Sex-Dependent Differential Expression of Lipidic Mediators Associated with Inflammation Resolution in Patients with Pulmonary Tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Serum Collection and Processing
2.3. Immunoassays
2.4. Statistics
3. Results
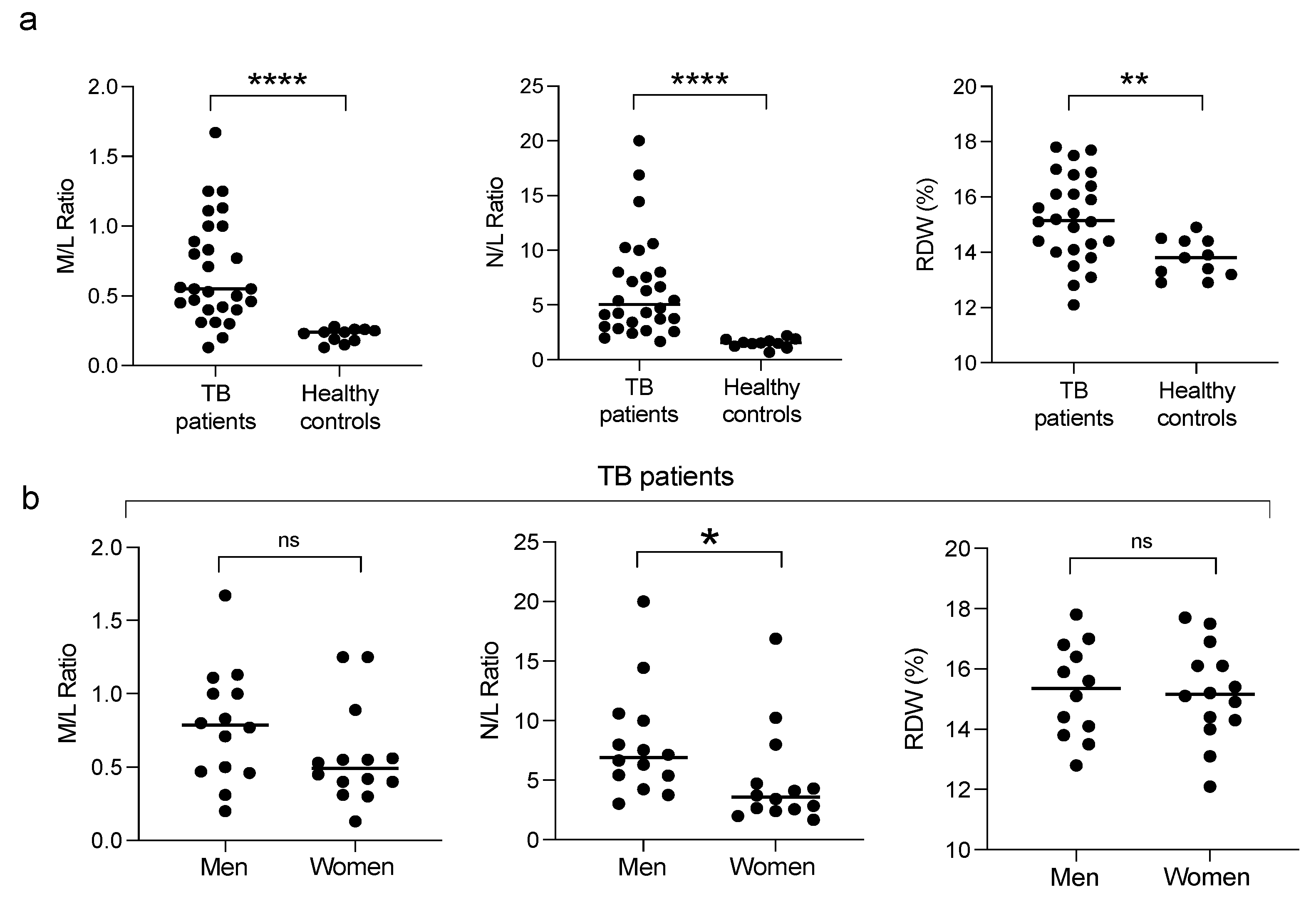
3.1. Clinical Characteristics of the Participants
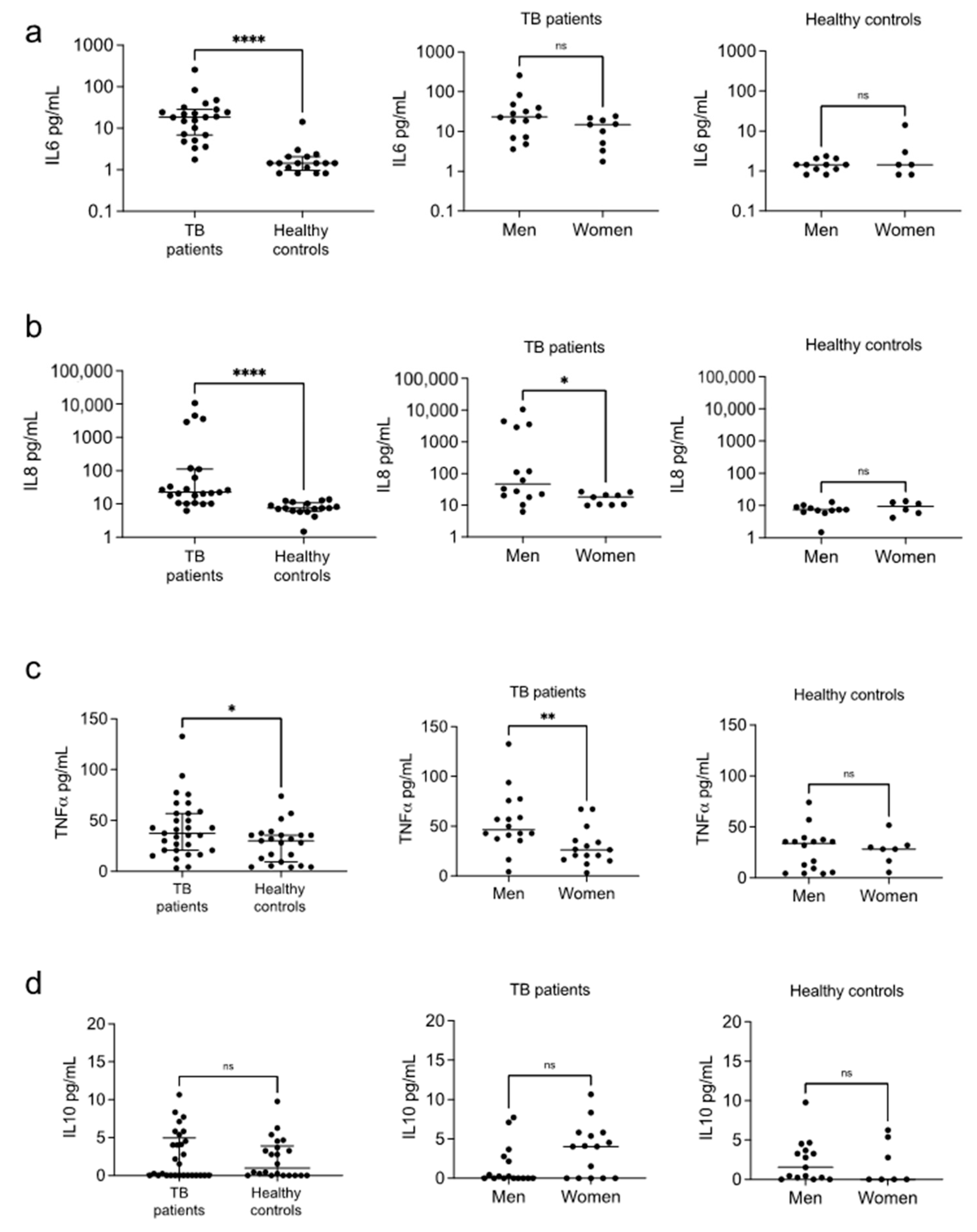
3.2. Cytokines
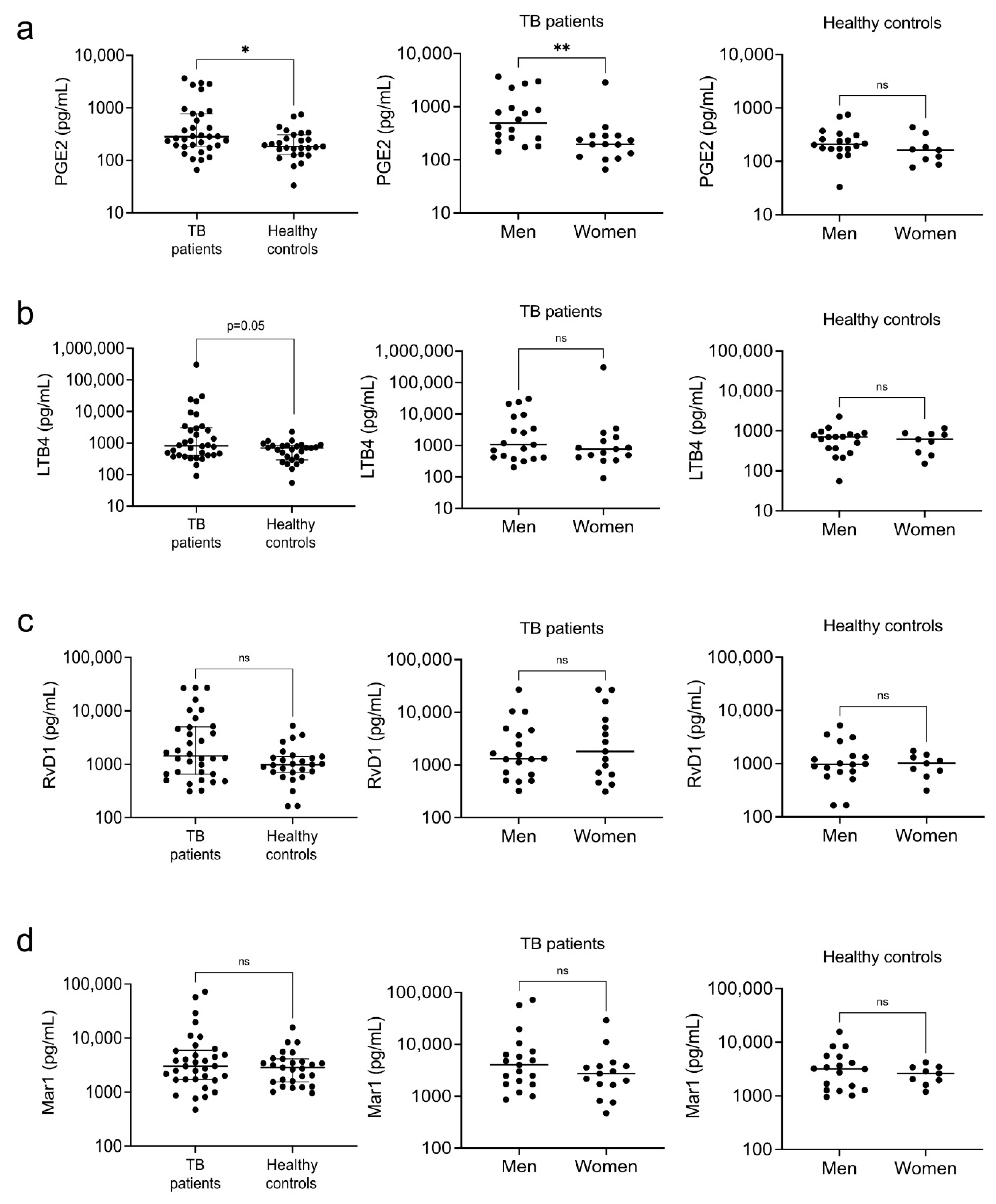
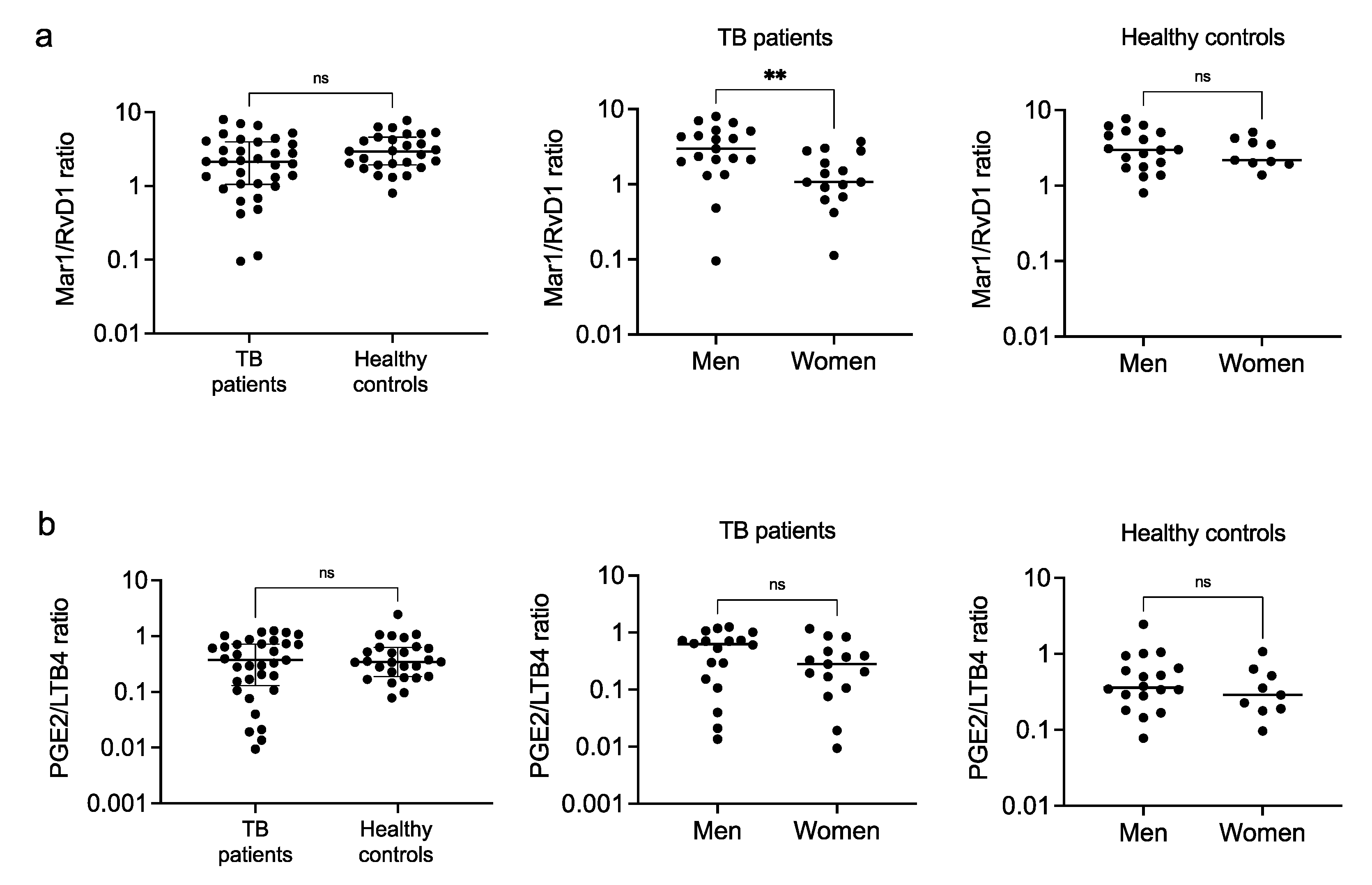
3.3. Eicosanoids
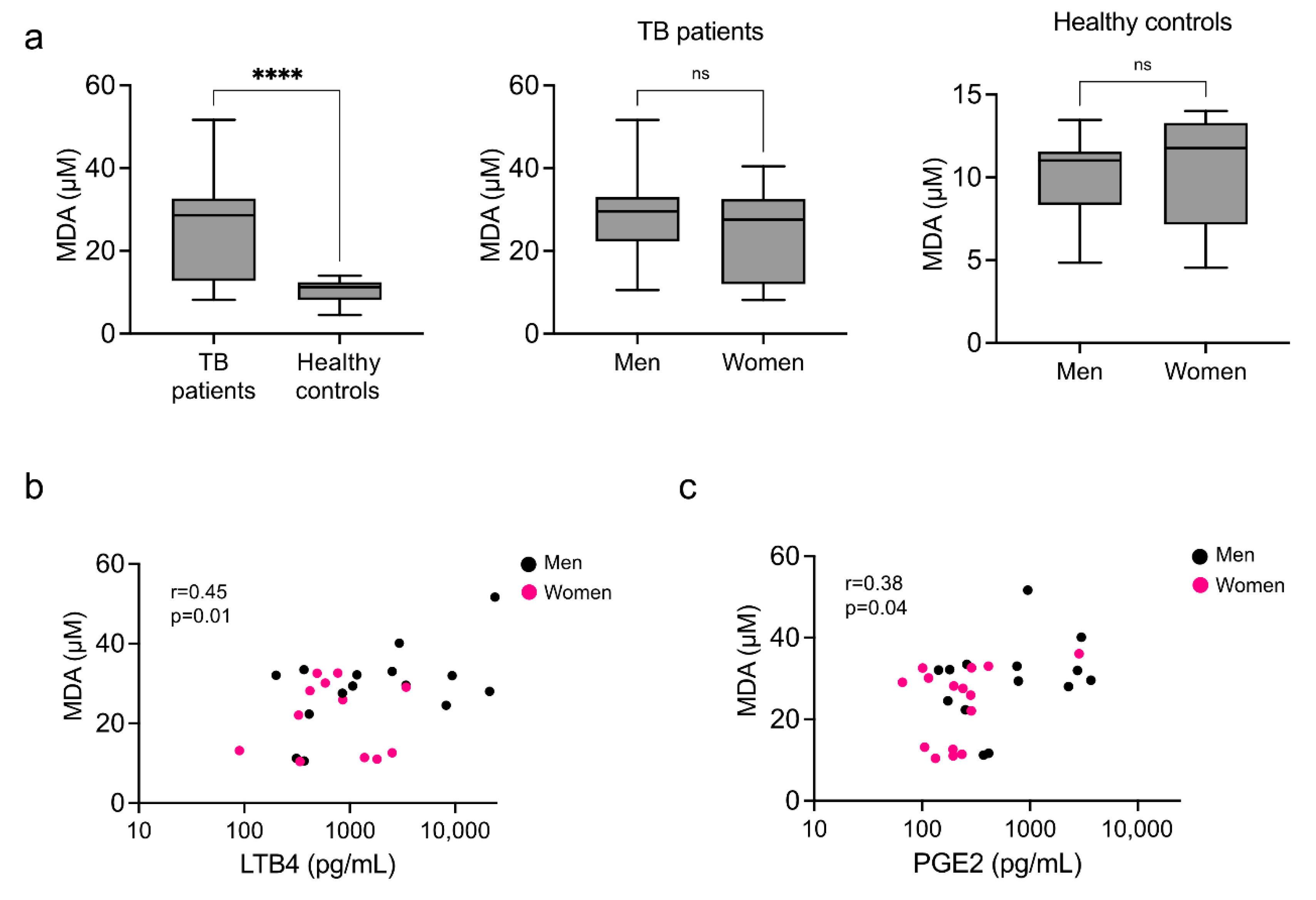
3.4. Systemic Marker of Lipid Peroxidation
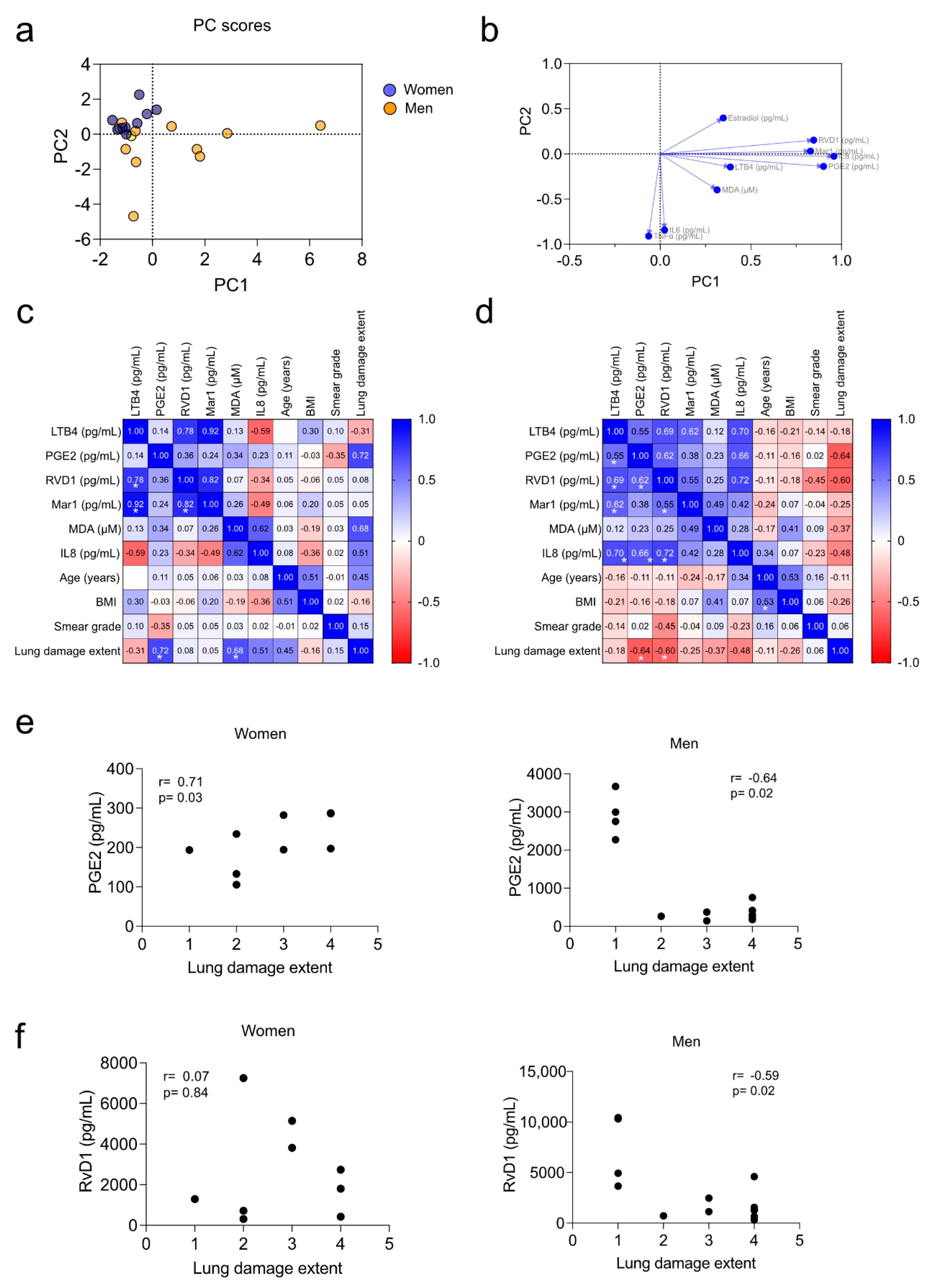
3.5. Correlation of Pro-Inflammatory Markers with the Severity of the Disease
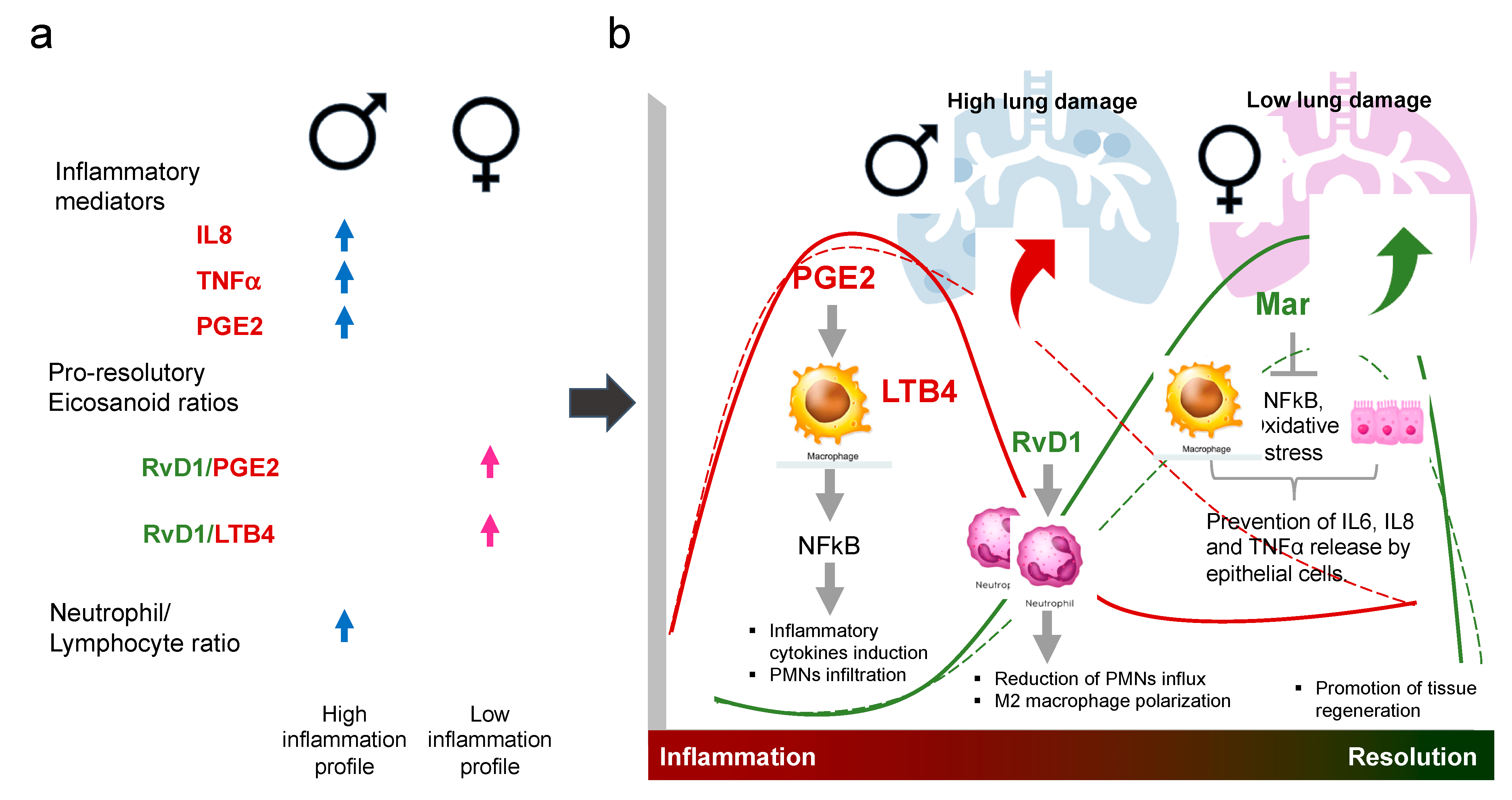
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaufmann, S.H.E.; Dorhoi, A. Inflammation in tuberculosis: Interactions, imbalances and interventions. Curr. Opin. Immunol. 2013, 25, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Neyrolles, O.; Quintana-Murci, L. Sexual inequality in tuberculosis. PLoS Med. 2009, 6, e1000199. [Google Scholar] [CrossRef]
- Amiri, M.R.J.; Siami, R.; Khaledi, A. Tuberculosis Status and Coinfection of Pulmonary Fungal Infections in Patients Referred to Reference Laboratory of Health Centers Ghaemshahr City during 2007–2017. Ethiop. J. Health Sci. 2018, 28, 683–690. [Google Scholar] [CrossRef]
- Chidambaram, V.; Tun, N.L.; Majella, M.G.; Ruelas Castillo, J.; Ayeh, S.K.; Kumar, A.; Neupane, P.; Sivakumar, R.K.; Win, E.P.; Abbey, E.J.; et al. Male sex is associated with worse microbiological and clinical outcomes following tuberculosis treatment: A retrospective cohort study, a systematic review of the literature, and meta-analysis. Clin. Infect. Dis. 2021, 73, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2021. Available online: https://www.who.int/publications/i/item/9789240037021.
- Rhines, A.S. The role of sex differences in the prevalence and transmission of tuberculosis. Tuberculosis 2013, 93, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Bothamley, G.H. Male Sex Bias in Immune Biomarkers for Tuberculosis. Front. Immunol. 2021, 12, 674. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Chen, M.; Divangahi, M.; Gan, H.; Shin, D.S.J.; Hong, S.; Lee, D.M.; Serhan, C.N.; Behar, S.M.; Remold, H.G. Lipid mediators in innate immunity against tuberculosis: Opposing roles of PGE 2 and LXA 4 in the induction of macrophage death. J. Exp. Med. 2008, 205, 2791–2801. [Google Scholar] [CrossRef]
- Kaul, V.; Bhattacharya, D.; Singh, Y.; Van Kaer, L.; Peters-Golden, M.; Bishai, W.R.; Das, G. An important role of prostanoid receptor EP2 in host resistance to mycobacterium tuberculosis infection in mice. J. Infect. Dis. 2012, 206, 1816–1825. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Sher, A. Cytokine and lipid mediator networks in tuberculosis. Immunol. Rev. 2015, 264, 264–275. [Google Scholar] [CrossRef]
- Nore, K.G.; Jørgensen, M.J.; Dyrhol-Riise, A.M.; Jenum, S.; Tonby, K. Elevated Levels of Anti-Inflammatory Eicosanoids and Monocyte Heterogeneity in Mycobacterium tuberculosis Infection and Disease. Front. Immunol. 2020, 11, 2938. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.L.; Coffman, T.M.; Koller, B.H. Mixed messages: Modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Investig. 2001, 108, 15–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fullerton, J.N.; O’Brien, A.J.; Gilroy, D.W. Lipid mediators in immune dysfunction after severe inflammation. Trends Immunol. 2014, 35, 12–21. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Pace, S.; Pergola, C.; Dehm, F.; Rossi, A.; Gerstmeier, J.; Troisi, F.; Pein, H.; Schaible, A.M.; Weinigel, C.; Rummler, S.; et al. Androgen-mediated sex bias impairs efficiency of leukotriene biosynthesis inhibitors in males. J. Clin. Investig. 2017, 127, 3167–3176. [Google Scholar] [CrossRef]
- Vinhaes, C.L.; Oliveira-de-Souza, D.; Silveira-Mattos, P.S.; Nogueira, B.; Shi, R.; Wei, W.; Yuan, X.; Zhang, G.; Cai, Y.; Barry, C.E.; et al. Changes in inflammatory protein and lipid mediator profiles persist after antitubercular treatment of pulmonary and extrapulmonary tuberculosis: A prospective cohort study. Cytokine 2019, 123, 154759. [Google Scholar] [CrossRef] [PubMed]
- Flamand, N.; Mancuso, P.; Serezani, C.H.C.; Brock, T.G. Leukotrienes: Mediators that have been typecast as villains. Cell. Mol. Life Sci. 2007, 64, 2657–2670. [Google Scholar] [CrossRef]
- O’Meara, S.J.; Rodgers, K.; Godson, C. Lipoxins: Update and impact of endogenous pro-resolution lipid mediators. Rev. Physiol. Biochem. Pharmacol. 2008, 160, 47–70. [Google Scholar] [CrossRef]
- Ruiz, A.; Sarabia, C.; Torres, M.; Juárez, E. Resolvin D1 (RvD1) and maresin 1 (Mar1) contribute to human macrophage control of M. tuberculosis infection while resolving inflammation. Int. Immunopharmacol. 2019, 74, 105694. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Andrade, B.B.; Oland, S.D.; Amaral, E.P.; Barber, D.L.; Gonzales, J.; Derrick, S.C.; Shi, R.; Kumar, N.P.; Wei, W.; et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2014, 511, 99–103. [Google Scholar] [CrossRef]
- Pavan Kumar, N.; Moideen, K.; Nancy, A.; Viswanathan, V.; Shruthi, B.S.; Shanmugam, S.; Hissar, S.; Kornfeld, H.; Babu, S. Plasma Eicosanoid Levels in Tuberculosis and Tuberculosis-Diabetes Co-morbidity Are Associated With Lung Pathology and Bacterial Burden. Front. Cell. Infect. Microbiol. 2019, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Báez-saldaña, R.; López-arteaga, Y.; Bizarrón-muro, A.; Ferreira-guerrero, E.; Ferreyra-reyes, L.; Delgado-sánchez, G.; Cruz-hervert, L.P.; Mongua-, N. A Novel Scoring System to Measure Radiographic Abnormalities and Related Spirometric Values in Cured Pulmonary Tuberculosis. PLoS ONE 2013, 8, e78926. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Beltrán, S.; Carreto-Binaghi, L.E.; Carranza, C.; Torres, M.; Gonzalez, Y.; Muñoz-Torrico, M.; Juárez, E. Oxidative stress and inflammatory mediators in exhaled breath condensate of patients with pulmonary tuberculosis. A pilot study with a biomarker perspective. Antioxidants 2021, 10, 1572. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.A.; Synder, L.M. Wallach’s Interpretation of Diagnostic Tests. Pathways to Arriving at a Clinical Diagnosis, 10th ed.; Wolters Kluwer: Pasadena, CA, USA, 2015; ISBN 9781451191769. [Google Scholar]
- Cirillo, M.; Anastasio, P.; De Santo, N.G. Relationship of gender, age, and body mass index to errors in predicted kidney function. Nephrol. Dial. Transplant. 2005, 20, 1791–1798. [Google Scholar] [CrossRef]
- Abakay, O.; Abakay, A.; Sen, H.S.; Tanrikulu, A.C. The Relationship Between Inflammatory Marker Levels and Pulmonary Tuberculosis Severity. Inflammation 2015, 38, 691–696. [Google Scholar] [CrossRef]
- Buttle, T.S.; Hummerstone, C.Y.; Billahalli, T.; Ward, R.J.B.; Barnes, K.E.; Marshall, N.J.; Spong, V.C.; Bothamley, G.H. The monocyte-to-lymphocyte ratio: Sex-specific differences in the tuberculosis disease spectrum, diagnostic indices and defining normal ranges. PLoS ONE 2021, 16, e0247745. [Google Scholar] [CrossRef]
- Bafica, A.; Scanga, C.A.; Serhan, C.; Machado, F.; White, S.; Sher, A.; Aliberti, J. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J. Clin. Investig. 2005, 115, 1601–1606. [Google Scholar] [CrossRef]
- Horton, K.C.; Hoey, A.L.; Béraud, G.; Corbett, E.L.; White, R.G. Systematic review and meta-analysis of sex differences in social contact patterns and implications for tuberculosis transmission and control. Emerg. Infect. Dis. 2020, 26, 910–919. [Google Scholar] [CrossRef]
- Chikovore, J.; Pai, M.; Horton, K.C.; Daftary, A.; Kumwenda, M.K.; Hart, G.; Corbett, E.L. Missing men with tuberculosis: The need to address structural influences and implement targeted and multidimensional interventions. BMJ Glob. Health 2020, 5, e002255. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Pizzol, D.; Cebola, B.; Stubbs, B.; Monno, L.; Saracino, A.; Luchini, C.; Solmi, M.; Segafredo, G.; Putoto, G.; et al. Social determinants of therapy failure and multi drug resistance among people with tuberculosis: A review. Tuberculosis 2017, 103, 44–51. [Google Scholar] [CrossRef]
- Shaweno, D.; Horton, K.C.; Hayes, R.J.; Dodd, P.J. Assortative social mixing and sex disparities in tuberculosis burden. Sci. Rep. 2021, 11, 7530. [Google Scholar] [CrossRef] [PubMed]
- Atomsa, D.; Abebe, G.; Sewunet, T. Immunological markers and hematological parameters among newly diagnosed tuberculosis patients at Jimma University Specialized Hospital. Ethiop. J. Health Sci. 2014, 24, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Wang, D.; Wang, H.; Liao, Y.; Song, Y.; Li, Y.; Zhang, Y.; Fan, G.; Zhong, X.; Ju, Y.; et al. Clinical features in pulmonary tuberculosis patients combined with diabetes mellitus in China: An observational study. Clin. Respir. J. 2021, 15, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Ryndak, M.B.; Laal, S. Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells. Front. Cell. Infect. Microbiol. 2019, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.J.; Dong, J.J.; Dong, J.Z.; Chen, Y.; Lin, Z.; Song, M.; Wang, Y.Q.; Chen, Y.P.; Shi, K.Q.; Zhou, M.T. A nomogram for predicting prognostic value of inflammatory response biomarkers in decompensated cirrhotic patients without acute-on-chronic liver failure. Aliment. Pharmacol. Ther. 2017, 45, 1413–1426. [Google Scholar] [CrossRef]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Fushiya, N.; Koike, K.; Nishino, H.; Tajiri, H. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br. J. Cancer 2012, 107, 988–993. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Feng, L.; Guo, R. Comparison of the prognostic values of inflammation markers in patients with acute pancreatitis: A retrospective cohort study. BMJ Open 2017, 7, e013206. [Google Scholar] [CrossRef]
- Miyahara, R.; Piyaworawong, S.; Naranbhai, V.; Prachamat, P.; Kriengwatanapong, P.; Tsuchiya, N.; Wongyai, J.; Bupachat, S.; Yamada, N.; Summanapan, S.; et al. Predicting the risk of pulmonary tuberculosis based on the neutrophil-to-lymphocyte ratio at TB screening in HIV-infected individuals. BMC Infect. Dis. 2019, 19, 667. [Google Scholar] [CrossRef]
- Han, Y.; Kim, S.J.; Lee, S.H.; Sim, Y.S.; Ryu, Y.J.; Chang, J.H.; Shim, S.S.; Kim, Y.; Lee, J.H. High blood neutrophil-lymphocyte ratio associated with poor outcomes in miliary tuberculosis. J. Thorac. Dis. 2018, 10, 339–346. [Google Scholar] [CrossRef]
- Nakao, M.; Muramatsu, H.; Arakawa, S.; Sakai, Y.; Suzuki, Y.; Fujita, K.; Sato, H. Immunonutritional status and pulmonary cavitation in patients with tuberculosis: A revisit with an assessment of neutrophil/lymphocyte ratio. Respir. Investig. 2019, 57, 60–66. [Google Scholar] [CrossRef]
- Sukson, P.; Liwsrisakun, C.; Inchai, J.; Trongtrakul, K.; Tajarernmuang, P. Peripheral Blood Monocyte to Lymphocyte Ratio for Prediction of Tuberculous Pleuritis: Monocyte to Lymphocyte Ratio in Tuberculous Pleuritis. Int. J. Infect. Dis. 2021, 112, 212–216. [Google Scholar] [CrossRef]
- Sibley, L.; Gooch, K.; Wareham, A.; Gray, S.; Chancellor, A.; Dowall, S.; Bate, S.; Marriott, A.; Dennis, M.; White, A.D.; et al. Differences in monocyte: Lymphocyte ratio and Tuberculosis disease progression in genetically distinct populations of macaques. Sci. Rep. 2019, 9, 3340. [Google Scholar] [CrossRef] [PubMed]
- Sampath, P.; Moideen, K.; Ranganathan, U.D.; Bethunaickan, R. Monocyte Subsets: Phenotypes and Function in Tuberculosis Infection. Front. Immunol. 2018, 9, 1726. [Google Scholar] [CrossRef] [PubMed]
- Deveci, F.; Handan Akbulut, H.; Turgut, T.; Hamdi Muz, M. Changes in serum cytokine levels in active tuberculosis with treatment. Mediat. Inflamm. 2005, 2005, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Fortes, A.; Pereira, K.; Antas, P.R.Z.; Franken, C.L.M.C.; Dalcolmo, M.; Ribeiro-Carvalho, M.M.; Cunha, K.S.; Geluk, A.; Kritski, A.; Kolk, A.; et al. Detection of in vitro interferon-γ and serum tumour necrosis factor-α in multidrug-resistant tuberculosis patients. Clin. Exp. Immunol. 2005, 141, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.R.; García, I.E.; De La Luz García Hernández, M.; Leon, D.A.; Marquez, R.; Pando, R.H. The role of prostaglandin E2 in the immunopathogenesis of experimental pulmonary tuberculosis. Immunology 2002, 106, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Harizi, H.; Juzan, M.; Moreau, J.-F.; Gualde, N. Prostaglandins Inhibit 5-Lipoxygenase-Activating Protein Expression and Leukotriene B 4 Production from Dendritic Cells Via an IL-10-Dependent Mechanism. J. Immunol. 2003, 170, 139–146. [Google Scholar] [CrossRef]
- Chan-Yeung, M.; Noertjojo, K.; Chan, S.L.; Tam, C.M. Sex differences in tuberculosis in Hong Kong. Int. J. Tuberc. Lung Dis. 2002, 6, 11–18. [Google Scholar]
- Dutta, N.K.; Schneider, B.E. Are There Sex-Specific Differences in Response to Adjunctive Host-Directed Therapies for Tuberculosis? Front. Immunol. 2020, 11, 1465. [Google Scholar] [CrossRef]

| Healthy Controls | Patients | |||||
|---|---|---|---|---|---|---|
| Characteristic | Women, n = 9 | Men, n = 18 | p Value | Women, n = 15 | Men, n = 19 | p Value |
| Age, years, median (range) | 33 (22–62) | 31 (23–58) | 0.7328 | 43 (19–62) | 47 (18–64) | 0.8304 |
| BMI, median (range) | 23.8 (20–27.5) | 24.1 (20.7–30) | 0.8503 | 21.8 (12.7–39.6) | 21.7 (15.8–33.3) | 0.7320 |
| AINES consumption the previous week, n (%) | 1 (11.1) | 0 (0) | 0.3333 | 6 (40) | 7 (36.8) | >0.9999 |
| Smoking history, n (%) | 3 (33.3) | 2 (40) | 0.2950 | 0 (0) | 2 (10.5) | 0.4920 |
| Diabetes, n (%) | 0 (0) | 0 (0) | >0.9999 | 9 (60) | 11 (57.8) | >0.9999 |
| Hypertension, n (%) | 0 (0) | 0 (0) | >0.9999 | 1 (6.7) | 1 (5.2) | >0.9999 |
| Leukocytes (103/mm3), median (range) | 8 (3.6–10.6) | 6.3 (4.5–7.7) | 0.0681 | 8.4 (4.1–15) | 9.6 (5.9–15.2) | 0.1259 |
| Neutrophils (103/mm3), median (range) | 4.3 (1.2–7.6) | 3.5 (2.1–4.7) | 0.0816 | 5.7 (2.2–13.5) | 7.3 (4.4–13) | 0.0569 |
| Lymphocytes (103/mm3), median (range) | 2.4 (1.6–3) | 2.2 (1.3–5.3) | 0.8838 | 1.7 (0.8–2.4) | 1.2 (0.5–2.4) | 0.0586 |
| Monocytes (103/mm3), median (range) | 0.5 (0.4–0.5) | 0.5 (0.3–0.7) | 0.8848 | 0.8 (0.5–1) | 0.95 (0.1–1.5) | 0.3173 |
| Eosinophils (103/mm3), median (range) | 0.2 (0–0.4) | 0.1 (0.1–0.5) | 0.9000 | 0.1 (0–0.2) | 0.05 (0–10.1) | 0.4900 |
| Basophils (103/mm3), median (range) | 0 (0–0.1) | 0 (0–0.1) | >0.9999 | 0 (0–0.1) | 0.05 (0–0.2) | 0.4484 |
| Erythrocytes (106/mm3), median (range) | 4.6 (4.2–4.8) | 5.4 (5–6) | <0.0001 | 4.2 (3.8–5.1) | 4.4 (2.9–5.5) | 0.8291 |
| Hemoglobin (gr/dL), median (range) | 13.7 (12.6–15.8) | 16.1 (15.1–17.5) | <0.0001 | 12.4 (9.4–16.7) | 12.6 (7.9–17.7) | 0.9795 |
| Hematocrit (%), median (range) | 41.1 (37.7–45.6) | 48.8 (42.9–52.1) | <0.0001 | 37.7 (27.9–48.8) | 37.7 (24–52.8) | 0.8574 |
| Platelets (103/mm3), median (range) | 234 (157–379) | 219 (151–320) | 0.7512 | 344 (133–525) | 327 (152–662) | 0.9387 |
| Glucose (mg/dL), median (range) | 93.3 (86.7–109.9) | 96 (81–117) | 0.8427 | 128 (84–274) | 138 (41–525) | >0.9999 |
| Urea (mg/dL), median (range) | 21.3 (12.84–36.4) | 25.6 (17.12–42) | 0.4990 | 23 (19–56.6) | 27.8 (10.7–57) | 0.5468 |
| BUN (mg/dL), median (range) | 9.9 (6–17) | 12 (8–19.63) | 0.5329 | 10.8 (9–26.4) | 12.1 (5–23.9) | 0.8377 |
| Uric acid (mg/dL), median (range) | 3.3 (3.3–3.3) | 6.2 (4.5–10.4) | <0.0001 | 4.5 (2.6–7.9) | 5.1 (1.4–13.69) | 0.6848 |
| Creatinine (mg/dL), median (range) | 0.7 (0.5–0.8) | 0.9 (0.7–1.2) | 0.0006 | 0.5 (0.2–1.1) | 0.8 (0.4–1.4) | 0.0266 |
| Characteristic | Women, n = 15 | Men, n = 19 | p Value |
|---|---|---|---|
| Bilateral, yes n (%) | 7 (77.7) | 8 (57.1) | 0.3998 |
| Cavities, yes n (%) | 3 (33.3) | 8 (57.1) | 0.4003 |
| Score of radiographic abnormalities, median (range) | 8 (2–16) | 10.5 (2–18) | 0.6305 |
| Smear grade, n (%) | |||
| 0 | 4 (26.7) | 3 (15.7) | 0.6722 |
| 1+ | 4 (26.7) | 8 (42.1) | 0.4764 |
| 2+ | 2 (13.3) | 2 (10.5) | >0.9999 |
| 3+ | 5 (33.3) | 3 (15.7) | 0.4172 |
| 4+ | 0 (0) | 3 (15.7) | 0.2380 |
| MDR, n (%) | 6 (40) | 7 (36.8) | >0.9999 |
| Outcome, n (%) | |||
| Unknown | 7 (46.6) | 8 (42.1) | >0.9999 |
| Treatment success, n (% of known) | 7 (87.5) | 9 (81.8) | >0.9999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carranza, C.; Carreto-Binaghi, L.E.; Guzmán-Beltrán, S.; Muñoz-Torrico, M.; Torres, M.; González, Y.; Juárez, E. Sex-Dependent Differential Expression of Lipidic Mediators Associated with Inflammation Resolution in Patients with Pulmonary Tuberculosis. Biomolecules 2022, 12, 490. https://doi.org/10.3390/biom12040490
Carranza C, Carreto-Binaghi LE, Guzmán-Beltrán S, Muñoz-Torrico M, Torres M, González Y, Juárez E. Sex-Dependent Differential Expression of Lipidic Mediators Associated with Inflammation Resolution in Patients with Pulmonary Tuberculosis. Biomolecules. 2022; 12(4):490. https://doi.org/10.3390/biom12040490
Chicago/Turabian StyleCarranza, Claudia, Laura Elena Carreto-Binaghi, Silvia Guzmán-Beltrán, Marcela Muñoz-Torrico, Martha Torres, Yolanda González, and Esmeralda Juárez. 2022. "Sex-Dependent Differential Expression of Lipidic Mediators Associated with Inflammation Resolution in Patients with Pulmonary Tuberculosis" Biomolecules 12, no. 4: 490. https://doi.org/10.3390/biom12040490
APA StyleCarranza, C., Carreto-Binaghi, L. E., Guzmán-Beltrán, S., Muñoz-Torrico, M., Torres, M., González, Y., & Juárez, E. (2022). Sex-Dependent Differential Expression of Lipidic Mediators Associated with Inflammation Resolution in Patients with Pulmonary Tuberculosis. Biomolecules, 12(4), 490. https://doi.org/10.3390/biom12040490






