Bioactive Peptides and Proteins from Wasp Venoms
Abstract
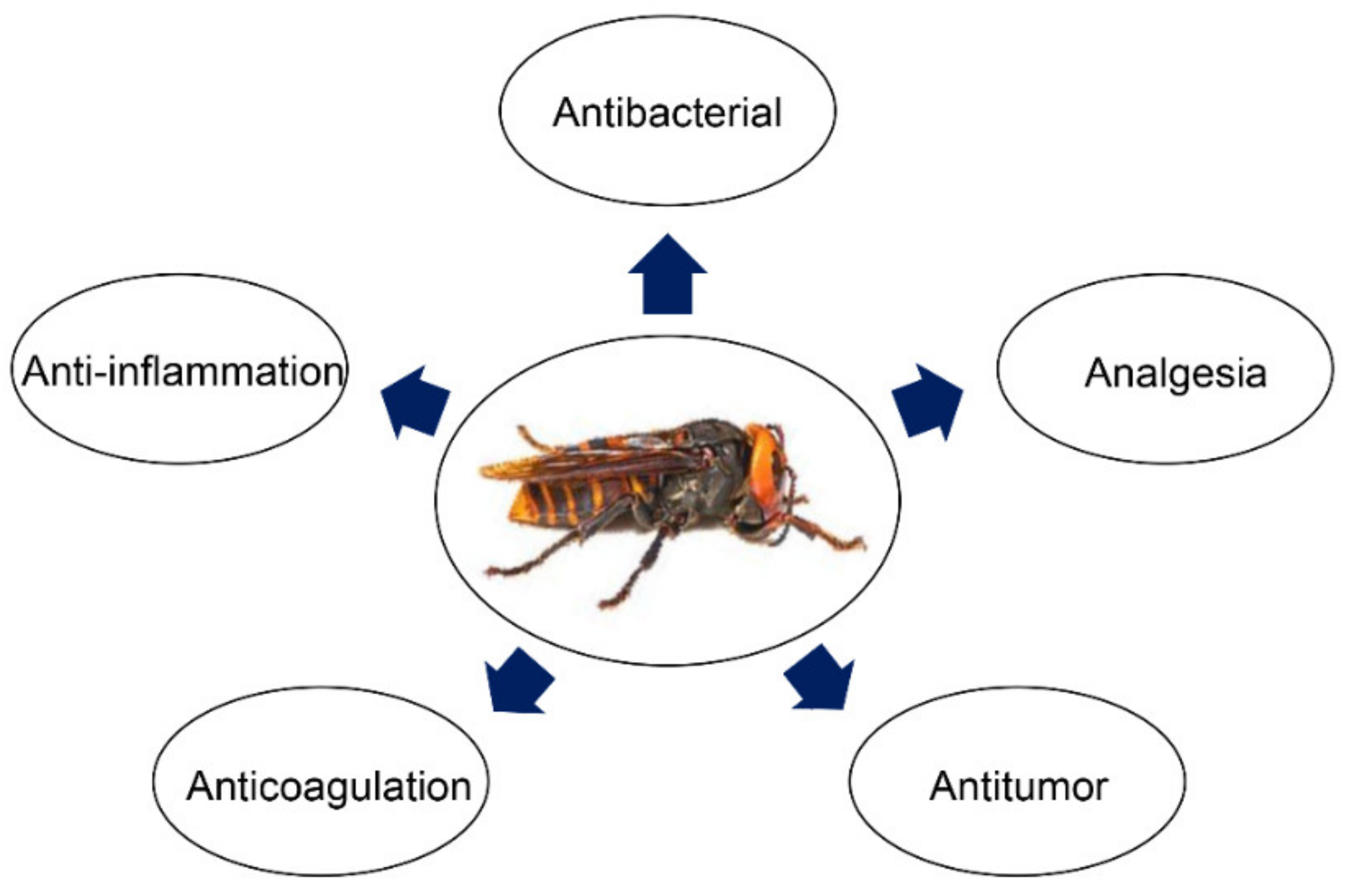
1. Introduction
2. General Origins and Properties of Wasp Venoms
2.1. Wasp Venoms Components
2.2. Peptidic Components
2.2.1. Neurotoxic Peptides
| Wasp-Scientific Name | Isolated Peptides | AA Sequence | Reference |
|---|---|---|---|
| Sphex argentatus | Sa12b | EDVDHVFLRF | [33] |
| Anoplius samariensis, Batozonellus maculifrons | α-PMTXs | RIKIGLFDQLSKL | [28] |
| Anoplius samariensis, Batozonellus maculifrons | β-PMTXs | RIKIGLFDQRSKL | [28] |
| Batozonellus maculifrons | β-PMTXs | RIKIGLFDQLSRL | [5] |
| Agelaia vicina | Agelaiatoxin-8 (AVTx8) | INWKLGKALNALL | [34] |
2.2.2. Kinins
2.2.3. Mastoparans
| Wasp-Scientific Name | Isolated Peptides | AA Sequence | References |
|---|---|---|---|
| Vespa xanthoptera Vespula lewisii | Mastoparan | INWKGIAAMAKKLL | [46,57] |
| Vespula vulgaris | Mastoparan V1 | INWKKIKSIIKAAMN | [57,58,59] |
| Vespa magnifica | Peptide 12a | INWKGIAAMAKKLL | [53] |
| Vespa magnifica | Peptide 12b | INWKGIAAMKKLL | [53] |
| Vespa magnifica | Peptide 12d | INLKAIAAMAKKLL | [60] |
| Vespa basalis | Mastoparan B | INLKAIAAFAKKLL | [61] |
| Vespa tropica | Mastoparan-VT1 | INLKAIAALAKKLL | [62] |
| Vespa basalis | Mastoparan-A | IKWKAILDAVKKVI | [61] |
| Vespa affinis | Mastoparan-AF | INLKAIAALAKKLF | [63] |
| Vespa basalis | Mastoparan-B | LKLKSIVSWAKKVL | [64] |
| Vespa bicolor | Mastoparan-VB1 | INMKASAAVAKKLL | [65] |
| Vespa crabro | Mastoparan-C | LNLKALLAVAKKIL | [66] |
| Vespa ducalis | Mastoparan-D | INLKAIAAFAKKLL | [63] |
| Vespa velutina | Mastoparan-V | IAWKGIAAMAKKLL | [63] |
| Vespa xanthoptera | Mastoparan-X | INWKGIAAMAKKLL | [67] |
| Polybia paulista | Polybia-MP I | IDWKKLLDAAKQIL | [46] |
2.2.4. Chemotactic Peptides
| Wasp-Scientific Name | Isolated Peptides | AA Sequence | Reference |
|---|---|---|---|
| Vespa mandarinia | VESCP-M2 | FLPILAKILGGLL | [70] |
| Vespa magnifica | VCP-5h | FLPIIGKLLSGLL | [72] |
| Vespa bicolor | VESP-VB1 | FMPIIGRLMSGSL | [65] |
| Vespa xanthoptera | VesCP-X | FLPIIAKLLGGLL | [73] |
| Vespa magnifica | Peptide 5e | FLPIIAKLLGGLL | [53] |
| Vespa magnifica | Peptide 5f | FLPIPRPILLGLL | [53] |
| Vespa magnifica | Peptide 5g | FLIIRRPIVLGLL | [53] |
| Vespa magnifica | Peptide 5h | FLPIIGKLLSGLL | [53] |
| Vespa analis | VesCP-A | FLPMIAKLLGGLL | [73] |
| Vespa mandarinia | VesCP-M | FLPIIGKLLSGLL | [73] |
| Vespa orientalis | HR-II | FLPLILGKLVKGLL | [73] |
| Vespa tropica | VesCP-T | FLPILGKILGGLL | [73] |
| Vespa tropica | VCP-VT1 | FLPIIGKLLSGLL | [62] |
| Vespa tropica | VCP-VT2 | FLPIIGKLLSG | [62] |
| Vespa crabro | Crabrolin | FLPLILRKIVTAL | [74] |
| Polybia paulista | Polybia-CP | ILGTILGLLKSL | [46] |
| Orancistrocerus drewseni | Orancis-Protonectin | ILGIITSLLKSL | [71] |
2.2.5. Other Peptides
2.3. Proteins Components
2.3.1. Hyaluronidase
2.3.2. Phospholipases
2.3.3. Antigen 5
2.3.4. Other Protein Components
3. Pharmacological and Medical Application of Bioactive Peptides from Wasp Venom
3.1. Antimicrobial Activities
3.2. Antitumor Activities
3.3. Anti-Inflammatory Activities
3.4. Other Activities
4. Wasp Venom Allergy and Immunotherapy
5. Conclusions
6. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.Y.; Cao, L.J.; Chen, P.Y.; Chen, X.X.; van Achterberg, K.; Hoffmann, A.A.; Liu, J.X.; Wei, S.J. Comparative mitogenomics and phylogenetics of the stinging wasps (Hymenoptera: Aculeata). Mol. Phylogenet. Evol. 2021, 159, 107119. [Google Scholar] [CrossRef] [PubMed]
- Dowton, M. Simultaneous analysis of 16S, 28S, COI and morphology in the Hymenoptera: Apocrita-evolutionary transitions among parasitic wasps. Biol. J. Linn. Soc. 2001, 74, 87–111. [Google Scholar]
- Evans, D.L.; Schmidt, J.O.; Evans, D.L.; Schmidt, J.O. Insect Defenses: Adaptive Mechanisms and Strategies of Predators and Prey; Suny Press: Albany, NY, USA, 1990; pp. 1–482. [Google Scholar]
- Nakajima, T. Pharmacological biochemistry of vespid venoms. In Venoms of Hymenoptera; Elsevier: Amsterdam, The Netherlands, 1986; pp. 309–327. [Google Scholar]
- Konno, K.; Kazuma, K.; Nihei, K. Peptide Toxins in Solitary Wasp Venoms. Toxins 2016, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Senti, G.; Johansen, P.; Martinez Gomez, J.; Prinz Varicka, B.M.; Kundig, T.M. Efficacy and safety of allergen-specific immunotherapy in rhinitis, rhinoconjunctivitis, and bee/wasp venom allergies. Int. Rev. Immunol. 2005, 24, 519–531. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, M.; Gao, Y.; Wu, X.; Xiao, H.; Yang, D.; Zhao, Y. Vespakinin-M, a natural peptide from Vespa magnifica, promotes functional recovery in stroke mice. Commun. Biol. 2022, 5, 74. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, W.X.; Duan, X.M.; Ni, L.L.; Liu, H.; Zhao, H.R.; Xiao, H.; Zhang, C.G.; Yang, Z.B. Wasp Venom Possesses Potential Therapeutic Effect in Experimental Models of Rheumatoid Arthritis. Evid. Based Complement. Alternat. Med. 2020, 2020, 6394625. [Google Scholar] [CrossRef]
- Mortari, M.R.; Cunha, A.O.; de Oliveira, L.; Vieira, E.B.; Gelfuso, E.A.; Coutinho-Netto, J.; Ferreira dos Santos, W. Anticonvulsant and behavioural effects of the denatured venom of the social wasp Polybia occidentalis (Polistinae, Vespidae). Basic Clin. Pharmacol. Toxicol. 2005, 97, 289–295. [Google Scholar] [CrossRef]
- Khalil, W.K.; Assaf, N.; ElShebiney, S.A.; Salem, N.A. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem. Int. 2015, 80, 79–86. [Google Scholar] [CrossRef]
- Thathiah, A.; De Strooper, B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 73–87. [Google Scholar] [CrossRef]
- Moreno, M.; Giralt, E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: Melittin, apamin and mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef]
- Piek, T. Venoms of the Hymenoptera; Harcourt Brace Jovanovich: San Diego, CA, USA, 1986. [Google Scholar]
- Kini, R.M.; Clemetson, K.J.; Markland, F.S.; McLane, M.A.; Morita, T. Toxins and Hemostasis; Springer: Berlin/Heidelberg, Germany, 2010; Volume 20011, p. 600. [Google Scholar]
- Fry, B.G.; Roelants, K.; Norman, J.A. Tentacles of venom: Toxic protein convergence in the Kingdom Animalia. J. Mol. Evol. 2009, 68, 311–321. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Habermann, E. Bee and wasp venoms. Science 1972, 177, 314–322. [Google Scholar] [CrossRef]
- Monteiro, M.C.; Romao, P.R.; Soares, A.M. Pharmacological perspectives of wasp venom. Protein Pept. Lett. 2009, 16, 944–952. [Google Scholar] [CrossRef]
- Piek, T. Pharmacology of Hymenoptera Venom in Handbook of Natural Toxins; Tu, A.T., Ed.; Marcel Dekker: New York, NY, USA, 1984; Volume 2, pp. 135–185. [Google Scholar]
- Lai, R.; Liu, C. Bioactive Peptides and Proteins from Wasp Venoms. In Toxins and Hemostasis: From Bench to Bedside; Kini, R.M., Clemetson, K.J., Markland, F.S., McLane, M.A., Morita, T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 83–95. [Google Scholar]
- Herrera, C.; Leza, M.; Martinez-Lopez, E. Diversity of compounds in Vespa spp. venom and the epidemiology of its sting: A global appraisal. Arch Toxicol. 2020, 94, 3609–3627. [Google Scholar] [CrossRef]
- Dos Santos-Pinto, J.R.A.; Perez-Riverol, A.; Lasa, A.M.; Palma, M.S. Diversity of peptidic and proteinaceous toxins from social Hymenoptera venoms. Toxicon 2018, 148, 172–196. [Google Scholar] [CrossRef]
- Piek, T.; Hue, B.; Pelhate, M.; Mony, L. The venom of the wasp Campsomeris sexmaculata (F.) blocks synaptic transmission in insect CNS. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1987, 87, 283–286. [Google Scholar] [CrossRef]
- Hue, B.; Piek, T. Irreversible presynaptic activation-induced block of transmission in the insect cns by hemicholinium-3 and threonine-6-bradykinin. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1989, 93, 87–89. [Google Scholar] [CrossRef]
- Piek, T. Neurotoxic kinins from wasp and ant venoms. Toxicon 1991, 29, 139–149. [Google Scholar] [CrossRef]
- Piek, T.; Hue, B.; Mantel, P.; Nakajima, T.; Pelhate, M.; Yasuhara, T. Threonine6-bradykinin in the venom of the wasp Colpa interrupta (F.) presynaptically blocks nicotinic synaptic transmission in the insect CNS. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1990, 96, 157–162. [Google Scholar] [CrossRef]
- Konno, K.; Palma, M.S.; Hitara, I.Y.; Juliano, M.A.; Juliano, L.; Yasuhara, T. Identification of bradykinins in solitary wasp venoms. Toxicon 2002, 40, 309–312. [Google Scholar] [CrossRef]
- Konno, K.; Hisada, M.; Itagaki, Y.; Naoki, H.; Kawai, N.; Miwa, A.; Yasuhara, T.; Takayama, H. Isolation and structure of pompilidotoxins, novel peptide neurotoxins in solitary wasp venoms. Biochem. Biophys. Res. Commun. 1998, 250, 612–616. [Google Scholar] [CrossRef]
- Konno, K.; Miwa, A.; Takayama, H.; Hisada, M.; Itagaki, Y.; Naoki, H.; Yasuhara, T.; Kawai, N. Alpha-pompilidotoxin (alpha-PMTX), a novel neurotoxin from the venom of a solitary wasp, facilitates transmission in the crustacean neuromuscular synapse. Neurosci. Lett. 1997, 238, 99–102. [Google Scholar] [CrossRef]
- Sahara, Y.; Gotoh, M.; Konno, K.; Miwa, A.; Tsubokawa, H.; Robinson, H.P.; Kawai, N. A new class of neurotoxin from wasp venom slows inactivation of sodium current. Eur. J. Neurosci. 2000, 12, 1961–1970. [Google Scholar] [CrossRef]
- De Oliveira, L.; Cunha, A.O.; Mortari, M.R.; Pizzo, A.B.; Miranda, A.; Coimbra, N.C.; dos Santos, W.F. Effects of microinjections of neurotoxin AvTx8, isolated from the social wasp Agelaia vicina (Hymenoptera, Vespidae) venom, on GABAergic nigrotectal pathways. Brain Res. 2005, 1031, 74–81. [Google Scholar] [CrossRef]
- Pizzo, A.B.; Beleboni, R.O.; Fontana, A.C.; Ribeiro, A.M.; Miranda, A.; Coutinho-Netto, J.; dos Santos, W.F. Characterization of the actions of AvTx 7 isolated from Agelaia vicina (Hymenoptera: Vespidae) wasp venom on synaptosomal glutamate uptake and release. J. Biochem. Mol. Toxicol. 2004, 18, 61–68. [Google Scholar] [CrossRef]
- Hernandez, C.; Konno, K.; Salceda, E.; Vega, R.; Zaharenko, A.J.; Soto, E. Sa12b Peptide from Solitary Wasp Inhibits ASIC Currents in Rat Dorsal Root Ganglion Neurons. Toxins 2019, 11, 585. [Google Scholar] [CrossRef]
- Pizzo, A.B.; Beleboni, R.O.; Gomes Carolino, R.O.; de Oliveira, L.; Miranda, A.; Coutinho-Netto, J.; Fontana, A.C.K.; Dos Santos, W.F. Isolation and chemical characterization of agelaiatoxin8 (AvTx8) from Agelaia vicina wasp venom and its biological effects on GABA neurotransmission. J. Biochem. Mol. Toxicol. 2017, 31, e21941. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.S.M.; Beraldo, W.T.; Rosenfeld, G. Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin. Am. J. Physiol. 1949, 156, 261–273. [Google Scholar]
- Piek, T. Wasp kinins and kinin analogues. In Animal Toxins; Birkhäuser Verlag: Basel, Switzerland, 2000; pp. 99–115. [Google Scholar]
- Podvin, S.; Bundey, R.; Toneff, T.; Ziegler, M.; Hook, V. Profiles of secreted neuropeptides and catecholamines illustrate similarities and differences in response to stimulation by distinct secretagogues. Mol. Cell Neurosci. 2015, 68, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Picolo, G.; Hisada, M.; Moura, A.B.; Machado, M.F.; Sciani, J.M.; Conceicao, I.M.; Melo, R.L.; Oliveira, V.; Lima-Landman, M.T.; Cury, Y.; et al. Bradykinin-related peptides in the venom of the solitary wasp Cyphononyx fulvognathus. Biochem. Pharmacol. 2010, 79, 478–486. [Google Scholar] [CrossRef]
- Mortari, M.R.; Cunha, A.O.; Carolino, R.O.; Coutinho-Netto, J.; Tomaz, J.C.; Lopes, N.P.; Coimbra, N.C.; Dos Santos, W.F. Inhibition of acute nociceptive responses in rats after i.c.v. injection of Thr6-bradykinin, isolated from the venom of the social wasp, Polybia occidentalis. Br. J. Pharmacol. 2007, 151, 860–869. [Google Scholar] [CrossRef]
- Konno, K.; Hisada, M.; Naoki, H.; Itagaki, Y.; Yasuhara, T.; Juliano, M.A.; Juliano, L.; Palma, M.S.; Yamane, T.; Nakajima, T. Isolation and sequence determination of peptides in the venom of the spider wasp (Cyphononyx dorsalis) guided by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry. Toxicon 2001, 39, 1257–1260. [Google Scholar] [CrossRef]
- Yoon, K.A.; Kim, K.; Phuong, N.; Seo, J.B.; Park, Y.H.; Kim, K.-G.; Seo, H.-y.; Koh, Y.H.; Lee, S.H. Comparative functional venomics of social hornets Vespa crabro and Vespa analis. J. Asia Pac. Entomol. 2015, 18, 815–823. [Google Scholar] [CrossRef]
- Kishimura, H.; Yasuhara, T.; Yoshida, H.; Nakajima, T. Vespakinin-M, a novel bradykinin analogue containing hydroxyproline, in the venom of Vespa mandarinia Smith. Chem. Pharm. Bull. 1976, 24, 2896–2897. [Google Scholar] [CrossRef]
- Yasuhara, T.; Yoshida, H.; Nakajima, T. Chemical investigation of the hornet (Vespa xanthoptera Cameron) venom. The structure of a new bradykinin analogue “vespakinin-X”. Chem. Pharm. Bull. 1977, 25, 936–941. [Google Scholar] [CrossRef]
- Gobbo, M.; Biondi, L.; Filira, F.; Rocchi, R.; Piek, T. Cyclic analogues of wasp kinins from Vespa analis and Vespa tropica. Int. J. Pept. Protein Res. 1995, 45, 282–289. [Google Scholar] [CrossRef]
- Piek, T.; Mantel, P.; Van Ginkel, C.J. Megascoliakinin, a bradykinin-like compound in the venom of Megascolia flavifrons Fab. (Hymenoptera: Scoliidae). Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1984, 78, 473–474. [Google Scholar] [CrossRef]
- Dias, N.B.; de Souza, B.M.; Gomes, P.C.; Brigatte, P.; Palma, M.S. Peptidome profiling of venom from the social wasp Polybia paulista. Toxicon 2015, 107, 290–303. [Google Scholar] [CrossRef]
- Hirai, Y.; Yasuhara, T.; Yoshida, H.; Nakajima, T.; Fujino, M.; Kitada, C. A new mast cell degranulating peptide “mastoparan” in the venom of Vespula lewisii. Chem. Pharm. Bull. 1979, 27, 1942–1944. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Higashijima, T.; Uzu, S.; Nakajima, T.; Ross, E.M. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J. Biol. Chem. 1988, 263, 6491–6494. [Google Scholar] [CrossRef]
- Baptista-Saidemberg, N.B.; Saidemberg, D.M.; Ribeiro, R.A.; Arcuri, H.A.; Palma, M.S.; Carneiro, E.M. Agelaia MP-I: A peptide isolated from the venom of the social wasp, Agelaia pallipes pallipes, enhances insulin secretion in mice pancreatic islets. Toxicon 2012, 60, 596–602. [Google Scholar] [CrossRef]
- Kurihara, H.; Kitajima, K.; Senda, T.; Fujita, H.; Nakajima, T. Multigranular exocytosis induced by phospholipase A2-activators, melittin and mastoparan, in rat anterior pituitary cells. Cell Tissue Res. 1986, 243, 311–316. [Google Scholar] [CrossRef]
- Baek, J.H.; Ji, Y.; Shin, J.S.; Lee, S.; Lee, S.H. Venom peptides from solitary hunting wasps induce feeding disorder in lepidopteran larvae. Peptides 2011, 32, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, J.; Lu, Q.; Yang, H.; Zhang, Y.; Lai, R. Two families of antimicrobial peptides from wasp (Vespa magnifica) venom. Toxicon 2006, 47, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.; de Souza, B.M.; Palma, M.S.; da Cruz-Hofling, M.A. Myotoxic effects of mastoparan from Polybia paulista (Hymenoptera, Epiponini) wasp venom in mice skeletal muscle. Toxicon 2007, 50, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Monge-Fuentes, V.; Gomes, F.; Lopes, K.; dos Anjos, L.; Campos, G.; Arenas, C.; Biolchi, A.; Goncalves, J.; Galante, P.; et al. Pharmacological Alternatives for the Treatment of Neurodegenerative Disorders: Wasp and Bee Venoms and Their Components as New Neuroactive Tools. Toxins 2015, 7, 3179–3209. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Zurita, E.; Giralt, E. Delivering wasp venom for cancer therapy. J. Control. Release 2014, 182, 13–21. [Google Scholar] [CrossRef]
- Da Silva, A.M.B.; Silva-Goncalves, L.C.; Oliveira, F.A.; Arcisio-Miranda, M. Pro-necrotic Activity of Cationic Mastoparan Peptides in Human Glioblastoma Multiforme Cells Via Membranolytic Action. Mol. Neurobiol. 2018, 55, 5490–5504. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.; Yosri, N.; Sakr, H.H.; Du, M.; Algethami, A.F.M.; Zhao, C.; Abdelazeem, A.H.; Tahir, H.E.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Wasp Venom Biochemical Components and Their Potential in Biological Applications and Nanotechnological Interventions. Toxins 2021, 13, 206. [Google Scholar] [CrossRef]
- Kim, Y.; Son, M.; Noh, E.Y.; Kim, S.; Kim, C.; Yeo, J.H.; Park, C.; Lee, K.W.; Bang, W.Y. MP-V1 from the Venom of Social Wasp Vespula vulgaris Is a de Novo Type of Mastoparan that Displays Superior Antimicrobial Activities. Molecules 2016, 21, 512. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Yu, H.; Li, J.; Lai, R. The mastoparanogen from wasp. Peptides 2006, 27, 3053–3057. [Google Scholar] [CrossRef]
- Park, N.G.; Yamato, Y.; Lee, S.; Sugihara, G. Interaction of mastoparan-B from venom of a hornet in Taiwan with phospholipid bilayers and its antimicrobial activity. Biopolymers 1995, 36, 793–801. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Lee, W.H.; Zhang, Y. Antimicrobial peptides from the venom gland of the social wasp Vespa tropica. Toxicon 2013, 74, 151–157. [Google Scholar] [CrossRef]
- Lin, C.H.; Tzen, J.T.; Shyu, C.L.; Yang, M.J.; Tu, W.C. Structural and biological characterization of mastoparans in the venom of Vespa species in Taiwan. Peptides 2011, 32, 2027–2036. [Google Scholar] [CrossRef]
- Ho, C.L.; Shih, Y.P.; Wang, K.T.; Yu, H.M. Enancing the hypotensive effect and diminishing the cytolytic activity of hornet mastoparan B by D-amino acid substitution. Toxicon 2001, 39, 1561–1566. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; Yang, X.; Zhai, L.; Lu, Z.; Liu, J.; Yu, H. Antimicrobial peptides from the venoms of Vespa bicolor Fabricius. Peptides 2008, 29, 1887–1892. [Google Scholar] [CrossRef]
- Argiolas, A.; Pisano, J.J. Isolation and characterization of two new peptides, mastoparan C and crabrolin, from the venom of the European hornet, Vespa crabro. J. Biol. Chem. 1984, 259, 10106–10111. [Google Scholar] [CrossRef]
- Todokoro, Y.; Yumen, I.; Fukushima, K.; Kang, S.W.; Park, J.S.; Kohno, T.; Wakamatsu, K.; Akutsu, H.; Fujiwara, T. Structure of tightly membrane-bound mastoparan-X, a G-protein-activating peptide, determined by solid-state NMR. Biophys. J. 2006, 91, 1368–1379. [Google Scholar] [CrossRef]
- Kastin, A.J. Handbook of Biologically Active Peptides; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Lee, S.H.; Baek, J.H.; Yoon, K.A. Differential Properties of Venom Peptides and Proteins in Solitary vs. Social Hunting Wasps. Toxins 2016, 8, 32. [Google Scholar] [CrossRef]
- Ombati, R.; Wang, Y.; Du, C.; Lu, X.; Li, B.; Nyachieo, A.; Li, Y.; Yang, S.; Lai, R. A membrane disrupting toxin from wasp venom underlies the molecular mechanism of tissue damage. Toxicon 2018, 148, 56–63. [Google Scholar] [CrossRef]
- Murata, K.; Shinada, T.; Ohfune, Y.; Hisada, M.; Yasuda, A.; Naoki, H.; Nakajima, T. Novel mastoparan and protonectin analogs isolated from a solitary wasp, Orancistrocerus drewseni drewseni. Amino Acids 2009, 37, 389–394. [Google Scholar] [CrossRef]
- Yu, H.; Yang, H.; Ma, D.; Lv, Y.; Liu, T.; Zhang, K.; Lai, R.; Liu, J. Vespid chemotactic peptide precursor from the wasp, Vespa magnifica (Smith). Toxicon 2007, 50, 377–382. [Google Scholar] [CrossRef]
- Nakajima, T.; Yasuhara, T.; Uzu, S.; Wakamatsu, K.; Miyazawa, T.; Fukuda, K.; Tsukamoto, Y. Wasp venom peptides; wasp kinins, new cytotrophic peptide families and their physico-chemical properties. Peptides 1985, 6 (Suppl. S3), 425–430. [Google Scholar] [CrossRef]
- Krishnakumari, V.; Nagaraj, R. Antimicrobial and hemolytic activities of crabrolin, a 13-residue peptide from the venom of the European hornet, Vespa crabro, and its analogs. J. Pept. Res. 1997, 50, 88–93. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Yang, H.; Lai, R. A novel bioactive peptide from wasp venom. J. Venom. Res. 2010, 1, 43–47. [Google Scholar]
- Rungsa, P.; Janpan, P.; Saengkun, Y.; Jangpromma, N.; Klaynongsruang, S.; Patramanon, R.; Uawonggul, N.; Daduang, J.; Daduang, S. Heterologous expression and mutagenesis of recombinant Vespa affinis hyaluronidase protein (rVesA2). J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190030. [Google Scholar] [CrossRef]
- An, S.; Chen, L.; Wei, J.F.; Yang, X.; Ma, D.; Xu, X.; Xu, X.; He, S.; Lu, J.; Lai, R. Purification and characterization of two new allergens from the venom of Vespa magnifica. PLoS ONE 2012, 7, e31920. [Google Scholar] [CrossRef]
- Rungsa, P.; Incamnoi, P.; Sukprasert, S.; Uawonggul, N.; Klaynongsruang, S.; Daduang, J.; Patramanon, R.; Roytrakul, S.; Daduang, S. Cloning, structural modelling and characterization of VesT2s, a wasp venom hyaluronidase (HAase) from Vespa tropica. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 28. [Google Scholar] [CrossRef]
- Sukprasert, S.; Rungsa, P.; Uawonggul, N.; Incamnoi, P.; Thammasirirak, S.; Daduang, J.; Daduang, S. Purification and structural characterisation of phospholipase A1 (Vespapase, Ves a 1) from Thai banded tiger wasp (Vespa affinis) venom. Toxicon 2013, 61, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.D.; Santos, K.S.; de Souza, B.M.; Arcuri, H.A.; Cunha-Neto, E.; Castro, F.M.; Kalil, J.E.; Palma, M.S. Purification, sequencing and structural characterization of the phospholipase A1 from the venom of the social wasp Polybia paulista (Hymenoptera, Vespidae). Toxicon 2007, 50, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Inoue, A.; Makide, K.; Saiki, N.; Arai, H. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie 2007, 89, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.H.; Chuang, C.Y.; Ko, T.P.; Hu, N.J.; Chou, C.C.; Shih, Y.P.; Ho, C.L.; Wang, A.H. Crystal structure of vespid phospholipase A(1) reveals insights into the mechanism for cause of membrane dysfunction. Insect Biochem. Mol. Biol. 2016, 68, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, X.; Ma, D.; Zhang, K.; Lai, R. A phospholipase A1 platelet activator from the wasp venom of Vespa magnifica (Smith). Toxicon 2008, 51, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Korneev, A.S.; Salikhov, S.I.; Tuichibaev, M.U. Amino acid sequence of orientotoxins I and II from the venom of the hornet Vespa orientalis. Bioorg. Khim. 1989, 15, 127–129. [Google Scholar] [PubMed]
- Ho, C.L.; Lin, Y.L.; Li, S.F. Three toxins with phospholipase activity isolated from the yellow-legged hornet (Vespa verutina) venom. Toxicon 1999, 37, 1015–1024. [Google Scholar] [CrossRef]
- Monsalve, R.I.; Gutierrez, R.; Hoof, I.; Lombardero, M. Purification and molecular characterization of phospholipase, antigen 5 and hyaluronidases from the venom of the Asian hornet (Vespa velutina). PLoS ONE 2020, 15, e0225672. [Google Scholar] [CrossRef]
- Abe, T.; Sugita, M.; Fujikura, T.; Hiyoshi, J.; Akasu, M. Giant hornet (Vespa mandarinia) venomous phospholipases. The purification, characterization and inhibitory properties by biscoclaurine alkaloids. Toxicon 2000, 38, 1803–1816. [Google Scholar] [CrossRef]
- Hoffman, D.R. Allergens in Hymenoptera venom. XXV: The amino acid sequences of antigen 5 molecules and the structural basis of antigenic cross-reactivity. J. Allergy Clin. Immunol. 1993, 92, 707–716. [Google Scholar] [CrossRef]
- Dos Santos-Pinto, J.R.; Dos Santos, L.D.; Andrade Arcuri, H.; Castro, F.M.; Kalil, J.E.; Palma, M.S. Using proteomic strategies for sequencing and post-translational modifications assignment of antigen-5, a major allergen from the venom of the social wasp Polybia paulista. J. Proteome Res. 2014, 13, 855–865. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Zhou, Y.; Xie, C.; Zhu, B.; Zhu, H.; Liu, S.; Wang, W.; Chen, H.; Ji, Y. Deciphering the venomic transcriptome of killer-wasp Vespa velutina. Sci. Rep. 2015, 5, 9454. [Google Scholar] [CrossRef]
- Rungsa, P.; Incamnoi, P.; Sukprasert, S.; Uawonggul, N.; Klaynongsruang, S.; Daduang, J.; Patramanon, R.; Roytrakul, S.; Daduang, S. Comparative proteomic analysis of two wasps venom, Vespa tropica and Vespa affinis. Toxicon 2016, 119, 159–167. [Google Scholar] [CrossRef]
- Lee, V.S.; Tu, W.C.; Jinn, T.R.; Peng, C.C.; Lin, L.J.; Tzen, J.T. Molecular cloning of the precursor polypeptide of mastoparan B and its putative processing enzyme, dipeptidyl peptidase IV, from the black-bellied hornet, Vespa basalis. Insect Mol. Biol. 2007, 16, 231–237. [Google Scholar] [CrossRef]
- Han, J.; You, D.; Xu, X.; Han, W.; Lu, Y.; Lai, R.; Meng, Q. An anticoagulant serine protease from the wasp venom of Vespa magnifica. Toxicon 2008, 51, 914–922. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Lu, Z.; Zhai, L.; Jiang, J.; Liu, J.; Yu, H. A novel serine protease inhibitor from the venom of Vespa bicolor Fabricius. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 153, 116–120. [Google Scholar] [CrossRef]
- Haim, B.; Rimon, A.; Ishay, J.S.; Rimon, S. Purification, characterization and anticoagulant activity of a proteolytic enzyme from Vespa orientalis venom. Toxicon 1999, 37, 825–829. [Google Scholar] [CrossRef]
- Kasahara, M.; Gutknecht, J.; Brew, K.; Spurr, N.; Goodfellow, P.N. Cloning and mapping of a testis-specific gene with sequence similarity to a sperm-coating glycoprotein gene. Genomics 1989, 5, 527–534. [Google Scholar] [CrossRef]
- Kolarich, D.; Leonard, R.; Hemmer, W.; Altmann, F. The N-glycans of yellow jacket venom hyaluronidases and the protein sequence of its major isoform in Vespula vulgaris. FEBS J. 2005, 272, 5182–5190. [Google Scholar] [CrossRef]
- Soldatova, L.N.; Crameri, R.; Gmachl, M.; Kemeny, D.M.; Schmidt, M.; Weber, M.; Mueller, U.R. Superior biologic activity of the recombinant bee venom allergen hyaluronidase expressed in baculovirus-infected insect cells as compared with Escherichia coli. J. Allergy Clin. Immunol. 1998, 101, 691–698. [Google Scholar] [CrossRef]
- Wypych, J.I.; Abeyounis, C.J.; Reisman, R.E. Analysis of differing patterns of cross-reactivity of honeybee and yellow jacket venom-specific IgE: Use of purified venom fractions. Int. Arch Allergy Appl. Immunol. 1989, 89, 60–66. [Google Scholar] [CrossRef]
- Bazon, M.L.; Silveira, L.H.; Simioni, P.U.; Brochetto-Braga, M.R. Current Advances in Immunological Studies on the Vespidae Venom Antigen 5: Therapeutic and Prophylaxis to Hypersensitivity Responses. Toxins 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, A.; Lasa, A.M.; Dos Santos-Pinto, J.R.A.; Palma, M.S. Insect venom phospholipases A1 and A2: Roles in the envenoming process and allergy. Insect Biochem. Mol. Biol. 2019, 105, 10–24. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Sakell, R.H.; Schmidt, M. Sol i 1, the phospholipase allergen of imported fire ant venom. J. Allergy Clin. Immunol. 2005, 115, 611–616. [Google Scholar] [CrossRef]
- Sanchez, F.; Blanca, M.; Miranda, A.; Carmona, M.J.; Garcia, J.; Fernandez, J.; Torres, M.J.; Rondon, M.C.; Juarez, C. Comparison of Vespula germanica venoms obtained from different sources. Int. Arch Allergy Immunol. 1994, 104, 385–389. [Google Scholar] [CrossRef]
- Henriksen, A.; King, T.P.; Mirza, O.; Monsalve, R.I.; Meno, K.; Ipsen, H.; Larsen, J.N.; Gajhede, M.; Spangfort, M.D. Major venom allergen of yellow jackets, Ves v 5: Structural characterization of a pathogenesis-related protein superfamily. Proteins 2001, 45, 438–448. [Google Scholar] [CrossRef]
- Reisman, R.E.; Dvorin, D.J.; Randolph, C.C.; Georgitis, J.W. Stinging insect allergy: Natural history and modification with venom immunotherapy. J. Allergy Clin. Immunol. 1985, 75, 735–740. [Google Scholar] [CrossRef]
- Tankersley, M.S.; Ledford, D.K. Stinging insect allergy: State of the art 2015. J. Allergy Clin. Immunol. Pract. 2015, 3, 315–322. [Google Scholar] [CrossRef]
- Hoffman, D.R. Allergens in Hymenoptera venom XV: The immunologic basis of vespid venom cross-reactivity. J. Allergy Clin. Immunol. 1985, 75, 611–613. [Google Scholar] [CrossRef]
- King, T.P.; Guralnick, M. Hymenoptera allergens. Clin. Allergy Immunol. 2004, 18, 339–353. [Google Scholar]
- Hawdon, J.M.; Jones, B.F.; Hoffman, D.R.; Hotez, P.J. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J. Biol. Chem. 1996, 271, 6672–6678. [Google Scholar] [CrossRef]
- Morissette, J.; Cote, G.; Anctil, J.L.; Plante, M.; Amyot, M.; Heon, E.; Trope, G.E.; Weissenbach, J.; Raymond, V. A common gene for juvenile and adult-onset primary open-angle glaucomas confined on chromosome 1q. Am. J. Hum. Genet. 1995, 56, 1431–1442. [Google Scholar] [PubMed]
- Lu, G.; Kochoumian, L.; King, T.P. Sequence identity and antigenic cross-reactivity of white face hornet venom allergen, also a hyaluronidase, with other proteins. J. Biol. Chem. 1995, 270, 4457–4465. [Google Scholar] [CrossRef] [PubMed]
- Tomalski, M.D.; King, T.P.; Miller, L.K. Expression of hornet genes encoding venom allergen antigen 5 in insects. Arch Insect Biochem. Physiol. 1993, 22, 303–313. [Google Scholar] [CrossRef]
- Yamamoto, T.; Arimoto, H.; Kinumi, T.; Oba, Y.; Uemura, D. Identification of proteins from venom of the paralytic spider wasp, Cyphononyx dorsalis. Insect Biochem. Mol. Biol. 2007, 37, 278–286. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, R.J.; Cheng, Y.; Du, J.; Volovych, O.; Han, L.B.; Li, J.C.; Hu, Y.; Lu, Z.Y.; Lu, Z.; et al. Insights into the venom protein components of Microplitis mediator, an endoparasitoid wasp. Insect Biochem. Mol. Biol. 2019, 105, 33–42. [Google Scholar] [CrossRef]
- Li, W.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J.D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021, 50, 4932–4973. [Google Scholar] [CrossRef]
- Sampson, H.A.; Munoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F., Jr.; Bock, S.A.; Branum, A.; Brown, S.G.; Camargo, C.A., Jr.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397. [Google Scholar] [CrossRef]
- Bulet, P.; Stocklin, R.; Menin, L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2004, 198, 169–184. [Google Scholar] [CrossRef]
- Rydlo, T.; Miltz, J.; Mor, A. Eukaryotic antimicrobial peptides: Promises and premises in food safety. J. Food Sci. 2006, 71, R125–R135. [Google Scholar] [CrossRef]
- Reddy, K.V.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Alford, M.A.; Haney, E.F. Antibiofilm activity of host defence peptides: Complexity provides opportunities. Nat. Rev. Microbiol. 2021, 19, 786–797. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Wu, Y.; Wang, L.; Ma, C.; Xi, X.; Bininda-Emonds, O.R.P.; Shaw, C.; Chen, T.; Zhou, M. Evaluation of the bioactivity of a mastoparan peptide from wasp venom and of its analogues designed through targeted engineering. Int. J. Biol. Sci. 2018, 14, 599–607. [Google Scholar] [CrossRef]
- Silva, J.C.; Neto, L.M.; Neves, R.C.; Goncalves, J.C.; Trentini, M.M.; Mucury-Filho, R.; Smidt, K.S.; Fensterseifer, I.C.; Silva, O.N.; Lima, L.D.; et al. Evaluation of the antimicrobial activity of the mastoparan Polybia-MPII isolated from venom of the social wasp Pseudopolybia vespiceps testacea (Vespidae, Hymenoptera). Int. J. Antimicrob. Agents 2017, 49, 167–175. [Google Scholar] [CrossRef]
- Henriksen, J.R.; Etzerodt, T.; Gjetting, T.; Andresen, T.L. Side chain hydrophobicity modulates therapeutic activity and membrane selectivity of antimicrobial peptide mastoparan-X. PLoS ONE 2014, 9, e91007. [Google Scholar]
- Yamada, Y.; Shinohara, Y.; Kakudo, T.; Chaki, S.; Futaki, S.; Kamiya, H.; Harashima, H. Mitochondrial delivery of mastoparan with transferrin liposomes equipped with a pH-sensitive fusogenic peptide for selective cancer therapy. Int. J. Pharm. 2005, 303, 1–7. [Google Scholar] [CrossRef]
- Yoon, K.A.; Kim, K.; Phuong, N.; Seo, J.B.; Park, Y.H.; Kim, K.-G.; Seo, H.-y.; Koh, Y.H.; Lee, S.H. Comparative bioactivities of mastoparans from social hornets Vespa crabro and Vespa analis. J. Asia Pac. Entomol. 2015, 18, 825–829. [Google Scholar] [CrossRef]
- Wang, K.R.; Zhang, B.Z.; Zhang, W.; Yan, J.X.; Li, J.; Wang, R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides 2008, 29, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.R.; Yan, J.X.; Zhang, B.Z.; Song, J.J.; Jia, P.F.; Wang, R. Novel mode of action of polybia-MPI, a novel antimicrobial peptide, in multi-drug resistant leukemic cells. Cancer Lett. 2009, 278, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Leite, N.B.; Aufderhorst-Roberts, A.; Palma, M.S.; Connell, S.D.; Ruggiero Neto, J.; Beales, P.A. PE and PS Lipids Synergistically Enhance Membrane Poration by a Peptide with Anticancer Properties. Biophys. J. 2015, 109, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.L.; Duc, T.D.; Thuy, A.M.; Chau, P.M.; Tung, T.T. Chemical approaches in the development of natural nontoxic peptide Polybia-MP1 as a potential dual antimicrobial and antitumor agent. Amino Acids 2021, 53, 843–852. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Liu, L.W.; Wang, K.R.; Song, J.J.; Yan, J.X.; Li, Z.Y.; Zhang, B.Z.; Wang, R. A novel analog of antimicrobial peptide Polybia-MPI, with thioamide bond substitution, exhibits increased therapeutic efficacy against cancer and diminished toxicity in mice. Peptides 2010, 31, 1832–1838. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Andrade, G.P.; Sato, R.H.; Pedron, C.N.; Manieri, T.M.; Cerchiaro, G.; Ribeiro, A.O.; de la Fuente-Nunez, C.; Oliveira, V.X., Jr. Natural and redesigned wasp venom peptides with selective antitumoral activity. Beilstein J. Org. Chem. 2018, 14, 1693–1703. [Google Scholar] [CrossRef]
- He, P.P.; Li, X.D.; Wang, L.; Wang, H. Bispyrene-Based Self-Assembled Nanomaterials: In Vivo Self-Assembly, Transformation, and Biomedical Effects. Acc. Chem. Res. 2019, 52, 367–378. [Google Scholar] [CrossRef]
- Carvajal, L.A.; Neriah, D.B.; Senecal, A.; Benard, L.; Thiruthuvanathan, V.; Yatsenko, T.; Narayanagari, S.R.; Wheat, J.C.; Todorova, T.I.; Mitchell, K.; et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci. Transl. Med. 2018, 10, eaao3003. [Google Scholar] [CrossRef]
- Ng, S.Y.; Yoshida, N.; Christie, A.L.; Ghandi, M.; Dharia, N.V.; Dempster, J.; Murakami, M.; Shigemori, K.; Morrow, S.N.; Van Scoyk, A.; et al. Targetable vulnerabilities in T- and NK-cell lymphomas identified through preclinical models. Nat. Commun. 2018, 9, 2024. [Google Scholar] [CrossRef]
- Yibin, G.; Jiang, Z.; Hong, Z.; Gengfa, L.; Liangxi, W.; Guo, W.; Yongling, L. A synthesized cationic tetradecapeptide from hornet venom kills bacteria and neutralizes lipopolysaccharide in vivo and in vitro. Biochem. Pharmacol. 2005, 70, 209–219. [Google Scholar] [CrossRef]
- Kaushik, D.K.; Thounaojam, M.C.; Mitra, A.; Basu, A. Vespa tropica venom suppresses lipopolysaccharide-mediated secretion of pro-inflammatory cyto-chemokines by abrogating nuclear factor-kappa B activation in microglia. Inflamm. Res. 2014, 63, 657–665. [Google Scholar] [CrossRef]
- Danneels, E.L.; Formesyn, E.M.; de Graaf, D.C. Exploring the Potential of Venom from Nasonia vitripennis as Therapeutic Agent with High-Throughput Screening Tools. Toxins 2015, 7, 2051–2070. [Google Scholar] [CrossRef]
- Schmidt, J.O.; Blum, M.S.; Overal, W.L. Comparative enzymology of venoms from stinging Hymenoptera. Toxicon 1986, 24, 907–921. [Google Scholar] [CrossRef]
- Tan, N.H.; Ponnudurai, G. Comparative study of the enzymatic, hemorrhagic, procoagulant and anticoagulant activities of some animal venoms. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1992, 103, 299–302. [Google Scholar]
- Czaikoski, P.G.; Menaldo, D.L.; Marcussi, S.; Baseggio, A.L.; Fuly, A.L.; Paula, R.C.; Quadros, A.U.; Romao, P.R.; Buschini, M.L.; Cunha, F.Q.; et al. Anticoagulant and fibrinogenolytic properties of the venom of Polybia occidentalis social wasp. Blood Coagul. Fibrinolysis 2010, 21, 653–659. [Google Scholar] [CrossRef]
- Goncalves, J.; Rangel, M.; Biolchi, A.; Alves, E.; Moreira, K.; Silva, L.; Mortari, M. Antinociceptive properties of the mastoparan peptide Agelaia-MPI isolated from social wasps. Toxicon 2016, 120, 15–21. [Google Scholar] [CrossRef]
- Mendes, M.A.; Palma, M.S. Two new bradykinin-related peptides from the venom of the social wasp Protopolybia exigua (Saussure). Peptides 2006, 27, 2632–2639. [Google Scholar] [CrossRef]
- Schiener, M.; Graessel, A.; Ollert, M.; Schmidt-Weber, C.B.; Blank, S. Allergen-specific immunotherapy of Hymenoptera venom allergy-also a matter of diagnosis. Hum. Vaccines Immunother. 2017, 13, 2467–2481. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.D.; Santos, K.S.; Pinto, J.R.; Dias, N.B.; de Souza, B.M.; dos Santos, M.F.; Perales, J.; Domont, G.B.; Castro, F.M.; Kalil, J.E.; et al. Profiling the proteome of the venom from the social wasp Polybia paulista: A clue to understand the envenoming mechanism. J. Proteome Res. 2010, 9, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Yang, X.; Liu, M.; Liu, C.; Han, L.; Guo, H.; Li, C.; Sun, Y.; Li, X.; Xiao, M.; et al. Biopanning of allergens from wasp sting patients. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, U.M.; Durham, S.R. Hymenoptera Venom Allergy: How Does Venom Immunotherapy Prevent Anaphylaxis From Bee and Wasp Stings? Front. Immunol. 2019, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.T.; Ekerljung, L.; Bjerg, A.; Van, T.T.N.; Lundback, B.; Ronmark, E. Sensitization to airborne allergens among adults and its impact on allergic symptoms: A population survey in northern Vietnam. Clin. Transl. Allergy 2014, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Severino, M.; Bonadonna, P.; Passalacqua, G. Large local reactions from stinging insects: From epidemiology to management. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Antonicelli, L.; Bilo, M.B.; Bonifazi, F. Epidemiology of Hymenoptera allergy. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 341–346. [Google Scholar] [CrossRef]
- Golden, D.B. Anaphylaxis to insect stings. Immunol. Allergy Clin. N. Am. 2015, 35, 287–302. [Google Scholar] [CrossRef]
- Barnard, J.H. Studies of 400 Hymenoptera sting deaths in the United States. J. Allergy Clin. Immunol. 1973, 52, 259–264. [Google Scholar] [CrossRef]
- Bilo, B.M.; Bonifazi, F. Epidemiology of insect-venom anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 330–337. [Google Scholar] [CrossRef]
- Sainte-Laudy, J.; Sabbah, A.; Drouet, M.; Lauret, M.G.; Loiry, M. Diagnosis of venom allergy by flow cytometry. Correlation with clinical history, skin tests, specific IgE, histamine and leukotriene C4 release. Clin. Exp. Allergy 2000, 30, 1166–1171. [Google Scholar] [CrossRef]
- Eberlein, B.; Krischan, L.; Darsow, U.; Ollert, M.; Ring, J. Double positivity to bee and wasp venom: Improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J. Allergy Clin. Immunol. 2012, 130, 155–161. [Google Scholar] [CrossRef]
- Gattinger, P.; Lupinek, C.; Kalogiros, L.; Silar, M.; Zidarn, M.; Korosec, P.; Koessler, C.; Novak, N.; Valenta, R.; Mittermann, I. The culprit insect but not severity of allergic reactions to bee and wasp venom can be determined by molecular diagnosis. PLoS ONE 2018, 13, e0199250. [Google Scholar] [CrossRef]
- Kolaczek, A.; Skorupa, D.; Antczak-Marczak, M.; Kuna, P.; Kupczyk, M. Safety and efficacy of venom immunotherapy: A real life study. Postepy Derm. Alergol. 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Pospischil, I.M.; Kagerer, M.; Cozzio, A.; Angelova-Fischer, I.; Guenova, E.; Ballmer-Weber, B.; Hoetzenecker, W. Comparison of the Safety Profiles of 3 Different Hymenoptera Venom Immunotherapy Protocols: A Retrospective 2-Center Study of 143 Patients. Int. Arch Allergy Immunol. 2020, 181, 783–789. [Google Scholar] [CrossRef]
- Kohli-Wiesner, A.; Stahlberger, L.; Bieli, C.; Stricker, T.; Lauener, R. Induction of specific immunotherapy with hymenoptera venoms using ultrarush regimen in children: Safety and tolerance. J. Allergy 2012, 2012, 790910. [Google Scholar] [CrossRef][Green Version]
- Aalberse, R.C.; Akkerdaas, J.; van Ree, R. Cross-reactivity of IgE antibodies to allergens. Allergy 2001, 56, 478–490. [Google Scholar] [CrossRef]
- Spillner, E.; Blank, S.; Jakob, T. Hymenoptera allergens: From venom to “venome”. Front. Immunol. 2014, 5, 77. [Google Scholar] [CrossRef]

| Wasp-Scientific Name | Isolated Peptides | AA Sequence | References |
|---|---|---|---|
| Vespa mandarinia | Vespakinin-M | GRPXGFSPFRID | [42] |
| Vespa magnifica | Vespakinin-M | GRPPGFSPFRID | [41] |
| Vespa xanthoptera | Vespakinin-X | ARPPGFSPFRIV | [43] |
| Vespa analis | Vespakinin-A | GRPPGFSPFRVI | [44] |
| Vespa tropica | Vespakinin-T | GRPPGFSPFRVV | [44] |
| Polybia occidentalis | Thr6-bradykinin | RPPGFTPFR | [39] |
| Megascolia flavifrons, and Colpa interrupta | Thr6-bradykinin-Lys-Ala | RPPGFTPFRKA | [44,45] |
| Cyphononyx fulvognathus and Polybia paulista | RA-Thr6-Bradykinin | RARPPGFTPFR | [46] |
| Protein | Isolated Protein | Wasp-Scientific Name | References |
|---|---|---|---|
| Hyaluronidase | VesA2 | Vespa affinis | [76] |
| Vesp ma 2 | Vespa magnifica | [77] | |
| VesT2a | Vespa tropica | [78] | |
| VesT2b | Vespa tropica | [76] | |
| Vesp v 2A | Vespa velutina | [76] | |
| Vesp v 2B | Vespa velutina | [76] | |
| Phospholipase | Vesp a 1.1 | Vespa affinis | [79] |
| Vesp a 1.2 | Vespa affinis | [79] | |
| Phospholipase A1 | Polybia paulista | [80,81] | |
| vPLA2 | Vespa basalis | [82] | |
| Phospholipase A1(Ves v 1) | Polybia paulista | [80] | |
| Magnifin (PLA1) | Vespa magnifica | [83] | |
| Orientotoxin I | Vespa orientalis | [84] | |
| Orientotoxin II | Vespa orientalis | [84] | |
| VT 1 | Vespa velutina | [85] | |
| Vesp v 1 | Vespa velutina | [86] | |
| PLB I | Vespa xanthoptera | [87] | |
| PLB II | Vespa xanthoptera | [87] | |
| Antigen 5 | Vesp c 5.01 | Vespa crabro | [88] |
| Vesp c 5.02 | Vespa crabro | [88] | |
| Vesp m 5 | Vespa mandarinia | [88] | |
| Vesp ma 5 | Vespa magnifica | [89] | |
| Vesp v 5 | Vespa velutina | [77] | |
| Magnvesin | Vespa magnifica | [86] | |
| Dipeptidyl Peptidase IV | No name | Vespa affinis | [90] |
| No name | Vespa basalis | [91] | |
| No name | Vespa tropica | [92] | |
| Serine Protease | Bicolin | Vespa bicolor | [93] |
| Protease I | Vespa orientalis | [94] | |
| No name | Vespa velutina | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Kamau, P.M.; Lai, R. Bioactive Peptides and Proteins from Wasp Venoms. Biomolecules 2022, 12, 527. https://doi.org/10.3390/biom12040527
Luo L, Kamau PM, Lai R. Bioactive Peptides and Proteins from Wasp Venoms. Biomolecules. 2022; 12(4):527. https://doi.org/10.3390/biom12040527
Chicago/Turabian StyleLuo, Lei, Peter Muiruri Kamau, and Ren Lai. 2022. "Bioactive Peptides and Proteins from Wasp Venoms" Biomolecules 12, no. 4: 527. https://doi.org/10.3390/biom12040527
APA StyleLuo, L., Kamau, P. M., & Lai, R. (2022). Bioactive Peptides and Proteins from Wasp Venoms. Biomolecules, 12(4), 527. https://doi.org/10.3390/biom12040527







