Alteration of the Neuromuscular Junction and Modifications of Muscle Metabolism in Response to Neuron-Restricted Expression of the CHMP2Bintron5 Mutant in a Mouse Model of ALS-FTD Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
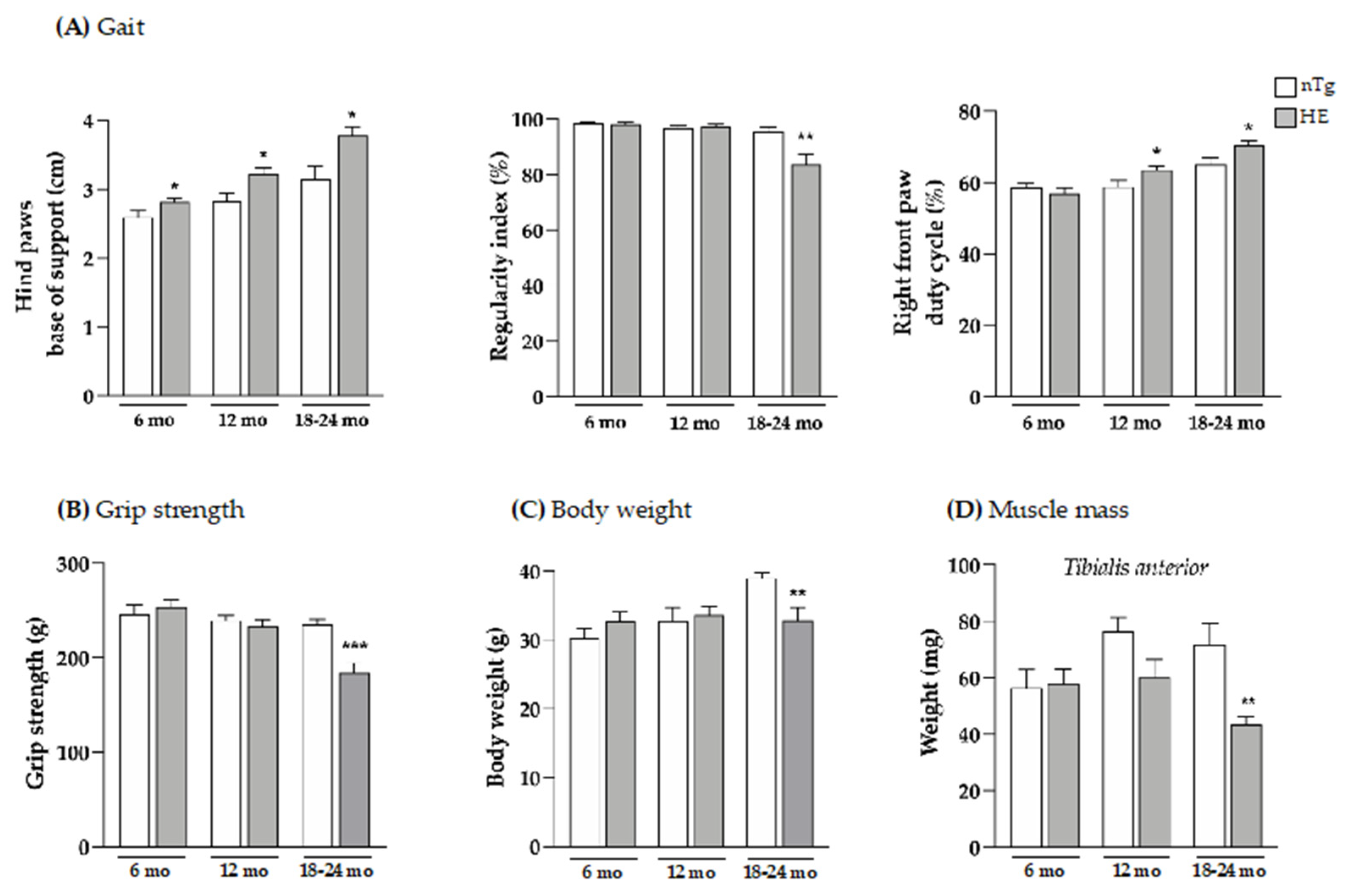
2.3. Gait and Muscle Strength Analysis
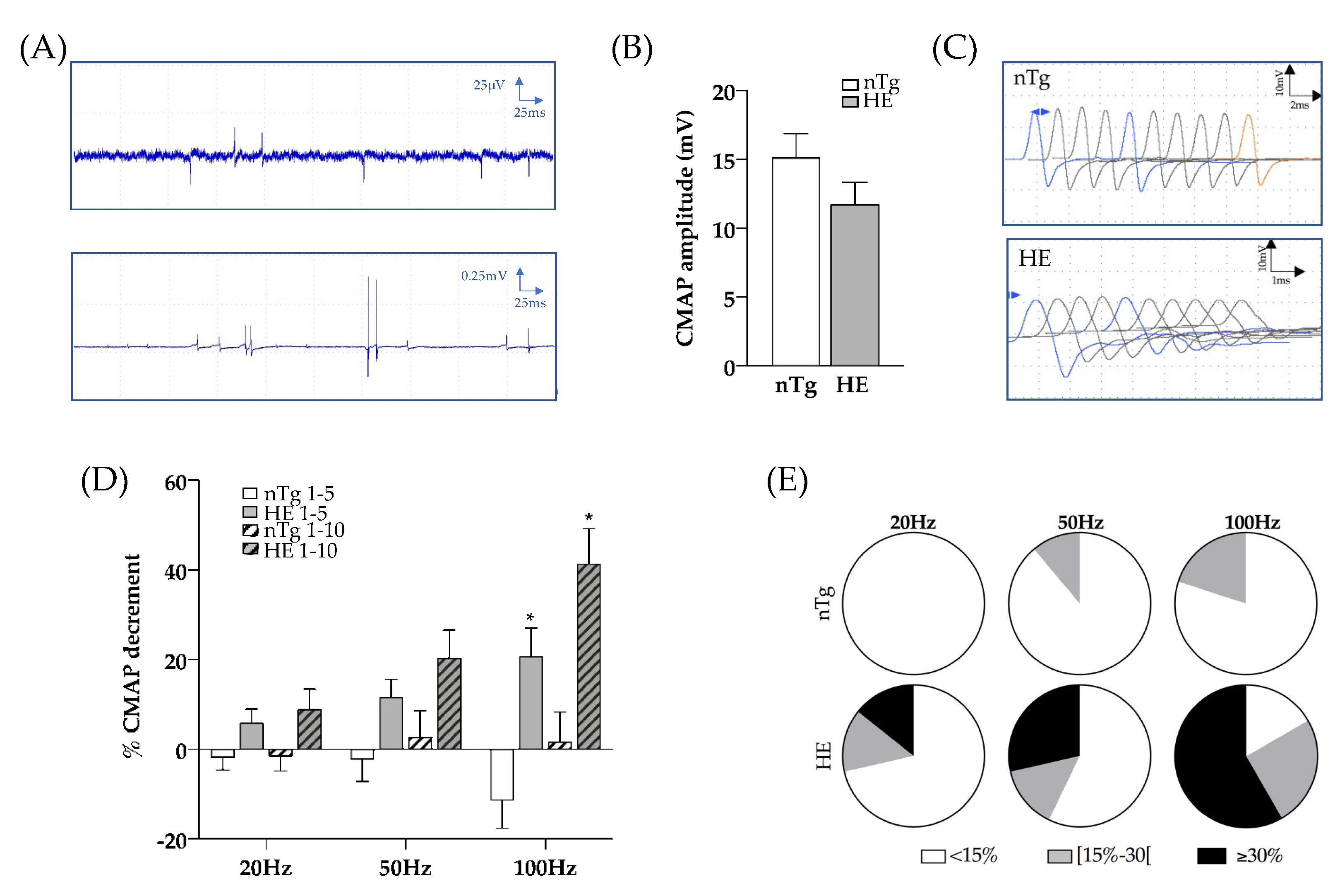
2.4. Electrophysiology
2.5. Tissues Preparation
2.6. Neuromuscular Junctions
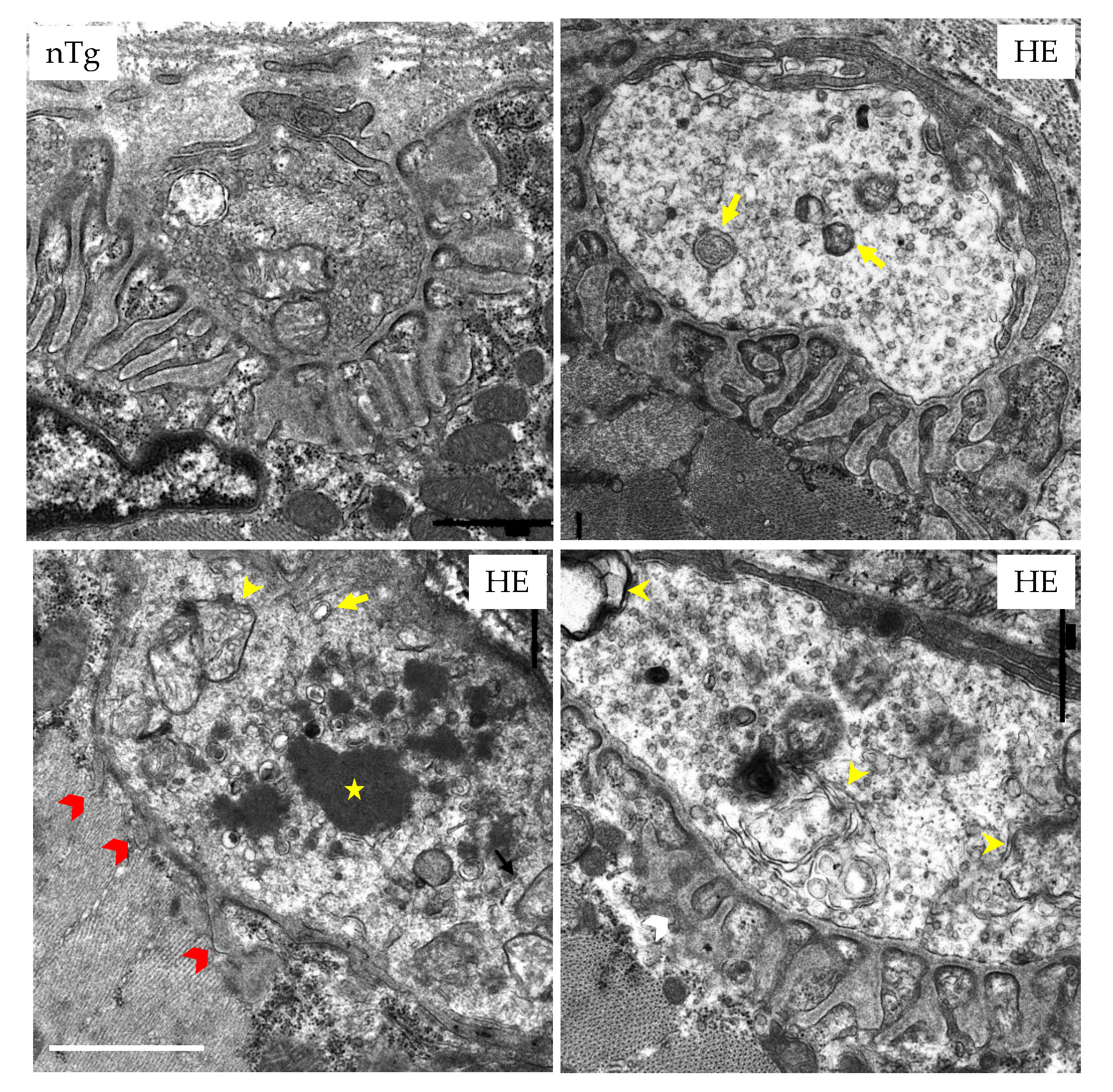
2.7. Electron Microscopy
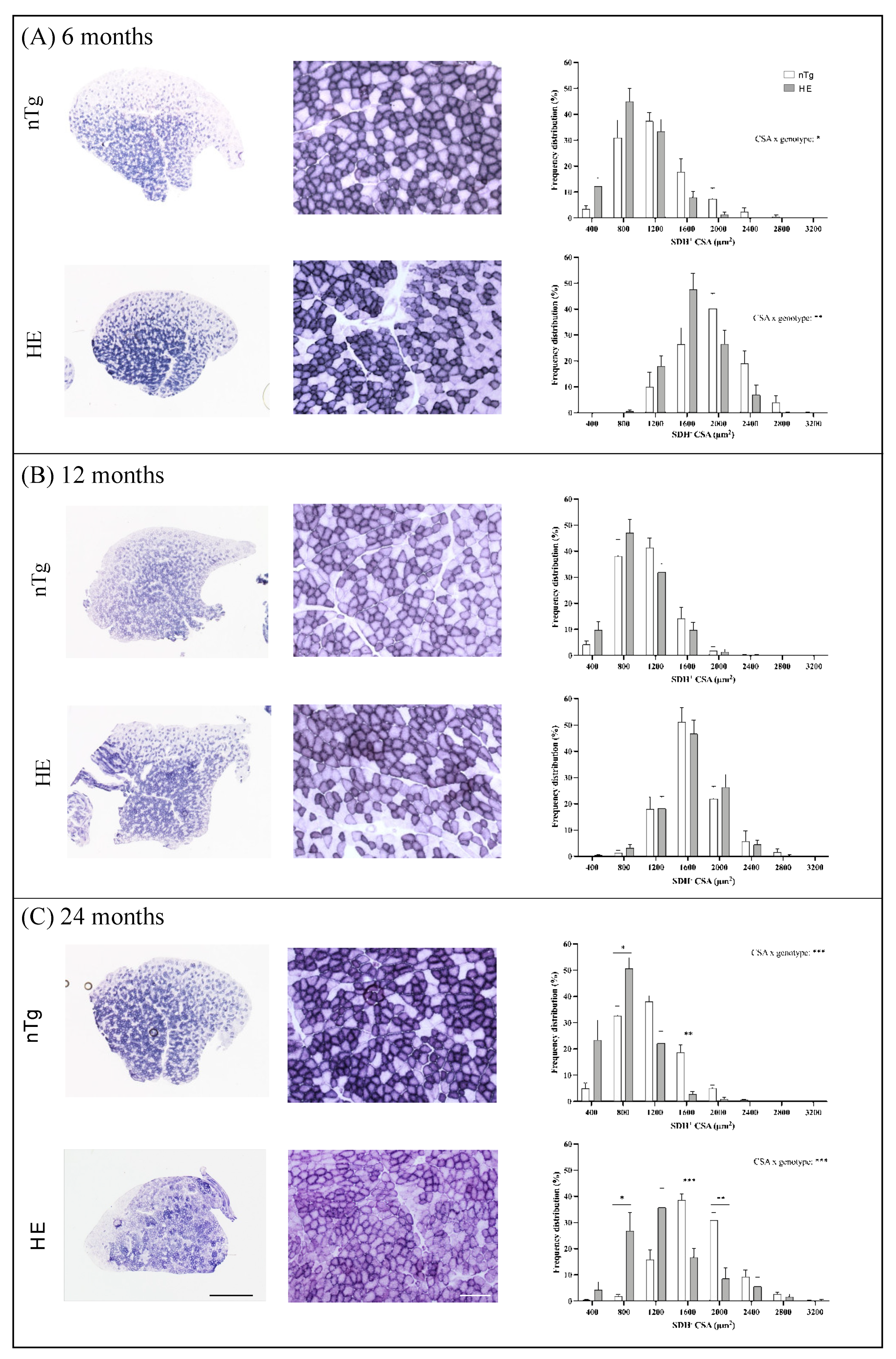
2.8. Histochemistry
2.9. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
2.10. Statistical Analysis
3. Results
3.1. Neuronal Expression of the CHMP2Bintron5 Mutant Is Sufficient to Trigger Alterations in Gait and Motor Coordination
3.2. Expression of CHMP2intron5 Results in Structural and Functional Alterations of the Neuromuscular Junction
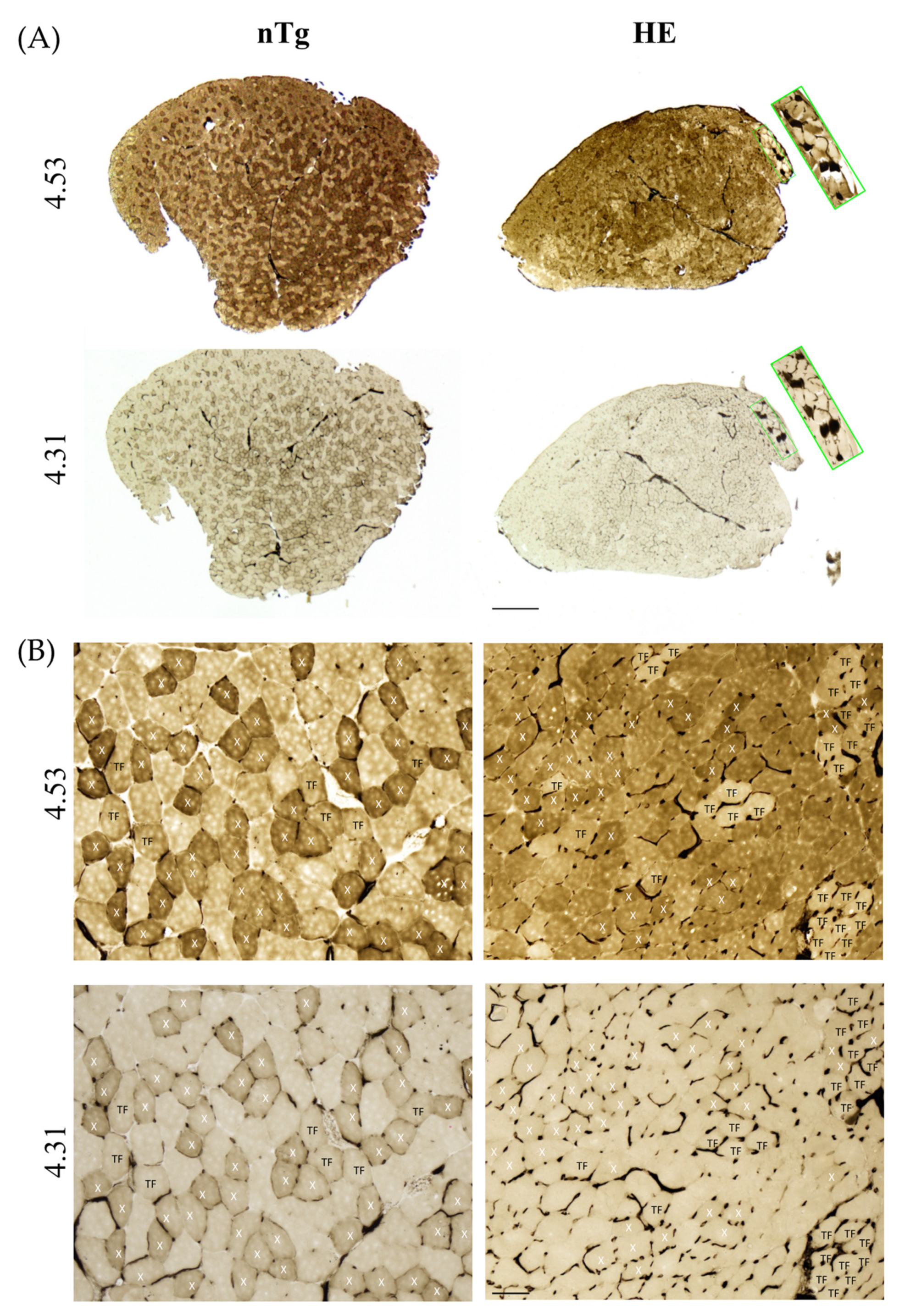
3.3. Neuronal Expression of CHMP2Bintron5 Leads to Impaired Muscle Metabolism and Myosin Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lømo, T. Nerve-muscle interactions. In Clinical Neurophysiology of Disorders of Muscle and the Neuromuscular Junction in Adults and Children. IFSCN Handbook of Clinical Neurophysiology; Stålberg, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 47–65. [Google Scholar]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 5, 17071. [Google Scholar]
- Dengler, A.; Konstanzer, A.; Küther, G.; Hesse, S.; Wolf, W.; Struppler, A. Amyotrophic lateral sclerosis: Macro-EMG and twitch forces of single motor units. Muscle Nerve 1990, 13, 545–550. [Google Scholar] [CrossRef]
- Maselli, R.A.; Wollman, R.L.; Leung, C.; Distad, B.; Palombi, S.; Richman, D.P.; Salazar-Grueso, E.F.; Roos, R.P. Neuromuscular transmission in amyotrophic lateral sclerosis. Muscle Nerve 1993, 16, 1193–1203. [Google Scholar]
- Fischer, L.R.; Culver, D.G.; Tennant, P.; Davis, A.A.; Wang, M.; Castellano-Sanchez, A.; Khan, J.; Polak, M.A.; Glass, J.D. Amyotrophic lateral sclerosis is a distal axonopathy: Evidence in mice and man. Exp. Neurol. 2004, 185, 232–240. [Google Scholar]
- Bruneteau, G.; Bauché, S.; de Aguilar, J.L.G.; Brochier, G.; Mandjee, N.; Tanguy, M.L.; Hussain, G.; Behin, A.; Khiami, F.; Sariali, E.; et al. Endplate denervation correlates with Nogo-A muscle expression in amyotrophic lateral sclerosis patients. Ann. Clin. Transl. Neurol. 2015, 2, 362–372. [Google Scholar]
- Frey, D.; Schneider, C.; Xu, L.; Borg, J.; Spooren, W.; Caroni, P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J. Neurosci. 2000, 20, 2534–2542. [Google Scholar]
- Cappello, V.; Vezzoli, E.; Righi, M.; Fossati, M.; Mariotti, R.; Crespi, A.; Patruno, M.; Bentivoglio, M.; Pietrini, G.; Francolini, M. Analysis of neuromuscular junctions and effects of anabolic steroid administration in the SOD1G93A mouse model of ALS. Mol. Cell. Neurosci. 2012, 51, 12–21. [Google Scholar]
- Telerman-Toppet, N.; Coërs, C. Motor innervation and fiber type pattern in amyotrophic lateral sclerosis and in Charcot-Marie-Tooth disease. Muscle Nerve 1978, 1, 133–139. [Google Scholar]
- Sharp, P.S.; Dick, J.R.; Greensmith, L. The effect of peripheral nerve injury on disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neuroscience 2005, 130, 897–910. [Google Scholar]
- Palamiuc, L.; Schlagowski, A.; Ngo, S.T.; Vernay, A.; Dirrig-Grosch, S.; Henriques, A.; Boutillier, A.L.; Zoll, J.; Echaniz-Laguna, A.; Loeffler, J.P.; et al. A metabolic switch toward lipid use in glycolytic muscle is an early pathologic event in a mouse model of amyotrophic lateral sclerosis. EMBO Mol. Med. 2015, 7, 526–546. [Google Scholar]
- Echaniz-Laguna, A.; Zoll, J.; Ponsot, E.; N'guessan, B.; Tranchant, C.; Loeffler, J.P.; Lampert, E. Muscular mitochondrial function in amyotrophic lateral sclerosis is progressively altered as the disease develops: A temporal study in man. Exp. Neurol. 2006, 198, 25–30. [Google Scholar]
- Steyn, F.J.; Li, R.; Kirk, S.E.; Tefera, T.W.; Xie, T.Y.; Tracey, T.J.; Kelk, D.; Wimberger, E.; Garton, F.C.; Roberts, L.; et al. Altered skeletal muscle glucose-fatty acid flux in amyotrophic lateral sclerosis. Brain Commun. 2020, 2, fcaa154. [Google Scholar]
- Scaricamazza, S.; Salvatori, I.; Amadio, S.; Nesci, V.; Torcinaro, A.; Giacovazzo, G.; Primiano, A.; Gloriani, M.; Candelise, N.; Pieroni, L.; et al. Repurposing of Trimetazidine for Amyotrophic Lateral Sclerosis: A study in SOD1G93A mice. Br. J. Pharmacol. 2021, 179, 1732–1752. [Google Scholar] [CrossRef]
- Jaarsma, D.; Teuling, E.; Haasdijk, E.D.; De Zeeuw, C.I.; Hoogenraad, C.C. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J. Neurosci. 2008, 28, 2075–2088. [Google Scholar]
- Wang, L.; Sharma, K.; Deng, H.X.; Siddique, T.; Grisotti, G.; Liu, E.; Roos, R.P. Restricted expression of mutant SOD1 in spinal motor neurons and interneurons induces motor neuron pathology. Neurobiol. Dis. 2008, 29, 400–408. [Google Scholar]
- Wong, M.; Martin, L.J. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum. Mol. Genet. 2010, 19, 2284–2302. [Google Scholar]
- Dobrowolny, G.; Lepore, E.; Martini, M.; Barberi, L.; Nunn, A.; Scicchitano, B.M.; Musarò, A. Metabolic Changes Associated With Muscle Expression of SOD1G93A. Front. Physiol. 2018, 9, 831. [Google Scholar]
- Martin, L.J.; Wong, M. Skeletal Muscle-Restricted Expression of Human SOD1 in Transgenic Mice Causes a Fatal ALS-Like Syndrome. Front. Neurol. 2020, 11, 592851. [Google Scholar]
- Chia, R.; Chiò, A.; Traynor, B.J. Novel genes associated with amyotrophic lateral sclerosis: Diagnostic and clinical implications. Lancet Neurol. 2018, 17, 94–102. [Google Scholar]
- Skibinski, G.; Parkinson, N.J.; Brown, J.M.; Chakrabarti, L.; Lloyd, S.L.; Hummerich, H.; Nielsen, J.E.; Hodges, J.R.; Spillantini, M.G.; Thusgaard, T.; et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 2005, 37, 806–808. [Google Scholar]
- Parkinson, N.; Ince, P.G.; Smith, M.O.; Highley, R.; Skibinski, G.; Andersen, P.M.; Morrison, K.E.; Pall, H.S.; Hardiman, O.; Collinge, J.; et al. MRC Proteomics in ALS Study; FReJA Consortium. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 2006, 67, 1074–1077. [Google Scholar]
- van der Zee, J.; Urwin, H.; Engelborghs, S.; Bruyland, M.; Vandenberghe, R.; Dermaut, B.; De Pooter, T.; Peeters, K.; Santens, P.; De Deyn, P.P.; et al. CHMP2B C-truncating mutations in frontotemporal lobar degeneration are associated with an aberrant endosomal phenotype in vitro. Hum. Mol. Genet. 2008, 17, 313–322. [Google Scholar]
- Cox, L.E.; Ferraiuolo, L.; Goodall, E.F.; Heath, P.R.; Higginbottom, A.; Mortiboys, H.; Hollinger, H.C.; Hartley, J.A.; Brockington, A.; Burness, C.E.; et al. Mutations in CHMP2B in lower motor neuron predominant amyotrophic lateral sclerosis (ALS). PLoS ONE 2010, 5, e9872. [Google Scholar]
- Ghanim, M.; Guillot-Noel, L.; Pasquier, F.; Jornea, L.; Deramecourt, V.; Dubois, B.; Le Ber, I.; Brice, A.; French Research Network on FTD and FTD/MND. CHMP2B mutations are rare in French families with frontotemporal lobar degeneration. J. Neurol. 2010, 257, 2032–2036. [Google Scholar]
- van Blitterswijk, M.; Vlam, L.; van Es, M.A.; van der Pol, W.L.; Hennekam, E.A.; Dooijes, D.; Schelhaas, H.J.; van der Kooi, A.J.; de Visser, M.; Veldink, J.H.; et al. Genetic overlap between apparently sporadic motor neuron diseases. PLoS ONE 2012, 7, e48983. [Google Scholar]
- Narain, P.; Pandey, A.; Gupta, S.; Gomes, J.; Bhatia, R.; Vivekanandan, P. Targeted next-generation sequencing reveals novel and rare variants in Indian patients with amyotrophic lateral sclerosis. Neurobiol. Aging 2018, 71, 265.e9–265.e14. [Google Scholar]
- Puppala, G.K.; Gorthi, S.P.; Chandran, V.; Gundabolu, G. Frontotemporal Dementia—Current Concepts. Neurol. India 2021, 69, 1144–1152. [Google Scholar]
- Sadoul, R.; Laporte, M.H.; Chassefeyre, R.; Chi, K.I.; Goldberg, Y.; Chatellard, C.; Hemming, F.J.; Fraboulet, S. The role of ESCRT during development and functioning of the nervous system. Semin. Cell Dev. Biol. 2018, 74, 40–49. [Google Scholar]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar]
- Ghazi-Noori, S.; Froud, K.E.; Mizielinska, S.; Powell, C.; Smidak, M.; de Marco, M.F.; O'Malley, C.; Farmer, M.; Parkinson, N.; Fisher, E.M.; et al. Progressive neuronal inclusion formation and axonal degeneration in CHMP2B mutant transgenic mice. Brain 2012, 135 Pt 3, 819–832. [Google Scholar]
- Vernay, A.; Therreau, L.; Blot, B.; Risson, V.; Dirrig-Grosch, S.; Waegaert, R.; Lequeu, T.; Sellal, F.; Schaeffer, L.; Sadoul, R.; et al. A transgenic mouse expressing CHMP2Bintron5 mutant in neurons develops histological and behavioural features of amyotrophic lateral sclerosis and frontotemporal dementia. Hum. Mol. Genet. 2016, 25, 3341–3360. [Google Scholar]
- Clayton, E.L.; Mancuso, R.; Nielsen, T.T.; Mizielinska, S.; Holmes, H.; Powell, N.; Norona, F.; Larsen, J.O.; Milioto, C.; Wilson, K.M.; et al. Early microgliosis precedes neuronal loss and behavioural impairment in mice with a frontotemporal dementia-causing CHMP2B mutation. Hum. Mol. Genet. 2017, 26, 873–887. [Google Scholar]
- Feng, G.; Mellor, R.H.; Bernstein, M.; Keller-Peck, C.; Nguyen, Q.T.; Wallace, M.; Nerbonne, J.M.; Lichtman, J.W.; Sanes, J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neurotechnique 2000, 28, 41–51. [Google Scholar]
- Hamers, F.P.T.; Koopmans, G.C.; Joosten, E.A.J. CatWalk assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma 2006, 24, 537–548. [Google Scholar]
- Górska, T.; Zmysłowski, W.; Majczyński, H. Overground locomotion in intact rats: Interlimb coordination, support patterns and support phases duration. Acta Neurobiol. Exp. (Wars) 1999, 59, 131–144. [Google Scholar]
- Dupuis, L.; Gonzalez de Aguilar, J.L.; Echaniz-Laguna, A.; Eschbach, J.; Rene, F.; Oudart, H.; Halter, B.; Huze, C.; Schaeffer, L.; Bouillaud, F.; et al. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS ONE 2009, 4, e5390. [Google Scholar]
- Echaniz-Laguna, A.; Rene, F.; Marcel, C.; Bangratz, M.; Fontaine, B.; Loeffler, J.P.; Nicole, S. Electrophysiological studies in a mouse model of Schwartz- Jampel syndrome demonstrate muscle fiber hyperactivity of peripheral nerve origin. Muscle Nerve 2009, 40, 55–61. [Google Scholar]
- Oosthuyse, B.; Moons, L.; Storkebaum, E.; Beck, H.; Nuyens, D.; Brusselmans, K.; Van Dorpe, J.; Hellings, P.; Gorselink, M.; Heymans, S.; et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 2001, 28, 131–138. [Google Scholar]
- Pestronk, G.J.; Kaiser, K.K.; Brooke, M.H. ATPase stain in muscle histochemistry. Muscle Nerve 1992, 15, 258. [Google Scholar]
- Hämäläinen, N.; Pette, D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J. Histochem. Cytochem. 1993, 41, 733–743. [Google Scholar]
- Pinheiro-Dardis, C.M.; Erbereli, B.T.; Gigo-Benato, D.; Castro, P.A.T.S.; Russo, T.L. Electrical stimulation delays reinnervation in denervated rat muscle. Muscle Nerve 2017, 56, E108–E118. [Google Scholar]
- Duclert, A.; Changeux, J.P. Acetylcholine receptor gene expression at the developing neuromuscular junction. Physiol. Rev. 1995, 75, 339–368. [Google Scholar]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar]
- Lee, J.A.; Beigneux, A.; Ahmad, S.T.; Young, S.G.; Gao, F.B. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr. Biol. 2007, 17, 1561–1567. [Google Scholar]
- Belly, A.; Bodon, G.; Blot, B.; Bouron, A.; Sadoul, R.; Goldberg, Y. CHMP2B mutants linked to frontotemporal dementia impair maturation of dendritic spines. J. Cell Sci. 2010, 123 Pt 17, 2943–2954. [Google Scholar]
- Gascon, E.; Lynch, K.; Ruan, H.; Almeida, S.; Verheyden, J.M.; Seeley, W.W.; Dickson, D.W.; Petrucelli, L.; Sun, D.; Jiao, J.; et al. Alterations in microRNA-124 and AMPA receptors contribute to social behavioral deficits in frontotemporal dementia. Nat. Med. 2014, 20, 1444–1451. [Google Scholar]
- Chassefeyre, R.; Martínez-Hernández, J.; Bertaso, F.; Bouquier, N.; Blot, B.; Laporte, M.; Fraboulet, S.; Couté, Y.; Devoy, A.; Isaacs, A.M.; et al. Regulation of postsynaptic function by the dementia-related ESCRT-III subunit CHMP2B. J. Neurosci. 2015, 35, 3155–3173. [Google Scholar]
- Ugbode, C.; West, R.J.H. Lessons learned from CHMP2B, implications for frontotemporal dementia and amyotrophic lateral sclerosis. Neurobiol. Dis. 2021, 147, 105144. [Google Scholar]
- Pun, S.; Santos, A.F.; Saxena, S.; Xu, L.; Caroni, P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat. Neurosci. 2006, 9, 408–419. [Google Scholar]
- Caroni, P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J. Neurosci. Methods 1997, 71, 3–9. [Google Scholar]
- Léger, B.; Vergani, L.; Sorarù, G.; Hespel, P.; Derave, W.; Gobelet, C.; D'Ascenzio, C.; Angelini, C.; Russell, A.P. Human skeletal muscle atrophy in amyotrophic lateral sclerosis reveals a reduction in Akt and an increase in atrogin-1. FASEB J. 2006, 20, 583–585. [Google Scholar] [PubMed]
- Kimura, N.; Kumamoto, T.; Oniki, T.; Nomura, M.; Nakamura, K.; Abe, Y.; Hazama, Y.; Ueyama, H. Role of ubiquitin-proteasome proteolysis in muscle fiber destruction in experimental chloroquine-induced myopathy. Muscle Nerve 2009, 39, 521–528. [Google Scholar] [PubMed]
- Lima, S.C.; Caierão, Q.M.; Peviani, S.M.; Russo, T.L.; Somazz, M.C.; Salvini, T.F.; Teodori, R.M.; Minamoto, V.B. Muscle and nerve responses after different intervals of electrical stimulation sessions on denervated rat muscle. Am. J. Phys. Med. Rehabil. 2009, 88, 126–135. [Google Scholar]
- Meinen, S.; Lin, S.; Rüegg, M.A.; Punga, A.R. Fatigue and muscle atrophy in a mouse model of myasthenia gravis is paralleled by loss of sarcolemmal nNOS. PLoS ONE 2012, 7, e44148. [Google Scholar]
- Adams, L.; Carlson, B.M.; Henderson, L.; Goldman, D. Adaptation of nicotinic acetylcholine receptor, myogenin, and MRF4 gene expression to long-term muscle denervation. J. Cell Biol. 1995, 131, 1341–1349. [Google Scholar] [PubMed] [Green Version]
- Ma, J.; Shen, J.; Lee, C.A.; Elsaidi, G.A.; Smith, T.L.; Walker, F.O.; Rushing, J.T.; Tan, K.H.; Koman, L.A.; Smith, B.P. Gene expression of nAChR, SNAP-25 and GAP-43 in skeletal muscles following botulinum toxin A injection: A study in rats. J. Orthop. Res. 2005, 23, 302–309. [Google Scholar] [PubMed]
- Halter, B.; de Aguilar, J.L.G.; Rene, F.; Petri, S.; Fricker, B.; Echaniz-Laguna, A.; Dupuis, L.; Larmet, Y.; Loeffler, J.P. Oxidative stress in skeletal muscle stimulates early expression of Rad in a mouse model of amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2010, 48, 915–923. [Google Scholar] [PubMed]
- Mori, A.; Yamashita, S.; Nakajima, M.; Hori, H.; Tawara, A.; Matsuo, Y.; Misumi, Y.; Ando, Y. CMAP decrement as a potential diagnostic marker for ALS. Acta Neurol. Scand. 2016, 134, 49–53. [Google Scholar]
- Alanazy, M.H.; Hegedus, J.; White, C.; Korngut, L. Decremental responses in patients with motor neuron disease. Brain Behav. 2017, 7, e00846. [Google Scholar]
- Sun, X.S.; Liu, W.X.; Chen, Z.H.; Ling, L.; Yang, F.; Wang, H.F.; Cui, F.; Huang, X.S. Repetitive Nerve Stimulation in Amyotrophic Lateral Sclerosis. Chin. Med. J. 2018, 131, 2146–2151. [Google Scholar]
- Souayah, N.; Coakley, K.M.; Chen, R.; Ende, N.; McArdle, J.J. Defective neuromuscular transmission in the SOD1 G93A transgenic mouse improves after administration of human umbilical cord blood cells. Stem Cell Rev. Rep. 2012, 8, 224–228. [Google Scholar]
- Sharma, A.; Lyashchenko, A.K.; Lu, L.; Nasrabady, S.E.; Elmaleh, M.; Mendelsohn, M.; Nemes, A.; Tapia, J.C.; Mentis, G.Z.; Shneider, N.A. ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function. Nat. Commun. 2016, 7, 10465. [Google Scholar]
- Vinsant, S.; Mansfield, C.; Jimenez-Moreno, R.; Del Gaizo Moore, V.; Yoshikawa, M.; Hampton, T.G.; Prevette, D.; Caress, J.; Oppenheim, R.W.; Milligan, C. Characterization of early pathogenesis in the SOD1(G93A) mouse model of ALS: Part I, background and methods. Brain Behav. 2013, 3, 335–350. [Google Scholar]
- So, E.; Mitchell, J.C.; Memmi, C.; Chennell, G.; Vizcay-Barrena, G.; Allison, L.; Shaw, C.E.; Vance, C. Mitochondrial abnormalities and disruption of the neuromuscular junction precede the clinical phenotype and motor neuron loss in hFUSWT transgenic mice. Hum. Mol. Genet. 2018, 27, 463–474. [Google Scholar]
- Clayton, E.L.; Bonnycastle, K.; Isaacs, A.M.; Cousin, M.A.; Schorge, S. A novel synaptopathy-defective synaptic vesicle protein trafficking in the mutant CHMP2B mouse model of frontotemporal dementia. J. Neurochem. 2021, 160, 412–425. [Google Scholar] [CrossRef]
- Zhu, L.J.; Zhang, C.; Chen, C. Research progress on vesicle cycle and neurological disorders. J. Pharm. Pharm. Sci. 2021, 24, 400–441. [Google Scholar]
- Tremblay, E.; Martineau, É.; Robitaille, R. Opposite Synaptic Alterations at the Neuromuscular Junction in an ALS Mouse Model: When Motor Units Matter. J. Neurosci. 2017, 37, 8901–8918. [Google Scholar]
- Chand, K.K.; Lee, K.M.; Lee, J.D.; Qiu, H.; Willis, E.F.; Lavidis, N.A.; Hilliard, M.A.; Noakes, P.G. Defects in synaptic transmission at the neuromuscular junction precede motor deficits in a TDP-43Q331K transgenic mouse model of amyotrophic lateral sclerosis. FASEB J. 2018, 32, 2676–2689. [Google Scholar]
- Lee, J.A.; Liu, L.; Gao, F.B. Autophagy defects contribute to neurodegeneration induced by dysfunctional ESCRT-III. Autophagy 2009, 5, 1070–1072. [Google Scholar]
- Nielsen, T.T.; Mizielinska, S.; Hasholt, L.; Isaacs, A.M.; Nielsen, J.E.; FReJA Consortium. Reversal of pathology in CHMP2B-mediated frontotemporal dementia patient cells using RNA interference. J. Gene Med. 2012, 14, 521–529. [Google Scholar]
- Zhang, Y.; Schmid, B.; Nikolaisen, N.K.; Rasmussen, M.A.; Aldana, B.I.; Agger, M.; Calloe, K.; Stummann, T.C.; Larsen, H.M.; Nielsen, T.T.; et al. Patient iPSC-Derived Neurons for Disease Modeling of Frontotemporal Dementia with Mutation in CHMP2B. Stem Cell Rep. 2017, 8, 648–658. [Google Scholar]
- West, R.J.H.; Ugbode, C.; Fort-Aznar, L.; Sweeney, S.T. Neuroprotective activity of ursodeoxycholic acid in CHMP2B(Intron5) models of frontotemporal dementia. Neurobiol. Dis. 2020, 144, 105047. [Google Scholar]
- Urwin, H.; Authier, A.; Nielsen, J.E.; Metcalf, D.; Powell, C.; Froud, K.; Malcolm, D.S.; Holm, I.; Johannsen, P.; Brown, J.; et al. Disruption of endocytic trafficking in frontotemporal dementia with CHMP2B mutations. Hum. Mol. Genet. 2010, 19, 2228–2238. [Google Scholar]
- Amin, A.; Perera, N.D.; Beart, P.M.; Turner, B.J.; Shabanpoor, F. Amyotrophic Lateral Sclerosis and Autophagy: Dysfunction and Therapeutic Targeting. Cells 2020, 9, 2413. [Google Scholar]
- Pette, D.; Vrbová, G. What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve 1999, 6, 666–677. [Google Scholar]
- Hegedus, J.; Putman, C.T.; Gordon, T. Time course of preferential motor unit loss in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2007, 28, 154–164. [Google Scholar]
- Deforges, S.; Branchu, J.; Biondi, O.; Grondard, C.; Pariset, C.; Lécolle, S.; Lopes, P.; Vidal, P.P.; Chanoine, C.; Charbonnier, F. Motoneuron survival is promoted by specific exercise in a mouse model of amyotrophic lateral sclerosis. J. Physiol. 2009, 587 Pt 14, 3561–3572. [Google Scholar]
- Feige, J.N.; Auwerx, J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007, 17, 292–301. [Google Scholar]
- Gordon, T.; Hegedus, J.; Tam, S.L. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol. Res. 2004, 26, 174–185. [Google Scholar]
- Baloh, R.H.; Rakowicz, W.; Gardner, R.; Pestronk, A. Frequent atrophic groups with mixed-type myofibers is distinctive to motor neuron syndromes. Muscle Nerve 2007, 36, 107–110. [Google Scholar]
- Chivet, M.; Marchioretti, C.; Pirazzini, M.; Piol, D.; Scaramuzzino, C.; Polanco, M.J.; Romanello, V.; Zuccaro, E.; Parodi, S.; D'Antonio, M.; et al. Polyglutamine-Expanded Androgen Receptor Alteration of Skeletal Muscle Homeostasis and Myonuclear Aggregation Are Affected by Sex, Age and Muscle Metabolism. Cells 2020, 9, 325. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waegaert, R.; Dirrig-Grosch, S.; Liu, H.; Boutry, M.; Luan, P.; Loeffler, J.-P.; René, F. Alteration of the Neuromuscular Junction and Modifications of Muscle Metabolism in Response to Neuron-Restricted Expression of the CHMP2Bintron5 Mutant in a Mouse Model of ALS-FTD Syndrome. Biomolecules 2022, 12, 497. https://doi.org/10.3390/biom12040497
Waegaert R, Dirrig-Grosch S, Liu H, Boutry M, Luan P, Loeffler J-P, René F. Alteration of the Neuromuscular Junction and Modifications of Muscle Metabolism in Response to Neuron-Restricted Expression of the CHMP2Bintron5 Mutant in a Mouse Model of ALS-FTD Syndrome. Biomolecules. 2022; 12(4):497. https://doi.org/10.3390/biom12040497
Chicago/Turabian StyleWaegaert, Robin, Sylvie Dirrig-Grosch, Haoyi Liu, Marion Boutry, Ping Luan, Jean-Philippe Loeffler, and Frédérique René. 2022. "Alteration of the Neuromuscular Junction and Modifications of Muscle Metabolism in Response to Neuron-Restricted Expression of the CHMP2Bintron5 Mutant in a Mouse Model of ALS-FTD Syndrome" Biomolecules 12, no. 4: 497. https://doi.org/10.3390/biom12040497
APA StyleWaegaert, R., Dirrig-Grosch, S., Liu, H., Boutry, M., Luan, P., Loeffler, J.-P., & René, F. (2022). Alteration of the Neuromuscular Junction and Modifications of Muscle Metabolism in Response to Neuron-Restricted Expression of the CHMP2Bintron5 Mutant in a Mouse Model of ALS-FTD Syndrome. Biomolecules, 12(4), 497. https://doi.org/10.3390/biom12040497




