Essential Oils of Duguetia Species A. St. Hill (Annonaceae): Chemical Diversity and Pharmacological Potential
Abstract
1. Introduction
2. Chemical Constituents of Duguetia Species EOs
3. Pharmacological Properties of Duguetia Species EO
3.1. Anti-Inflammatory Activity
3.2. Antinociceptive Activity
3.3. Antibacterial and Antifungal Activities
3.4. Trypanocidal Activity
3.5. Antioxidant Activity
3.6. Cytotoxic and Antitumor Activity
3.7. Other Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EC50 | effective concentration of 50% |
| ED50 | effective dose of 50% |
| EOs | essential oils |
| IC50 | inhibitory concentration of 50% |
| LC50 | lethal concentration of 50% |
| LPS | lipopolysaccharides |
| MIC | minimum inhibitory concentration |
References
- Couvreur, T.L.P.; Pirie, M.D.; Chatrou, L.W.; Saunders, R.M.K.; SU, Y.C.F.; Richardson, J.E.; Erkens, R.H.J. Early evolutionary history of the flowering plant family Annonaceae: Steady diversification and boreotropical geodispersal. J. Biogeogr. 2011, 38, 664–680. [Google Scholar] [CrossRef]
- Lobão, A.Q.; Lopes, J.C.; Erkens, R.H.J.; Mendes-Silva, I.; Pontes Pires, A.F.; Silva, L.V.; Oliveira, M.L.B.; Johnson, D.; Mello-Silva, R. (In Memoriam). Annonaceae in Flora do Brasil 2020. Rio de Janeiro: Jardim Botânico do Rio de Janeiro. 2020. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB110219 (accessed on 11 August 2021).
- Maas, P.J.M.; Rainer, H.; Lobão, A.Q. Annonaceae in Lista de Espécies da Flora do Brasil. Rio de Janeiro: Jardim Botânico do Rio de Janeiro. 2013. Available online: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB110219 (accessed on 12 August 2021).
- Maas, P.J.M.; Maas, H.; Miralha, J.M.S. Flora da Reserva Ducke, Amazonas, Brasil: Annonaceae. Rodriguesia 2007, 58, 617–662. [Google Scholar] [CrossRef]
- Lopes, J.C.; Silva, R.M. Annonaceae da Reserva Natural Vale, Linhares, Espírito Santo. Rodriguesia 2014, 65, 599–635. [Google Scholar] [CrossRef]
- Perez, E.G.; Cassels, B.K. Alkaloids from the Genus Duguetia. Alkaloids Chem. Biol. 2010, 68, 83–156. [Google Scholar]
- Gonçalves, G.L.P.; Domingues, V.C.; Ribeiro, L.P.; Fernandes, J.B.; Fernandes, M.F.G.; Forim, M.R.; Vendramim, J.D. Compounds from Duguetia lanceolata St.-Hil. (Annonaceae) bioactive against Zabrotes subfasciatus (Boheman) (Coleoptera: Chrysomelidae: Bruchinae). Ind. Crops Prod. 2017, 97, 360–367. [Google Scholar] [CrossRef]
- Lobão, A.Q. Duguetia in Flora do Brasil 2020. Rio de Janeiro: Jardim Botânico do Rio de Janeiro. 2020. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB110296 (accessed on 15 August 2021).
- Nardelli, V.B.; Souza, C.A.S.; Chaar, J.S.; Koolen, H.H.F.; Silva, F.M.A.; Costa, E.V. Isoquinoline-derived alkaloids and one terpene lactone from the leaves of Duguetia pycnastera (Annonaceae). Biochem. Syst. Ecol. 2021, 94, 23–26. [Google Scholar] [CrossRef]
- Bevalot, F.; Leboeuf, M.; Bouquet, A.; Cavé, A. Dosage de I’allantoine. Ann. Pharm. Fr. 1977, 35, 65. [Google Scholar]
- Silberbauer-Gottsberger, I. O cerrado como potencial de plantas medicinais e tóxicas. Oréades 1981, 8, 15–30. [Google Scholar]
- Navarro, V.R.; Sette, I.M.F.; Da Cunha, E.V.L.; Silva, M.S.; Barbosa-Fiho, J.M.; Maia, J.G.S. Alcaloides de Duguetia flagellaris Huber (Annonaceae). Rev. Bras. Pl. Med. 2001, 3, 23–29. [Google Scholar]
- Pott, A.; Pott, V.J. Plantas do Pantanal; EMBRAPA: Corumba, Brazil, 1994. [Google Scholar]
- Defilipps, R.A.; Maina, S.L.; Crepin, J. Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana); Department of Botany, National Museum of Natural History, Smithsonian Institution: Washington, DC, USA, 2004; pp. 20013–27012. [Google Scholar]
- Almeida, J.R.G.S.; Oliveira, M.R.; Guimarães, A.L.; Oliveira, A.P.; Ribeiro, L.A.A.; Lúcio, A.S.S.C.; Quintans, L.J., Jr. Phenolic quantification and antioxidant activity of Anaxagorea dolichocarpa and Duguetia chrysocarpa (Annonaceae). Int. J. Pharma Bio Sci. 2011, 2, 367–374. [Google Scholar]
- Almeida, J.R.G.S.; Araújo, E.C.C.; Ribeiro, L.A.A.; De Lima, J.T.; Nunes, X.P.; Lúcio, A.S.S.C.; Agra, M.F.; Filho, J.M.B. Antinociceptive activity of ethanol extract from Duguetia chrysocarpa Maas (Annonaceae). Sci. World J. 2012, 2012, 859210. [Google Scholar] [CrossRef]
- Saldanha, A.A.; Vieira, L.; Maia, D.S.S.; Oliveira, F.M.; Ribeiro, R.I.M.A.; Thomé, R.G.; Santos, H.B.; Lopes, D.O.; Carollo, C.A.; Silva, D.B.; et al. Anti-inflammatory and antinociceptive activities of a phenylpropanoid-enriched fraction of Duguetia furfuracea. Inflammopharmacology 2021, 29, 409–422. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Carvalho-Filho, J.L.S.; Blank, A.F.; Alves, P.B.; Ehlert, P.A.D.; Melo, A.S.; Cavalcanti, S.C.H.; Arrigoni-Blank, M.F.; Silva-Mann, R. Influence of the harvesting time, temperature and drying period on basil (Ocimum basilicum L.) essential oil. Rev. Bras. Farmacogn. 2006, 16, 24–30. [Google Scholar] [CrossRef]
- Silva, T.B.; Menezes, L.R.A.; Sampaio, M.F.C.; Meira, C.S.; Guimarães, E.T.; Soares, M.B.P.; Prata, A.P.N.; Nogueira, P.C.L.; Costa, E.V. Chemical composition and anti-Trypanosoma cruzi activity of essential oils obtained from leaves of Xylopia frutescens and Xylopia laevigata (Annonaceae). Nat. Prod. Commun. 2013, 8, 403–406. [Google Scholar] [CrossRef]
- Jürgens, A.; Webber, A.C.; Gottsberger, G. Floral scent compounds of Amazonian Annonaceae species pollinated by small beetles and thrips. Phytochemistry 2000, 55, 551–558. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; Carreira, L.M.M.; Oliveira, J. Essential oil composition from Duguetia species (annonaceae). J. Essent. Oil Res. 2006, 18, 60–63. [Google Scholar] [CrossRef]
- Fechine, I.M.; Navarro, V.R.; Da-Cunha, E.V.L.; Silva, M.S. Alkaloids and volatile constituents from Duguetia flagellaris. Biochem. Syst. Ecol. 2002, 30, 267–269. [Google Scholar] [CrossRef]
- Silva, D.B.; Tulli, E.C.O.; Garcez, W.S.; Nascimento, E.A.; Siqueira, J.M. Chemical constituents of the underground stem bark of Duguetia furfuracea (Annonaceae). J. Braz. Chem. Soc. 2007, 18, 1560–1565. [Google Scholar] [CrossRef]
- Vidotto, C.; Silva, D.B.; Patussi, R.; Gardini, L.F.; Tibúrcio, J.D.; Alves, S.N.; Siqueira, J.M. Brine shrimp lethality test as a biological model for preliminary selection of pediculicidal components from natural source. Biosci. J. 2013, 29, 255–263. [Google Scholar]
- Saldanha, A.A.; Vieira, L.; Ribeiro, R.I.M.A.; Thomé, R.G.; Santos, H.B.; Silva, D.B.; Carollo, C.A.; Oliveira, F.M.; Lopes, D.O.; Siqueira, J.M.; et al. Chemical composition and evaluation of the anti-inflammatory and antinociceptive activities of Duguetia furfuracea essential oil: Effect on edema, leukocyte recruitment, tumor necrosis factor alpha production, iNOS expression, and adenosinergic and opioid. J. Ethnopharmacol. 2019, 231, 325–336. [Google Scholar] [CrossRef]
- Valter, J.L.; Alencar, K.M.C.; Sartori, A.L.B.; Nascimento, E.A.; Chang, R.; De Morais, S.A.L.; Laura, V.A.; Yoshida, N.C.; Carollo, C.A.; Da Silva, D.B.; et al. Variação química no óleo essencial das folhas de seis indivíduos de Duguetia furfuracea (Annonaceae). Rev. Bras. Farmacogn. 2008, 18, 373–378. [Google Scholar] [CrossRef][Green Version]
- Almeida, J.R.G.; Facanali, R.; Vieira, M.A.R.; Marques, M.O.M.; Lúcio, A.S.S.C.; Lima, E.O.; Agra, M.F.; Barbosa-Filho, J.M. Composition and antimicrobial activity of the leaf essential oils of Duguetia gardneriana Mart. and Duguetia moricandiana Mart. (Annonaceae). J. Essent. Oil Res. 2010, 22, 275–278. [Google Scholar] [CrossRef]
- Rodrigues, A.C.B.C.; Bomfim, L.M.; Neves, S.P.; Menezes, L.R.A.; Dias, R.B.; Soares, M.B.P.; Prata, A.P.N.; Rocha, C.A.G.; Costa, E.V.; Bezerra, D.P. Antitumor Properties of the Essential Oil from the Leaves of Duguetia gardneriana. Planta Med. 2015, 81, 798–803. [Google Scholar] [CrossRef]
- Siqueira, J.M.; Müller, L.; Carollo, C.A.; Garcez, W.S.; Boaventura, M.A.D.; Nascimento, E.A. Aromadendrane sesquiterpenoids from the essential oil of leaves of Duguetia glabriuscula—Annonaceae. J. Chil. Chem. Soc. 2003, 48, 89–93. [Google Scholar] [CrossRef]
- Sousa, O.V.; Del-Vechio-Vieira, G.; Alves, M.S.; Araújo, A.A.L.; Pinto, M.A.O.; Amaral, M.P.H.; Rodarte, M.P.; Kaplan, M.A. Chemical composition and biological activities of the essential oils from Duguetia lanceolata St. Hil. barks. Molecules 2012, 17, 11056–11066. [Google Scholar] [CrossRef]
- Ribeiro, L.P.; Domingues, V.C.; Gonçalves, G.L.P.; Fernandes, J.B.; Glória, E.M.; Vendramim, J.D. Essential oil from Duguetia lanceolata St.-Hil. (Annonaceae): Suppression of spoilers of stored-grain. Food Biosci. 2020, 36, 100653. [Google Scholar] [CrossRef]
- Sousa, O.V.; Del-Vechio-Vieira, G.; Santos, B.C.S.; Yamamoto, C.H.; Araújo, A.L.S.M.; Araújo, A.L.A.; Pinto, M.A.O.; Rodarte, M.P.; Alves, M.S. In-vivo and vitro bioactivities of the essential oil of Duguetia lanceolata branches. Afr. J. Pharm. Pharmacol. 2016, 10, 298–310. [Google Scholar]
- Bay, M.; Oliveira, J.V.S.; Junior, P.A.S.; Murta, S.M.F.; Santos, A.R.; Bastos, I.S.; Orlandi, P.P.; Junior, P.T.S. In vitro trypanocidal and antibacterial activities of essential oils from four species of the family Annonaceae. Chem. Biodivers. 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C. Plant chemophenetics—A new term for plant chemosystematics/plant chemotaxonomy in the macro-molecular era. Phytochemistry 2019, 163, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Fournier, G.; Hadjiakhoondi, A.; Leboeuf, M.; Cavé, A.; Charles, B. Essential oils of Annonaceae. Part VIII. Volatile constituents of the essential oils from three Guatteria species. J. Essent. Oil Res. 1997, 9, 275–278. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; Carreira, L.M.M.; Oliveira, J.; Araújo, J.S. Essential oil of the Amazon Guatteria and Guatteriopsis species. Flavour Fragr. J. 2005, 20, 478–480. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; Silva, A.C.M.; Oliveira, J.; Carreira, L.M.M.; Araújo, J.S. Leaf essential oil from four Xylopia species. Flavour Fragr. J. 2005, 20, 474–477. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Kuruppu, A.I.; Paranagama, P.; Goonasekara, C. Medicinal plants commonly used against cancer in traditional medicine formulae in Sri Lanka. Saudi Pharm. J. 2019, 27, 565–573. [Google Scholar] [CrossRef]
- Sousa, O.V.; Soares Júnior, D.T.; Del-Vechio, G.; Mattosinhos, R.G.; Gattass, C.R.; Kaplan, M.A.C. Atividades antinociceptiva e antiinflamatória do óleo essencial de cascas de Duguetia lanceolata St. Hil., Annonaceae. Rev. Bras. Farmacogn. 2004, 14, 11–14. [Google Scholar] [CrossRef]
- Matos, M.F.C.; Leite, L.I.S.P.; Brustolim, D.; De Siqueira, J.M.; Carollo, C.A.; Hellmann, A.R.; Pereira, N.F.G.; Da Silva, D.B. Antineoplastic activity of selected constituents of Duguetia glabriuscula. Fitoterapia 2006, 77, 227–229. [Google Scholar] [CrossRef]
- Mery, B.; Guy, J.B.; Vallard, A.; Espenel, S.; Ardail, D.; Rodriguez-Lafrasse, C.; Rancoule, C.; Magné, N. In vitro cell death determination for drug discovery: A landscape review of real issues. J. Cell Death 2017, 10, 1179670717691251. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. In vitro assays and techniques utilized in anticancer drug discovery. J. Appl. Toxicol. 2019, 39, 38–71. [Google Scholar] [CrossRef] [PubMed]
- Cascaes, M.M.; Carneiro, O.S.; Nascimento, L.D.; de Moraes, A.A.B.; Oliveira, M.S.; Cruz, J.N.; Guilhon, G.M.S.P.; Andrade, E.H.d.A. Essential Oils from Annonaceae Species from Brazil: A Systematic Review of Their Phytochemistry, and Biological Activities. Int. J. Mol. Sci. 2021, 22, 12140. [Google Scholar] [CrossRef] [PubMed]
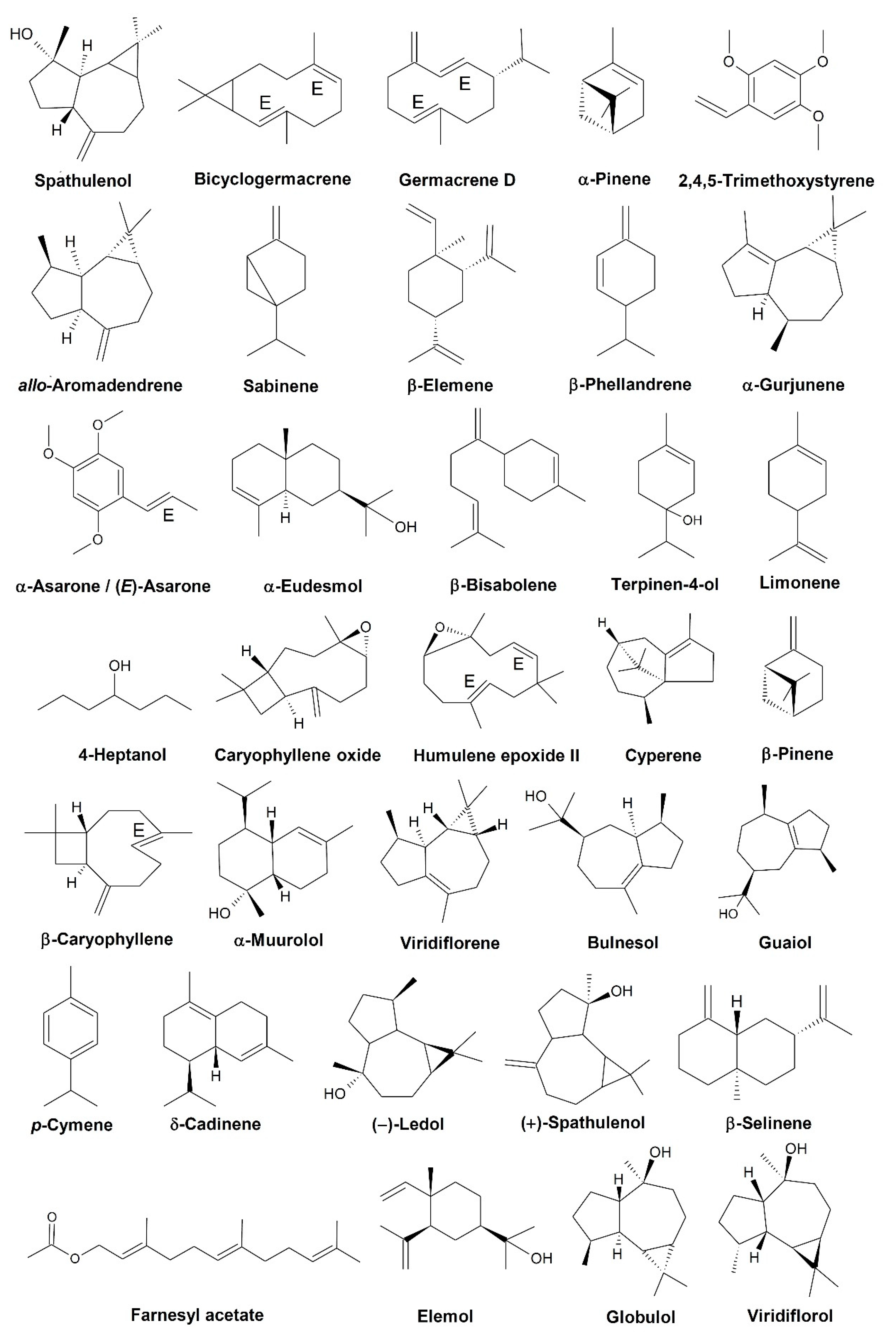
| Species | Popular Name in Brazil | Part Used | Popular Uses | References |
|---|---|---|---|---|
| D. chrysocarpa | Pindaíba-da-mata | Leaves; branch | Inflammatory diseases; gastrointestinal ulcers | [15,16] |
| D. furfuracea | Pinha do campo | Leaves | Renal colic | [17] |
| Root | Stomachache | [11] | ||
| Rheumatism | [12] | |||
| Seeds | Against lice | [11] | ||
| D. flagellaris | Caniceiro preto | - | Rheumatism | [13] |
| D. confinis | Unknown | - | Cough; Toothache | [10] |
| D. staudtii | Unknown | Bark | Gastrointestinal pain; Breathing difficulties | [6] |
| D. pycnastera | Envira preta | Bark | Muscle pain | [14] |
| Cough | ||||
| Leaves | Fever | |||
| Cold sweat |
| Species (Popular Name in Brazil) | Part Used | Total Components | Major Constituents 1 | References |
|---|---|---|---|---|
| D. asterotricha (envira, envira-surucucu-da-mata, envireira) | Flowers | 9 (69.3%) | Limonene (14.1%), p-cymene (5.5%), and α-pinene (4.2%) | [23] |
| D. eximia (unknown) | Leaves and stem | 76 (4) | α-Eudesmol (80.3%) and spathulenol (5.0%) | [24] |
| D. flagellaris (ameju-preto, caniceiro-preto, pindaíva, pindaíba) | Bark | 76 (4) | Germacrene D (16.5%), cyperene (10.6%), α-muurolol (8.6%), humulene epoxide II (5.3%), spathulenol (5.0%), caryophyllene oxide (5.0%), δ-cadinene (4.3%), α-muurolene (4.2%), and β-elemene (4.0%) | [24] |
| Stem | 3 (4) | Germacrene D (16.5%), cyperene (10.6%) and α-muurolol (8.6%) | [25] | |
| Leaves and Stem | 76 (4) | Spathulenol (58.7%), α-muurolol (6.2%) and humulene epoxide II (4.3%) | [24] | |
| Branches | 2 (4) | Spathulenol (58.7%), and α-muurolol (6.2%) | [25] | |
| D. furfuracea (araticum, ata brava, pinha do campo, pindaúva do campo, marolinho-do-cerrado, pinha-de-guará) | Stem | 19 (4) | 2,4,5-Trimethoxystyrene (29.2%), α-asarone (23.8%), bicyclogermacrene (8.6%), epi-globulol (6.4%), and spathulenol (4.7%) | [26] |
| 39 (4) | α-Gurjunene (22.2%), 2,4,5-trimethoxystyrene (19.7%), cyperene (16.0%), α-asarone (10.1%), and trans-m-mentha-4,8-diene (6.5%) | [27] | ||
| 24 (4) | (E)-Asarone (21.9%), bicyclogermacrene (16.7%), 2,4,5-trimethoxystyrene (16.1%), α-gurjunene (15.0%), cyperene (7.8%), and (E)-caryophyllene (4.6%) | [28] | ||
| 8 (4) | α-Asarone (36.4%), 2,4,5-trimethoxystyrene (27.8%), bicyclogermacrene (11.1%), α-gurjunene (10.5%), and cyperene (5.8%) | [17] | ||
| Leaves 2 | 17 (99.4%) | β-Phellandrene (42.2%), bicyclogermacrene (20.7%), myrcene (6.8%), spathulenol (5.5%), α-phellandrene (4.6%), and sabinene (4.3%) | [29] | |
| 17 (99.7%) | Sabinene (25.1%), terpinen-4-ol (16.2%), p-cymene (8.3%), caryophyllene oxide (7.7%), and spathulenol (5.1%) | |||
| 18 (99.2%) | Bicyclogermacrene (29.1%), spathulenol (18.3%), germacrene D (9.6%), trans-caryophyllene (9.3%), δ-cadinene (5.5%), and caryophyllene oxide (5.3%) | |||
| 18 (100%) | Bicyclogermacrene (24%), germacrene D (15%), trans-caryophyllene (12.9%), spathulenol (12.4%), and caryophyllene oxide (6.8%) | |||
| 19 (100%) | Bicyclogermacrene (21.4%), germacrene D (13.6%), spathulenol (12.2%), and caryophyllene oxide (5.2%) | |||
| 20 (99.7%) | Terpinen-4-ol (21.6%), spathulenol (20.9%), sabinene (17.3%), and p-cymene (5.6%) | |||
| 31 (4) | Spathulenol (17.8%), bicyclogermacrene (16.2%), germacrene D (13.0%), β-caryophyllene (11.5%), and viridiflorol (4.0%) | [27] | ||
| D. gardneriana (jaquinha) | Leaves | 33 (91.4%) | Germacrene D (28.1%), viridiflorene (24.0%), β-pinene (12.6%), α-pinene (9.1%), and β-caryophyllene (5.6%) | [30] |
| 4 (96%) | β-Bisabolene (81.0%), elemicin (8.0%), and germacrene D (4.2%) | [31] | ||
| D. glabriuscula (unknown) | Leaves | 18 (4) | allo-Aromadendrene (22.6%), viridiflorene (13.3%), (–)-ledol (10.6%), α-santaleno (7.5%), (+)-spathulenol (5.8%), allo-aromadendrene-14β-al (5.0%), and farnesyl acetate (4.2%) | [32] |
| Leaves | 24 (4) | allo-Aromadendrene (16.2%), (–)-ledol (13.4%), (+)-spathulenol (12.1%), farnesyl acetate (5.9%), and viridiflorol (4.8%) | ||
| D. lanceolata (araticum-bravo, pinha-brava, embireira, embira, pindaíba, pindaúba, pindaíva) | Bark 3 | 72 (99.6%) | β-Elemene (12.7%), caryophyllene oxide (12.4%), β-selinene (8.4%), humulene epoxide II (7.4%), and β-eudesmol (6.8%) | [33] |
| 51 (99.3%) | β-elemene (14.9%), caryophyllene oxide (10.7%) β-selinene (10.4%), β-eudesmol (7.9%), humulene epoxide II (6.8%), β-sinensal (5.4%), and khusinol (5.0%) | |||
| Leaves | 5 (4) | β-Bisabolene (56.2%), 2,4,5-trimethoxystyrene (19.1%), trans-muurola-4(14),5-diene (12.2%), and 3,4,5-trimethoxystyrene (8.6%) | [34] | |
| Branches | 37 (92.9%) | β-Elemene (8.3%), caryophyllene oxide (7.7%), β-eudesmol (7.2%), β-selinene (7.1%), β-caryophyllene (6.2%), δ-cadinene (5.5%), cadina-1,4-dien-3-ol (5.2%), cadalene (4.8%), and δ-elemene (4.1%) | [35] | |
| D. moricandiana (unknown) | Leaves | 33 (95.5%) | Germacrene D (44.3%), α-pinene (13.0%), viridiflorene (9.3%), β-pinene (9.2%), and β-caryophyllene (6.8%) | [30] |
| D. pycnastera (ata, envira, envira-preta, envira-surucucu) | Leaves and stem | 76 (4) | Spathulenol (52.2%), allo-aromadendrene (9.1%), germacrene D (7.1%), elemol (5.1%), and bicyclogermacrene (4.8%) | [24] |
| D. quitarensis (ameju) | Aerial parts | 20 (97.3%) | 4-Heptanol (33.8%), α-thujene (18.4%), (E)-caryophyllene (14.4%), germacrene D (6.3%), and α-copaene (5.3%) | [36] |
| D. riparia (araticu da mata, envira-preta, makahymyra) | Leaves and stem | 76 (4) | Spathulenol (46.5%), caryophyllene oxide (28.9%), and α-pinene (6.1%) | [24] |
| D. trunciflora (envireira, envira, invira) | Leaves and stem | 76 (4) | α-Pinene (21.1%), bicyclogermacrene (17.6%), bulnesol (10.6%), spathulenol (10.5%), guaiol (8.1%), globulol (5.7%), humulene epoxide II (5.0%), and β-pinene (4.2%) | [24] |
| Bark | 76 (4) | β-Phellandrene (45.7%), guaiol (8.3%), α-cadinol (7.4%), (Z)-β-farnesene (4.8%), 7-epi-sesquithujene (4.5%), and bulnesol (4.2%) |
| Pharmacological Effects | Part Used | Actions | References |
|---|---|---|---|
| Anti-inflammatory | |||
| D. furfuracea | Stem | After 6 h, EO inhibited paw edema induced by LPS by 92.4%. | [28] |
| Stem | After 2 h of LPS injection, doses of 3 and 10 mg/kg of EO inhibited paw edema by 90.9% and 92.42%, respectively. After 4 h, there was a significant reduction effect, with percentages of 77.8% (3 mg/kg) and 81.5% (10 mg/kg). | [17] | |
| D. lanceolata | Bark | EO at doses of 50, 100 and 200 mg/kg significantly reduced paw edema caused by carrageenan in 20.8%, 36.5% and 49.0%, respectively. | [43] |
| Branches | After 4 h, EO reduced the formation of paw edema caused by carrageenan by 18.3% (50 mg/kg), 32.3% (100 mg/kg) and 44.1% (200 mg/kg). | [35] | |
| Antinociceptive | |||
| D. furfuracea | Stem | EO inhibited formalin-induced activity, and caffeine (10 mg/kg) and naloxone (5 mg/kg) administration reversed the EO’s antinociceptive activity. | [28] |
| Stem | Inhibition of 43.4% and 44.1% of formalin-induced activity was observed during the 1st phase at doses of 10 and 30 mg/kg, respectively. In the 2nd phase, there was also reduction in licking time at doses of 10 mg/kg (30.9%) and 30 mg/kg (39.8%). | [17] | |
| D. lanceolata | Bark | Number of abdominal contractions (ED50 = 21.8 mg/kg) and paw-licking time 1st phase (ED50 = 5.3 mg/kg) and 2nd phase (ED50 = 1.4 mg/kg) were reduced in the formalin test. | [43] |
| Branches | In the formalin test, EO caused significant and time-dependent inhibition of paw licking at doses of 50, 100 and 200 mg/kg at 1st and 2nd phases. | [35] | |
| Antibacterial and Antifungal | |||
| D. gardneriana | Leaves | EO showed weak activity against Staphylococcus aureus and Candida guilliermondii. | [30] |
| D. lanceolata | Bark | EO inhibited the growth of Staphylococcus pyogenes, Escherichia coli and Candida albicans with MIC values ranging from 20 to 125 µg/mL. | [33] |
| D. moricandiana | Leaves | EO was active against Staphylococcus aureus and Candida albicans | [30] |
| D. quitarensis | Aerial parts | EO was active for Gram-positive microorganisms Streptococcus mutans and Streptococcus pyogenes with MIC of 37.5 µg/mL. | [37] |
| Trypanocidal | |||
| D. quitarensis | Aerial parts | EO showed trypanocidal activity against the amastigote and trypomastigote forms of Trypanosoma cruzi with IC50 values of 0.26 and 0.54 µg/mL, respectively. | [36] |
| Antioxidant | |||
| D. lanceolata | Branches | EO presented antioxidant effect, demonstrated through the DPPH radical, potency reduction and β-carotene assays. It inhibited lipid peroxidation by 41.5% (EC50 equal to 159.4 µg/mL). | [35] |
| Cytotoxic and Antitumor | |||
| D. furfuracea | Stem | EO was active against A. salina with LC50 values of 2.6 µg/mL. | [26] |
| Stem | EO was active against A. salina with LC50 of 715.2 mg/cm3. | [27] | |
| D. lanceolata | Bark | EO was cytotoxic against A. salina with LC50 of 49.0 and 60.7 µg/mL, corresponding to different times of extraction. | [36] |
| D. gabriuscula | Leaves | EO exhibited cytotoxicity towards tumor cell lines and showed IC50 value of 11.6 µg/mL for human larynx carcinoma (Hep2) cell line. | [44] |
| Leaves | EO was toxic to A. salina with LC50 of 1.6 mg/mL. | [32] | |
| D. gardneriana | Leaves | EO exhibited cytotoxic effect with IC50 values of 16.9, 19.2, 13.1 and 19.3 µg/mL against B16-F10, HepG2, HL-60 and K562 cell lines, respectively. In the in vivo experiment, tumor growth was reduced by 5.4 and 37.5% at doses of 40 and 80 mg/kg, respectively. | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.C.d.; Nogueira, M.L.; Oliveira, F.P.d.; Costa, E.V.; Bezerra, D.P. Essential Oils of Duguetia Species A. St. Hill (Annonaceae): Chemical Diversity and Pharmacological Potential. Biomolecules 2022, 12, 615. https://doi.org/10.3390/biom12050615
Santos ACd, Nogueira ML, Oliveira FPd, Costa EV, Bezerra DP. Essential Oils of Duguetia Species A. St. Hill (Annonaceae): Chemical Diversity and Pharmacological Potential. Biomolecules. 2022; 12(5):615. https://doi.org/10.3390/biom12050615
Chicago/Turabian StyleSantos, Albert C. dos, Mateus L. Nogueira, Felipe P. de Oliveira, Emmanoel V. Costa, and Daniel P. Bezerra. 2022. "Essential Oils of Duguetia Species A. St. Hill (Annonaceae): Chemical Diversity and Pharmacological Potential" Biomolecules 12, no. 5: 615. https://doi.org/10.3390/biom12050615
APA StyleSantos, A. C. d., Nogueira, M. L., Oliveira, F. P. d., Costa, E. V., & Bezerra, D. P. (2022). Essential Oils of Duguetia Species A. St. Hill (Annonaceae): Chemical Diversity and Pharmacological Potential. Biomolecules, 12(5), 615. https://doi.org/10.3390/biom12050615









