Flavonoid Biosynthesis Genes in Triticum aestivum L.: Methylation Patterns in Cis-Regulatory Regions of the Duplicated CHI and F3H Genes
Abstract
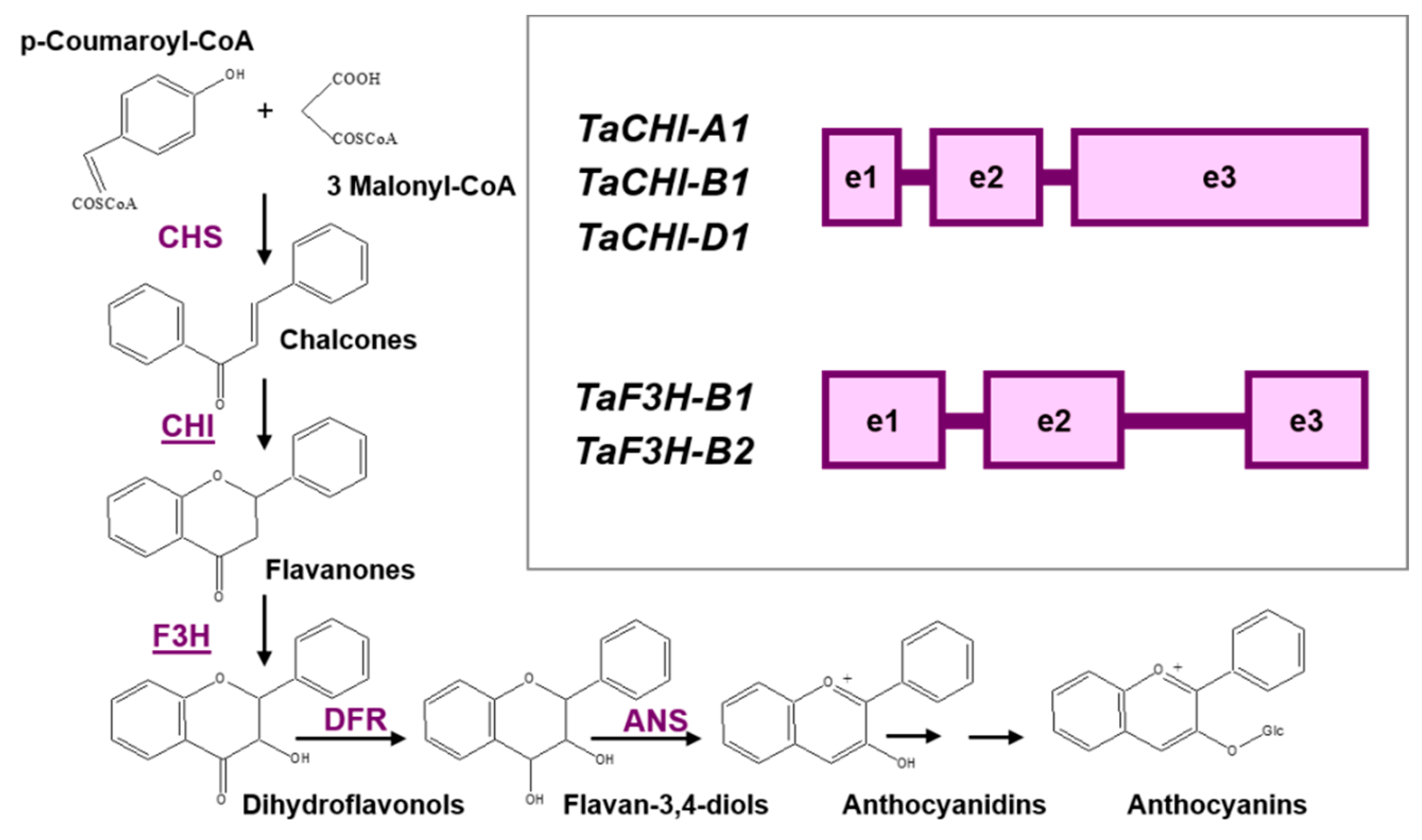
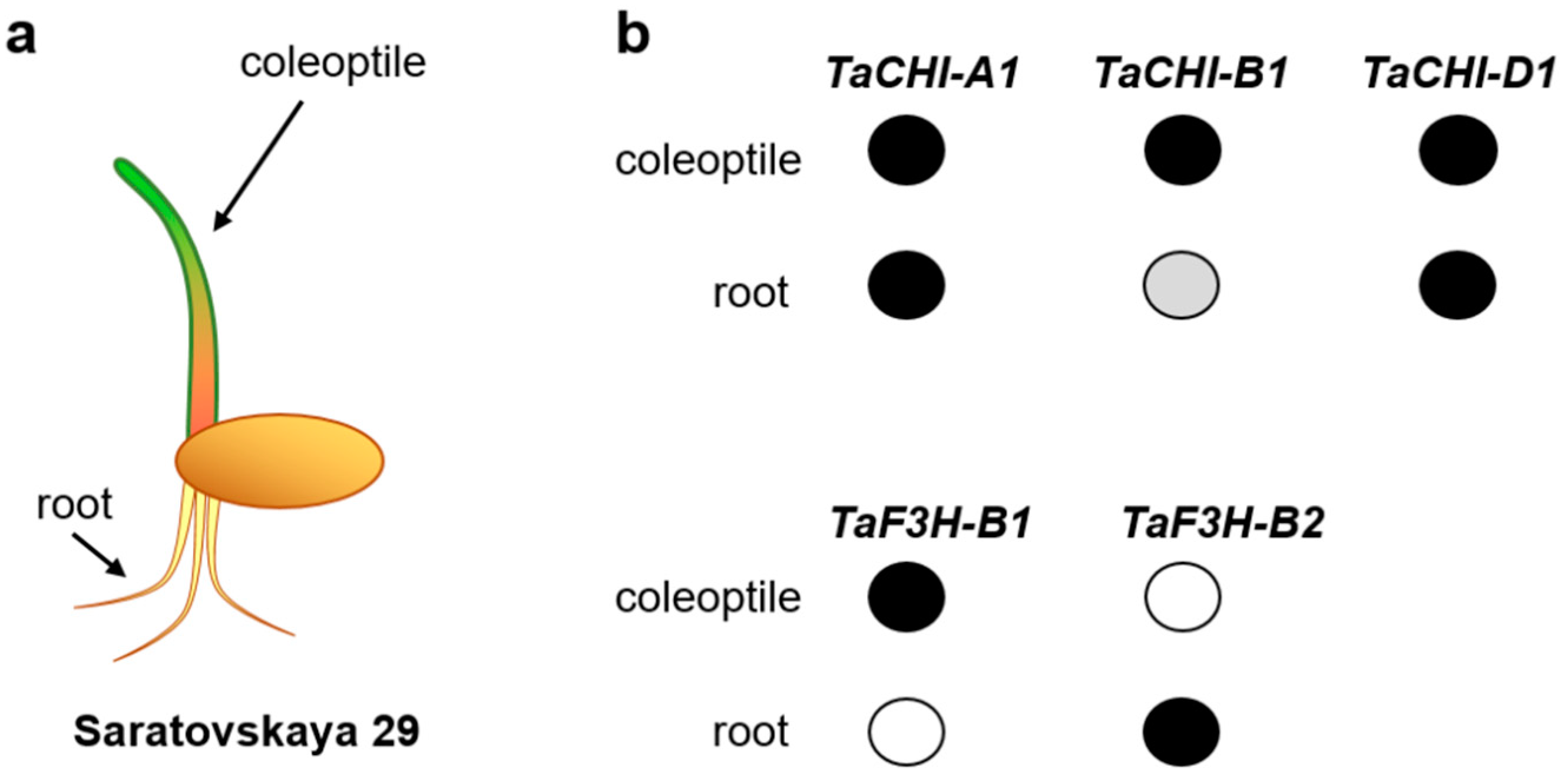
:1. Introduction
2. Materials and Methods
2.1. Plant Material, DNA Isolation, and Sodium Bisulfite Treatment
2.2. In Silico Promoter Analysis, and Primers’ Design
2.3. PCR, Electrophoretic Analysis, Extraction, and Purification
2.4. Cloning and Sequencing the Amplified PCR Fragments
3. Results
3.1. Prediction of CpG Islands
3.2. Analysis of Promoters
3.3. DNA Methylation in the Promoters of the TaCHI and TaF3H Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glover, N.M.; Redestig, H.; Dessimoz, C. Homoeologs: What Are They and How Do We Infer Them? Trends Plant Sci. 2016, 21, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.J. Genetic and Epigenetic Mechanisms for Gene Expression and Phenotypic Variation in Plant Polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.J.; Birchler, J.A. Polyploid and Hybrid Genomics; Chen, Z.J., Birchler, J.A., Eds.; John Wiley & Sons, Inc.: Oxford, UK, 2013; ISBN 9781118552872. [Google Scholar]
- Kaufmann, K.; Pajoro, A.; Angenent, G.C. Regulation of transcription in plants: Mechanisms controlling developmental switches. Nat. Rev. Genet. 2010, 11, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, L.-J.; Quinton-Tulloch, M.; Olohan, L.; Price, J.; Hall, N.; Hall, A. A genome-wide survey of DNA methylation in hexaploid wheat. Genome Biol. 2015, 16, 273. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Jiao, W.; Liu, Y.; Ye, W.; Wang, X.; Liu, B.; Song, Q.; Chen, Z.J. Dynamic and reversible DNA methylation changes induced by genome separation and merger of polyploid wheat. BMC Biol. 2020, 18, 171. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Strygina, K.V. Synthesis of Flavonoid Pigments in Grain of Representatives of Poaceae: General Patterns and Exceptions in N.I. Vavilov’s Homologous Series. Russ. J. Genet. 2020, 56, 1345–1358. [Google Scholar] [CrossRef]
- Himi, E.; Maekawa, M.; Noda, K. Differential Expression of Three Flavanone 3-Hydroxylase Genes in Grains and Coleoptiles of Wheat. Int. J. Plant Genomics 2011, 2011, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himi, E.; Noda, K. Isolation and location of three homoeologous dihydroflavonol-4-reductase (DFR) genes of wheat and their tissue-dependent expression. J. Exp. Bot. 2004, 55, 365–375. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Ivanisenko, N.V.; Khlestkina, E.K. Organization and evolution of the chalcone synthase gene family in bread wheat and relative species. BMC Genet. 2019, 20, 30. [Google Scholar] [CrossRef] [Green Version]
- Khlestkina, E.K.; Röder, M.S.; Salina, E.A. Relationship between homoeologous regulatory and structural genes in allopolyploid genome—A case study in bread wheat. BMC Plant Biol. 2008, 8, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khlestkina, E.K.; Tereshchenko, O.Y.; Salina, E.A. Anthocyanin biosynthesis genes location and expression in wheat–rye hybrids. Mol. Genet. Genomics 2009, 282, 475. [Google Scholar] [CrossRef] [PubMed]
- Khlestkina, E.K.; Dobrovolskaya, O.B.; Leonova, I.N.; Salina, E.A. Diversification of the Duplicated F3h Genes in Triticeae. J. Mol. Evol. 2013, 76, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Shoeva, O.Y.; Khlestkina, E.K.; Berges, H.; Salina, E.A. The homoeologous genes encoding chalcone–flavanone isomerase in Triticum aestivum L.: Structural characterization and expression in different parts of wheat plant. Gene 2014, 538, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; Li, B.; Gao, J.W.; Liu, J.Z.; Zhao, X.Q.; Zheng, Q.; Tong, Y.P.; Li, Z.S.; Yang, G.H.; Li, B.; et al. Cloning and expression of two chalcone synthase and a flavonoid 3’5’-hydroxylase 3’-end cDNAs from developing seeds of blue-grained wheat involved in anthocyanin biosynthetic pathway. Acta Bot. Sin. 2004, 46, 588–594. [Google Scholar]
- Shoeva, O.Y.; Khlestkina, E.K. F3h gene expression in various organs of wheat. Mol. Biol. 2013, 47, 901–903. [Google Scholar] [CrossRef]
- Khlestkina, E. The adaptive role of flavonoids: Emphasis on cereals. Cereal Res. Commun. 2013, 41, 185–198. [Google Scholar] [CrossRef]
- Himi, E.; Taketa, S. Barley Ant17, encoding flavanone 3-hydroxylase (F3H), is a promising target locus for attaining anthocyanin/proanthocyanidin-free plants without pleiotropic reduction of grain dormancy. Genome 2015, 58, 43–53. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Dobrovolskaya, O.B.; Leonova, I.N.; Salina, E.A.; Khlestkina, E.K. The B-, G- and S-genomic Chi genes in family Triticeae. Biol. Plant. 2016, 60, 279–284. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, T.; Nan, W.; Jeewani, D.C.; Niu, Y.; Li, C.; Wang, Y.; Shi, X.; Wang, C.; Wang, J.; et al. Two transcription factors TaPpm1 and TaPpb1 co-regulate anthocyanin biosynthesis in purple pericarps of wheat. J. Exp. Bot. 2018, 69, 2555–2567. [Google Scholar] [CrossRef] [Green Version]
- Presinszka, M.; Stiasna, K.; Vyhnanek, T.; Trojan, V.; Schweiger, W.; Bartos, J.A.N.; Havel, L. Primer efficiency determination for flavanone 3-hydroxylase gene in wheat (Triticum aestivum L.). In Proceedings of the International Ph.D. Students Conference on MendelNet 2014At: At Fac Agron, Brno, Czech Republic, 19–20 November 2014; pp. 469–473. [Google Scholar]
- Liu, D.; Li, S.; Chen, W.; Zhang, B.; Liu, D.; Liu, B.; Zhang, H. Transcriptome Analysis of Purple Pericarps in Common Wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, X.; Hou, H.; Ma, X.; Sun, S.; Wang, H.; Kong, L. Metabolomics and gene expression analysis reveal the accumulation patterns of phenylpropanoids and flavonoids in different colored-grain wheats (Triticum aestivum L.). Food Res. Int. 2020, 138, 109711. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-C.; Lee, T.-Y.; Huang, H.-D.; Huang, H.-Y.; Pan, R.-L. PlantPAN: Plant promoter analysis navigator, for identifying combinatorial cis-regulatory elements with distance constraint in plant gene groups. BMC Genomics 2008, 9, 561. [Google Scholar] [CrossRef] [Green Version]
- Chow, C.-N.; Zheng, H.-Q.; Wu, N.-Y.; Chien, C.-H.; Huang, H.-D.; Lee, T.-Y.; Chiang-Hsieh, Y.-F.; Hou, P.-F.; Yang, T.-Y.; Chang, W.-C. PlantPAN 2.0: An update of plant promoter analysis navigator for reconstructing transcriptional regulatory networks in plants. Nucleic Acids Res. 2016, 44, D1154–D1160. [Google Scholar] [CrossRef] [Green Version]
- Chow, C.-N.; Lee, T.-Y.; Hung, Y.-C.; Li, G.-Z.; Tseng, K.-C.; Liu, Y.-H.; Kuo, P.-L.; Zheng, H.-Q.; Chang, W.-C. PlantPAN3.0: A new and updated resource for reconstructing transcriptional regulatory networks from ChIP-seq experiments in plants. Nucleic Acids Res. 2019, 47, D1155–D1163. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-C.; Dahiya, R. MethPrimer: Designing primers for methylation PCRs. Bioinformatics 2002, 18, 1427–1431. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, W. OLIGO 7 primer analysis software. Methods Mol. Biol. 2007, 402, 35–59. [Google Scholar] [CrossRef]
- Gu, Z.; Rifkin, S.A.; White, K.P.; Li, W.-H. Duplicate genes increase gene expression diversity within and between species. Nat. Genet. 2004, 36, 577–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teichmann, S.A.; Babu, M.M. Gene regulatory network growth by duplication. Nat. Genet. 2004, 36, 492–496. [Google Scholar] [CrossRef]
- Voordeckers, K.; Pougach, K.; Verstrepen, K.J. How do regulatory networks evolve and expand throughout evolution? Curr. Opin. Biotechnol. 2015, 34, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strygina, K.V.; Khlestkina, E.K. Myc-like transcriptional factors in wheat: Structural and functional organization of the subfamily I members. BMC Plant Biol. 2019, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Strygina, K.V.; Börner, A.; Khlestkina, E.K. Identification and characterization of regulatory network components for anthocyanin synthesis in barley aleurone. BMC Plant Biol. 2017, 17, 184. [Google Scholar] [CrossRef] [Green Version]
- Strygina, K.V.; Khlestkina, E.K. Structural and functional divergence of the Mpc1 genes in wheat and barley. BMC Evol. Biol. 2019, 19, 45. [Google Scholar] [CrossRef]
- Gehring, M.; Henikoff, S. DNA methylation dynamics in plant genomes. Biochim. Biophys. Acta-Gene Struct. Expr. 2007, 1769, 276–286. [Google Scholar] [CrossRef]
- Zilberman, D.; Gehring, M.; Tran, R.K.; Ballinger, T.; Henikoff, S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat. Genet. 2007, 39, 61–69. [Google Scholar] [CrossRef]
- Bewick, A.J.; Schmitz, R.J. Gene body DNA methylation in plants. Curr. Opin. Plant Biol. 2017, 36, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yazaki, J.; Sundaresan, A.; Cokus, S.; Chan, S.W.L.; Chen, H.; Henderson, I.R.; Shinn, P.; Pellegrini, M.; Jacobsen, S.E.; et al. Genome-wide High-Resolution Mapping and Functional Analysis of DNA Methylation in Arabidopsis. Cell 2006, 126, 1189–1201. [Google Scholar] [CrossRef] [Green Version]
- To, T.K.; Saze, H.; Kakutani, T. DNA Methylation within Transcribed Regions. Plant Physiol. 2015, 168, 1219–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shitsukawa, N.; Tahira, C.; Kassai, K.-I.; Hirabayashi, C.; Shimizu, T.; Takumi, S.; Mochida, K.; Kawaura, K.; Ogihara, Y.; Murai, K. Genetic and Epigenetic Alteration among Three Homoeologous Genes of a Class E MADS Box Gene in Hexaploid Wheat. Plant Cell 2007, 19, 1723–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz-Ares, J.; Ghosal, D.; Wienand, U.; Peterson, P.A.; Saedler, H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987, 6, 3553. [Google Scholar] [CrossRef]
- Himi, E.; Taketa, S. Isolation of candidate genes for the barley Ant1 and wheat Rc genes controlling anthocyanin pigmentation in different vegetative tissues. Mol. Genet. Genomics 2015, 290, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Shoeva, O.Y.; Kukoeva, T.V.; Börner, A.; Khlestkina, E.K. Barley Ant1 is a homolog of maize C1 and its product is part of the regulatory machinery governing anthocyanin synthesis in the leaf sheath. Plant Breed. 2015, 134, 400–405. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, M.-G.; Kang, C.-S.; Park, C.-S.; Choi, S.-B.; Park, Y.-I. A wheat R2R3-MYB protein PURPLE PLANT1 (TaPL1) functions as a positive regulator of anthocyanin biosynthesis. Biochem. Biophys. Res. Commun. 2016, 469, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, E.I.; Shoeva, O.Y.; Khlestkina, E.K. Marker-assisted development of bread wheat near-isogenic lines carrying various combinations of purple pericarp (Pp) alleles. Euphytica 2015, 203, 469–476. [Google Scholar] [CrossRef]
- Shoeva, O.; Gordeeva, E.; Khlestkina, E. The Regulation of Anthocyanin Synthesis in the Wheat Pericarp. Molecules 2014, 19, 20266–20279. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Punetha, A.; Grover, A.; Sundar, D. Insights into the key enzymes of secondary metabolites biosynthesis in Camellia sinensis. J. Bioinforma. Seq. Anal. 2010, 2, 53–68. [Google Scholar]
- Tereshchenko, O.Y.; Arbuzova, V.S.; Khlestkina, E.K. Allelic state of the genes conferring purple pigmentation in different wheat organs predetermines transcriptional activity of the anthocyanin biosynthesis structural genes. J. Cereal Sci. 2013, 57, 10–13. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer | PCR Product Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| TaCHI-A1 | 5′ TTATTAATTAAGTAGAAAAGAATTGTTTAGTTAA 3′ | 5′ TAAAAACRTAATTAATAATAATTAAAAAAAACAC 3′ | 374 | 50 |
| 5′ GTTTTTGYGTGAGTTGAATGGTAG 3′ | 5′ ACRAAATAATCAACTAAAATAATAATACC 3′ | 298 | 50 | |
| 5′ GGAGAGGGTATTATTATTTTAGTTG 3′ | 5′ AAACCRTTAACTAAATAATCACCTAC 3′ | 273 | 50 | |
| TaCHI-B1 | 5′ TTTGATTATTGTTATTTTTATTTTAAATATGTATGT 3′ | 5′ ATATAAAAACRTAATTAATAATAATTAAAAAAAACA 3′ | 436 | 50 |
| 5′ TYGTAGGGTGGTTTTTTTTGAGA 3′ | 5′ TAACRAACAAACACTTATATTAATAAAAC 3′ | 464 | 50 | |
| 5′ AGTTGTTGYGGTGTTATATAGGG 3′ | 5′ ACCAAATACRTTAAAACAAAATCTAAAATC 3′ | 343 | 53 | |
| TaCHI-D1 | 5′ TATATTTTATAAGGTGGTTTTTTTAATTTTGTTGAG 3′ | 5′ TAAAAACRTAATTAATAATAATTAAAAAAAACAC 3′ | 320 | 53 |
| 5′ GTGYGTGAGTTGAATGGTAGTTTG 3′ | 5′ TAATAATACRTACCCCCCCTATATAACACC 3′ | 294 | 55 | |
| 5′ GYGTAGGTTAGAGAATTAGATTAG 3′ | 5′ AAATAAACRTACTCRAAACCCRAC 3′ | 326 | 50 | |
| TaF3H-B1 | 5′ ATGATGTATAGGTTTTAGATATTGGG 3′ | 5′ ATATACRCACAACACACACATCAC 3′ | 400 | 53 |
| 5′ ATATGAGYGTTTGTATTTGGATTGTG 3′ | 5′ CTCRTATAATAATTTATTCCTTAATAAAAAACTC 3′ | 365 | 53 | |
| 5′ TAAAYGGTYGAGTTTTTTATTAAGGAATAAATTATT 3′ | 5′ TCRAAAAAAACRTCTCGTTACTCACC 3′ | 125 | 53 | |
| TaF3H-B2 | 5′ GYGAGTTTAGATGGTTAGATATTTTTTGT 3′ | 5′ CACTAAATAAACATCACCAAAAAATCTAAAAT 3′ | 410 | 53 |
| 5′ TTATTTTAGATTTTTTGGTGATGTTTATTTA 3′ | 5′ AAACAAAAACATCCTATTACTCAC 3′ | 222 | 50 |
| Gene | Tissue | Methylation Sites in Colonies | ||
|---|---|---|---|---|
| CpG | CpHpG | CpHpH | ||
| TaCHI-A1 | coleoptile | 0/60 in 10 | 0/54 in 10 | 0/125 in 10 |
| root | 0/60 in 8 11/60 in 2 | 0/54 in 8 7/54 in 2 | 0/125 in 8 15/125 in 2 | |
| TaCHI-B1 | coleoptile | 0/50 in 10 | 0/42 in 10 | 0/118 in 10 |
| root | 7/50 in 10 | 2/42 in 10 | 1/118 in 10 | |
| TaCHI-D1 | coleoptile | 0/44 in 10 | 0/46 in 10 | 0/112 in 10 |
| root | 0/44 in 10 | 0/46 in 10 | 0/112 in 10 | |
| TaF3H-B1 | coleoptile | 25/59 in 4 26/59 in 6 | 4/41 in 4 5/41 in 1 7/41 in 4 8/41 in 1 | 12/72 in 2 13/72 in 1 14/72 in 1 18/72 in 1 25/72 in 2 26/72 in 2 27/72 in 1 |
| root | 25/59 in 4 26/59 in 6 | 5/41 in 2 7/41 in 8 | 17/72 in 1 18/72 in 1 24/72 in 3 25/72 in 5 | |
| TaF3H-B2 | coleoptile | 0/12 in 6 1/12 in 1 3/12 in 1 4/12 in 3 | 0/13 in 6 2/13 in 4 | 0/76 in 6 3/76 in 1 4/76 in 3 |
| root | 0/12 in 7 4/12 in 3 | 0/13 in 7 2/13 in 3 | 0/76 in 7 6/76 in 3 | |
| Methylation Status | ||||||
|---|---|---|---|---|---|---|
| Gene | Motif Name | Sequence | Function | Reference | Coleoptile | Root |
| TaCHI-A1 | ABRELATERD1 | ACGTG | Element required for etiolation-induced expression of the gene responsive to dehydration | S000414 | - | + |
| ACGTATERD1 | ACGT | Sequence required for etiolation-induced expression | S000415 | - | + | |
| CBFHV | RYCGAC | Dehydration-responsive element | S000497 | - | + | |
| CGACGOSAMY3 | CGACG | Coupling element for the G box element | S000205 | - | + | |
| CGCGBOXAT | VCGCGB | Element in promoters of many genes | S000501 | - | + | |
| CRTDREHVCBF2 | GTCGAC | Core CRT/DRE motif | S000411 | - | + | |
| HEXAMERATH4 | CCGTCG | Hexamer motif of the Arabidopsis thaliana histone H4 promoter | S000146 | - | + | |
| MYBCORE | CNGTTR | Binding site for animal and plant MYB proteins | S000176 | - | + | |
| SORLIP2AT | GGGCC | Sequences over-represented in light-induced promoters | S000483 | - | + | |
| SURECOREATSULTR11 | GAGAC | Core of sulfur-responsive element | S000499 | - | + | |
| TaCHI-B1 | CBFHV | RYCGAC | Dehydration-responsive element | S000497 | - | + |
| DRECRTCOREAT | RCCGAC | Core motif of a dehydration-responsive element/C-repeat cis-acting element | S000418 | - | + | |
| LTRECOREATCOR15 | CCGAC | Core of low temperature-responsive element | S000153 | - | + | |
| MYB2CONSENSUSAT | YAACKG | MYB recognition site | S000409 | - | + | |
| MYBCORE | CNGTTR | Binding site for animal and plant MYB proteins | S000176 | - | + | |
| MYBCOREATCYCB1 | AACGG | Myb core | S000502 | - | + | |
| PALBOXAPC | CCGTCC | Box A | S000137 | - | + | |
| TaF3H-B1 | ABRELATERD1 | ACGTG | Element required for etiolation-induced expression of the gene responsive to dehydration | S000414 | + | + |
| ABRERATCAL | MACGYGB | “Repeated sequence motifs” identified DE in the upstream regions of Ca(2+)-responsive upregulated DE genes | S000507 | + | + | |
| ARFAT | TGTCTC | ARF (auxin response factor) binding site found in the promoters DE of primary/early auxin response genes in Arabidopsis thaliana | S000270 | + | + | |
| ACGTATERD1 | ACGT | Sequence required for etiolation-induced expression | S000415 | + | + | |
| CBFHV | RYCGAC | Dehydration-responsive element | S000497 | + | + | |
| CGACGOSAMY3 | CGACG | Coupling element for the G box element | S000205 | + | + | |
| CGCGBOXAT | VCGCGB | Element in promoters of many genes | S000501 | + | + | |
| CRTDREHVCBF2 | GTCGAC | Core CRT/DRE motif | S000411 | + | + | |
| CURECORECR | GTAC | Core of a copper-response element | S000493 | + | + | |
| DOFCOREZM | AAAG | Core site required for binding of Dof | S000265 | + | + | |
| DPBFCOREDCDC3 | ACACNNG | Binding core sequence of f-bZIP transcription factors | S000292 | + | + | |
| GATABOX | GATA | Motif required for high-level, light-regulated, and tissue-specific expression | S000039 | + | + | |
| LTRE1HVBLT49 | CCGAAA | Low-temperature-responsive element | S000250 | + | + | |
| MYBCOREATCYCB1 | AACGG | Myb core | S000502 | + | + | |
| MYBST1 | GGATA | Core motif of MYB binding site | S000180 | + | + | |
| NODCON2GM | CTCTT | Putative nodulin consensus sequences | S000462 | + | + | |
| OSE2ROOTNODULE | CTCTT | One of the consensus sequence motifs of organ-specific elements DE (OSE) characteristic of the promoters activated in infected cells DE of root nodules | S000468 | + | + | |
| RHERPATEXPA7 | KCACGW | Right part of RHEs (Root Hair-specific cis-Elements) | S000512 | + | + | |
| SEBFCONSSTPR10A | YTGTCWC | Binding site of the potato silencing element binding factor DE (SEBF) gene found in the promoter of the pathogenesis-related gene DE (PR-10a) | S000391 | + | + | |
| SURECOREATSULTR11 | GAGAC | Core of sulfur-responsive element | S000499 | + | + | |
| TaF3H-B2 | CURECORECR | GTAC | Core motif of a CuRE (copper-response element) | S000493 | + | + |
| DPBFCOREDCDC3 | ACACNNG | bZIP transcription factor binding core sequence | S000292 | - | + | |
| ERELEE4 | AWTTCAAA | Ethylene responsive element | S000037 | + | - | |
| GATABOX | GATA | Motif required for high-level, light-regulated, and tissue-specific expression | S000039 | + | + | |
| REALPHALGLHCB21 | AACCAA | Motif required for phytochrome regulation; the DNA binding activity is high in etiolated plants | S000362 | + | + | |
| RHERPATEXPA7 | KCACGW | Right part of RHEs | S000512 | - | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strygina, K.; Khlestkina, E. Flavonoid Biosynthesis Genes in Triticum aestivum L.: Methylation Patterns in Cis-Regulatory Regions of the Duplicated CHI and F3H Genes. Biomolecules 2022, 12, 689. https://doi.org/10.3390/biom12050689
Strygina K, Khlestkina E. Flavonoid Biosynthesis Genes in Triticum aestivum L.: Methylation Patterns in Cis-Regulatory Regions of the Duplicated CHI and F3H Genes. Biomolecules. 2022; 12(5):689. https://doi.org/10.3390/biom12050689
Chicago/Turabian StyleStrygina, Ksenia, and Elena Khlestkina. 2022. "Flavonoid Biosynthesis Genes in Triticum aestivum L.: Methylation Patterns in Cis-Regulatory Regions of the Duplicated CHI and F3H Genes" Biomolecules 12, no. 5: 689. https://doi.org/10.3390/biom12050689
APA StyleStrygina, K., & Khlestkina, E. (2022). Flavonoid Biosynthesis Genes in Triticum aestivum L.: Methylation Patterns in Cis-Regulatory Regions of the Duplicated CHI and F3H Genes. Biomolecules, 12(5), 689. https://doi.org/10.3390/biom12050689







