A. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell. Mol. Neurobiol. 2005, 25, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nagai, N.; Umemura, K. A review of the mechanisms of blood-brain barrier permeability by tissue-type plasminogen activator treatment for cerebral ischemia. Front. Cell. Neurosci. 2016, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Kurmann, L.; Okoniewski, M.; Dubey, R.K. Transcryptomic analysis of human brain -microvascular endothelial cell driven changes in -vascular pericytes. Cells 2021, 10, 1784. [Google Scholar] [CrossRef]
- Miller, D.S.; Bauer, B.; Hartz, A.M. Modulation of P-glycoprotein at the blood-brain barrier: Opportunities to improve central nervous system pharmacotherapy. Pharmacol. Rev. 2008, 60, 196–209. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Xie, Y.; Chen, Y. Effect of Neuroinflammation on ABC Transporters: Possible Contribution to Refractory Epilepsy. CNS Neurol. Disord. Drug Targets 2018, 17, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, B.; Li, X.; Liu, G.; Liu, R.; Guo, J.; Xu, B.; Li, Y.; Fang, W. Significance and Mechanisms of P-glycoprotein in Central Nervous System Diseases. Curr. Cancer Drug Targets 2019, 20, 1141–1155. [Google Scholar] [CrossRef]
- Hayashi, K.; Pu, H.; Tian, J.; Andras, I.E.; Lee, Y.W.; Hennig, B.; Toborek, M. HIV-Tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J. Neurochem. 2005, 93, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Hennig, B.; Toborek, M. Intact lipid rafts regulate HIV-1 Tat protein-induced activation of the Rho signaling and upregulation of P-glycoprotein in brain endothelial cells. J. Cereb. Blood Flow Metab. 2010, 30, 522–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, U.; Bulot, C.; Honer zu Bentrup, K.; Mondal, D. Specific increase in MDR1 mediated drug-efflux in human brain endothelial cells following co-exposure to HIV-1 and saquinavir. PLoS ONE 2013, 8, e75374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, G.N.; Patel, R.; Cummins, C.L.; Bendayan, R. Induction of P-glycoprotein by several antiretroviral drugs in human brain microvessel endothelial cells. Antimicrob. Agents Chemother. 2013, 57, 4481–4488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne, O.; Peyravian, N.; Nair, M.; Daunert, S.; Toborek, M. The paradox of HIV blood-brain barrier penetrance and antiretroviral drug delivery deficiencies. Trends Neurosci. 2020, 43, 695–708. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T cell development, localization, and function throughout life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, L.; Cho, H.J.; Toborek, M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain 2019, 142, 502–511. [Google Scholar] [CrossRef]
- Li, G.-H.; Henderson, L.; Nath, A. Astrocytes as an HIV reservoir: Mechanism of HIV infection. Curr. HIV Res. 2016, 14, 373–381. [Google Scholar] [CrossRef]
- Joseph, S.B.; Arrildt, K.T.; Sturdevant, C.B.; Swanstrom, R. HIV-1 target cells in the CNS. J. NeuroVirology 2014, 21, 276–289. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Toborek, M. HIV-1-induced alterations of Claudin-5 expression at the blood-brain barrier level. Methods Mol. Biol. 2011, 762, 355–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, S.; Castro, V.; Toborek, M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J. Cell. Mol. Med. 2012, 16, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Minato, N. Myeloid cells. Int. J. Biochem. Cell Biol. 2004, 36, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, L.; Dygert, L.; Toborek, M. Antiretroviral treatment with Efavirenz disrupts the blood-brain barrier integrity and increases stroke severity. Sci. Rep. 2016, 6, 39738. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, B.; Eum, S.Y.; Toborek, M. HIV-1 TAT triggers nuclear localization of ZO-1 via rho signaling and camp response element-binding protein activation. J. Neurosci. 2012, 32, 143–150. [Google Scholar] [CrossRef] [Green Version]
- András, I.E.; Pu, H.; Tian, J.; Deli, M.A.; Nath, A.; Hennig, B.; Toborek, M. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J. Cereb. Blood Flow Metab. 2005, 25, 1159–1170. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.W.; Eum, S.Y.; Nath, A.; Toborek, M. Estrogen-mediated protection against HIV Tat protein-induced inflammatory pathways in human vascular endothelial cells. Cardiovasc. Res. 2004, 63, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Clifford, D.B.; Ances, B.M. HIV-associated neurocognitive disorder. Lancet Infect. Dis. 2013, 13, 976–986. [Google Scholar] [CrossRef] [Green Version]
- Marincowitz, C.; Genis, A.; Goswami, N.; De Boever, P.; Nawrot, T.S.; Strijdom, H. Vascular endothelial dysfunction in the wake of HIV and art. FEBS J. 2018, 286, 1256–1270. [Google Scholar] [CrossRef] [Green Version]
- Henderson, L.J.; Reoma, L.B.; Kovacs, J.A.; Nath, A. Advances toward curing HIV-1 infection in tissue reservoirs. J. Virol. 2020, 94, e00375-19. [Google Scholar] [CrossRef]
- Arenaccio, C.; Anticoli, S.; Manfredi, F.; Chiozzini, C.; Olivetta, E.; Federico, M. Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1. Retrovirology 2015, 12, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial cells: The main HIV-1 reservoir in the brain. Front. Cell. Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Carbonell, D.; Ye, F.; Ramanath, N.; Garcia-Mesa, Y.; Knapp, P.E.; Hauser, K.F.; Karn, J. Cross-talk between microglia and neurons regulates HIV latency. PLoS Pathog. 2019, 15, e1008249. [Google Scholar] [CrossRef] [Green Version]
- Bruner, K.M.; Wang, Z.; Simonetti, F.R.; Bender, A.M.; Kwon, K.J.; Sengupta, S.; Fray, E.J.; Beg, S.A.; Antar, A.A.; Jenike, K.M.; et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019, 566, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Nixon, C.C.; Mavigner, M.; Sampey, G.C.; Brooks, A.D.; Spagnuolo, R.A.; Irlbeck, D.M.; Mattingly, C.; Ho, P.T.; Schoof, N.; Cammon, C.G.; et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 2020, 578, 160–165. [Google Scholar] [CrossRef]
- Honeycutt, J.B.; Thayer, W.O.; Baker, C.E.; Ribeiro, R.M.; Lada, S.M.; Cao, Y.; Cleary, R.A.; Hudgens, M.G.; Richman, D.D.; Garcia, J.V. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat. Med. 2017, 23, 638–643. [Google Scholar] [CrossRef]
- Gu, C.J.; Borjabad, A.; Hadas, E.; Kelschenbach, J.; Kim, B.H.; Chao, W.; Arancio, O.; Suh, J.; Polsky, B.; McMillan, J.; et al. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog. 2018, 14, e1007061. [Google Scholar] [CrossRef] [Green Version]
- Delery, E.C.; MacLean, A.G. Culture Model for Non-human Primate Choroid Plexus. Front. Cell. Neurosci. 2019, 13, 396. [Google Scholar] [CrossRef]
- Meeker, R.B.; Williams, K.; Killebrew, D.A.; Hudson, L.C. Cell trafficking through the choroid plexus. Cell Adhes. Migr. 2012, 6, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Burkala, E.J.; He, J.; West, J.T.; Wood, C.; Petito, C.K. Compartmentalization of HIV-1 in the central nervous system: Role of the choroid plexus. AIDS 2005, 19, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.V.; Dahlheimer, J.L.; Bardgett, M.E.; Snyder, A.Z.; Finch, R.A.; Sartorelli, A.C.; Piwnica-Worms, D. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc. Natl. Acad. Sci. USA 1999, 96, 3900–3905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, L.; Nair, M.; Toborek, M. Solving the blood-brain barrier challenge for the effective treatment of HIV replication in the central nervous system. Curr. Pharm. Des. 2016, 22, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Kwon, K.J.; Farber, D.L.; Siliciano, R.F. The Latent Reservoir for HIV-1: How immunologic memory and clonal expansion contribute to HIV-1 persistence. J. Immunol. 2016, 197, 407–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, H.-H.; Lichterfeld, M. Recent progress in understanding HIV reservoirs. Curr. Opin. HIV AIDS 2018, 13, 137–142. [Google Scholar] [CrossRef]
- Darcis, G.; Maes, N.; Pasternak, A.O.; Sauvage, A.-S.; Frippiat, F.; Meuris, C.; Uurlings, F.; Lecomte, M.; Léonard, P.; Elmoussaoui, M.; et al. Detectability of HIV residual viremia despite therapy is highly associated with treatment with a protease inhibitor-based combination antiretroviral therapy. Antimicrob. Agents Chemother. 2020, 64, e01902–e01919. [Google Scholar] [CrossRef]
- Sörstedt, E.; Nilsson, S.; Blaxhult, A.; Gisslén, M.; Flamholc, L.; Sönnerborg, A.; Yilmaz, A. Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels. BMC Infect. Dis. 2016, 16, 305. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, N.; von Siebenthal, C.; Vongrad, V.; Turk, T.; Neumann, K.; Beerenwinkel, N.; Bogojeska, J.; Fellay, J.; Roth, V.; Kok, Y.L.; et al. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive art. Nat. Commun. 2019, 10, 3193. [Google Scholar] [CrossRef] [Green Version]
- Avalos, C.R.; Abreu, C.M.; Queen, S.E.; Li, M.; Price, S.; Shirk, E.N.; Engle, E.L.; Forsyth, E.; Bullock, B.T.; Mac Gabhann, F.; et al. Brain macrophages in simian immunodeficiency virus-infected, antiretroviral-suppressed macaques: A functional latent reservoir. MBio 2017, 8, e01186-17. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, L.; Méroth, F.; Tournebize, M.; Leda, A.R.; Sun, E.; Toborek, M. Targeting the HIV-infected brain to improve ischemic stroke outcome. Nat. Commun. 2019, 10, 2009. [Google Scholar] [CrossRef] [Green Version]
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013, 39, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vouri, S.; Kebodeaux, C.; Wilson, A.; Smith, D. A review of cardiovascular and Renal Function Monitoring: A consideration of older adults with HIV. HIV/AIDS Res. Palliat. Care 2013, 263, 263–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lake, J.E.; Currier, J.S. Metabolic disease in HIV infection. Lancet Infect. Dis. 2013, 13, 964–975. [Google Scholar] [CrossRef]
- Bhatia, N.S.; Chow, F.C. Neurologic complications in treated HIV-1 infection. Curr. Neurol. Neurosci. Rep. 2016, 16. [Google Scholar] [CrossRef]
- Martin-Iguacel, R.; Negredo, E.; Peck, R.; Friis-Møller, N. Hypertension is a key feature of the metabolic syndrome in subjects aging with HIV. Curr. Hypertens. Rep. 2016, 18, 46. [Google Scholar] [CrossRef] [Green Version]
- Nasi, M.; De Biasi, S.; Gibellini, L.; Bianchini, E.; Pecorini, S.; Bacca, V.; Guaraldi, G.; Mussini, C.; Pinti, M.; Cossarizza, A. Ageing and inflammation in patients with HIV infection. Clin. Exp. Immunol. 2016, 187, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Nduka, C.U.; Stranges, S.; Sarki, A.M.; Kimani, P.K.; Uthman, O.A. Evidence of increased blood pressure and hypertension risk among people living with HIV on antiretroviral therapy: A systematic review with meta-analysis. J. Hum. Hypertens. 2015, 30, 355–362. [Google Scholar] [CrossRef]
- d’Ettorre, G.; Ceccarelli, G.; Pavone, P.; Vittozzi, P.; De Girolamo, G.; Schietroma, I.; Serafino, S.; Giustini, N.; Vullo, V. What happens to cardiovascular system behind the undetectable level of HIV viremia? AIDS Res. Ther. 2016, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Peltenburg, N.C.; Schoeman, J.C.; Hou, J.; Mora, F.; Harms, A.C.; Lowe, S.H.; Bierau, J.; Bakker, J.A.; Verbon, A.; Hankemeier, T.; et al. Persistent metabolic changes in HIV-infected patients during the first year of combination antiretroviral therapy. Sci. Rep. 2018, 8, 16947. [Google Scholar] [CrossRef] [Green Version]
- Robertson, K.; Liner, J.; Meeker, R.B. Antiretroviral neurotoxicity. J. NeuroVirology 2012, 18, 388–399. [Google Scholar] [CrossRef]
- Wang, P.; Tian, X.; Tang, J.; Duan, X.; Wang, J.; Cao, H.; Qiu, X.; Wang, W.; Mai, M.; Yang, Q.; et al. Artemisinin protects endothelial function and vasodilation from oxidative damage via activation of PI3K/AKT/Enos pathway. Exp. Gerontol. 2021, 147, 111270. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, I.; Trunfio, M.; Guastamacchia, G.; Bonora, S.; Calcagno, A. A review of the potential mechanisms of neuronal toxicity associated with antiretroviral drugs. J. Neuro.Virol. 2020, 26, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; D’Agostino, L.; Tuzer, F.; Torres, C. HIV antiretroviral therapy drugs induce premature senescence and altered physiology in HUVECS. Mech. Ageing Dev. 2018, 175, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Peyravian, N.; Dikici, E.; Deo, S.; Toborek, M.; Daunert, S. Opioid antagonists as potential therapeutics for ischemic stroke. Prog. Neurobiol. 2019, 182, 101679. [Google Scholar] [CrossRef]
- Voirin, A.-C.; Perek, N.; Roche, F. Inflammatory stress induced by a combination of cytokines (IL-6, IL-17, TNF-α) leads to a loss of integrity on bend.3 endothelial cells in vitro BBB model. Brain Res. 2020, 1730, 146647. [Google Scholar] [CrossRef]
- Nooka, S.; Ghorpade, A. HIV-1-associated inflammation and antiretroviral therapy regulate astrocyte endoplasmic reticulum stress responses. Cell Death Discov. 2017, 3, 17061. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, L.; Toborek, M. Dysregulation of endoplasmic reticulum stress and autophagic responses by the Antiretroviral Drug Efavirenz. Mol. Pharmacol. 2015, 88, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-F.; Dugas, T.R. Endothelial mitochondrial senescence accelerates cardiovascular disease in antiretroviral-receiving HIV patients. Toxicol. Lett. 2019, 317, 13–23. [Google Scholar] [CrossRef]
- Guha, D.; Mukerji, S.S.; Chettimada, S.; Misra, V.; Lorenz, D.R.; Morgello, S.; Gabuzda, D. Cerebrospinal fluid extracellular vesicles and neurofilament light protein as biomarkers of central nervous system injury in HIV-infected patients on antiretroviral therapy. AIDS 2019, 33, 615–625. [Google Scholar] [CrossRef]
- Blas-Garcia, A.; Polo, M.; Alegre, F.; Funes, H.A.; Martinez, E.; Apostolova, N.; Esplugues, J.V. Lack of mitochondrial toxicity of darunavir, raltegravir and rilpivirine in neurons and hepatocytes: A comparison with Efavirenz. J. Antimicrob. Chemother. 2014, 69, 2995–3000. [Google Scholar] [CrossRef] [Green Version]
- Tan, I.L.; McArthur, J.C. HIV-associated neurological disorders. CNS Drugs 2012, 26, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Grimmig, B.; Izzo, J.; Brown, L.A.; Hudson, C.; Smith, A.J.; Tan, J.; Bickford, P.C.; Giunta, B. HIV non-nucleoside reverse transcriptase inhibitor Efavirenz reduces neural stem cell proliferation in vitro and in vivo. Cell Transplant. 2016, 25, 1967–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Wang, Y.; Qin, Z.; Qiu, L.; Zhang, M.; Huang, Y.; Zheng, J.C. Combined medication of antiretroviral drugs tenofovir disoproxil fumarate, emtricitabine, and raltegravir reduces neural progenitor cell proliferation in vivo and in vitro. J. Neuroimmune Pharmacol. 2017, 12, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Festa, L.; Roth, L.M.; KJensen, B.; Geiger, J.D.; Jordan-Sciutto, K.L.; Grinspan, J.B. Protease inhibitors, saquinavir and darunavir, inhibit oligodendrocyte maturation: Implications for lysosomal stress. J. Neuroimmune Pharmacol. 2019, 16, 169–180. [Google Scholar] [CrossRef]
- Shah, A.; Gangwani, M.R.; Chaudhari, N.S.; Glazyrin, A.; Bhat, H.K.; Kumar, A. Neurotoxicity in the post-HAART era: Caution for the antiretroviral therapeutics. Neurotox. Res. 2016, 30, 677–697. [Google Scholar] [CrossRef]
- Persidsky, Y.; Hill, J.; Zhang, M.; Dykstra, H.; Winfield, M.; Reichenbach, N.L.; Potula, R.; Mukherjee, A.; Ramirez, S.H.; Rom, S. Dysfunction of brain pericytes in chronic neuroinflammation. J. Cereb. Blood Flow Metab. 2015, 36, 794–807. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shim, E.; Crespo-Mejias, Y.; Nguyen, P.G.; Gibbons, A.; Liu, D.; Shide, E.; Poirier, M.C. Cardiomyocytes are protected from antiretroviral nucleoside analog-induced mitochondrial toxicity by overexpression of pgc-1α. Cardiovasc. Toxicol. 2014, 15, 224–231. [Google Scholar] [CrossRef]
- Smith, R.L.; de Boer, R.; Brul, S.; Budovskaya, Y.; van Spek, H. Premature and accelerated aging: HIV or Haart? Front. Genet. 2013, 3, 328. [Google Scholar] [CrossRef] [Green Version]
- Bañó, M.; Morén, C.; Barroso, S.; Juárez, D.L.; Guitart-Mampel, M.; González-Casacuberta, I.; Canto-Santos, J.; Lozano, E.; León, A.; Pedrol, E.; et al. Mitochondrial toxicogenomics for antiretroviral management: HIV Post-exposure prophylaxis in uninfected patients. Front. Genet. 2020, 11, 497. [Google Scholar] [CrossRef]
- Hukezalie, K.R.; Thumati, N.R.; Côté, H.C.; Wong, J.M. In vitro and ex vivo inhibition of human telomerase by Anti-HIV nucleoside reverse transcriptase inhibitors (nrtis) but not by Non-NRTIs. PLoS ONE 2012, 7, e47505. [Google Scholar] [CrossRef] [Green Version]
- Leeansyah, E.; Cameron, P.U.; Solomon, A.; Tennakoon, S.; Velayudham, P.; Gouillou, M.; Spelman, T.; Hearps, A.; Fairley, C.; Smit, D.V.; et al. Inhibition of telomerase activity by human immunodeficiency virus (HIV) nucleos(t)ide reverse transcriptase inhibitors: A potential factor contributing to HIV-associated accelerated aging. J. Infect. Dis. 2013, 207, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, F.M. Telomerase inhibition may contribute to accelerated mitochondrial aging induced by anti-retroviral HIV treatment. Med. Hypotheses 2013, 81, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Oeck, S.; West, A.P.; Mangalhara, K.C.; Sainz, A.G.; Newman, L.E.; Zhang, X.-O.; Wu, L.; Yan, Q.; Bosenberg, M.; et al. Mitochondrial DNA stress signalling protects the nuclear genome. Nat. Metab. 2019, 1, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Yarosz, E.L.; Chang, C.-H. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw. 2018, 18. [Google Scholar] [CrossRef]
- Stefanatos, R.; Sanz, A. The role of mitochondrial Ros in the aging brain. FEBS Lett. 2017, 592, 743–758. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, C.; LI, Z.; Kim, G.; Jeevanandam, V.; Uriel, N. Molecular mechanism of the association between Atrial Fibrillation and heart failure includes energy metabolic dysregulation due to mitochondrial dysfunction. J. Card. Fail. 2019, 25, 911–920. [Google Scholar] [CrossRef]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y.; et al. Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4356386. [Google Scholar] [CrossRef]
- Anasooya Shaji, C.; Robinson, B.D.; Yeager, A.; Beeram, M.R.; Davis, M.L.; Isbell, C.L.; Huang, J.H.; Tharakan, B. The tri-phasic role of hydrogen peroxide in blood-brain barrier endothelial cells. Sci. Rep. 2019, 9, 133. [Google Scholar] [CrossRef]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef]
- Sulhan, S.; Lyon, K.A.; Shapiro, L.A.; Huang, J.H. Neuroinflammation and blood-brain barrier disruption following traumatic brain injury: Pathophysiology and potential therapeutic targets. J. Neurosci. Res. 2018, 98, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Diwanji, N.; Bergmann, A. Basement membrane damage by ros- and JNK-mediated MMP2 activation drives macrophage recruitment to overgrown tissue. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.-R.; Kim, J.Y.; Hyun, H.-W.; Kim, J.-E. Endothelial NOS activation induces the blood-brain barrier disruption via ER stress following status epilepticus. Brain Res. 2015, 1622, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.; Bertrand, L.; Luethen, M.; Dabrowski, S.; Lombardi, J.; Morgan, L.; Sharova, N.; Stevenson, M.; Blasig, I.E.; Toborek, M. Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation. FASEB J. 2015, 30, 1234–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, V.; Skowronska, M.; Lombardi, J.; He, J.; Seth, N.; Velichkovska, M.; Toborek, M. Occludin regulates glucose uptake and ATP production in pericytes by influencing AMP-activated protein kinase activity. J. Cereb. Blood Flow Metab. 2017, 38, 317–332. [Google Scholar] [CrossRef] [Green Version]
- Apostolova, N.; Gomez-Sucerquia, L.J.; Alegre, F.; Funes, H.A.; Victor, V.M.; Barrachina, M.D.; Blas-Garcia, A.; Esplugues, J.V. ER stress in human hepatic cells treated with Efavirenz: Mitochondria again. J. Hepatol. 2013, 59, 780–789. [Google Scholar] [CrossRef]
- Donnelly, N.; Gorman, A.M.; Gupta, S.; Samali, A. The eIF2α kinases: Their structures and functions. Cell. Mol. Life Sci. 2013, 70, 3493–3511. [Google Scholar] [CrossRef]
- Kawabata, T.; Yoshimori, T. Autophagosome Biogenesis and human health. Cell Discov. 2020, 6, 33. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. The J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.A.; Masliah, E.; Vinters, H.V.; Beizai, P.; Moore, D.J.; Achim, C.L. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 2005, 19, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.A.; Swinton, M.K.; Soontornniyomkij, B.; Carson, A.; Achim, C.L. Beta amyloid levels in cerebrospinal fluid of HIV-infected people vary by exposure to antiretroviral therapy. AIDS 2020, 34, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Achim, C.L.; Adame, A.; Dumaop, W.; Everall, I.P.; Masliah, E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J. Neuroimmune Pharmacol. 2009, 4, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Gross, A.M.; Jaeger, P.A.; Kreisberg, J.F.; Licon, K.; Jepsen, K.L.; Khosroheidari, M.; Morsey, B.M.; Swindells, S.; Shen, H.; Ng, C.T.; et al. Methylome-wide Analysis of Chronic HIV Infection Reveals Five-Year Increase in Biological Age and Epigenetic Targeting of HLA. Mol. Cell 2016, 62, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Choi, M.-S.; Inn, K.-S.; Kim, B.-J. Inhibition of HIV-1 reactivation by a telomerase-derived peptide in a hsp90-dependent manner. Sci. Rep. 2016, 6, 28896. [Google Scholar] [CrossRef] [Green Version]
- Gavegnano, C.; Detorio, M.; Montero, C.; Bosque, A.; Planelles, V.; Schinazi, R.F. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus ReactivationIn Vitro. Antimicrob. Agents Chemother. 2014, 58, 1977–1986. [Google Scholar] [CrossRef] [Green Version]
- Gavegnano, C.; Brehm, J.H.; Dupuy, F.P.; Talla, A.; Ribeiro, S.P.; Kulpa, D.A.; Cameron, C.; Santos, S.; Hurwitz, S.J.; Marconi, V.C.; et al. Novel mechanisms to inhibit HIV reservoir seeding using JAK inhibitors. PLoS Pathog. 2017, 13, e1006740. [Google Scholar] [CrossRef] [Green Version]
- Lichterfeld, M. Reactivation of latent HIV moves shock-and-kill treatments forward. Nature 2020, 578, 42–43. [Google Scholar] [CrossRef]
- Jiang, G.; Mendes, E.A.; Kaiser, P.; Wong, D.P.; Tang, Y.; Cai, I.; Fenton, A.; Melcher, G.P.; Hildreth, J.E.; Thompson, G.R.; et al. Synergistic reactivation of latent HIV expression by ingenol-3-angelate, PEP005, targeted NF-KB signaling in combination with JQ1 induced P-tefb activation. PLoS Pathog. 2015, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBrien, J.B.; Mavigner, M.; Franchitti, L.; Smith, S.A.; White, E.; Tharp, G.K.; Walum, H.; Busman-Sahay, K.; Aguilera-Sandoval, C.R.; Thayer, W.O.; et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8+ cells. Nature 2020, 578, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, E.K.; Spicer, L.; Smith, S.A.; Lee, D.; Fast, R.; Paganini, S.; Lawson, B.O.; Nega, M.; Easley, K.; Schmitz, J.E.; et al. Cd8 + lymphocytes are required for maintaining viral suppression in siv-infected macaques treated with short-term antiretroviral therapy. Immunity 2016, 45, 656–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, R.; Conway, J.M.; Margolis, D.M.; Perelson, A.S. Determinants of the efficacy of HIV latency-reversing agents and implications for drug and treatment design. JCI Insight 2018, 3, e123052. [Google Scholar] [CrossRef]
- Vansant, G.; Bruggemans, A.; Janssens, J.; Debyser, Z. Block-and-lock strategies to cure HIV infection. Viruses 2020, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Kessing, C.F.; Nixon, C.C.; Li, C.; Tsai, P.; Takata, H.; Mousseau, G.; Ho, P.T.; Honeycutt, J.B.; Fallahi, M.; Trautmann, L.; et al. In vivo suppression of HIV rebound by Didehydro-cortistatin a, a “block-and-lock” strategy for HIV-1 treatment. Cell Rep. 2017, 21, 600–611. [Google Scholar] [CrossRef] [Green Version]
- Besnard, E.; Hakre, S.; Kampmann, M.; Lim, H.W.; Hosmane, N.N.; Martin, A.; Bassik, M.C.; Verschueren, E.; Battivelli, E.; Chan, J.; et al. The mTOR complex controls HIV latency. Cell Host Microbe 2016, 20, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Vargas, B.; Giacobbi, N.S.; Sanyal, A.; Venkatachari, N.J.; Han, F.; Gupta, P.; Sluis-Cremer, N. Inhibitors of signaling pathways that block reversal of HIV-1 latency. Antimicrob. Agents Chemother. 2019, 63, e01744-18. [Google Scholar] [CrossRef] [Green Version]
- Niu, Q.; Liu, Z.; Alamer, E.; Fan, X.; Chen, H.; Endsley, J.; Gelman, B.B.; Tian, B.; Kim, J.H.; Michael, N.L.; et al. Structure-guided drug design identifies a BRD4-selective small molecule that suppresses HIV. J. Clin. Investig. 2019, 129, 3361–3373. [Google Scholar] [CrossRef]
- Kaushik, A.; Yndart, A.; Atluri, V.; Tiwari, S.; Tomitaka, A.; Gupta, P.; Jayant, R.D.; Alvarez-Carbonell, D.; Khalili, K.; Nair, M. Magnetically guided non-invasive CRISPR-Cas9/grna delivery across blood-brain barrier to eradicate latent HIV-1 infection. Sci. Rep. 2019, 9, 3928. [Google Scholar] [CrossRef] [Green Version]
- Dash, P.K.; Kaminski, R.; Bella, R.; Su, H.; Mathews, S.; Ahooyi, T.M.; Chen, C.; Mancuso, P.; Sariyer, R.; Ferrante, P.; et al. Sequential Laser Art and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat. Commun. 2019, 10, 2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Lei, R.; Le Duff, Y.; Li, J.; Guo, F.; Wainberg, M.A.; Liang, C. The CRISPR/cas9 system inactivates latent HIV-1 proviral DNA. Retrovirology 2015, 12, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halper-Stromberg, A.; Lu, C.-L.; Klein, F.; Horwitz, J.A.; Bournazos, S.; Nogueira, L.; Eisenreich, T.R.; Liu, C.; Gazumyan, A.; Schaefer, U.; et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 2014, 158, 989–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, R.; Berges, B.K.; Solis-Leal, A.; Igbinedion, O.; Strong, C.L.; Schiller, M.R. Talen gene editing takes aim on HIV. Hum. Genet. 2016, 135, 1059–1070. [Google Scholar] [CrossRef] [Green Version]
- Rathore, A.; Iketani, S.; Wang, P.; Jia, M.; Sahi, V.; Ho, D.D. CRISPR-based gene knockout screens reveal deubiquitinases involved in HIV-1 latency in two Jurkat cell models. Sci. Rep. 2020, 10, 5350. [Google Scholar] [CrossRef] [Green Version]
- Yukl, S.A.; Boritz, E.; Busch, M.; Bentsen, C.; Chun, T.-W.; Douek, D.; Eisele, E.; Haase, A.; Ho, Y.-C.; Hütter, G.; et al. Challenges in detecting HIV persistence during potentially curative interventions: A study of the berlin patient. PLoS Pathog. 2013, 9, e1003347. [Google Scholar] [CrossRef]
- Gupta, R.K.; Peppa, D.; Hill, A.L.; Gálvez, C.; Salgado, M.; Pace, M.; McCoy, L.E.; Griffith, S.A.; Thornhill, J.; Alrubayyi, A.; et al. Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: A case report. Lancet HIV 2020, 7, e340–e347. [Google Scholar] [CrossRef] [Green Version]
- Skundric, D.S.; Tse, H.Y.; Montgomery, P.C. Functional phenotypes of CCR5 on CD4+ T cells of relevance to its genetic and epigenetic associations with HIV infection. Cell. Mol. Immunol. 2020, 17, 680–681. [Google Scholar] [CrossRef]
- Peterson, C.W.; Kiem, H.-P. Lessons from London and Berlin: Designing A scalable gene therapy approach for HIV cure. Cell Stem Cell 2019, 24, 685–687. [Google Scholar] [CrossRef]
- Gupta, R.K.; Abdul-Jawad, S.; McCoy, L.E.; Mok, H.P.; Peppa, D.; Salgado, M.; Martinez-Picado, J.; Nijhuis, M.; Wensing, A.M.; Lee, H.; et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Berg, C.; Daugvilaite, V.; Steen, A.; Jørgensen, A.S.; Våbenø, J.; Rosenkilde, M.M. Inhibition of HIV fusion by small molecule agonists through efficacy-engineering of CXCR4. ACS Chem. Biol. 2018, 13, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-S.; Yin, J.; Leng, F.; Teng, R.-F.; Xu, C.; Xia, X.-Y.; Pan, X.-M. HIV coreceptor tropism determination and mutational pattern identification. Sci. Rep. 2016, 6, 21280. [Google Scholar] [CrossRef] [PubMed]
- ElZohary, L.; Weglicki, W.B.; Chmielinska, J.J.; Kramer, J.H.; Mak, I.T. MG-supplementation attenuated lipogenic and oxidative/nitrosative gene expression caused by combination antiretroviral therapy (CART) in HIV-1-transgenic rats. PLoS ONE 2019, 14, e0210107. [Google Scholar] [CrossRef] [Green Version]
- Coulibaly, F.S.; Ezoulin, M.J.; Purohit, S.S.; Ayon, N.J.; Oyler, N.A.; Youan, B.-B.C. Layer-by-layer engineered microbicide drug delivery system targeting HIV-1 gp120: Physicochemical and biological properties. Mol. Pharm. 2017, 14, 3512–3527. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, F.S.; Thomas, D.N.; Youan, B.-B.C. Anti-HIV lectins and current delivery strategies. AIMS Mol. Sci. 2018, 5, 96–116. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Ferro, S.; De Luca, L.; Monforte, A.M.; Romeo, R.; Buemi, M.R.; Pannecouque, C. Graphene quantum dots based systems as HIV inhibitors. Bioconjugate Chem. 2018, 29, 3084–3093. [Google Scholar] [CrossRef] [PubMed]
- Surnar, B.; Shah, A.S.; Park, M.; Kalathil, A.A.; Kamran, M.Z.; Ramirez Jaime, R.; Toborek, M.; Nair, M.; Kolishetti, N.; Dhar, S. Brain-Accumulating Nanoparticles for Assisting Astrocytes to Reduce Human Immunodeficiency Virus and Drug Abuse-Induced Neuroinflammation and Oxidative Stress. ACS Nano 2021, 15, 15741–15753. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).