Biomarker Characterization and Prediction of Virulence and Antibiotic Resistance from Helicobacter pylori Next Generation Sequencing Data
Abstract
1. Introduction
2. Virulence Factors
2.1. Cytotoxin-Associated Gene Pathogenicity Island (cagPAI)
2.2. Vacuolating Cytotoxin A

2.3. Integrative and Conjugative Elements (ICEs)
2.4. Other Virulence factors
2.4.1. Urease
2.4.2. Flagella Chemotaxis System
2.4.3. Outer Membrane Proteins
AlpA/AlpB
BabA
HomB
HopQ
OipA
SabA
3. Genes Involved in Antibiotic Resistance
3.1. Clarithromycin
3.2. Amoxicillin
3.3. Metronidazole
3.4. Levofloxacin
3.5. Tetracyclines
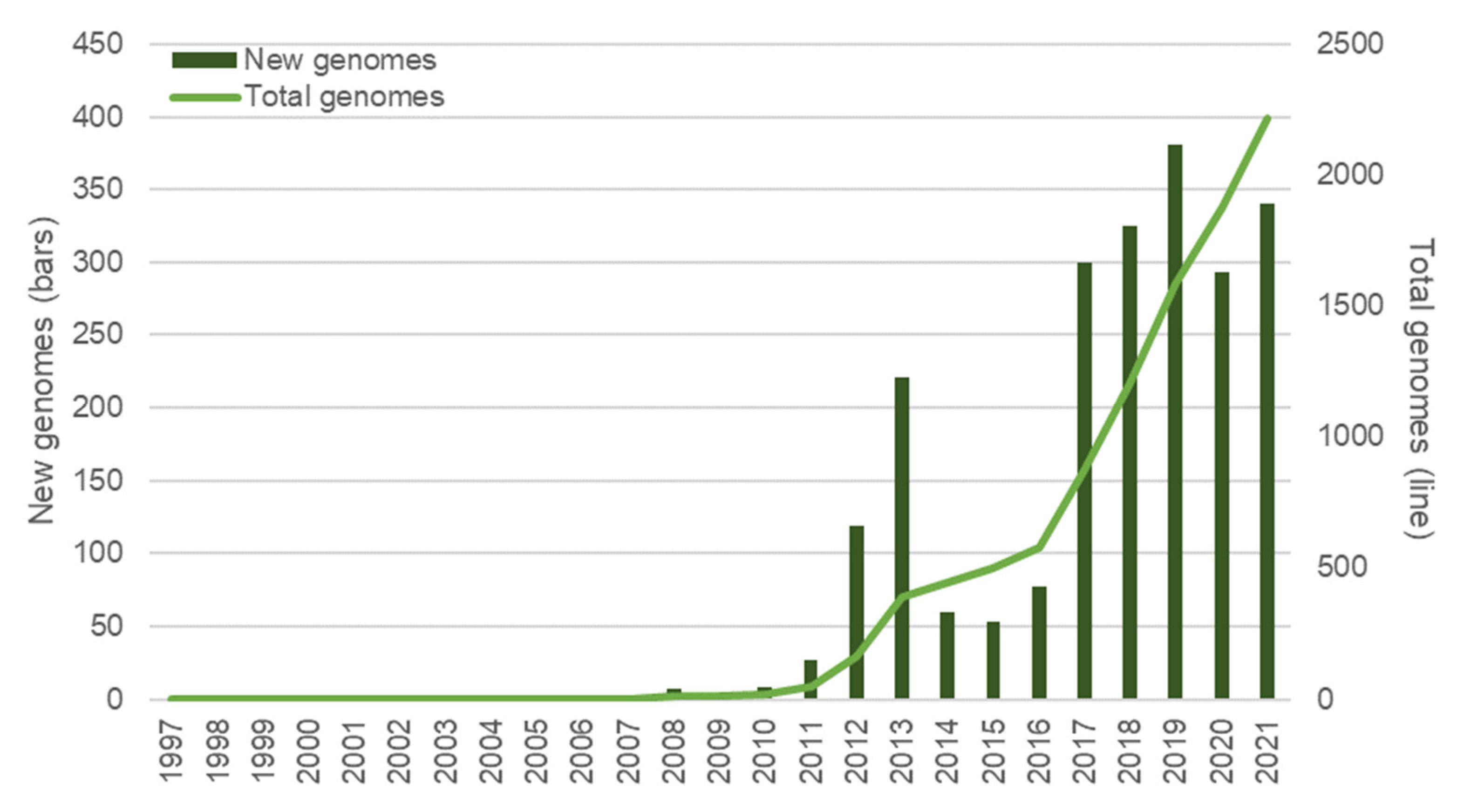
4. Current Technologies Used for H. pylori Marker Detection
5. New Approaches to H. pylori Biomarker Detection
5.1. Sequencing Technologies
5.2. Virulence Potential Evaluation and Antibiotic-Resistance Profiling
5.3. Current Tools as Possible Limiting Factors
5.4. New Applications: The Example of Methylome Analysis
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 16, 1311–1315. [Google Scholar] [CrossRef]
- Polk, D.B.; Peek, R.M. Helicobacter pylori: Gastric Cancer and Beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Oleastro, M.; Rocha, R.; Vale, F.F. Population Genetic Structure of Helicobacter pylori Strains from Portuguese-Speaking Countries. Helicobacter 2017, 22, e12382. [Google Scholar] [CrossRef] [PubMed]
- Mégraud, F.; Lehours, P.; Vale, F.F. The History of Helicobacter pylori: From Phylogeography to Paleomicrobiology. Clin. Microbiol. Infect. 2016, 22, 922–927. [Google Scholar] [CrossRef]
- Dastmalchi, N.; Safaralizadeh, R.; Banan Khojasteh, S.M. The Correlation between MicroRNAs and Helicobacter pylori in Gastric Cancer. Pathog. Dis. 2019, 77, ftz039. [Google Scholar] [CrossRef]
- Ki, O.M.; Eun, J.S.; Hi, J.K.; Eui, J.L.; Won, I.K.; Chang, S.K.; Kim, K.M. Methylation of P16INK4A and P57KIP2 Are Involved in the Development and Progression of Gastric MALT Lymphomas. Mod. Pathol. 2006, 19, 141–148. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer; World Health Organization. Schistosomes, Liver Flukes and Helicobacter Pylori; International Agency for Research on Cancer (IARC): Lyon, France, 1994; ISBN 9283212614.
- Muhammad, J.; Zaidi, S.; Saeed, S.; Ishaq, M. Current Status of Helicobacter pylori Association with Haematological Andcardiovascular Diseases: A Mini Review. J. Pak. Med. Assoc. 2017, 67, 907–911. [Google Scholar]
- Franceschi, F.; Covino, M.; Roubaud Baudron, C. Review: Helicobacter pylori and Extragastric Diseases. Helicobacter 2019, 24, e12636. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Achtman, M.; Azuma, T.; Berg, D.E.; Ito, Y.; Morelli, G.; Pan, Z.-J.; Suerbaum, S.; Thompson, S.A.; van der Ende, A.; van Doorn, L.-J. Recombination and Clonal Groupings within Helicobacter pylori from Different Geographical Regions. Mol. Microbiol. 1999, 32, 459–470. [Google Scholar] [CrossRef]
- Björkholm, B.M.; Oh, J.D.; Falk, P.G.; Engstrand, L.G.; Gordon, J.I. Genomics and Proteomics Converge on Helicobacter pylori. Curr. Opin. Microbiol. 2001, 4, 237–245. [Google Scholar] [CrossRef]
- Morelli, G.; Didelot, X.; Kusecek, B.; Schwarz, S.; Bahlawane, C.; Falush, D.; Suerbaum, S.; Achtman, M. Microevolution of Helicobacter pylori during Prolonged Infection of Single Hosts and within Families. PLoS Genet. 2010, 6, e1001036. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.K.; Furuta, Y.; Yahara, K.; Fukuyo, M.; Shiwa, Y.; Nishiumi, S.; Yoshida, M.; Azuma, T.; Yoshikawa, H.; Kobayashi, I. Population Evolution of Helicobacter pylori through Diversification in DNA Methylation and Interstrain Sequence Homogenization. Mol. Biol. Evol. 2016, 33, 2848–2859. [Google Scholar] [CrossRef] [PubMed]
- Yahara, K.; Lehours, P.; Vale, F.F. Analysis of Genetic Recombination and the Pan-Genome of a Highly Recombinogenic Bacteriophage Species. Microb. Genom. 2019, 5, e000282. [Google Scholar] [CrossRef] [PubMed]
- Waskito, L.A.; Salama, N.R.; Yamaoka, Y. Pathogenesis of Helicobacter pylori Infection. Helicobacter 2018, 23, e12516. [Google Scholar] [CrossRef] [PubMed]
- Šterbenc, A.; Jarc, E.; Poljak, M.; Homan, M. Helicobacter pylori Virulence Genes. World J. Gastroenterol. 2019, 25, 4870–4884. [Google Scholar] [CrossRef]
- Tohidpour, A. CagA-Mediated Pathogenesis of Helicobacter pylori. Microb. Pathog. 2016, 93, 44–55. [Google Scholar] [CrossRef]
- Chauhan, N.; Tay, A.C.Y.; Marshall, B.J.; Jain, U. Helicobacter pylori VacA, a Distinct Toxin Exerts Diverse Functionalities in Numerous Cells: An Overview. Helicobacter 2019, 24, e12544. [Google Scholar] [CrossRef]
- Mucito-Varela, E.; Castillo-Rojas, G.; Calva, J.J.; López-Vidal, Y. Integrative and Conjugative Elements of Helicobacter pylori Are Hypothetical Virulence Factors Associated with Gastric Cancer. Front. Cell. Infect. Microbiol. 2020, 10, 525335. [Google Scholar] [CrossRef]
- Chang, W.-L.; Yeh, Y.-C.; Sheu, B.-S. The Impacts of H. pylori Virulence Factors on the Development of Gastroduodenal Diseases. J. Biomed. Sci. 2018, 25, 68. [Google Scholar] [CrossRef]
- Olbermann, P.; Josenhans, C.; Moodley, Y.; Uhr, M.; Stamer, C.; Vauterin, M.; Suerbaum, S.; Achtman, M.; Linz, B. A Global Overview of the Genetic and Functional Diversity in the Helicobacter pylori Cag Pathogenicity Island. PLoS Genet. 2010, 6, e1001069. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Tegtmeyer, N.; Fischer, W. Composition, Structure and Function of the Helicobacter pylori Cag Pathogenicity Island Encoded Type IV Secretion System. Future Microbiol. 2015, 10, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and Its Pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Blaser, M.J. The Role of CagA in the Gastric Biology of Helicobacter pylori. Cancer Res. 2016, 76, 4028–4031. [Google Scholar] [CrossRef]
- Phuc, B.H.; Tuan, V.P.; Dung, H.D.Q.; Binh, T.T.; Tung, P.H.; Tri, T.D.; Thuan, N.P.M.; van Khien, V.; Trang, T.T.H.; Akada, J.; et al. Helicobacter pylori Type 4 Secretion Systems as Gastroduodenal Disease Markers. Sci. Rep. 2021, 11, 4584. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Shaffer, C.L.; Rettberg, L.A.; Ghosal, D.; Jensen, G.J. In Vivo Structures of the Helicobacter pylori Cag Type IV Secretion System. Cell Rep. 2018, 23, 673–681. [Google Scholar] [CrossRef]
- Hu, B.; Khara, P.; Song, L.; Soe Lin, A.; Frick-Cheng, A.E.; Lorena Harvey, M.; Cover, T.L.; Christie, P.J.; Scott Hultgren, E.J. In situ Molecular Architecture of the Helicobacter pylori cag Type IV Secretion System. mBio 2019, 10, e00849-19. [Google Scholar] [CrossRef]
- Frick-Cheng, A.E.; Pyburn, T.M.; Voss, B.J.; McDonald, W.H.; Ohi, M.D.; Cover, T.L. Molecular and Structural Analysis of the Helicobacter pylori cag Type IV Secretion System Core Complex. mBio 2016, 7, e02001-15. [Google Scholar] [CrossRef]
- Hatakeyama, M. Helicobacter pylori CagA and Gastric Cancer: A Paradigm for Hit-and-Run Carcinogenesis. Cell Host Microbe 2014, 15, 306–316. [Google Scholar] [CrossRef]
- Cover, T.L.; Lacy, D.B.; Ohi, M.D. The Helicobacter pylori cag Type IV Secretion System. Trends Microbiol. 2020, 28, 682–695. [Google Scholar] [CrossRef]
- Schindele, F.; Weiss, E.; Haas, R.; Fischer, W. Quantitative Analysis of CagA Type IV Secretion by Helicobacter pylori Reveals Substrate Recognition and Translocation Requirements. Mol. Microbiol. 2016, 100, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeyer, N.; Neddermann, M.; Asche, C.I.; Backert, S. Subversion of Host Kinases: A Key Network in Cellular Signaling Hijacked by Helicobacter pylori CagA. Mol. Microbiol. 2017, 105, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Oncogenic Mechanisms of the Helicobacter pylori CagA Protein. Nat. Rev. Cancer 2004, 4, 688–694. [Google Scholar] [CrossRef]
- Xia, Y.; Yamaoka, Y.; Zhu, Q.; Matha, I.; Gao, X. A Comprehensive Sequence and Disease Correlation Analyses for the C-Terminal Region of CagA Protein of Helicobacter pylori. PLoS ONE 2009, 4, e7736. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, N.; Yuasa, H.; Tanaka, S.; Sawa, H.; Miura, M.; Matsui, A.; Higashi, H.; Musashi, M.; Iwabuchi, K.; Suzuki, M.; et al. Transgenic Expression of Helicobacter pylori CagA Induces Gastrointestinal and Hematopoietic Neoplasms in Mouse. Proc. Natl. Acad. Sci. USA 2008, 105, 1003–1008. [Google Scholar] [CrossRef]
- Wandler, A.M.; Guillemin, K. Transgenic Expression of the Helicobacter pylori Virulence Factor CagA Promotes Apoptosis or Tumorigenesis through JNK Activation in Drosophila. PLoS Pathog. 2012, 8, e1002939. [Google Scholar] [CrossRef][Green Version]
- Neal, J.T.; Peterson, T.S.; Kent, M.L.; Guillemin, K.H. pylori Virulence Factor CagA Increases Intestinal Cell Proliferation by Wnt Pathway Activation in a Transgenic Zebrafish Model. Dis. Models Mech. 2013, 6, 802–810. [Google Scholar] [CrossRef]
- Jones, K.R.; Whitmire, J.M.; Merrell, D.S. A Tale of Two Toxins: Helicobacter pylori CagA and VacA Modulate Host Pathways That Impact Disease. Front. Microbiol. 2010, 1, 115. [Google Scholar] [CrossRef]
- dos Santos Pereira, E.; Magalhães Albuquerque, L.; de Queiroz Balbino, V.; da Silva Junior, W.J.; Rodriguez Burbano, R.M.; Pordeus Gomes, J.P.; Barem Rabenhorst, S.H. Helicobacter pylori CagE, CagG, and CagM Can Be a Prognostic Marker for Intestinal and Diffuse Gastric Cancer. Infect. Genet. Evol. 2020, 84, e104477. [Google Scholar] [CrossRef]
- Akeel, M.; Shehata, A.; Elhafey, A.; Elmakki, E.; Aboshouk, T.; Ageely, H.; Mahfouz, M. Helicobacter pylori VacA, CagA and IceA Genotypes in Dyspeptic Patients from Southwestern Region, Saudi Arabia: Distribution and Association with Clinical Outcomes and Histopathological Changes. BMC Gastroenterol. 2019, 19, 16. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Sheu, B.-S.; Wu, J.-J. Helicobacter pylori Infection: An Overview of Bacterial Virulence Factors and Pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y. Pathogenesis of Helicobacter pylori-related Gastroduodenal Diseases from Molecular Epidemiological Studies. Gastroenterol. Res. Pract. 2012, 2012, 371503. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Forman, D.; Waskito, L.; Yamaoka, Y.; Crabtree, J. Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer. Toxins 2018, 10, 163. [Google Scholar] [CrossRef]
- Yamaoka, Y. Mechanisms of Disease: Helicobacter pylori Virulence Factors. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 629–641. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Reddy, R.; Graham, D.Y. Helicobacter pylori Virulence Factor Genotypes in Children in the United States: Clues about Genotype and Outcome Relationships. J. Clin. Microbiol. 2010, 48, 2550–2551. [Google Scholar] [CrossRef] [PubMed]
- Homan, M.; Luzar, B.; Kocjan, B.J.; Orel, R.; Močilnik, T.; Shrestha, M.; Kveder, M.; Poljak, M. Prevalence and Clinical Relevance of CagA, VacA, and IceA Genotypes of Helicobacter pylori Isolated from Slovenian Children. J. Pediatric Gastroenterol. Nutr. 2009, 49, 289–296. [Google Scholar] [CrossRef]
- Biernat, M.M.; Gościniak, G.; Iwańczak, B. Prevalence of Helicobacter pylori CagA, VacA, IceA, BabA2 Genotypes in Polish Children and Adolescents with Gastroduodenal Disease. Postępy Hig. Med. Doświadczalnej 2014, 68, 1015–1021. [Google Scholar] [CrossRef]
- Oleastro, M.; Gerhard, M.; Lopes, A.I.; Ramalho, P.; Cabral, J.; Sousa Guerreiro, A.; Monteiro, L. Helicobacter pylori Virulence Genotypes in Portuguese Children and Adults with Gastroduodenal Pathology. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 85–91. [Google Scholar] [CrossRef]
- Ko, J.S.; Kim, K.M.; Oh, Y.L.; Seo, J.K. CagA, VacA, and IceA Genotypes of Helicobacter pylori in Korean Children. Pediatrics Int. 2008, 50, 628–631. [Google Scholar] [CrossRef]
- Azuma, T.; Kato, S.; Zhou, W.; Yamazaki, S.; Yamakawa, A.; Ohtani, M.; Fujiwara, S.; Minoura, T.; Iinuma, K.; Kato, T. Diversity of VacA and CagA Genes of Helicobacter pylori in Japanese Children. Aliment. Pharmacol. Ther. 2004, 20, 7–12. [Google Scholar] [CrossRef]
- Oleastro, M.; Cordeiro, R.; Ferrand, J.; Nunes, B.; Lehours, P.; Carvalho-Oliveira, I.; Mendes, A.I.; Penque, D.; Monteiro, L.; Mégraud, F.; et al. Evaluation of the Clinical Significance of HomB, a Novel Candidate Marker of Helicobacter pylori Strains Associated with Peptic Ulcer Disease. J. Infect. Dis. 2008, 198, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, N.; Caston, R.; McClain, M.; Ohi, M.; Cover, T. An Overview of Helicobacter pylori VacA Toxin Biology. Toxins 2016, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- McClain, M.S.; Cao, P.; Cover, T.L. Amino-Terminal Hydrophobic Region of Helicobacter pylori Vacuolating Cytotoxin (VacA) Mediates Transmembrane Protein Dimerization. Infect. Immun. 2001, 69, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Gangwer, K.A.; Mushrush, D.J.; Stauff, D.L.; Spiller, B.; McClain, M.S.; Cover, T.L.; Lacy, D.B. Crystal Structure of the Helicobacter pylori Vacuolating Toxin P55 Domain. Proc. Natl. Acad. Sci. USA 2007, 104, 16293–16298. [Google Scholar] [CrossRef]
- Soyfoo, D.M.; Doomah, Y.H.; Xu, D.; Zhang, C.; Sang, H.M.; Liu, Y.Y.; Zhang, G.X.; Jiang, J.X.; Xu, S.F. New Genotypes of Helicobacter pylori VacA D-Region Identified from Global Strains. BMC Mol. Cell Biol. 2021, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Bakhti, S.Z.; Latifi-Navid, S.; Mohammadi, S.; Zahri, S.; Bakhti, F.S.; Feizi, F.; Yazdanbod, A.; Siavoshi, F. Relevance of Helicobacter pylori vacA 3′-End Region Polymorphism to Gastric Cancer. Helicobacter 2016, 21, 305–316. [Google Scholar] [CrossRef]
- Thi Huyen Trang, T.; Thanh Binh, T.; Yamaoka, Y. Relationship between VacA Types and Development of Gastroduodenal Diseases. Toxins 2016, 8, 182. [Google Scholar] [CrossRef]
- Gauthier, N.C.; Monzo, P.; Kaddai, V.; Doye, A.; Ricci, V.; Boquet, P. Helicobacter pylori VacA Cytotoxin: A Probe for a Clathrin-Independent and Cdc42-Dependent Pinocytic Pathway Routed to Late Endosomes. Mol. Biol. Cell 2005, 16, 4852–4866. [Google Scholar] [CrossRef]
- Palframan, S.L.; Kwok, T.; Gabriel, K. Vacuolating Cytotoxin A (VacA), a Key Toxin for Helicobacter pylori Pathogenesis. Front. Cell. Infect. Microbiol. 2012, 2, 92. [Google Scholar] [CrossRef]
- Djekic, A.; Müller, A. The Immunomodulator VacA Promotes Immune Tolerance and Persistent Helicobacter pylori Infection through Its Activities on T-Cells and Antigen-Presenting Cells. Toxins 2016, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Xue, J.; Zhang, Z.J.; Jia, Y.P.; Tong, Y.N.; Han, D.; Li, Q.; Xiang, Y.; Mao, X.H.; Tang, B. Helicobacter pylori VacA Induces Autophagic Cell Death in Gastric Epithelial Cells via the Endoplasmic Reticulum Stress Pathway Article. Cell Death Dis. 2017, 8, 3207. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, J.; Gong, Y.; Yuan, Y. Serum VacA Antibody Is Associated with Risks of Peptic Ulcer and Gastric Cancer: A Meta-Analysis. Microb. Pathog. 2016, 99, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Greenfield, L.K.; Bronte-Tinkew, D.; Capurro, M.I.; Rizzuti, D.; Jones, N.L. VacA Promotes CagA Accumulation in Gastric Epithelial Cells during Helicobacter pylori Infection. Sci. Rep. 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Breithaupt, U.; Kern, B.; Smith, S.I.; Spicher, C.; Haas, R. A Comprehensive Analysis of Helicobacter pylori Plasticity Zones Reveals That They Are Integrating Conjugative Elements with Intermediate Integration Specificity. BMC Genom. 2014, 15, 310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delahay, R.M.; Croxall, N.J.; Stephens, A.D. Phylogeographic Diversity and Mosaicism of the Helicobacter pylori Tfs Integrative and Conjugative Elements. Mob. DNA 2018, 9, 5. [Google Scholar] [CrossRef]
- Kersulyte, D.; Velapatiño, B.; Mukhopadhyay, A.K.; Cahuayme, L.; Bussalleu, A.; Combe, J.; Gilman, R.H.; Berg, D.E. Cluster of Type IV Secretion Genes in Helicobacter pylori’s Plasticity Zone. J. Bacteriol. 2003, 185, 3764–3772. [Google Scholar] [CrossRef]
- Kersulyte, D.; Lee, W.; Subramaniam, D.; Anant, S.; Herrera, P.; Cabrera, L.; Balqui, J.; Barabas, O.; Kalia, A.; Gilman, R.H.; et al. Helicobacter pylori’s Plasticity Zones Are Novel Transposable Elements. PLoS ONE 2009, 4, e6859. [Google Scholar] [CrossRef]
- Fischer, W.; Windhager, L.; Rohrer, S.; Zeiller, M.; Karnholz, A.; Hoffmann, R.; Zimmer, R.; Haas, R. Strain-Specific Genes of Helicobacter pylori: Genome Evolution Driven by a Novel Type IV Secretion System and Genomic Island Transfer. Nucleic Acids Res. 2010, 38, 6089–6101. [Google Scholar] [CrossRef]
- Silva, B.; Nunes, A.; Vale, F.F.; Rocha, R.; Gomes, J.P.; Dias, R.; Oleastro, M. The Expression of Helicobacter pylori Tfs Plasticity Zone Cluster Is Regulated by pH and Adherence, and Its Composition Is Associated with Differential Gastric IL-8 Secretion. Helicobacter 2017, 22, e12390. [Google Scholar] [CrossRef]
- Alandiyjany, M.N.; Croxall, N.J.; Grove, J.I.; Delahay, R.M. A Role for the Tfs3 ICE-Encoded Type IV Secretion System in pro-Inflammatory Signalling by the Helicobacter pylori Ser/Thr Kinase, CtkA. PLoS ONE 2017, 12, e0182144. [Google Scholar] [CrossRef] [PubMed]
- Tenguria, S.; Ansari, S.A.; Khan, N.; Ranjan, A.; Devi, S.; Tegtmeyer, N.; Lind, J.; Backert, S.; Ahmed, N. Helicobacter pylori Cell Translocating Kinase (CtkA/JHP0940) Is pro-Apoptotic in Mouse Macrophages and Acts as Auto-Phosphorylating Tyrosine Kinase. Int. J. Med. Microbiol. 2014, 304, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Park, K.-S.; Kim, J.-H.; Yang, S.-H.; Yoon, J.Y.; Han, B.-G.; Kim, H.S.; Lee, S.J.; Jang, J.Y.; Kim, K.H.; et al. Helicobacter pylori Proinflammatory Protein up-Regulates NF-kB as a Cell-Translocating Ser/Thr Kinase. Proc. Natl. Acad. Sci. USA 2010, 107, 21418–21423. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, J.; Abbas, Z.; Naz, S.; Islam, M.; Abid, S.; Jafri, W. Associations between the Plasticity Region Genes of Helicobacter pylori and Gastroduodenal Diseases in a High-Prevalence Area. Gut Liver 2010, 4, 345–350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alam, J.; Sarkar, A.; Karmakar, B.C.; Ganguly, M.; Paul, S.; Mukhopadhyay, A.K. Novel Virulence Factor dupA of Helicobacter pylori as an Important Risk Determinant for Disease Manifestation: An Overview. World J. Gastroenterol. 2020, 26, 4739–4752. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, C.; Shao, C.; Wang, S.; Wang, A.; Yang, Y.; Yuan, X.; Shao, S. Intact Long-Type DupA Protein in Helicobacter pylori Is an ATPase Involved in Multifunctional Biological Activities. Microb. Pathog. 2015, 81, 53–59. [Google Scholar] [CrossRef]
- Shiota, S.; Nguyen, L.T.; Murakami, K.; Kuroda, A.; Mizukami, K.; Okimoto, T.; Kodama, M.; Fujioka, T.; Yamaoka, Y. Association of Helicobacter pylori DupA with the Failure of Primary Eradication. J. Clin. Gastroenterol. 2012, 46, 297–301. [Google Scholar] [CrossRef]
- Jung, S.W.; Sugimoto, M.; Shiota, S.; Graham, D.Y.; Yamaoka, Y. The Intact dupA Cluster Is a More Reliable Helicobacter pylori Virulence Marker than dupA Alone. Infect. Immun. 2012, 80, 381–387. [Google Scholar] [CrossRef]
- Marshall, B.J.; Barrett, L.J.; Prakash, C.; McCallum, R.W.; Guerrant, R.L. Urea Protects Helicobacter (Campylobacter) pylori from the Bactericidal Effect of Acid. Gastroenterology 1990, 99, 697–702. [Google Scholar] [CrossRef]
- Gu, H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr. Microbiol. 2017, 74, 863–869. [Google Scholar] [CrossRef]
- Schmees, C.; Prinz, C.; Treptau, T.; Rad, R.; Hengst, L.; Voland, P.; Bauer, S.; Brenner, L.; Schmid, R.M.; Gerhard, M. Inhibition of T-Cell Proliferation by Helicobacter pylori γ-Glutamyl Transpeptidase. Gastroenterology 2007, 132, 1820–1833. [Google Scholar] [CrossRef] [PubMed]
- Oertli, M.; Noben, M.; Engler, D.B.; Semper, R.P.; Reuter, S.; Maxeiner, J.; Gerhard, M.; Taube, C.; Müller, A. Helicobacter pylori γ-Glutamyl Transpeptidase and Vacuolating Cytotoxin Promote Gastric Persistence and Immune Tolerance. Proc. Natl. Acad. Sci. USA 2013, 110, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, C.; Thiberge, J.-M.; Ferrero, R.L.; Labigne, A. Essential Role of Helicobacter pylori γ-Glutamyltranspeptidase for the Colonization of the Gastric Mucosa of Mice. Mol. Microbiol. 1999, 31, 1359–1372. [Google Scholar] [CrossRef]
- Kim, K.M.; Lee, S.G.; Kim, J.M.; Kim, D.S.; Song, J.Y.; Kang, H.L.; Lee, W.K.; Cho, M.J.; Rhee, K.H.; Youn, H.S.; et al. Helicobacter pylori γ-Glutamyltranspeptidase Induces Cell Cycle Arrest at the G1-S Phase Transition. J. Microbiol. 2010, 48, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Boonyanugomol, W.; Chomvarin, C.; Song, J.Y.; Kim, K.M.; Kim, J.M.; Cho, M.J.; Lee, W.K.; Kang, H.L.; Rhee, K.H.; Sripa, B.; et al. Effects of Helicobacter pylori γ-Glutamyltranspeptidase on Apoptosis and Inflammation in Human Biliary Cells. Dig. Dis. Sci. 2012, 57, 2615–2624. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, R.; Duan, G.; Wang, C.; Sun, N.; Zhang, L.; Chen, S.; Fan, Q.; Xi, Y. Production and Delivery of Helicobacter pylori NapA in Lactococcus Lactis and Its Protective Efficacy and Immune Modulatory Activity. Sci. Rep. 2018, 8, 6435. [Google Scholar] [CrossRef]
- Ramachandran, M.; Jin, C.; Yu, D.; Eriksson, F.; Essand, M. Vector-Encoded Helicobacter pylori Neutrophil-Activating Protein Promotes Maturation of Dendritic Cells with TH1 Polarization and Improved Migration. J. Immunol. 2014, 193, 2287–2296. [Google Scholar] [CrossRef]
- Pachathundikandi, S.K.; Lind, J.; Tegtmeyer, N.; El-Omar, E.M.; Backert, S. Interplay of the Gastric Pathogen Helicobacter pylori with Toll-like Receptors. BioMed Res. Int. 2015, 2015, 192420. [Google Scholar] [CrossRef]
- Evans, D.J.; Evans, D.G.; Takemura, T.; Nakano, H.; Lampert, H.C.; Graham, D.Y.; Granger, D.N.; Kvietys, P.R. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect. Immun. 1995, 63, 2213–2220. [Google Scholar] [CrossRef]
- Brisslert, M.; Enarsson, K.; Lundin, S.; Karlsson, A.; Kusters, J.G.; Svennerholm, A.M.; Backert, S.; Quiding-Järbrink, M. Helicobacter pylori Induce Neutrophil Transendothelial Migration: Role of the Bacterial HP-NAP. FEMS Microbiol. Lett. 2005, 249, 95–103. [Google Scholar] [CrossRef]
- Godlewska, R.; Pawlowski, M.; Dzwonek, A.; Mikula, M.; Ostrowski, J.; Drela, N.; Jagusztyn-Krynicka, E.K. Tip-α (Hp0596 Gene Product) Is a Highly Immunogenic Helicobacter pylori Protein Involved in Colonization of Mouse Gastric Mucosa. Curr. Microbiol. 2008, 56, 279–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuzuhara, T.; Suganuma, M.; Oka, K.; Fujiki, H. DNA-Binding Activity of TNF-α Inducing Protein from Helicobacter pylori. Biochem. Biophys. Res. Commun. 2007, 362, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Yamaguchi, K.; Ono, Y.; Matsumoto, H.; Hayashi, T.; Ogawa, T.; Imai, K.; Kuzuhara, T.; Nishizono, A.; Fujiki, H. TNF-α-Inducing Protein, a Carcinogenic Factor Secreted from H. pylori, Enters Gastric Cancer Cells. Int. J. Cancer 2008, 123, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hirano, K.; Takahashi, A.; Yamaguchi, K.; Beppu, M.; Fujiki, H.; Suganuma, M. Nucleolin on the Cell Surface as a New Molecular Target for Gastric Cancer Treatment. Biol. Pharm. Bull. 2010, 33, 796–803. [Google Scholar] [CrossRef]
- Senkovich, O.A.; Yin, J.; Ekshyyan, V.; Conant, C.; Traylor, J.; Adegboyega, P.; McGee, D.J.; Rhoads, R.E.; Slepenkov, S.; Testerman, T.L. Helicobacter pylori AlpA and AlpB Bind Host Laminin and Influence Gastric Inflammation in Gerbils. Infect. Immun. 2011, 79, 3106–3116. [Google Scholar] [CrossRef]
- Yonezawa, H.; Osaki, T.; Fukutomi, T.; Hanawa, T.; Kurata, S.; Zaman, C.; Hojo, F.; Kamiya, S. Diversification of the AlpB Outer Membrane Protein of Helicobacter pylori Affects Biofilm Formation and Cellular Adhesion. J. Bacteriol. 2017, 199, e00729-16. [Google Scholar] [CrossRef]
- Ilver, D.; Arnqvist, A.; Ögren, J.; Frick, I.M.; Kersulyte, D.; Incecik, E.T.; Berg, D.E.; Covacci, A.; Engstrand, L.; Borén, T. Helicobacter pylori Adhesin Binding Fucosylated Histo-Blood Group Antigens Revealed by Retagging. Science 1998, 279, 373–377. [Google Scholar] [CrossRef]
- Walz, A.; Odenbreit, S.; Stühler, K.; Wattenberg, A.; Meyer, H.E.; Mahdavi, J.; Borén, T.; Ruhl, S. Identification of Glycoprotein Receptors within the Human Salivary Proteome for the Lectin-like BabA and SabA Adhesins of Helicobacter pylori by Fluorescence-Based 2-D Bacterial Overlay. Proteomics 2009, 9, 1582–1592. [Google Scholar] [CrossRef]
- Aspholm-Hurtig, M.; Dailide, G.; Lahmann, M.; Kalia, A.; Ilver, D.; Roche, N.; Vikström, S.; Sjöström, R.; Lindén, S.; Bäckström, A.; et al. Functional Adaptation of BabA the H. pylori ABO Blood Group Antigen Binding Adhesin. Science 2004, 305, 519–522. [Google Scholar] [CrossRef]
- Servetas, S.L.; Kim, A.; Su, H.; Cha, J.H.; Merrell, D.S. Comparative Analysis of the Hom Family of Outer Membrane Proteins in Isolates from Two Geographically Distinct Regions: The United States and South Korea. Helicobacter 2018, 23, e12461. [Google Scholar] [CrossRef]
- Servetas, S.L.; Doster, R.S.; Kim, A.; Windham, I.H.; Cha, J.H.; Gaddy, J.A.; Merrell, D.S. ArsRS-Dependent Regulation of HomB Contributes to Helicobacter pylori Biofilm Formation. Front. Microbiol. 2018, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Oleastro, M.; Monteiro, L.; Lehours, P.; Mégraud, F.; Ménard, A. Identification of Markers for Helicobacter pylori Strains Isolated from Children with Peptic Ulcer Disease by Suppressive Subtractive Hybridization. Infect. Immun. 2006, 74, 4064–4074. [Google Scholar] [CrossRef] [PubMed]
- Belogolova, E.; Bauer, B.; Pompaiah, M.; Asakura, H.; Brinkman, V.; Ertl, C.; Bartfeld, S.; Nechitaylo, T.Y.; Haas, R.; Machuy, N.; et al. Helicobacter pylori Outer Membrane Protein HopQ Identified as a Novel T4SS-Associated Virulence Factors. Cell. Microbiol. 2013, 15, 1896–1912. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeyer, N.; Harrer, A.; Schmitt, V.; Singer, B.B.; Backert, S. Expression of CEACAM1 or CEACAM5 in AZ-521 Cells Restores the Type IV Secretion Deficiency for Translocation of CagA by Helicobacter pylori. Cell. Microbiol. 2019, 21, e12965. [Google Scholar] [CrossRef] [PubMed]
- Kennemann, L.; Brenneke, B.; Andres, S.; Engstrand, L.; Meyer, T.F.; Aebischer, T.; Josenhans, C.; Suerbaum, S. In Vivo Sequence Variation in HopZ, a Phase-Variable Outer Membrane Protein of Helicobacter pylori. Infect. Immun. 2012, 80, 4364–4373. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Kikuchi, S.; El-Zimaity, H.M.T.; Gutierrez, O.; Osato, M.S.; Graham, D.Y. Importance of Helicobacter pylori OipA in Clinical Presentation, Gastric Inflammation, and Mucosal Interleukin 8 Production. Gastroenterology 2002, 123, 414–424. [Google Scholar] [CrossRef]
- Tabassam, F.H.; Graham, D.Y.; Yamaoka, Y. OipA Plays a Role in Helicobacter pylori-Induced Focal Adhesion Kinase Activation and Cytoskeletal Re-Organization. Cell. Microbiol. 2008, 10, 1008–1020. [Google Scholar] [CrossRef]
- Marcos, N.T.; Magalhães, A.; Ferreira, B.; Oliveira, M.J.; Carvalho, A.S.; Mendes, N.; Gilmartin, T.; Head, S.R.; Figueiredo, C.; David, L.; et al. Helicobacter pylori Induces Β3GnT5 in Human Gastric Cell Lines, Modulating Expression of the SabA Ligand Sialyl-Lewis x. J. Clin. Investig. 2008, 118, 2325–2336. [Google Scholar] [CrossRef]
- Walz, A.; Odenbreit, S.; Mahdavi, J.; Borén, T.; Ruhl, S. Identification and Characterization of Binding Properties of Helicobacter pylori by Glycoconjugate Arrays. Glycobiology 2005, 15, 700–708. [Google Scholar] [CrossRef]
- Mobley, R.M.; Garner, L.T.; Bauerfeind, P. Helicobacter pylori Nickel-Transport Gene NixA: Synthesis of Catalytically Active Urease in Escherichia coli Independent of Growth Conditions. Mol. Microbiol. 1995, 16, 97–109. [Google Scholar] [CrossRef]
- Cunha, E.S.; Chen, X.; Sanz-Gaitero, M.; Mills, D.J.; Luecke, H. Cryo-EM Structure of Helicobacter pylori Urease with an Inhibitor in the Active Site at 2.0 Å Resolution. Nat. Commun. 2021, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Weeks, D.L.; Eskandari, S.; Scott, D.R.; Sachs, G. A H+-Gated Urea Channel: The Link between Helicobacter pylori Urease and Gastric Colonization. Science 2000, 287, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Götz, M.; Beier, D. Histidine Residue 94 Is Involved in pH Sensing by Histidine Kinase ArsS of Helicobacte pylori. PLoS ONE 2009, 4, e6930. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.A.; Sachs, G.; Wen, Y.I.; Feng, J.; Scott, D.R. Role of the Helicobacter pylori Sensor Kinase ArsS in Protein Trafficking and Acid Acclimation. J. Bacteriol. 2012, 194, 5545–5551. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, A.H.M.; Kuipers, E.J.; Stoof, J.; Poppelaars, S.W.; Kusters, J.G. Acid-Responsive Gene Induction of Ammonia-Producing Enzymes in Helicobacter pylori Is Mediated via a Metal-Responsive Repressor Cascade. Infect. Immun. 2004, 72, 766–773. [Google Scholar] [CrossRef][Green Version]
- Bonis, M.; Ecobichon, C.; Guadagnini, S.; Prévost, M.C.; Boneca, I.G. A M23B Family Metallopeptidase of Helicobacter pylori Required for Cell Shape, Pole Formation and Virulence. Mol. Microbiol. 2010, 78, 809–819. [Google Scholar] [CrossRef]
- Suerbaum, S.; Josenhans, C.; Labigne1, A. Cloning and Genetic Characterization of the Helicobacter pylori and Helicobacter mustelae FlaB Flagellin Genes and Construction of H. pylori FlaA-and FlaB-Negative Mutants by Electroporation-Mediated Allelic Exchange. J. Bacteriol. 1993, 175, 3278–3288. [Google Scholar] [CrossRef][Green Version]
- Praszkier, J.; Sutton, P.; Ferrero, R.L. Virulence Mechanisms of Helicobacter pylori: An Overview. In Helicobacter pylori Research: From Bench to Bedside; Springer: Tokyo, Japan, 2016; pp. 57–88. ISBN 9784431559368. [Google Scholar]
- Terry, K.; Williams, S.M.; Connolly, L.; Ottemann, K.M. Chemotaxis Plays Multiple Roles during Helicobacter pylori Animal Infection. Infect. Immun. 2005, 73, 803–811. [Google Scholar] [CrossRef]
- Alm, R.A.; Bina, J.; Andrews, B.M.; Doig, P.; Hancock, R.E.W.; Trust, T.J. Comparative Genomics of Helicobacter pylori: Analysis of the Outer Membrane Protein Families. Infect. Immun. 2000, 68, 4155–4168. [Google Scholar] [CrossRef]
- Oleastro, M.; Ménard, A. The Role of Helicobacter pylori Outer Membrane Proteins in Adherence and Pathogenesis. Biology 2013, 2, 1110–1134. [Google Scholar] [CrossRef]
- de Jonge, R.; Durrani, Z.; Rijpkema, S.G.; Kuipers, E.J.; van Vliet, A.H.M.; Kusters, J.G. Role of the Helicobacter pylori Outer-Membrane Proteins AlpA and AlpB in Colonization of the Guinea Pig Stomach. J. Med. Microbiol. 2004, 53, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jeng, Y.W.; Beswick, E.J.; Ohno, T.; Odenbreit, S.; Haas, R.; Reyes, V.E.; Kita, M.; Graham, D.Y.; Yamaoka, Y. Functional and Intracellular Signaling Differences Associated with the Helicobacter pylori AlpAB Adhesin from Western and East Asian Strains. J. Biol. Chem. 2007, 282, 6242–6254. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.M.; Gideonsson, P.; Canfield, D.R.; Borén, T.; Solnick, J.V. Dynamic Expression of the BabA Adhesin and Its BabB Paralog during Helicobacter pylori Infection in Rhesus Macaques. Infect. Immun. 2017, 85, e00094-17. [Google Scholar] [CrossRef] [PubMed]
- Argent, R.H.; Kidd, M.; Owen, R.J.; Thomas, R.J.; Limb, M.C.; Atherton, J.C. Determinants and Consequences of Different Levels of CagA Phosphorylation for Clinical Isolates of Helicobacter pylori. Gastroenterology 2004, 127, 514–523. [Google Scholar] [CrossRef]
- Rad, R.; Gerhard, M.; Lang, R.; Schöniger, M.; Rösch, T.; Schepp, W.; Becker, I.; Wagner, H.; Prinz, C. The Helicobacter pylori Blood Group Antigen-Binding Adhesin Facilitates Bacterial Colonization and Augments a Nonspecific Immune Response. J. Immunol. 2002, 168, 3033–3041. [Google Scholar] [CrossRef]
- Moonens, K.; Gideonsson, P.; Subedi, S.; Bugaytsova, J.; Romaõ, E.; Mendez, M.; Nordén, J.; Fallah, M.; Rakhimova, L.; Shevtsova, A.; et al. Structural Insights into Polymorphic ABO Glycan Binding by Helicobacter pylori. Cell Host Microbe 2016, 19, 55–66. [Google Scholar] [CrossRef]
- Gerhard, M.; Lehn, N.; Neumayer, N.; Borén, T.; Rad, R.; Schepp, W.; Miehlke, S.; Classen, M.; Prinz, C. Clinical Relevance of the Helicobacter pylori Gene for Blood-Group Antigen-Binding Adhesin. Proc. Natl. Acad. Sci. USA 1999, 96, 12778–12783. [Google Scholar] [CrossRef]
- Abadi, A.T.B.; Rafiei, A.; Ajami, A.; Hosseini, V.; Taghvaei, T.; Jones, K.R.; Merrell, D.S. Helicobacter pylori HomB, but Not CagA, Is Associated with Gastric Cancer in Iran. J. Clin. Microbiol. 2011, 49, 3191–3197. [Google Scholar] [CrossRef]
- Kim, A.; Servetas, S.L.; Kang, J.; Kim, J.; Jang, S.; Cha, H.J.; Lee, W.J.; Kim, J.; Romero-Gallo, J.; Peek, R.M.; et al. Helicobacter pylori Bab Paralog Distribution and Association with CagA, VacA, and HomA/B Genotypes in American and South Korean Clinical Isolates. PLoS ONE 2015, 10, e0137078. [Google Scholar] [CrossRef]
- Cao, P.; Kerry, J.L.; Blaser, M.J.; Cover, T.L. Analysis of HopQ Alleles in East Asian and Western Strains of Helicobacter pylori. FEMS Microbiol. Lett. 2005, 251, 37–43. [Google Scholar] [CrossRef]
- Dossumbekova, A.; Prinz, C.; Mages, J.; Lang, R.; Kusters, J.G.; Van Vliet, A.H.M.; Reindl, W.; Backert, S.; Saur, D.; Schmid, R.M.; et al. Helicobacter pylori HopH (OipA) and Bacterial Pathogenicity: Genetic and Functional Genomic Analysis of hopH Gene Polymorphisms. J. Infect. Dis. 2006, 194, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Ojo, O.; Fujimoto, S.; Odenbreit, S.; Haas, R.; Gutierrez, O.; El-Zimaity, H.M.T.; Reddy, R.; Arnqvist, A.; Graham, D.Y. Helicobacter pylori Outer Membrane Proteins and Gastroduodenal Disease. Gut 2006, 55, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y. Increasing evidence of the role of Helicobacter pylori SabA in the pathogenesis of gastroduodenal disease. J. Infect. Dev. Ctries. 2008, 2, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Horridge, D.N.; Begley, A.A.; Kim, J.; Aravindan, N.; Fan, K.; Forsyth, M.H. Outer Inflammatory Protein a (OipA) of Helicobacter pylori Is Regulated by Host Cell Contact and Mediates CagA Translocation and Interleukin-8 Response Only in the Presence of a Functional Cag Pathogenicity Island Type IV Secretion System. Pathog. Dis. 2017, 75, ftx113. [Google Scholar] [CrossRef]
- Akanuma, M.; Maeda, S.; Ogura, K.; Mitsuno, Y.; Hirata, Y.; Ikenoue, T.; Otsuka, M.; Watanabe, T.; Yamaji, Y.; Yoshida, H.; et al. The Evaluation of Putative Virulence Factors of Helicobacter pylori for Gastroduodenal Disease by Use of a Short-Term Mongolian Gerbil Infection Model. J. Infect. Dis. 2002, 185, 341–347. [Google Scholar] [CrossRef]
- Franco, A.T.; Johnston, E.; Krishna, U.; Yamaoka, Y.; Israel, D.A.; Nagy, T.A.; Wroblewski, L.E.; Piazuelo, M.B.; Correa, P.; Peek, R.M. Regulation of Gastric Carcinogenesis by Helicobacter pylori Virulence Factors. Cancer Res. 2008, 68, 379–387. [Google Scholar] [CrossRef]
- Talarico, S.; Whitefield, S.E.; Fero, J.; Haas, R.; Salama, N.R. Regulation of Helicobacter pylori Adherence by Gene Conversion. Mol. Microbiol. 2012, 84, 1050–1061. [Google Scholar] [CrossRef]
- Doohan, D.; Rezkitha, Y.A.A.; Waskito, L.A.; Yamaoka, Y.; Miftahussurur, M. Helicobacter pylori BabA–SabA Key Roles in the Adherence Phase: The Synergic Mechanism for Successful Colonization and Disease Development. Toxins 2021, 13, 485. [Google Scholar] [CrossRef]
- Loh, J.T.; Gupta, S.S.; Friedman, D.B.; Krezel, A.M.; Cover, T.L. Analysis of Protein Expression Regulated by the Helicobacter pylori ArsRS Two-Component Signal Transduction System. J. Bacteriol. 2010, 192, 2034–2043. [Google Scholar] [CrossRef]
- Katelaris, P.; Hunt, R.; Bazzoli, F.; Cohen, H.; Fock, K.; Gemilyan, M.; Malfertheiner, P.; Mégraud, F.; Piscoya, A.; Quach, D.; et al. Helicobacter pylori. WGO Global Guidelines; World Gastroenterology Organisation: Milwaukee, WI, USA, 2021. [Google Scholar]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-Analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Marques, A.T.; Vítor, J.M.B.; Santos, A.; Oleastro, M.; Vale, F.F. Trends in Helicobacter pylori Resistance to Clarithromycin: From Phenotypic to Genomic Approaches. Microb. Genom. 2020, 6, e000344. [Google Scholar] [CrossRef] [PubMed]
- Ducournau, A.; Bénéjat, L.; Sifré, E.; Bessède, E.; Lehours, P.; Mégraud, F. Helicobacter pylori Resistance to Antibiotics in 2014 in France Detected by Phenotypic and Genotypic Methods. Clin. Microbiol. Infect. 2016, 22, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Jaka, H.; Rüttgerodt, N.; Bohne, W.; Mueller, A.; Gross, U.; Kasang, C.; Mshana, S.E. Helicobacter pylori Mutations Conferring Resistance to Fluoroquinolones and Clarithromycin among Dyspeptic Patients Attending a Tertiary Hospital, Tanzania. Can. J. Gastroenterol. Hepatol. 2019, 2019, 8481375. [Google Scholar] [CrossRef] [PubMed]
- Mégraud, F.; Alix, C.; Charron, P.; Bénéjat, L.; Ducournau, A.; Bessède, E.; Lehours, P. Survey of the Antimicrobial Resistance of Helicobacter pylori in France in 2018 and Evolution during the Previous 5 Years. Helicobacter 2021, 26, e12767. [Google Scholar] [CrossRef]
- Kocsmár, É.; Buzás, G.M.; Szirtes, I.; Kocsmár, I.; Kramer, Z.; Szijártó, A.; Fadgyas-Freyler, P.; Szénás, K.; Rugge, M.; Fassan, M.; et al. Primary and Secondary Clarithromycin Resistance in Helicobacter pylori and Mathematical Modeling of the Role of Macrolides. Nat. Commun. 2021, 12, 2255. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Shrestha, P.K.; Subsomwong, P.; Sharma, R.P.; Yamaoka, Y. Emerging Helicobacter pylori Levofloxacin Resistance and Novel Genetic Mutation in Nepal. BMC Microbiol. 2016, 16, 256. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Syam, A.F.; Nusi, I.A.; Makmun, D.; Waskito, L.A.; Zein, L.H.; Akil, F.; Uwan, W.B.; Simanjuntak, D.; Wibawa, I.D.N.; et al. Surveillance of Helicobacter pylori Antibiotic Susceptibility in Indonesia: Different Resistance Types among Regions and with Novel Genetic Mutations. PLoS ONE 2016, 11, e0166199. [Google Scholar] [CrossRef]
- Gong, M.; Han, Y.; Wang, X.; Tao, H.; Meng, F.; Hou, B.; Sun, B.B.; Wang, G. Effect of Temperature on Metronidazole Resistance in Helicobacter pylori. Front. Microbiol. 2021, 12, 681911. [Google Scholar] [CrossRef]
- Eed, E.M.; Hawash, Y.A.; Khalifa, A.S.; Alsharif, K.F.; Alghamdi, S.A.; Saber, T.; Ismail, K.A.; Shehab-Eldeen, S.A. Molecular Diagnosis of Helicobacter pylori Antibiotic Resistance in the Taif Region, Saudi Arabia. Microbiol. Immunol. 2019, 63, 199–205. [Google Scholar] [CrossRef]
- Farzi, N.; Yadegar, A.; Sadeghi, A.; Aghdaei, H.A.; Smith, S.M.; Raymond, J.; Suzuki, H.; Zali, M.R. High Prevalence of Antibiotic Resistance in Iranian Helicobacter pylori Isolates: Importance of Functional and Mutational Analysis of Resistance Genes and Virulence Genotyping. J. Clin. Med. 2019, 8, 2004. [Google Scholar] [CrossRef]
- Hulten, K.G.; Genta, R.M.; Kalfus, I.N.; Zhou, Y.; Zhang, H.; Graham, D.Y. Comparison of Culture With Antibiogram to Next-Generation Sequencing Using Bacterial Isolates and Formalin-Fixed, Paraffin-Embedded Gastric Biopsies. Gastroenterology 2021, 161, 1433–1442.e2. [Google Scholar] [CrossRef] [PubMed]
- Tuan, V.P.; Narith, D.; Tshibangu-Kabamba, E.; Dung, H.D.Q.; Viet, P.T.; Sokomoth, S.; Binh, T.T.; Sokhem, S.; Tri, T.D.; Ngov, S.; et al. A Next-Generation Sequencing-Based Approach to Identify Genetic Determinants of Antibiotic Resistance in Cambodian Helicobacter pylori Clinical Isolates. J. Clin. Med. 2019, 8, 858. [Google Scholar] [CrossRef] [PubMed]
- Saranathan, R.; Levi, M.H.; Wattam, A.R.; Malek, A.; Asare, E.; Behin, D.S.; Pan, D.H.; Jacobs, W.R.; Szymczak, W.A. Helicobacter pylori Infections in the Bronx, New York: Surveying Antibiotic Susceptibility and Strain Lineage by Whole-Genome Sequencing. J. Clin. Microbiol. 2020, 58, e01591-19. [Google Scholar] [CrossRef] [PubMed]
- Lauener, F.N.; Imkamp, F.; Lehours, P.; Buissonnière, A.; Benejat, L.; Zbinden, R.; Keller, P.M.; Wagner, K. Genetic Determinants and Prediction of Antibiotic Resistance Phenotypes in Helicobacter pylori. J. Clin. Med. 2019, 8, 53. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Kim, J.Y.; Kim, N.; Park, J.H.; Nam, R.H.; Lee, S.M.; Kim, J.W.; Kim, J.M.; Park, J.Y.; Lee, D.H. Specific Mutations of Penicillin-Binding Protein 1A in 77 Clinically Acquired Amoxicillin-Resistant Helicobacter pylori Strains in Comparison with 77 Amoxicillin-Susceptible Strains. Helicobacter 2017, 22, e12437. [Google Scholar] [CrossRef]
- Zerbetto De Palma, G.; Mendiondo, N.; Wonaga, A.; Viola, L.; Ibarra, D.; Campitelli, E.; Salim, N.; Corti, R.; Goldman, C.; Catalano, M. Occurrence of Mutations in the Antimicrobial Target Genes Related to Levofloxacin, Clarithromycin, and Amoxicillin Resistance in Helicobacter pylori Isolates from Buenos Aires City. Microb. Drug Resist. 2017, 23, 351–358. [Google Scholar] [CrossRef]
- Azzaya, D.; Gantuya, B.; Oyuntsetseg, K.; Davaadorj, D.; Matsumoto, T.; Akada, J.; Yamaoka, Y. High Antibiotic Resistance of Helicobacter pylori and Its Associated Novel Gene Mutations among the Mongolian Population. Microorganisms 2020, 8, 1062. [Google Scholar] [CrossRef]
- Nezami, B.G.; Jani, M.; Alouani, D. Helicobacter pylori Mutations Detected by Next-Generation-Sequencing in Formalin-Fixed, Paraffin-Embedded Gastric Biopsy Specimens Are Associated with Treatment Failure. J. Clin. Microbiol. 2019, 57, e01834-18. [Google Scholar] [CrossRef]
- Ontsira Ngoyi, E.N.; Atipo Ibara, B.I.; Moyen, R.; Ahoui Apendi, P.C.; Ibara, J.R.; Obengui, O.; Ossibi Ibara, R.B.; Nguimbi, E.; Niama, R.F.; Ouamba, J.M.; et al. Molecular Detection of Helicobacter pylori and Its Antimicrobial Resistance in Brazzaville, Congo. Helicobacter 2015, 20, 316–320. [Google Scholar] [CrossRef]
- Harrison, U.; Fowora, M.A.; Seriki, A.T.; Loell, E.; Mueller, S.; Ugo-Ijeh, M.; Onyekwere, C.A.; Lesi, O.A.; Otegbayo, J.A.; Akere, A.; et al. Helicobacter pylori Strains from a Nigerian Cohort Show Divergent Antibiotic Resistance Rates and a Uniform Pathogenicity Profile. PLoS ONE 2017, 12, e0176454. [Google Scholar] [CrossRef]
- Seriki, A.T.; Smith, S.I.; Adeleye, A.I.; Fowora, M.A. Molecular Analysis of Low-Level Tetracycline Resistance in Clinical Isolates of Helicobacter pylori among Dyspeptic Patients in South West Nigeria. J. Glob. Antimicrob. Resist. 2018, 13, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Cheng, H.; Hu, F.L.; Li, J. Distribution of gyrA Mutations in Fluoroquinolone-Resistant Helicobacter pylori Strains. World J. Gastroenterol. 2010, 16, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Egli, K.; Wagner, K.; Keller, P.M.; Risch, L.; Risch, M.; Bodmer, T. Comparison of the Diagnostic Performance of qPCR, Sanger Sequencing, and Whole-Genome Sequencing in Determining Clarithromycin and Levofloxacin Resistance in Helicobacter pylori. Front. Cell. Infect. Microbiol. 2020, 10, 596371. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Meng, F.; Mao, X.; Zhang, Y.; Wang, J.; Liu, Y.; Zhu, W.; Gu, B.; Huang, Q. Using Next-Generation Sequencing to Analyze Helicobacter pylori Clones with Different Levofloxacin Resistances from a Patient with Eradication Failure. Medicine 2020, 99, e20761. [Google Scholar] [CrossRef]
- Rhie, S.Y.; Park, J.Y.; Shin, T.S.; Kim, J.W.; Kim, B.J.; Kim, J.G. Discovery of a Novel Mutation in DNA Gyrase and Changes in the Fluoroquinolone Resistance of Helicobacter pylori over a 14-Year Period: A Single Center Study in Korea. Antibiotics 2020, 9, 287. [Google Scholar] [CrossRef]
- López-Gasca, M.; Peña, J.; García-Amado, M.A.; Michelangeli, F.; Contreras, M. Point Mutations at gyrA and gyrB Genes of Levofloxacin-Resistant Helicobacter pylori Isolates in the Esophageal Mucosa from a Venezuelan Population. Am. J. Trop. Med. Hyg. 2018, 98, 1051–1055. [Google Scholar] [CrossRef]
- Camorlinga-Ponce, M.; Gómez-Delgado, A.; Aguilar-Zamora, E.; Torres, R.C.; Giono-Cerezo, S.; Escobar-Ogaz, A.; Torres, J. Phenotypic and Genotypic Antibiotic Resistance Patterns in Helicobacter pylori Strains from Ethnically Diverse Population in México. Front. Cell. Infect. Microbiol. 2021, 10, 539115. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Lu, B.; Dai, J. Helicobacter pylori and Antibiotic Resistance, A Continuing and Intractable Problem. Helicobacter 2016, 21, 349–363. [Google Scholar] [CrossRef]
- Versalovic, J.; Shortridge, D.; Kibler, K.; Griffy, M.V.; Beyer, J.; Flamm, R.K.; Tanaka, S.K.; Graham, D.Y.; Go, M.F. Mutations in 23S rRNA Are Associated with Clarithromycin Resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 1996, 40, 477–480. [Google Scholar] [CrossRef]
- Versalovic, J.; Osato, M.S.; Spakovsky, K.; Dore, M.P.; Reddy, R.; Stone, G.G.; Shortridge, D.; Flamm, R.K.; Tanaka, S.K.; Graham, D.Y. Point Mutations in the 23S rRNA Gene of Helicobacter pylori Associated with Different Levels of Clarithromycin Resistance. J. Antimicrob. Chemother. 1997, 40, 283–286. [Google Scholar] [CrossRef][Green Version]
- Stone, G.G.; Shortridge, D.; Flamm, R.K.; Versalovic, J.; Beyer, J.; Idler, K.; Zulawinski, L.; Tanaka, S.K. Identification of a 23S rRNA Gene Mutation in Clarithromycin-Resistant Helicobacter pylori. Helicobacter 1996, 1, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Mégraud, F.H. pylori Antibiotic Resistance: Prevalence, Importance, and Advances in Testing. Gut 2004, 53, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Binh, T.T.; Shiota, S.; Suzuki, R.; Matsuda, M.; Trang, T.T.H.; Kwon, D.H.; Iwatani, S.; Yamaoka, Y. Discovery of Novel Mutations for Clarithromycin Resistance in Helicobacter pylori by Using Next-Generation Sequencing. J. Antimicrob. Chemother. 2014, 69, 1796–1803. [Google Scholar] [CrossRef]
- Van Amsterdam, K.; Bart, A.; Van Der Ende, A. A Helicobacter pylori TolC Efflux Pump Confers Resistance to Metronidazole. Antimicrob. Agents Chemother. 2005, 49, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Suzuki, H.; Nishizawa, T.; Tsugawa, H.; Muraoka, H.; Saito, Y.; Matsuzaki, J.; Hibi, T. Contribution of Efflux Pumps to Clarithromycin Resistance in Helicobacter pylori. J. Gastroenterol. Hepatol. 2010, 25, 75–79. [Google Scholar] [CrossRef]
- Cho, H.; Uehara, T.; Bernhardt, T.G. Beta-Lactam Antibiotics Induce a Lethal Malfunctioning of the Bacterial Cell Wall Synthesis Machinery. Cell 2014, 159, 1300–1311. [Google Scholar] [CrossRef]
- Tseng, Y.S.; Wu, D.C.; Chang, C.Y.; Kuo, C.H.; Yang, Y.C.; Jan, C.M.; Su, Y.C.; Kuo, F.C.; Chang, L.L. Amoxicillin Resistance with β-Lactamase Production in Helicobacter pylori. Eur. J. Clin. Investig. 2009, 39, 807–812. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Parlow, M.H.; Schneider, J.; Burroughs, S.; Wickland, C.; Vakil, N.B.; Dunn, B.E.; Phadnis, S.H. Identification of a Novel Penicillin-Binding Protein from Helicobacter pylori. J. Bacteriol. 1999, 181, 5107–5110. [Google Scholar] [CrossRef]
- Kwon, D.H.; Dore, M.P.; Kim, J.J.; Kato, M.; Lee, M.; Wu, J.Y.; Graham, D.Y. High-Level β-Lactam Resistance Associated with Acquired Multidrug Resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 2003, 47, 2169–2178. [Google Scholar] [CrossRef]
- Co, E.M.A.; Schiller, N.L. Resistance Mechanisms in an in vitro-selected Amoxicillin-Resistant Strain of Helicobacter pylori. Antimicrob. Agents Chemother. 2006, 50, 4174–4176. [Google Scholar] [CrossRef]
- Gerrits, M.M.; Schuijffel, D.; Van Zwet, A.A.; Kuipers, E.J.; Vandenbroucke-Grauls, C.M.J.E.; Kusters, J.G. Alterations in Penicillin-Binding Protein 1A Confer Resistance to β-Lactam Antibiotics in Helicobacter pylori. Antimicrob. Agents Chemother. 2002, 46, 2229–2233. [Google Scholar] [CrossRef] [PubMed]
- Matteo, M.J.; Granados, G.; Olmos, M.; Wonaga, A.; Catalano, M. Helicobacter pylori Amoxicillin Heteroresistance Due to Point Mutations in PBP-1A in Isogenic Isolates. J. Antimicrob. Chemother. 2008, 61, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Postius, S.; Melchers, K.; Schäfer, K.P. Mutations of the Helicobacter pylori Genes rdxA and pbp1 Cause Resistance against Metronidazole and Amoxicillin. Antimicrob. Agents Chemother. 2001, 45, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Rimbara, E.; Noguchi, N.; Kawai, T.; Sasatsu, M. Correlation between Substitutions in Penicillin-Binding Protein 1 and Amoxicillin Resistance in Helicobacter pylori. Microbiol. Immunol. 2007, 51, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Rimbara, E.; Noguchi, N.; Kawai, T.; Sasatsu, M. Mutations in Penicillin-Binding Proteins 1, 2 and 3 Are Responsible for Amoxicillin Resistance in Helicobacter pylori. J. Antimicrob. Chemother. 2008, 61, 995–998. [Google Scholar] [CrossRef]
- Cosar, C.; Julou, L. Activité de l’(Hydroxy-2′éthyl)-I Méthyl-2 Nitro-5 Imidazole (8.823 R.P.) Vis-a-Vis Des Infections Expérimentales a Trichomonas vaginalis. Ann. Inst. Pasteur 1959, 96, 238–241. [Google Scholar]
- Leitsch, D. A Review on Metronidazole: An Old Warhorse in Antimicrobial Chemotherapy. Parasitology 2019, 146, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An Update on Metabolism, Structure-Cytotoxicity and Resistance Mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef]
- Jorgensen, M.A.; Manos, J.; Mendz, G.L.; Hazell, S.L. The Mode of Action of Metronidazole in Helicobacter Pylori: Futile Cycling or Reduction? J. Antimicrob. Chemother. 1998, 41, 67–75. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Asencio, C.; Mégraud, F.; Mendz, G.L. A Redox Basis for Metronidazole Resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 2009, 53, 1884–1891. [Google Scholar] [CrossRef]
- Marais, A.; Bilardi, C.; Cantet, F.; Mendz, G.L.; Mégraud, F. Characterization of the Genes rdxA and frxA Involved in Metronidazole Resistance in Helicobacter pylori. Res. Microbiol. 2003, 154, 137–144. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, N.; Kwon, Y.H.; Nam, R.H.; Kim, J.M.; Park, J.Y.; Lee, Y.S.; Lee, D.H. RdxA, FrxA, and Efflux Pump in Metronidazole-Resistant Helicobacter pylori: Their Relation to Clinical Outcomes. J. Gastroenterol. Hepatol. 2018, 33, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; El-Zaatari, F.A.K.; Kato, M.; Osato, M.S.; Reddy, R.; Yamaoka, Y.; Graham, D.Y. Analysis of RdxA and Involvement of Additional Genes Encoding NAD(P)H Flavin Oxidoreductase (FrxA) and Ferredoxin-like Protein (FdxB) in Metronidazole Resistance of Helicobacter pylori. Antimicrob. Agents Chemother. 2000, 44, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Suzuki, H.; Satoh, K.; Hirata, K.; Matsuzaki, J.; Saito, Y.; Suematsu, M.; Hibi, T. Two Amino Acids Mutation of Ferric Uptake Regulator Determines Helicobacter pylori Resistance to Metronidazole. Antioxid. Redox Signal. 2011, 14, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Chivers, P.T.; Berg, D.E. Point Mutations in Helicobacter pylori’s fur Regulatory Gene That Alter Resistance to Metronidazole, a Prodrug Activated by Chemical Reduction. PLoS ONE 2011, 6, e18236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehrabadi, J.F.; Sirous, M.; Daryani, N.E.; Eshraghi, S.; Akbari, B.; Shirazi, M.H. Assessing the Role of the RND Efflux Pump in Metronidazole Resistance of Helicobacter pylori by RT-PCR Assay. J. Infect. Dev. Ctries. 2011, 5, 88–93. [Google Scholar] [CrossRef][Green Version]
- Puah, S.M.; Goh, K.L.; Ng, H.K.; Chua, K.H. Current Status of Helicobacter pylori Resistance to Clarithromycin and Levofloxacin in Malaysia-Findings from a Molecular Based Study. PeerJ 2021, 9, e11518. [Google Scholar] [CrossRef]
- Rimbara, E.; Noguchi, N.; Kawai, T.; Sasatsu, M. Fluoroquinolone Resistance in Helicobacter pylori: Role of Mutations at Position 87 and 91 of GyrA on the Level of Resistance and Identification of a Resistance Conferring Mutation in GyrB. Helicobacter 2012, 17, 36–42. [Google Scholar] [CrossRef]
- Francesco, V. De Mechanisms of Helicobacter pylori Antibiotic Resistance: An Updated Appraisal. World J. Gastrointest. Pathophysiol. 2011, 2, 35. [Google Scholar] [CrossRef]
- Gerrits, M.M.; De Zoete, M.R.; Arents, N.L.A.; Kuipers, E.J.; Kusters, J.G. 16S RRNA Mutation-Mediated Tetracycline Resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 2002, 46, 2996–3000. [Google Scholar] [CrossRef]
- Dadashzadeh, K.; Milani, M.; Rahmati, M.; Akbarzadeh, A. Real-Time PCR Detection of 16S rRNA Novel Mutations Associated with Helicobacter pylori Tetracycline Resistance in Iran. Asian Pac. J. Cancer Prev. 2014, 15, 8883–8886. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Dannelly, H.K. Inactivation of the Putative Tetracycline Resistance Gene HP1165 in Helicobacter pylori Led to Loss of Inducible Tetracycline Resistance. Arch. Microbiol. 2006, 185, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Qaria, M.; Kumar, N.; Hussain, A.; Qumar, S.; Doddam, S.; Sepe, L.; Ahmed, N. Roles of Cholesteryl-α-Glucoside Transferase and Cholesteryl Glucosides in Maintenance of Helicobacter pylori Morphology, Cell Wall Integrity, and Resistance to Antibiotics. mBio 2018, 9, e01523-18. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, X.; Wen, Y.; Chen, H.; She, F. A Newly Discovered Drug Resistance Gene Rfaf in Helicobacter pylori. Infect. Drug Resist. 2019, 12, 3507–3514. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, C.; Liu, Y.; Huang, Y.; Bai, Y.; Hang, X.; Zeng, L.; Zhu, D.; Bi, H. Armeniaspirol A: A Novel Anti-Helicobacter pylori Agent. Microb. Biotechnol. 2021, 15, 442–454. [Google Scholar] [CrossRef]
- Eftekharivash, L.; Hamedi, J.; Zarrini, G.; Bakhtiari, R. Acidophilic and Acid Tolerant Actinobacteria as New Sources of Antimicrobial Agents against Helicobacter pylori. Arch. Razi Inst. 2021, 76, 261–272. [Google Scholar] [CrossRef]
- Fauzia, K.A.; Miftahussurur, M.; Syam, A.F.; Waskito, L.A.; Doohan, D.; Rezkitha, Y.A.A.; Matsumoto, T.; Tuan, V.P.; Akada, J.; Yonezawa, H.; et al. Biofilm Formation and Antibiotic Resistance Phenotype of Helicobacter pylori Clinical Isolates. Toxins 2020, 12, 473. [Google Scholar] [CrossRef]
- Yonezawa, H.; Osaki, T.; Hojo, F.; Kamiya, S. Effect of Helicobacter pylori Biofilm Formation on Susceptibility to Amoxicillin, Metronidazole and Clarithromycin. Microb. Pathog. 2019, 132, 100–108. [Google Scholar] [CrossRef]
- Krzyżek, P.; Migdał, P.; Grande, R.; Gościniak, G. Biofilm Formation of Helicobacter pylori in Both Static and Microfluidic Conditions Is Associated with Resistance to Clarithromycin. Front. Cell. Infect. Microbiol. 2022, 12, 868905. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Fauzia, K.A.; Nusi, I.A.; Setiawan, P.B.; Syam, A.F.; Waskito, L.A.; Doohan, D.; Ratnasari, N.; Khomsan, A.; Adnyana, I.K.; et al. E-Test versus Agar Dilution for Antibiotic Susceptibility Testing of Helicobacter pylori: A Comparison Study. BMC Res. Notes 2020, 13, 22. [Google Scholar] [CrossRef]
- Mégraud, F.; Bénéjat, L.; Ontsira Ngoyi, E.N.; Lehours, P. Molecular Approaches to Identify Helicobacter pylori Antimicrobial Resistance. Gastroenterol. Clin. N. Am. 2015, 44, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.-Y.; Park, D.I.; Park, H.; Kim, M.-K.; Kim, D.H.; Kim, I.-S.; Kim, Y.J. Dual-Priming Oligonucleotide-Based Multiplex PCR for the Detection of Helicobacter pylori and Determination of Clarithromycin Resistance with Gastric Biopsy Specimens. Helicobacter 2009, 14, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Elviss, N.C.; Lawson, A.J.; Owen, R.J. Application of 3′-Mismatched Reverse Primer PCR Compared with Real-Time PCR and PCR-RFLP for the Rapid Detection of 23S rDNA Mutations Associated with Clarithromycin Resistance in Helicobacter pylori. Int. J. Antimicrob. Agents 2004, 23, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Ménard, A.; Santos, A.; Mégraud, F.; Oleastro, M. PCR-Restriction Fragment Length Polymorphism Can Also Detect Point Mutation A2142C in the 23S rRNA Gene, Associated with Helicobacter pylori Resistance to Clarithromycin. Antimicrob. Agents Chemother. 2002, 46, 1156–1157. [Google Scholar] [CrossRef]
- Oleastro, M.; Ménard, A.; Santos, A.; Lamouliatte, H.; Monteiro, L.; Barthélémy, P.; Mégraud, F. Real-Time PCR Assay for Rapid and Accurate Detection of Point Mutations Conferring Resistance to Clarithromycin in Helicobacter pylori. J. Clin. Microbiol. 2003, 41, 397–402. [Google Scholar] [CrossRef]
- Cambau, E.; Allerheiligen, V.; Coulon, C.; Corbel, C.; Lascols, C.; Deforges, L.; Soussy, C.J.; Delchier, J.C.; Megraud, F. Evaluation of a New Test, GenoType HelicoDR, for Molecular Detection of Antibiotic Resistance in Helicobacter pylori. J. Clin. Microbiol. 2009, 47, 3600–3607. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, N.; Nam, R.H.; Park, J.H.; Choi, Y.J.; Kim, J.M.; Kim, J.S.; Jung, H.C. GenoType HelicoDR Test in the Determination of Antimicrobial Resistance of Helicobacter pylori in Korea. Scand. J. Gastroenterol. 2014, 49, 1058–1067. [Google Scholar] [CrossRef]
- Pastukh, N.; Binyamin, D.; On, A.; Paritsky, M.; Peretz, A. GenoType® HelicoDR Test in Comparison with Histology and Culture for Helicobacter pylori Detection and Identification of Resistance Mutations to Clarithromycin and Fluoroquinolones. Helicobacter 2017, 22, e12447. [Google Scholar] [CrossRef]
- Tomb, J.F.; White, O.; Kerlavage, A.R.; Clayton, R.A.; Sutton, G.G.; Fleischmann, R.D.; Ketchum, K.A.; Klenk, H.P.; Gill, S.; Dougherty, B.A.; et al. The Complete Genome Sequence of the Gastric Pathogen Helicobacter pylori. Nature 1997, 388, 539–547. [Google Scholar] [CrossRef]
- Alm, R.A.; Ling, L.S.L.; Moir, D.T.; King, B.L.; Brown, E.D.; Doig, P.C.; Smith, D.R.; Noonan, B.; Guild, B.C.; DeJonge, B.L.; et al. Genomic-Sequence Comparison of Two Unrelated Isolates of the Human Gastric Pathogen Helicobacter pylori. Nature 1999, 397, 176–180. [Google Scholar] [CrossRef]
- Whittam, T.S.; Bumbaugh, A.C. Inferences from Whole-Genome Sequences of Bacterial Pathogens. Curr. Opin. Genet. Dev. 2002, 12, 719–725. [Google Scholar] [CrossRef]
- Berthenet, E.; Sheppard, S.; Vale, F.F. Recent “Omics” Advances in Helicobacter pylori. Helicobacter 2016, 21, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M.; et al. The PATRIC Bioinformatics Resource Center: Expanding Data and Analysis Capabilities. Nucleic Acids Res. 2020, 48, D606–D612. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Li, S.; Hu, N.; He, Y.; Pong, R.; Lin, D.; Lu, L.; Law, M. Comparison of Next-Generation Sequencing Systems. J. Biomed. Biotechnol. 2012, 2012, 251364. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, S.; Klaassen, K.; Stankovic, B.; Stojiljkovic, M.; Zukic, B. Next-Generation Sequencing: The Enabler and the Way Ahead. In Microbiomics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 175–200. [Google Scholar]
- Palau, M.; Piqué, N.; José Ramírez-Lázaro, M.; Lario, S.; Calvet, X.; Miñana-Galbis, D. Whole-Genome Sequencing and Comparative Genomics of Three Helicobacter pylori Strains Isolated from the Stomach of a Patient with Adenocarcinoma. Pathogens 2021, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Thorell, K.; Hosseini, S.; Palacios Gonzáles, R.V.P.; Chaotham, C.; Graham, D.Y.; Paszat, L.; Rabeneck, L.; Lundin, S.B.; Nookaew, I.; Sjöling, Å. Identification of a Latin American-Specific BabA Adhesin Variant through Whole Genome Sequencing of Helicobacter pylori Patient Isolates from Nicaragua. BMC Evol. Biol. 2016, 16, 53. [Google Scholar] [CrossRef]
- Imkamp, F.; Lauener, F.N.; Pohl, D.; Lehours, P.; Vale, F.F.; Jehanne, Q.; Zbinden, R.; Keller, P.M.; Wagner, K. Rapid Characterization of Virulence Determinants in Helicobacter pylori Isolated from Non-Atrophic Gastritis Patients by Next-Generation Sequencing. J. Clin. Med. 2019, 8, 30. [Google Scholar] [CrossRef]
- Gong, E.J.; Ahn, J.Y.; Kim, J.M.; Lee, S.M.; Na, H.K.; Lee, J.H.; Jung, K.W.; Choi, K.D.; Kim, D.H.; Song, H.J.; et al. Genotypic and Phenotypic Resistance to Clarithromycin in Helicobacter pylori Strains. J. Clin. Med. 2020, 9, 1930. [Google Scholar] [CrossRef]
- Iwamoto, A.; Tanahashi, T.; Okada, R.; Yoshida, Y.; Kikuchi, K.; Keida, Y.; Murakami, Y.; Yang, L.; Yamamoto, K.; Nishiumi, S.; et al. Whole-Genome Sequencing of Clarithromycin Resistant Helicobacter pylori Characterizes Unidentified Variants of Multidrug Resistant Efflux Pump Genes. Gut Pathog. 2014, 6, 27. [Google Scholar] [CrossRef]
- Watanabe, Y.; Oikawa, R.; Kodaka, Y.; Sato, Y.; Ono, S.; Kenmochi, T.; Suzuki, H.; Futagami, S.; Kato, M.; Yamamoto, H.; et al. Cancer-Related Genetic Variants of Helicobacter pylori Strains Determined Using Gastric Wash-Based Whole-Genome Analysis with Single-Molecule Real-Time Technology. Int. J. Cancer 2021, 148, 178–192. [Google Scholar] [CrossRef]
- Suzuki, R.; Satou, K.; Shiroma, A.; Shimoji, M.; Teruya, K.; Matsumoto, T.; Akada, J.; Hirano, T.; Yamaoka, Y. Genome-Wide Mutation Analysis of Helicobacter pylori after Inoculation to Mongolian Gerbils. Gut Pathog. 2019, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Satou, K.; Shiroma, A.; Teruya, K.; Shimoji, M.; Nakano, K.; Juan, A.; Tamotsu, H.; Terabayashi, Y.; Aoyama, M.; Teruya, M.; et al. Complete Genome Sequences of Eight Helicobacter pylori Strains with Different Virulence Factor Genotypes and Methylation Profiles, Isolated from Patients with Diverse Gastrointestinal Diseases on Okinawa Island, Japan, Determined Using PacBio Single-Molecule Real-Time Technology. Genome Announc. 2014, 2, e00286-14. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.; Dzink-Fox, J.; Shen, Z.; Piazuelo, M.B.; Wilson, K.T.; Correa, P.; Peek, R.M.; Constanza Camargo, M.; Fox, J.G. Helicobacter pylori Antimicrobial Resistance and Gene Variants in High-and Low-Gastric-Cancer-Risk Populations. J. Clin. Microbiol. 2021, 59, e03203-20. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, C.; Njoroge, S.; Tshibangu-Kabamba, E.; Moloo, Z.; Rajula, A.; Devani, S.; Matsumoto, T.; Nyerere, K.; Kariuki, S.; Revathi, G.; et al. Whole Genome Sequencing Reveals Virulence Potentials of Helicobacter pylori Strain KE21 Isolated from a Kenyan Patient with Gastric Signet Ring Cell Carcinoma. Toxins 2020, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.S.; Valk, P.L.; McClain, M.S.; Shaffer, C.L.; Metcalf, J.A.; Bordenstein, S.R.; Cover, T.L. Comparative Genomic Analysis of East Asian and Non-Asian Helicobacter pylori Strains Identifies Rapidly Evolving Genes. PLoS ONE 2013, 8, e0055120. [Google Scholar] [CrossRef]
- Mehrotra, T.; Devi, T.B.; Kumar, S.; Talukdar, D.; Karmakar, S.P.; Kothidar, A.; Verma, J.; Kumari, S.; Alexander, S.M.; Retnakumar, R.J.; et al. Antimicrobial Resistance and Virulence in Helicobacter pylori: Genomic Insights. Genomics 2021, 113, 3951–3966. [Google Scholar] [CrossRef]
- Hayashi, H.; Inoue, J.; Oyama, K.; Matsuoka, K.; Nishiumi, S.; Yoshida, M.; Yano, Y.; Kodama, Y. Detection of Novel Amino Acid Polymorphisms in the East Asian CagA of Helicobacter pylori with Full Sequencing Data. Kobe J. Med. Sci. 2020, 66, E22–E31. [Google Scholar] [CrossRef]
- Domanovich-Asor, T.; Craddock, H.A.; Motro, Y.; Khalfin, B.; Peretz, A.; Moran-Gilad, J. Unraveling Antimicrobial Resistance in Helicobacter pylori: Global Resistome Meets Global Phylogeny. Helicobacter 2021, 26, e12782. [Google Scholar] [CrossRef]
- Cui, R.; Song, Z.; Suo, B.; Tian, X.; Xue, Y.; Meng, L.; Niu, Z.; Jin, Z.; Zhang, H.; Zhou, L. Correlation Analysis among Genotype Resistance, Phenotype Resistance and Eradication Effect of Helicobacter pylori. Infect. Drug Resist. 2021, 14, 1747–1756. [Google Scholar] [CrossRef]
- Lang, J.; Zhu, R.; Sun, X.; Zhu, S.; Li, T.; Shi, X.; Sun, Y.; Yang, Z.; Wang, W.; Bing, P.; et al. Evaluation of the MGISEQ-2000 Sequencing Platform for Illumina Target Capture Sequencing Libraries. Front. Genet. 2021, 12, 730519. [Google Scholar] [CrossRef]
- Rizzato, C.; Torres, J.; Plummer, M.; Muñoz, N.; Franceschi, S.; Camorlinga-Ponce, M.; Fuentes-Pananá, E.M.; Canzian, F.; Kato, I. Variations in Helicobacter pylori Cytotoxin-Associated Genes and Their Influence in Progression to Gastric Cancer: Implications for Prevention. PLoS ONE 2012, 7, e0029605. [Google Scholar] [CrossRef] [PubMed]
- Canzian, F.; Rizzato, C.; Obazee, O.; Stein, A.; Flores-Luna, L.; Camorlinga-Ponce, M.; Mendez-Tenorio, A.; Vivas, J.; Trujillo, E.; Jang, H.; et al. Genetic Polymorphisms in the Cag Pathogenicity Island of Helicobacter pylori and Risk of Stomach Cancer and High-Grade Premalignant Gastric Lesions. Int. J. Cancer 2020, 147, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Aftab, H.; Miftahussurur, M.; Subsomwong, P.; Ahmed, F.; Khan, A.K.A.; Matsumoto, T.; Suzuki, R.; Yamaoka, Y. Two Populations of Less-Virulent Helicobacter pylori Genotypes in Bangladesh. PLoS ONE 2017, 12, e0182947. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Tuda, J.; Suzuki, R.; Kido, Y.; Kawamoto, F.; Matsuda, M.; Tantular, I.S.; Pusarawati, S.; Nasronudin; Harijanto, P.N.; et al. Extremely Low Helicobacter pylori Prevalence in North Sulawesi, Indonesia and Identification of a Maori-Tribe Type Strain: A Cross Sectional Study. Gut Pathog. 2014, 6, 42. [Google Scholar] [CrossRef]
- Binh, T.T.; Suzuki, R.; Trang, T.T.H.; Kwon, D.H.; Yamaoka, Y. Search for Novel Candidate Mutations for Metronidazole Resistance in Helicobacter pylori Using Next-Generation Sequencing. Antimicrob. Agents Chemother. 2015, 59, 2343–2348. [Google Scholar] [CrossRef]
- Hashinaga, M.; Suzuki, R.; Akada, J.; Matsumoto, T.; Kido, Y.; Okimoto, T.; Kodama, M.; Murakami, K.; Yamaoka, Y. Differences in Amino Acid Frequency in CagA and VacA Sequences of Helicobacter pylori Distinguish Gastric Cancer from Gastric MALT Lymphoma. Gut Pathog. 2016, 8, 54. [Google Scholar] [CrossRef]
- American Molecular Laboratories Inc. PyloriARTM/AmHPR® H. pylori Antibiotic Resistance Next Generation Sequencing Panel. Available online: http://amlaboratories.com/testing-services/helicobacter-pylori-detection-antibiotic-resistant-analysis/pyloriar-amhpr-h-pylori-antibiotic-resistance-next-generation-sequencing-panel/ (accessed on 28 March 2022).
- Tshibangu-Kabamba, E.; de Jesus Ngoma-Kisoko, P.; Tuan, V.P.; Matsumoto, T.; Akada, J.; Kido, Y.; Tshimpi-Wola, A.; Tshiamala-Kashala, P.; Ahuka-Mundeke, S.; Ngoy, D.M.; et al. Next-Generation Sequencing of the Whole Bacterial Genome for Tracking Molecular Insight into the Broad-Spectrum Antimicrobial Resistance of Helicobacter pylori Clinical Isolates from the Democratic Republic of Congo. Microorganisms 2020, 8, 887. [Google Scholar] [CrossRef]
- Boolchandani, M.; D’Souza, A.W.; Dantas, G. Sequencing-Based Methods and Resources to Study Antimicrobial Resistance. Nat. Rev. Genet. 2019, 20, 356–370. [Google Scholar] [CrossRef]
- Yusibova, M.; Hasman, H.; Clausen, P.T.L.C.; Imkamp, F.; Wagner, K.; Andersen, L.P. CRHP Finder, a Webtool for the Detection of Clarithromycin Resistance in Helicobacter pylori from Whole-Genome Sequencing Data. Helicobacter 2020, 25, e12752. [Google Scholar] [CrossRef]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A Novel Web Tool for WGS-Based Detection of Antimicrobial Resistance Associated with Chromosomal Point Mutations in Bacterial Pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Gorrell, R.; Kwok, T. The Helicobacter pylori Methylome: Roles in Gene Regulation and Virulence. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 400, pp. 105–127. ISBN 9783319505206. [Google Scholar]
- Roberts, R.J.; Vincze, T.; Posfai, J.; Macelis, D. REBASE—Enzymes and Genes for DNA Restriction and Modification. Nucleic Acids Res. 2007, 35, D269–D270. [Google Scholar] [CrossRef] [PubMed]
- Krebes, J.; Morgan, R.D.; Bunk, B.; Spröer, C.; Luong, K.; Parusel, R.; Anton, B.P.; König, C.; Josenhans, C.; Overmann, J.; et al. The Complex Methylome of the Human Gastric Pathogen Helicobacter pylori. Nucleic Acids Res. 2014, 42, 2415–2432. [Google Scholar] [CrossRef]
- Gauntlett, J.C.; Nilsson, H.O.; Fulurija, A.; Marshall, B.J.; Benghezal, M. Phase-Variable Restriction/Modification Systems Are Required for Helicobacter pylori Colonization. Gut Pathog. 2014, 6, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Anton, B.P.; Wang, S.; Baybayan, P.; Singh, S.; Ashby, M.; Chua, E.G.; Tay, C.Y.; Thirriot, F.; Loke, M.F.; et al. The Complete Methylome of Helicobacter pylori UM032. BMC Genom. 2015, 16, 424. [Google Scholar] [CrossRef]
- Lamichhane, B.; Chua, E.G.; Wise, M.J.; Laming, C.; Marshall, B.J.; Tay, C.Y. The Complete Genome and Methylome of Helicobacter pylori HpNEAfrica Strain HP14039. Gut Pathog. 2019, 11, 7. [Google Scholar] [CrossRef]

| Virulence Factor | Interaction Target | Target Location | Suggested Function | References |
|---|---|---|---|---|
| Urease | Urea | Gastric environment | Neutralize gastric acid | [80] |
| Flagella chemotaxis system | Not applied | Gastric environment | Bacterial movement to epithelial surface and deep gland | [81] |
| γ-glutamyl transpeptidase | Residues of Glutamine and Ammonia | T-cells, dendritic cells and epithelial cells | Adhesion, inhibition of T-cells, dendritic cells tolerization, apoptosis | [82,83,84,85,86] |
| Neutrophil-activating factor A | Unknown | Monocytes and dendritic cells | Induction of cytokines and TLR2 ligand | [87,88,89] |
| Neutrophils | Chemotaxis and transendothelial migration of leukocytes | [90,91] | ||
| Tumor necrosis factor-α-inducing protein α | Nucleolin | Gastric epithelia | Induction of cytokines and chemokine and cell migration | [92,93,94,95] |
| AlpA | Laminin | Gastric epithelia | Adhesion | [96] |
| AlpB | Laminin | Gastric epithelia | Adhesion | [96] |
| Unknown | Unknown | Biofilm formation | [97] | |
| BabA | Lewis B blood group antigens | Gastric epithelia | Adhesion, CagA translocation via the T4SS | [98] |
| Fucose residues on blood H antigen, A and B antigens salivary non-mucin glycoprotein | Gastric epithelia | Unknown | [99,100] | |
| HomB | Unknown | Unknown | Biofilm formation, increase in IL-8 secretion, Adhesion | [101,102,103] |
| HopQ | Carcinoembryonic antigen–related cell adhesion molecule family (1,3,5,6) | Leukocytes/endothelial and epithelial cells | Adhesion, CagA translocation via the T4SS | [104,105] |
| HopZ | Unknown | Epithelial cells | Adhesion | [106] |
| OipA | Unknown | Unknown | Adhesion, induction of inflammatory cytokine production, apoptosis | [107,108] |
| SabA | Sialyl-Lewis X, Sialyl-Lewis A, Lewis X | Gastric epithelia | T4SS assembly | [109] |
| Laminin (sialytaded moieties) | Gastric epithelia | Unknown | [110] | |
| Salivary glycoproteins (ex., heavy chain of secretory IgA1) | Saliva | Unknown | [99] |
| Antibiotic | Main Resistance Mechanism | Associated Biomarker (Gene Product) | References * |
|---|---|---|---|
| clarithromycin | structural changes on antibiotic target | mutated 23S rRNA gene | [144,145,146,147,148] |
| mutated rpl22 gene (ribosomal protein L22) | [149,150] | ||
| mutated infB gene (translation initiation factor IF-2) | [149,150] | ||
| metronidazole | inactivation/activity reduction of pro-drug activators | mutated rdxA gene (oxygen-insensitive NADPH nitroreductase) | [151,152,153,154,155] |
| mutated frxA gene (NAD(P)H-flavin oxidoreductase) | [151,152,153,156,157] | ||
| amoxicillin | structural changes on antibiotic target | mutated pbp1 gene (penicillin-binding protein 1) | [154,155,158,159,160] |
| tetracycline | structural changes on antibiotic target | mutated 16S rRNA gene | [155,161,162,163,164] |
| levofloxacin | structural changes on antibiotic target | mutated gyrA gene (DNA gyrase subunit A) | [146,152,165,166,167] |
| mutated gyrB gene (DNA gyrase subunit B) | [156,159,168,169,170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vital, J.S.; Tanoeiro, L.; Lopes-Oliveira, R.; Vale, F.F. Biomarker Characterization and Prediction of Virulence and Antibiotic Resistance from Helicobacter pylori Next Generation Sequencing Data. Biomolecules 2022, 12, 691. https://doi.org/10.3390/biom12050691
Vital JS, Tanoeiro L, Lopes-Oliveira R, Vale FF. Biomarker Characterization and Prediction of Virulence and Antibiotic Resistance from Helicobacter pylori Next Generation Sequencing Data. Biomolecules. 2022; 12(5):691. https://doi.org/10.3390/biom12050691
Chicago/Turabian StyleVital, Joana S., Luís Tanoeiro, Ricardo Lopes-Oliveira, and Filipa F. Vale. 2022. "Biomarker Characterization and Prediction of Virulence and Antibiotic Resistance from Helicobacter pylori Next Generation Sequencing Data" Biomolecules 12, no. 5: 691. https://doi.org/10.3390/biom12050691
APA StyleVital, J. S., Tanoeiro, L., Lopes-Oliveira, R., & Vale, F. F. (2022). Biomarker Characterization and Prediction of Virulence and Antibiotic Resistance from Helicobacter pylori Next Generation Sequencing Data. Biomolecules, 12(5), 691. https://doi.org/10.3390/biom12050691






