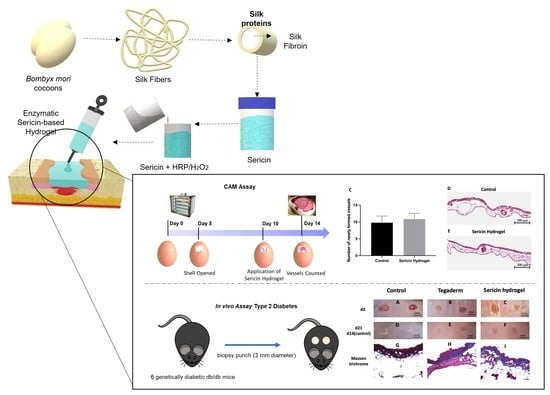
Exploring Silk Sericin for Diabetic Wounds: An In Situ-Forming Hydrogel to Protect against Oxidative Stress and Improve Tissue Healing and Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
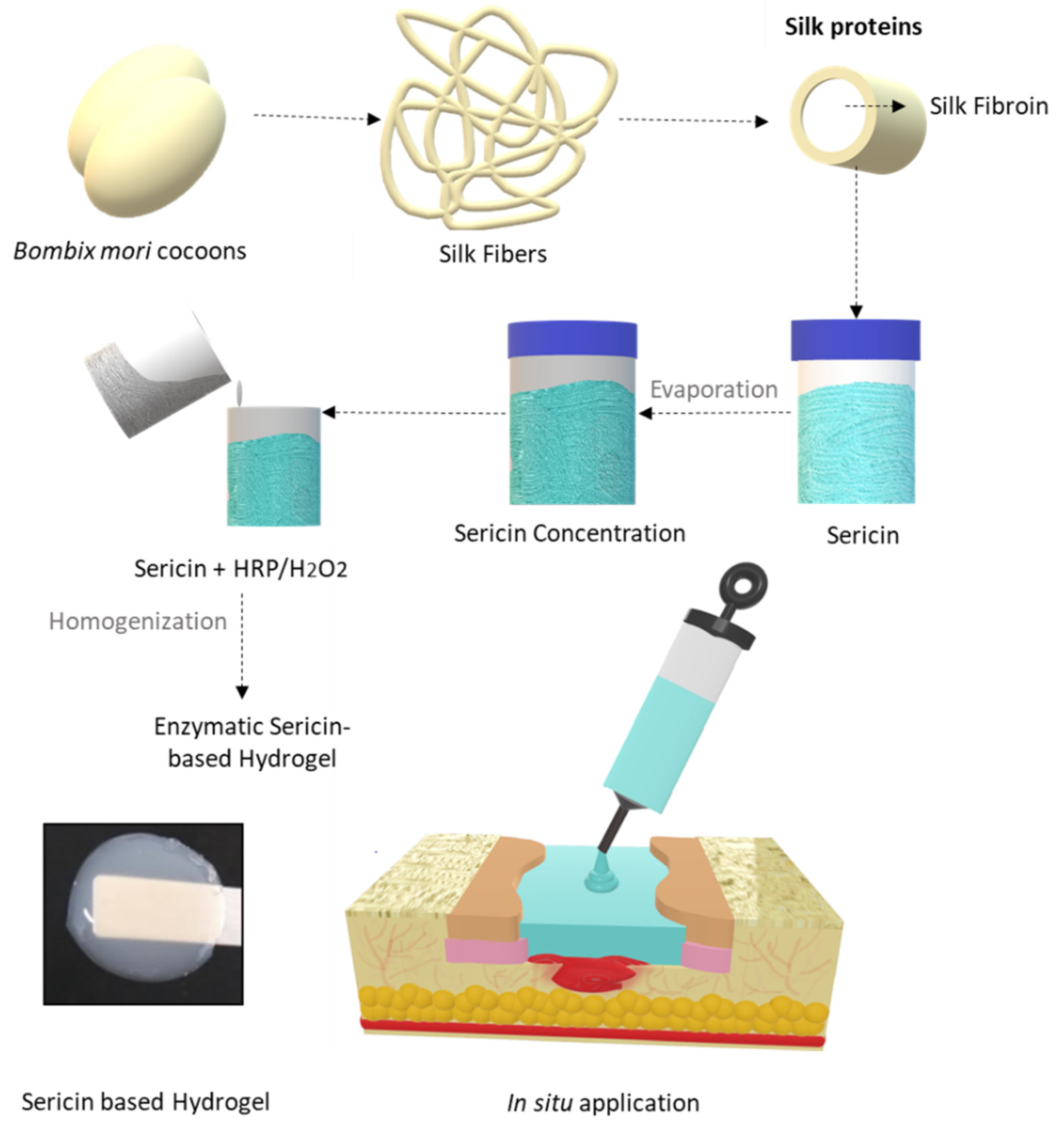
2.2. Silk Sericin Extraction and Hydrogel Preparation
2.3. Silk Sericin Hydrogel In Situ Experimental Design in a Wound Simulator
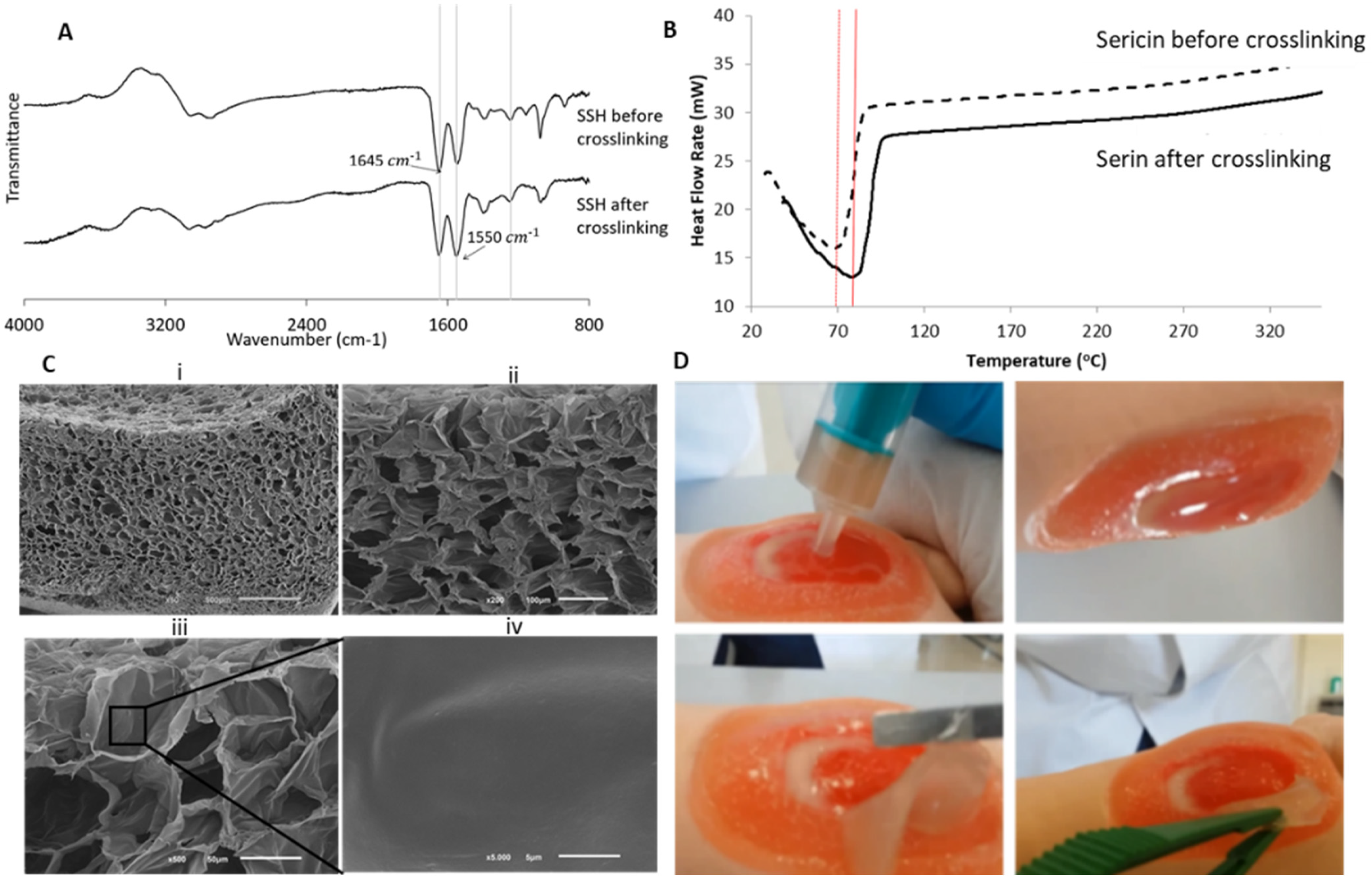
2.4. Differential Scanning Calorimetry Analysis
2.5. Fourier Transformed Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
2.6. Scanning Electron Microscopy
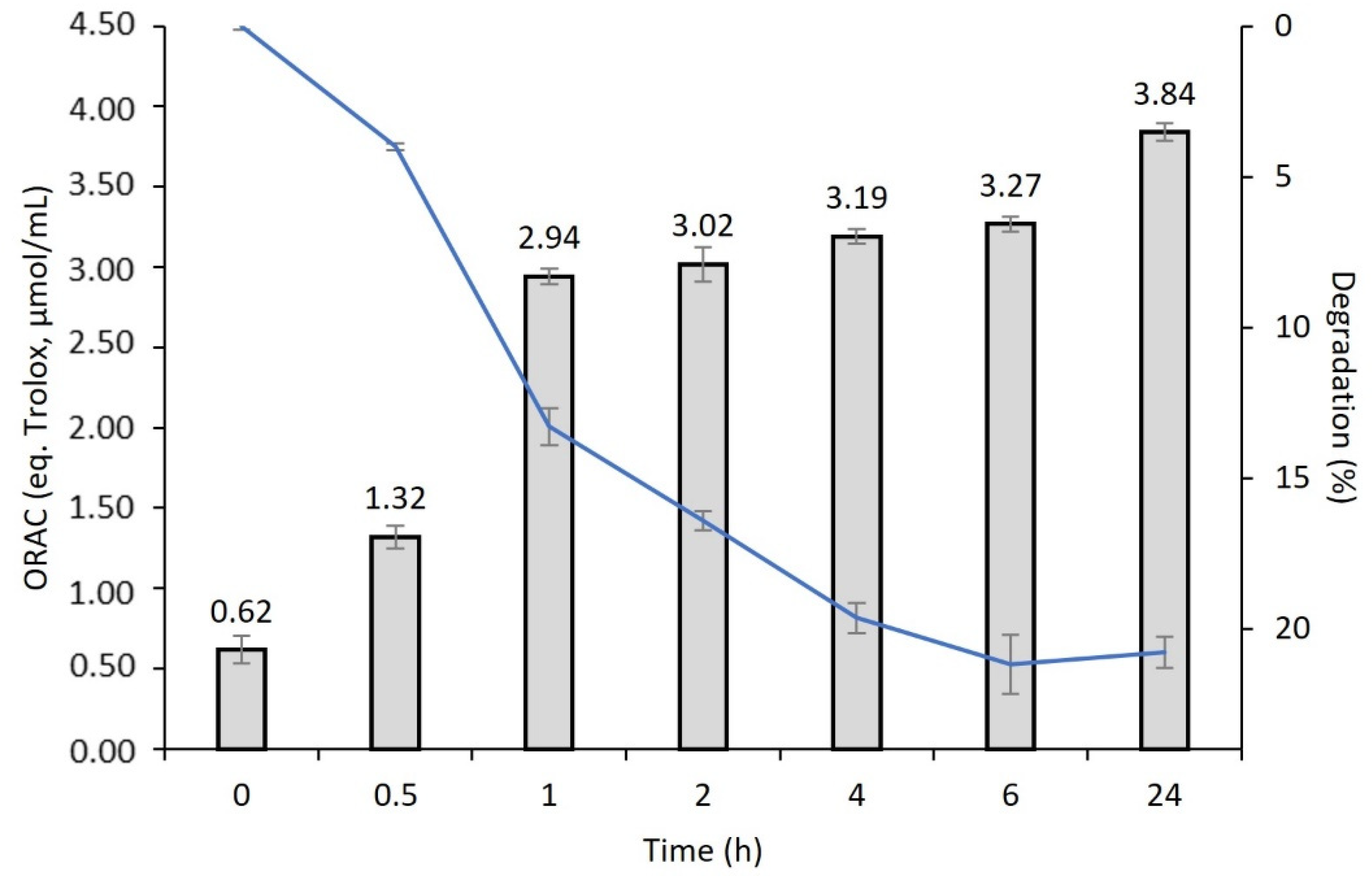
2.7. Degradation Behavior and Antioxidant Activity
2.8. Antimicrobial Activity
2.9. In Vivo Chicken Embryo Choriallantoic Membrane (CAM) Assay
2.10. Animal Model of Skin Wound Healing
2.11. Transmission Electron Microscopy
2.12. Superoxide Dismutase (SOD) Activity
2.13. Catalase Activity
2.14. Advanced Oxidation Protein Products (AOPP) Determination
2.15. Statistical Analysis
3. Results
3.1. Physicochemical Characterization
3.2. Antioxidant Activity vs. Degradation Behavior
3.3. Antimicrobial Activity
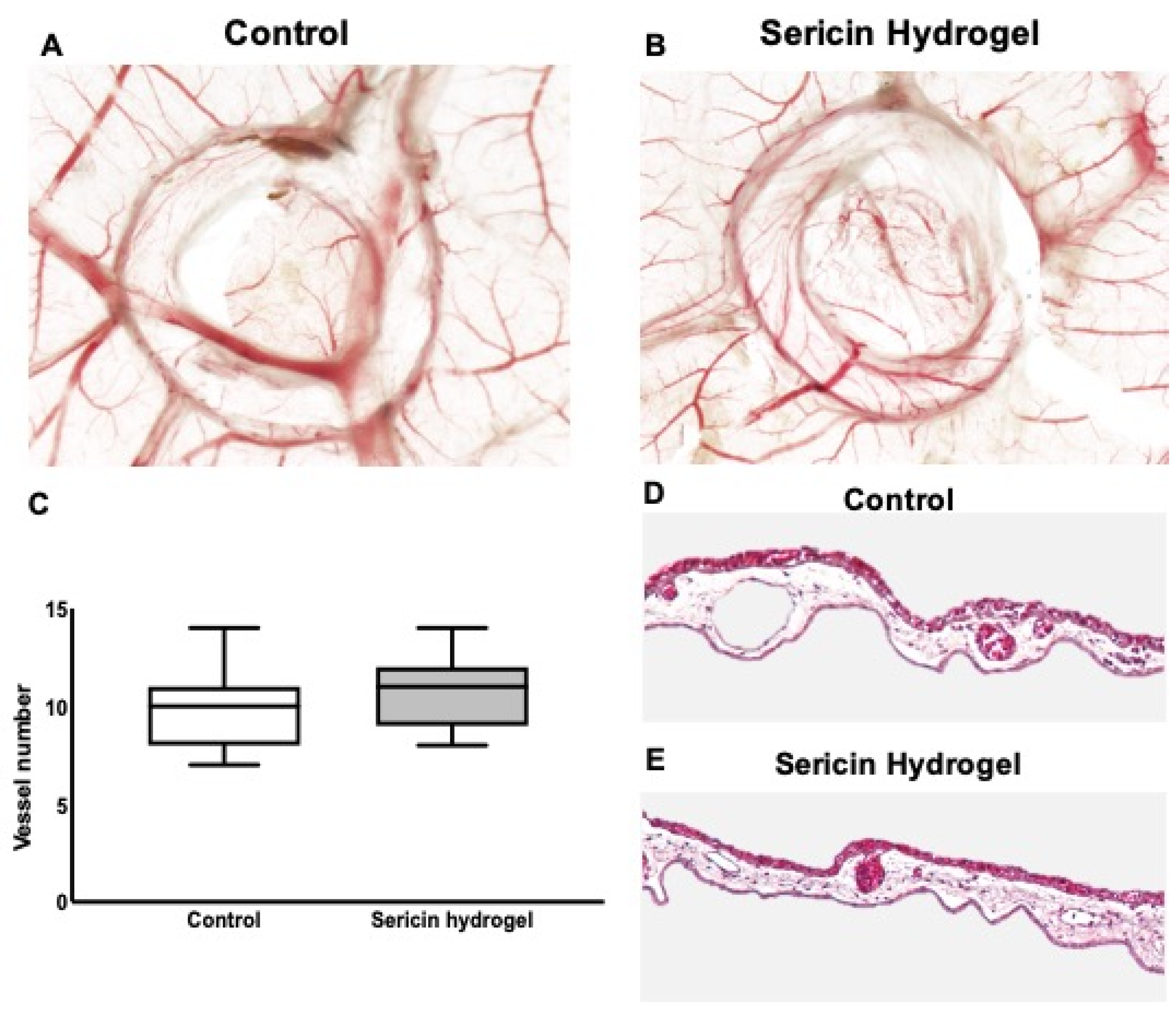
3.4. Chick Embryo Choriollantoic Membrane (CAM) Response to Sericin Hydrogel
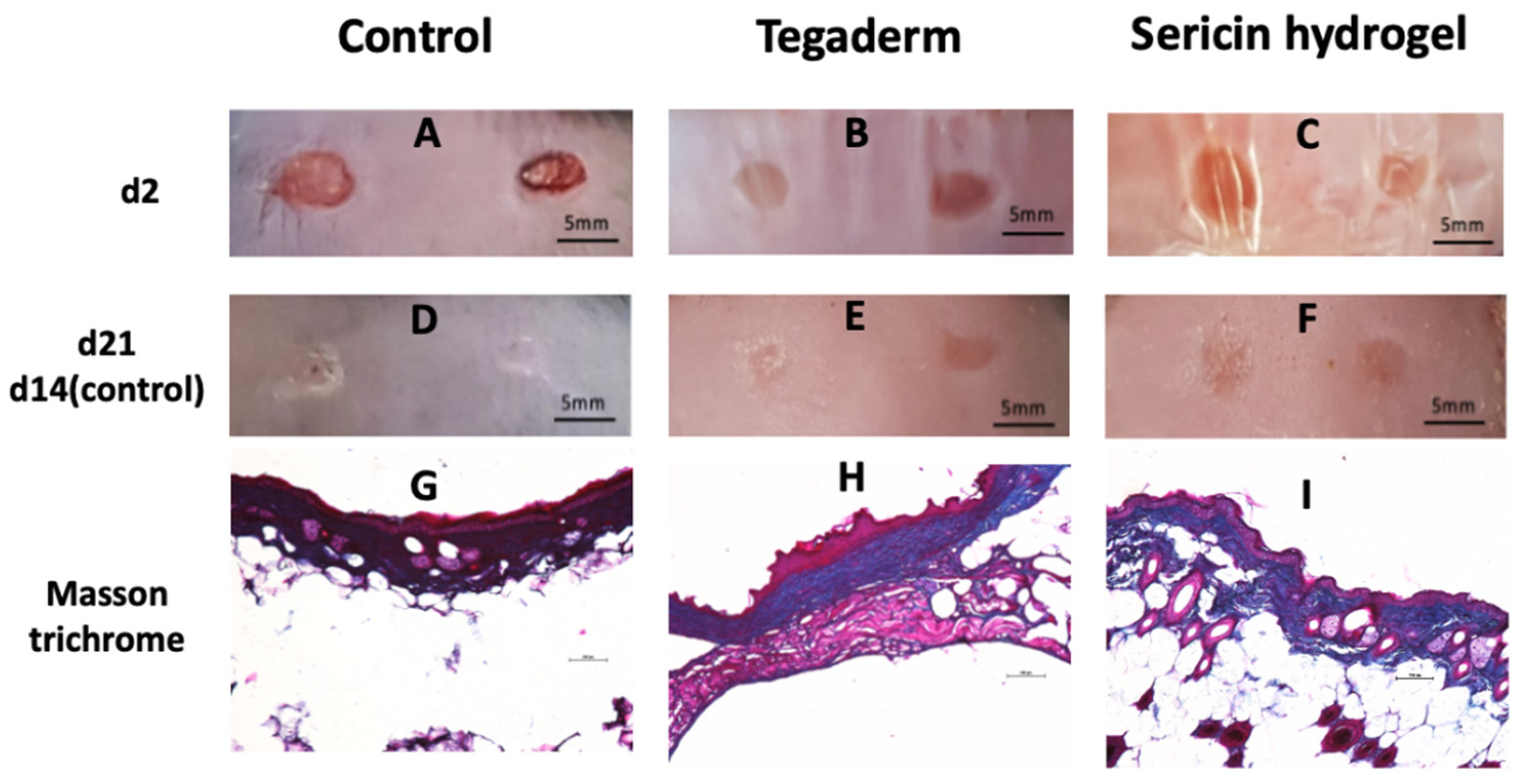
3.5. Histopathological Analysis of Chronic Wound Using an Animal Model of T2DM
3.6. In Vivo Assessment of Sericin-Based Hydrogel Antioxidant Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Rolling Updates on Coronavirus Disease (COVID-19). [updated 2020 July 31]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed on 22 April 2021).
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharm. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuri, D.; Barbalinardo, M.; Sotgiu, G.; Zamboni, R.; Nocchetti, M.; Donnadio, A.; Corticelli, F.; Valle, F.; Gennari, C.G.M.; Selmin, F.; et al. Nano-hybrid electrospun non-woven mats made of wool keratin and hydrotalcites as potential bio-active wound dressings. Nanoscale 2019, 11, 6422–6430. [Google Scholar] [CrossRef] [PubMed]
- Snyder, D.; Sullivan, N.; Margolis, D.; Schoelles, K. Skin Substitutes for Treating Chronic Wounds [Internet]; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2020.
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.M.C.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [Green Version]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2020, 8, 313–324. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Tao, G.; Cai, R.; Wang, Y.; Zuo, H.; He, H. Fabrication of antibacterial sericin based hydrogel as an injectable and mouldable wound dressing. Mater. Sci. Eng. C 2020, 119, 111597. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Mohammadi, A.A.; Moshiri, A. Healing potential of injectable Aloe vera hydrogel loaded by adipose-derived stem cell in skin tissue-engineering in a rat burn wound model. Cell Tissue Res. 2019, 377, 215–227. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, Y.; Zeng, Q.; Li, H.; Peng, J.; Xu, Y.; Chang, J. Injectable bioactive akermanite/alginate composite hydrogels for in situ skin tissue engineering. J. Mater. Chem. B 2017, 5, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liang, Y.; Zhang, D.; Sun, X.; Liang, L.; Li, J.; Liu, Y.-N. Gelatin-Based Hydrogels Blended with Gellan as an Injectable Wound Dressing. ACS Omega 2018, 3, 4766–4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Wei, J.; Qin, J.; Peng, W.; Lasaosa, F.L.; He, Y.; Mao, H.; et al. Injectable Adhesive Self-Healing Multicross-Linked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 57782–57797. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Jabbari, N.; Sharifi, R.; Ahmadi, M.; Vahhabi, A.; Seyedzadeh, S.J.; Nawaz, M.; Szafert, S.; Mahmoodi, M.; Jabbari, E.; et al. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci. 2020, 249, 117447. [Google Scholar] [CrossRef]
- Yang, M.; He, S.; Su, Z.; Yang, Z.; Liang, X.; Wu, Y. Thermosensitive Injectable Chitosan/Collagen/β-Glycerophosphate Composite Hydrogels for Enhancing Wound Healing by Encapsulating Mesenchymal Stem Cell Spheroids. ACS Omega 2020, 5, 21015–21023. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Yazdi, M.K.; Ghavami, S.B.; Farokhimanesh, S.; Amirabad, L.M.; Zarrintaj, P.; Saeb, M.R.; Hamblin, M.R.; Zare, M.; Mozafari, M. Mesenchymal Stem Cell Spheroids Embedded in an Injectable Thermosensitive Hydrogel: An In Situ Drug Formation Platform for Accelerated Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5096–5109. [Google Scholar] [CrossRef]
- Madl, A.C.; Madl, C.M.; Myung, D. Injectable Cucurbit [8]uril-Based Supramolecular Gelatin Hydrogels for Cell Encapsulation. ACS Macro Lett. 2020, 9, 619–626. [Google Scholar] [CrossRef]
- Ribeiro, V.; Almeida, L.R.; Martins, A.R.; Pashkuleva, I.; Marques, A.; Ribeiro, A.S.; Silva, C.; Bonifácio, G.; Sousa, R.; Reis, R.L.; et al. Influence of different surface modification treatments on silk biotextiles for tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 496–507. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2018, 8, e1800465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, N.; Fukuoka, Y.; Ishii, M.; Otake, H.; Yamamoto, T.; Taga, A.; Okamoto, N.; Shimomura, Y. Instillation of Sericin Enhances Corneal Wound Healing through the ERK Pathway in Rat Debrided Corneal Epithelium. Int. J. Mol. Sci. 2018, 19, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiga, A.; Castro, F.; Rocha, F.; Oliveira, A.L. Recent Advances in Silk Sericin/Calcium Phosphate Biomaterials. Front. Mater. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P.; Napavichayanum, S.; Pienpinijtham, P.; Rasmi, Y.; Bang, N. Antibiofilm activity and cytotoxicity of silk sericin against Streptococcus mutans bacteria in biofilm: An in vitro study. J. Wound Care 2020, 29, S25–S35. [Google Scholar] [CrossRef]
- Verma, J.; Kanoujia, J.; Parashar, P.; Tripathi, C.B.; Saraf, S.A. Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Deliv. Transl. Res. 2016, 7, 77–88. [Google Scholar] [CrossRef]
- Qi, C.; Liu, J.; Jin, Y.; Xu, L.; Wang, G.; Wang, Z.; Wang, L. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials 2018, 163, 89–104. [Google Scholar] [CrossRef]
- Jo, Y.-Y.; Kweon, H.; Oh, J.-H. Sericin for Tissue Engineering. Appl. Sci. 2020, 10, 8457. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, Ó.; Alves, P.; Granja, P.L.; Soares, R.; et al. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Sousa, P.; Borges, S.; Pintado, M. Enzymatic hydrolysis of insect Alphitobius diaperinus towards the development of bioactive peptide hydrolysates. Food Funct. 2020, 11, 3539–3548. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.; Torres, A.L.; Neto, T.; Aguiar, P.; Barrias, C.C.; Pinto, M.T.; Amaral, I.F. Conjugation of the T1 sequence from CCN1 to fibrin hydrogels for therapeutic vascularization. Mater. Sci. Eng. C 2019, 104, 109847. [Google Scholar] [CrossRef]
- Pinto, A.; Pinto, M.L.; Velho, S.; Pinto, M.T.; Cardoso, A.P.; Figueira, R.; Monteiro, A.; Marques, M.; Seruca, R.; Barbosa, M.A.; et al. Intricate Macrophage-Colorectal Cancer Cell Communication in Response to Radiation. PLoS ONE 2016, 11, e0160891. [Google Scholar] [CrossRef] [Green Version]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardão, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, R.; Costa, L.; Rodrigues, I.; Meireles, C.; Soares, R.; Tamagnini, P.; Mota, R. Biocompatibility of the Biopolymer Cyanoflan for Applications in Skin Wound Healing. Mar. Drugs 2021, 19, 147. [Google Scholar] [CrossRef]
- Costa, R.; Azevedo, D.; Barata, P.; Soares, R.; Guido, L.; Carvalho, D. Antiangiogenic and Antioxidant In Vitro Properties of Hydroethanolic Extract from açaí (Euterpe oleracea) Dietary Powder Supplement. Molecules 2021, 26, 2011. [Google Scholar] [CrossRef]
- Likitamporn, S.; Magaraphan, R. Mechanical and Thermal Properties of Sericin/PVA/Bentonite Scaffold: Comparison between Uncrosslinked and Crosslinked. Macromol. Symp. 2014, 337, 102–108. [Google Scholar] [CrossRef]
- Takechi, T.; Wada, R.; Fukuda, T.; Harada, K.; Takamura, H. Antioxidant activities of two sericin proteins extracted from cocoon of silkworm (Bombyx mori) measured by DPPH, chemiluminescence, ORAC and ESR methods. Biomed. Rep. 2014, 2, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Ustuner, O.; Anlas, C.; Bakirel, T.; Ustun-Alkan, F.; Sigirci, B.D.; Ak, S.; Akpulat, H.A.; Donmez, C.; Koca-Caliskan, U. In Vitro Evaluation of Antioxidant, Anti-Inflammatory, Antimicrobial and Wound Healing Potential of Thymus Sipyleus Boiss. Subsp. Rosulans (Borbas) Jalas. Molecules 2019, 24, 3353. [Google Scholar] [CrossRef] [Green Version]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doakhan, S.; Montazer, M.; Rashidi, A.; Moniri, R.; Moghadam, M. Influence of sericin/TiO2 nanocomposite on cotton fabric: Part 1. Enhanced antibacterial effect. Carbohydr. Polym. 2013, 94, 737–748. [Google Scholar] [CrossRef]
- Rajendran, R.; Balakumar, C.; Sivakumar, R.; Amruta, T.; Devaki, N. Extraction and application of natural silk protein sericin from Bombyx mori as antimicrobial finish for cotton fabrics. J. Text. Inst. 2012, 103, 458–462. [Google Scholar] [CrossRef]
- Manesa, K.C.; Kebede, T.G.; Dube, S.; Nindi, M.M. Profiling of Silk Sericin from Cocoons of Three Southern African Wild Silk Moths with a Focus on Their Antimicrobial and Antioxidant Properties. Materials 2020, 13, 5706. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Liu, Y.; Zhang, Q.; Liang, C.; Qin, H.; Liu, P.; Wang, K.; Zhang, X.; Chen, L.; Wei, Y. Shape Changes and Interaction Mechanism of Escherichia coli Cells Treated with Sericin and Use of a Sericin-Based Hydrogel for Wound Healing. Appl. Environ. Microbiol. 2016, 82, 4663–4672. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Rajkhowa, R.; Afrin, T.; Tsuzuki, T.; Wang, X. Facts and myths of antibacterial properties of silk. Biopolymers 2013, 101, 237–245. [Google Scholar] [CrossRef]
- Finnegan, S.; Percival, S. Clinical and Antibiofilm Efficacy of Antimicrobial Hydrogels. Adv. Wound Care 2015, 4, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Annese, T.; Tamma, R. The use of the chick embryo CAM assay in the study of angiogenic activiy of biomaterials. Microvasc. Res. 2020, 131, 104026. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doillon, C.J.; Dunn, M.G.; Bender, E.; Silver, F.H. Collagen Fiber Formation in Repair Tissue: Development of Strength and Toughness. Collagen Relat. Res. 1985, 5, 481–492. [Google Scholar] [CrossRef]
- Moura, L.; Dias, A.; Leal, E.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Chitosan-based dressings loaded with neurotensin—An efficient strategy to improve early diabetic wound healing. Acta Biomater. 2014, 10, 843–857. [Google Scholar] [CrossRef] [Green Version]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista-Silva, S.; Bernardes, B.G.; Borges, S.; Rodrigues, I.; Fernandes, R.; Gomes-Guerreiro, S.; Pinto, M.T.; Pintado, M.; Soares, R.; Costa, R.; et al. Exploring Silk Sericin for Diabetic Wounds: An In Situ-Forming Hydrogel to Protect against Oxidative Stress and Improve Tissue Healing and Regeneration. Biomolecules 2022, 12, 801. https://doi.org/10.3390/biom12060801
Baptista-Silva S, Bernardes BG, Borges S, Rodrigues I, Fernandes R, Gomes-Guerreiro S, Pinto MT, Pintado M, Soares R, Costa R, et al. Exploring Silk Sericin for Diabetic Wounds: An In Situ-Forming Hydrogel to Protect against Oxidative Stress and Improve Tissue Healing and Regeneration. Biomolecules. 2022; 12(6):801. https://doi.org/10.3390/biom12060801
Chicago/Turabian StyleBaptista-Silva, Sara, Beatriz G. Bernardes, Sandra Borges, Ilda Rodrigues, Rui Fernandes, Susana Gomes-Guerreiro, Marta Teixeira Pinto, Manuela Pintado, Raquel Soares, Raquel Costa, and et al. 2022. "Exploring Silk Sericin for Diabetic Wounds: An In Situ-Forming Hydrogel to Protect against Oxidative Stress and Improve Tissue Healing and Regeneration" Biomolecules 12, no. 6: 801. https://doi.org/10.3390/biom12060801
APA StyleBaptista-Silva, S., Bernardes, B. G., Borges, S., Rodrigues, I., Fernandes, R., Gomes-Guerreiro, S., Pinto, M. T., Pintado, M., Soares, R., Costa, R., & Oliveira, A. L. (2022). Exploring Silk Sericin for Diabetic Wounds: An In Situ-Forming Hydrogel to Protect against Oxidative Stress and Improve Tissue Healing and Regeneration. Biomolecules, 12(6), 801. https://doi.org/10.3390/biom12060801