Effect of Locally Delivered Minocycline on the Profile of Subgingival Bacterial Genera in Patients with Periodontitis: A Prospective Pilot Study
Abstract
1. Introduction
2. Materials and Methods
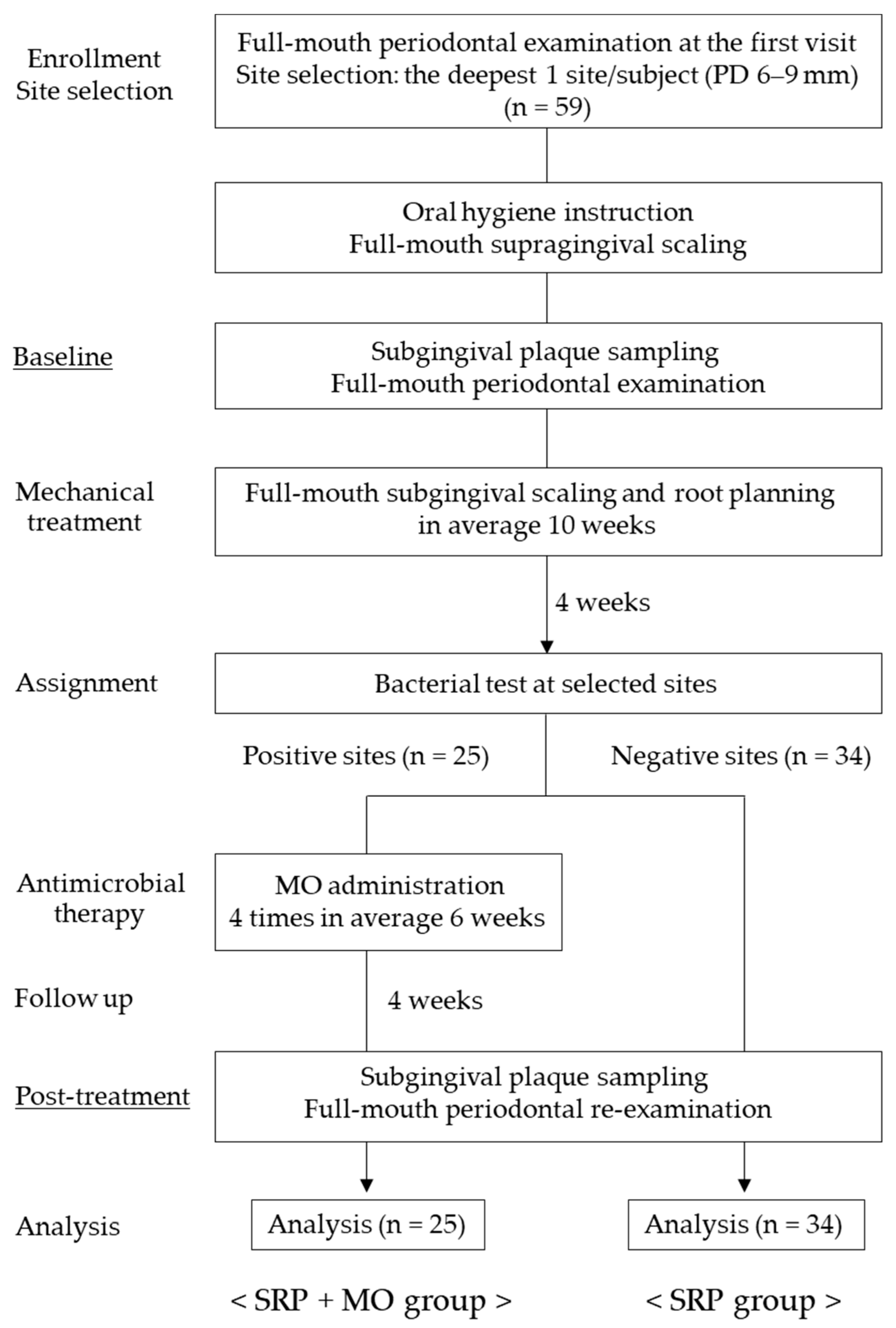
2.1. Study Population
2.2. Clinical Protocol
2.3. Clinical Assessment
2.4. Sample Collection
2.5. T-RFLP Analysis
2.6. Quantification of Periodontal Bacteria
2.7. Statistical Analysis
3. Results
3.1. Periodontal Parameters
3.2. Increase and Decrease in Subgingival Bacterial Genera
3.3. Subgingival Bacterial Genera
3.4. Periodontopathic Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Dyke, T.E. Pro-resolving mediators in the regulation of periodontal disease. Mol. Asp. Med. 2017, 58, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Cuttis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal diseases. Periodontol. 2000 2020, 83, 14–25. [Google Scholar]
- Kroes, I.; Lepp, P.W.; Relman, D.A. Bacterial diversity within the human subgingival crevice. Proc. Natl. Acad. Sci. USA 1999, 96, 14547–14552. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.P.; Haffajee, A.D.; Socransky, S.S. Microbiological goals of periodontal therapy. Periodontol 2000 2006, 42, 180–218. [Google Scholar] [CrossRef]
- Sanz, I.; Alonso, B.; Carasol, M.; Herrera, D.; Sanz, M. Nonsurgical treatment of periodontitis. J. Evid. Based Dent. Pract. 2012, 12 (Suppl. S3), 76–86. [Google Scholar] [CrossRef]
- Jepsen, S.; Deschner, J.; Braun, A.; Schwarz, F.; Eberhard, J. Calculus removal and the prevention of its formation. Periodontol 2000 2011, 55, 167–188. [Google Scholar] [CrossRef]
- Walker, C.; Karpinia, K. Rationale for use of antibiotics in periodontics. J. Periodontol. 2002, 73, 1188–1196. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000 2016, 71, 82–112. [Google Scholar] [CrossRef]
- Martu, M.-A.; Surlin, P.; Lazar, L.; Maftei, G.A.; Luchian, I.; Gheorghe, D.-N.; Rezus, E.; Toma, V.; Foia, L.-G. Evaluation of oxidative stress before and after using laser and photoactivation therapy as adjuvant of non-surgical periodontal treatment in patients with rheumatoid arthritis. Antioxidants 2021, 10, 226. [Google Scholar] [CrossRef]
- Nicolae, V.; Chiscop, I.; Cioranu, V.S.I.; Martu, M.A.; Luchian, A.I.; Martu, S.; Solomon, S.M. The use of photoactivated blue-o toluidine for periimplantitis treatment in patients with periodontal disease. Rev. Chim. (Buchar.) 2015, 66, 2121–2123. [Google Scholar]
- Santos, R.S.; Macedo, R.F.; Souza, E.A.; Soares, R.S.C.; Feitosa, D.S.; Sarmento, C.F.M. The use of systemic antibiotics in the treatment of refractory periodontitis: A systematic review. J. Am. Dent. Assoc. 2016, 147, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Pallasch, T.J. Antibiotic resistance. Dent. Clin. N. Am. 2003, 47, 623–639. [Google Scholar] [CrossRef]
- O’Connor, B.C.; Newman, H.N.; Wilson, M. Susceptibility and resistance of plaque bacteria to minocycline. J. Periodontol. 1990, 61, 228–233. [Google Scholar] [CrossRef]
- Golub, L.M.; Wolff, M.; Lee, H.M.; McNamara, T.F.; Ramamurthy, N.S.; Zambon, J.; Ciancio, S. Further evidence that tetracyclines inhibit collagenase activity in human crevicular fluid and from other mammalian sources. J. Periodont. Res. 1985, 20, 12–23. [Google Scholar] [CrossRef]
- Hokari, T.; Morozumi, T.; Komatsu, Y.; Shimizu, T.; Yoshino, T.; Tanaka, M.; Tanaka, Y.; Nohno, K.; Kubota, T.; Yoshie, H. Effects of antimicrobial photodynamic therapy and local administration of minocycline on clinical, microbiological, and inflammatory markers of periodontal pockets: A pilot study. Int. J. Dent. 2018, 2018, 1748584. [Google Scholar] [CrossRef]
- Miyazawa, H.; Nakajima, T.; Horimizu, M.; Okuda, K.; Sugita, N.; Yamazaki, K.; Li, L.; Hayashi-Okada, Y.; Arita, T.; Nishimoto, N.; et al. Impact of Local Drug Delivery of Minocycline on the Subgingival Microbiota during Supportive Periodontal Therapy: A Randomized Controlled Pilot Study. Dent. J. 2020, 8, 123. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 508–524.e5. [Google Scholar] [CrossRef]
- Cortelli, J.R.; Aquino, D.R.; Cortelli, S.C.; Carvalho-Filho, J.; Roman-Torres, C.V.G.; Costa, F.O. A double-blind randomized clinical trial of subgingival minocycline for chronic periodontitis. J. Oral. Sci. 2008, 50, 259–265. [Google Scholar] [CrossRef][Green Version]
- Soeroso, Y.; Akase, T.; Sunarto, H.; Kemal, Y.; Salim, R.; Octavia, M.; Viandita, A.; Setiawan, J.; Bachtiar, B.M. The risk reduction of recurrent periodontal pathogens of local application minocycline HCl 2% gel, used as an adjunct to scaling and root planing for chronic periodontitis treatment. Ther. Clin. Risk Manag. 2017, 13, 307–314. [Google Scholar] [CrossRef]
- Jünemann, S.; Prior, K.; Szczepanowski, R.; Harks, I.; Ehmke, B.; Goesmann, A.; Stoye, J.; Harmsen, D. Bacterial community shift in treated periodontitis patients revealed by ion torrent 16S rRNA gene amplicon sequencing. PLoS ONE 2012, 7, e41606. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Ranney, R.; Lamster, I.; Charles, A.; Chung, C.P.; Flemmig, T.; Kinane, D.; Listgarten, M.; Loe, H.; Schoor, R.; et al. 1999 International workshop for a classification of periodontal diseases and conditions. Consensus Report: Chronic periodontitis. Ann. Periodontol. 1999, 4, 38. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Takayama, S.; Saito, A.; Inoue, E.; Nakayama, Y.; Ogata, Y.; Shirakawa, S.; Nagano, T.; Gomi, K.; Morozumi, T.; et al. Evaluation of a novel immunochromatographic device for rapid and accurate clinical detection of Porphyromonas gingivalis in subgingival plaque. J. Microbiol. Methods 2015, 117, 4–10. [Google Scholar] [CrossRef]
- Nakayama, Y.; Ogata, Y.; Hiromatsu, Y.; Imamura, K.; Suzuki, E.; Saito, A.; Shirakawa, S.; Nagano, T.; Gomi, K.; Morozumi, T.; et al. Clinical usefulness of novel immunochromatographic detection device for Porphyromonas gingivalis in evaluating effects of scaling and root planing and local antimicrobial therapy. J. Periodontol. 2016, 87, 1238–1247. [Google Scholar] [CrossRef]
- Lu, H.K.; Chei, C.J. Efficacy of subgingivally applied minocycline in the treatment of chronic periodontitis. J. Periodont. Res. 2005, 40, 20–27. [Google Scholar] [CrossRef]
- Liu, W.-T.; Marsh, T.L.; Cheng, H.; Forney, L.J. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol. 1997, 63, 4516–4522. [Google Scholar] [CrossRef]
- Marsh, T.L. Terminal restriction fragment length polymorphism (T-RFLP): An emerging method for characterizing diversity among homologous populations of amplification products. Curr. Opin. Microbiol. 1999, 2, 323–327. [Google Scholar] [CrossRef]
- Nakano, Y.; Takeshita, T.; Kamio, N.; Shiota, S.; Shibata, Y.; Yasui, M.; Yamashita, Y. Development and application of a T-RFLP data analysis method using correlation coefficient matrices. J. Microbiol. Method 2008, 75, 501–505. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Sakamoto, M.; Takeuchi, Y.; Umeda, M.; Ishikawa, I.; Benno, Y. Application of terminal RFLP analysis to characterize oral bacterial flora in saliva of healthy subjects and patients with periodontitis. J. Med. Microbiol. 2003, 52, 79–89. [Google Scholar] [CrossRef]
- Sakamoto, M.; Huang, Y.; Ohnishi, M.; Umeda, M.; Ishikawa, I.; Benno, Y. Changes in oral microbial profiles after periodontal treatment as determined by molecular analysis of 16S rRNA genes. J. Med. Microbiol. 2004, 53, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Nakano, Y.; Yamashita, Y. Improved accuracy in terminal restriction fragment length polymorphism phylogenetic analysis using a novel internal size standard definition. Oral Microbiol. Immunol. 2007, 22, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Ushiroda, C.; Ohnogi, H.; Kudo, Y.; Yasui, M.; Inui, S.; et al. Protective effect of agaro-oligosaccharides on gut dysbiosis and colon tumorigenesis in high-fat diet-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G367–G375. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, K.; Yamaguchi, T.; Kawamura, K.; Shimizu, H.; Egashira, T.; Minabe, M.; Yoshino, T.; Oguhi, H. Rapid quantification of periodontitis-related bacteria using a novel modification of Invader PLUS technologies. Microbiol. Res. 2010, 165, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Takeuchi, H.; Shimizu, H.; Tadokoro, K.; Tanaka, K.; Kawamura, K.; Yamaguchi, T.; Egashira, T.; Nomura, Y.; Hanada, M. Quantification of periodontopathic bacteria in saliva using the invader assay. Jpn. J. Infect. Dis. 2012, 65, 415–423. [Google Scholar]
- Morozumi, T.; Nakagawa, T.; Nomura, Y.; Sugaya, T.; Kawanami, M.; Suzuki, F.; Takahashi, K.; Abe, Y.; Sato, S.; Makino-Oi, A.; et al. Salivary pathogen and serum antibody to assess the progression of chronic periodontitis: A 24-mo prospective multicenter cohort study. J. Periodont. Res. 2016, 51, 768–778. [Google Scholar] [CrossRef]
- Kakuta, E.; Nomura, Y.; Morozumi, T.; Nakagawa, T.; Nakamura, T.; Noguchi, K.; Yoshimura, A.; Hara, Y.; Fujise, O.; Nishimura, F.; et al. Assessing the progression of chronic periodontitis using subgingival pathogen levels: A 24-month prospective multicenter cohort study. BMC Oral Health 2017, 17, 46. [Google Scholar] [CrossRef][Green Version]
- Periasamy, S.; Kolenbrander, P.E. Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of enamel. J. Bacteriol. 2010, 192, 2965–2972. [Google Scholar] [CrossRef]
- Mashima, I.; Nakazawa, F. Interaction between Streptococcus spp. and Veillonella tobetsuensis in the early stage of oral iofilm formation. J. Bacteriol. 2015, 197, 2104–2111. [Google Scholar] [CrossRef]
- Goodson, J.M.; Gunsolley, J.C.; Grossi, S.G.; Bland, P.S.; Otomo-Corgel, J.; Doherty, F.; Comiskey, J. Minocycline HCl microspheres reduce red-complex bacteria in periodontal disease therapy. J. Periodontol. 2007, 78, 1568–1579. [Google Scholar] [CrossRef]
- Teles, F.R.F.; Lynch, M.C.; Patel, M.; Torresyap, G.; Martin, L. Bacterial resistance to minocyline after adjunctive minocycline microsheres during periodontal maintenance: A randomized clinical trial. J. Periodontol. 2021, 92, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Nyman, S.; Westfelt, E.; Socransky, S.S.; Haffajee, A. Critical probing depths in periodontal therapy. Compend. Contin. Educ. Dent. 1982, 3, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Wennström, J.L.; Heijl, L.; Lindhe, J. Periodontal surgery: Access therapy. In Clinical Periodontology and Implant Dentistry, 4th ed.; Lindhe, J., Karring, T., Lang, N.P., Eds.; Blackwell Munksgaard: Oxford, UK, 2003; pp. 519–560. [Google Scholar]
- Kamodyová, N.; Minárik, G.; Hodosy, J.; Celec, P. Single consumption of Bryndza cheese temporarily affects oral microbiota and salivary markers of oxidative stress. Curr. Microbiol. 2014, 69, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Martu, M.A.; Covasa, M. Clindamycin as an alternative option in optimizing periodontal therapy. Antibiotics 2021, 10, 814. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in non-surgical periodontal therapy: Clinical and microbiological aspects in a 6-month follow-up domiciliary protocol for oral hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home oral care of periodontal patients using antimicrobial gel with postbiotics, lactoferrin, and aloe barbadensis leaf juice powder vs. conventional chlorhexidine gel: A split-mouth randomized clinical trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]
| SRP + MO Group | SRP Group | Inter-Comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Baseline (n = 25) | Post-Treatment (n = 25) | Intra-Comparisons (p-Value) | Baseline (n = 34) | Post-Treatment (n = 34) | Intra-Comparisons (p-Value) | Baseline (p-Value) | Post-Treatment (p-Value) |
| PD-sampled sites (mm) | 6.96 ± 0.93 | 5.04 ± 2.01 | <0.0001 * | 6.79 ± 0.88 | 4.15 ± 1.21 | <0.0001 * | 0.492 | 0.052 |
| CAL-sampled sites (mm) | 8.40 ± 2.25 | 6.48 ± 2.63 | 0.001 * | 7.88 ± 1.89 | 5.74 ± 2.18 | <0.0001 * | 0.377 | 0.227 |
| BOP-sampled sites * | 0.72 ± 0.46 | 0.36 ± 0.49 | 0.007 * | 0.88 ± 0.33 | 0.26 ± 0.45 | <0.0001 * | 0.117 | 0.436 |
| PlI-sampled sites | 0.72 ± 0.68 | 0.76 ± 0.72 | 0.763 | 0.74 ± 0.75 | 0.62 ± 0.78 | 0.462 | 0.987 | 0.355 |
| GI-sampled sites | 1.72 ± 0.46 | 1.00 ± 0.91 | 0.002 * | 1.76 ± 0.61 | 0.85 ± 0.86 | <0.0001 * | 0.314 | 0.538 |
| SRP + MO Group (n = 25) | SRP Group (n = 34) | ||||
|---|---|---|---|---|---|
| Increase | Decrease | Increase | Decrease | p-Value | |
| Streptococcus | 19 (76%) | 6 (24%) | 22 (65%) | 12 (35%) | 0.352 |
| Eubacterium, Parvimonas | 10 (40%) | 15 (60%) | 14 (41%) | 20 (59%) | 0.928 |
| Eubacterium, Filifactor | 4 (16%) | 21 (84%) | 8 (24%) | 25 (74%) | 0.478 |
| Eubacterium | 11 (44%) | 12 (48%) | 18 (53%) | 15 (44%) | 0.621 |
| Veillonella | 10 (40%) | 13 (52%) | 24 (71%) | 10 (29%) | 0.033 * |
| Fusobacterium | 15 (60%) | 10 (40%) | 12 (35%) | 21 (62%) | 0.074 |
| Porphyromonas, Prevotella | 12 (48%) | 13 (52%) | 15 (44%) | 19 (56%) | 0.767 |
| Neisseria | 18 (72%) | 7 (28%) | 13 (38%) | 19 (56%) | 0.018 * |
| Unknown | 11 (44%) | 14 (56%) | 22 (65%) | 12 (35%) | 0.113 |
| SRP + MO Group (n = 25) | SRP Group (n = 34) | Inter-Comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Baseline | Post-Treatment | Intra-Comparisons (p-Value) | Baseline | Post-Treatment | Intra-Comparisons (p-Value) | Baseline (p-Value) | Post-Treatment (p-Value) |
| Total bacteria (log10) | 7.45 ± 0.50 | 6.74 ± 0.51 | <0.0001 * | 7.24 ± 0.57 | 6.83 ± 0.58 | <0.0001 * | 0.163 | 0.476 |
| Streptococcus (log10) | 5.54 ± 1.78 | 5.67 ± 0.58 | 0.253 | 5.73 ± 1.19 | 5.55 ± 1.21 | 0.407 | 0.753 | 0.514 |
| Eubacterium, Parvimonas (log10) | 5.93 ± 1.36 | 4.58 ± 2.14 | 0.001 * | 5.91 ± 0.82 | 5.34 ± 1.18 | 0.011 * | 0.273 | 0.338 |
| Eubacterium, Filifactor (log10) | 6.28 ± 1.42 | 4.15 ± 2.49 | 0.0001 * | 5.32 ± 2.10 | 4.12 ± 2.24 | <0.001 * | 0.006 * | 0.600 |
| Eubacterium (log10) | 4.46 ± 2.39 | 3.68 ± 2.39 | 0.042 * | 4.65 ± 2.05 | 4.71 ± 1.56 | 0.367 | 0.706 | 0.159 |
| Veillonella (log10) | 4.85 ± 1.95 | 4.01 ± 2.15 | 0.019 * | 4.31 ± 2.51 | 5.23 ± 1.19 | 0.11 | 0.741 | 0.01 * |
| Fusobacterium (log10) | 6.18 ± 0.93 | 4.92 ± 1.96 | 0.006 * | 5.65 ± 1.62 | 5.19 ± 1.82 | 0.031 * | 0.223 | 0.457 |
| Porphyromonas, Prevotella (log10) | 6.52 ± 0.71 | 5.77 ± 0.61 | 0.001 * | 6.38 ± 0.62 | 5.87 ± 0.64 | <0.0001 * | 0.361 | 0.565 |
| Neisseria (log10) | 4.23 ± 2.50 | 4.76 ± 1.60 | 0.914 | 4.78 ± 2.32 | 4.24 ± 2.27 | 0.054 | 0.118 | 0.951 |
| Unknown (log10) | 7.07 ± 0.48 | 6.32 ± 0.55 | <0.0001 * | 6.84 ± 0.63 | 6.45 ± 0.59 | p = 0.001 * | 0.145 | 0.425 |
| SRP + MO Group (n = 25) | SRP Group (n = 34) | Inter-Comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Baseline | Post-Treatment | Intra-Comparisons (p-Value) | Baseline | Post-Treatment | Intra-Comparisons (p-Value) | Baseline (p-Value) | Post-Treatment (p-Value) |
| P. gingivalis (log10) | 5.41 ± 1.29 | 1.64 ± 2.30 | <0.0001 * | 3.40 ± 2.65 | 0.48 ± 1.34 | <0.0001 * | 0.001 * | 0.022 * |
| A. actinomy- cetemcomitans (log10) | 0.33 ± 1.17 | 0.18 ± 0.88 | 0.593 | 0.49 ± 1.37 | 0.38 ± 1.24 | 0.686 | 0.64 | 0.481 |
| P. intermedia (log10) | 3.45 ± 2.70 | 1.30 ± 2.14 | 0.001 * | 2.39 ± 2.63 | 1.33 ± 2.13 | 0.016 * | 0.097 | 1.0 |
| T. forsythia (log10) | 5.59 ± 1.31 | 3.98 ± 1.65 | 0.001 * | 5.26 ± 1.21 | 3.31 ± 2.24 | <0.0001 * | 0.107 | 0.644 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozumi, T.; Nakayama, Y.; Shirakawa, S.; Imamura, K.; Nohno, K.; Nagano, T.; Miyazawa, H.; Hokari, T.; Takuma, R.; Sugihara, S.; et al. Effect of Locally Delivered Minocycline on the Profile of Subgingival Bacterial Genera in Patients with Periodontitis: A Prospective Pilot Study. Biomolecules 2022, 12, 719. https://doi.org/10.3390/biom12050719
Morozumi T, Nakayama Y, Shirakawa S, Imamura K, Nohno K, Nagano T, Miyazawa H, Hokari T, Takuma R, Sugihara S, et al. Effect of Locally Delivered Minocycline on the Profile of Subgingival Bacterial Genera in Patients with Periodontitis: A Prospective Pilot Study. Biomolecules. 2022; 12(5):719. https://doi.org/10.3390/biom12050719
Chicago/Turabian StyleMorozumi, Toshiya, Yohei Nakayama, Satoshi Shirakawa, Kentaro Imamura, Kaname Nohno, Takatoshi Nagano, Haruna Miyazawa, Takahiro Hokari, Ryo Takuma, Shuntaro Sugihara, and et al. 2022. "Effect of Locally Delivered Minocycline on the Profile of Subgingival Bacterial Genera in Patients with Periodontitis: A Prospective Pilot Study" Biomolecules 12, no. 5: 719. https://doi.org/10.3390/biom12050719
APA StyleMorozumi, T., Nakayama, Y., Shirakawa, S., Imamura, K., Nohno, K., Nagano, T., Miyazawa, H., Hokari, T., Takuma, R., Sugihara, S., Gomi, K., Saito, A., Ogata, Y., & Komaki, M. (2022). Effect of Locally Delivered Minocycline on the Profile of Subgingival Bacterial Genera in Patients with Periodontitis: A Prospective Pilot Study. Biomolecules, 12(5), 719. https://doi.org/10.3390/biom12050719












